Abstract
Recent evidence suggests that apical and basolateral endocytic pathways in epithelia converge in an apically located, pericentriolar endosomal compartment termed the apical recycling endosome. In this compartment, apically and basolaterally internalized membrane constituents are thought to be sorted for recycling back to their site of origin or for transcytosis to the opposite plasma membrane domain. We report here that in the epithelial cell line Madin–Darby Canine Kidney (MDCK), antibodies to Rab11a label an apical pericentriolar endosomal compartment that is dependent on intact microtubules for its integrity. Furthermore, this compartment is accessible to a membrane-bound marker (dimeric immunoglobulin A [IgA]) internalized from either the apical or basolateral pole, functionally defining it as the apical recycling endosome. We have also examined the role of a closely related epithelial-specific Rab, Rab25, in the regulation of membrane recycling and transcytosis in MDCK cells. When cDNA encoding Rab25 was transfected into MDCK cells, the protein colocalized with Rab11a in subapical vesicles. Rab25 transfection also altered the distribution of Rab11a, causing the coalescence of immunoreactivity into multiple denser vesicular structures not associated with the centrosome. Nevertheless, nocodazole still dispersed these vesicles, and dimeric IgA internalized from either the apical or basolateral membrane was detected in endosomes labeled with antibodies to both Rab11a and Rab25. Overexpression of Rab25 decreased the rate of IgA transcytosis and of apical, but not basolateral, recycling of internalized ligand. Conversely, expression of the dominant-negative Rab25T26N did not alter either apical recycling or transcytosis. These results indicate that both Rab11a and Rab25 associate with the apical recycling system of epithelial cells and suggest that Rab25 may selectively regulate the apical recycling and/or transcytotic pathways.
INTRODUCTION
Rab proteins constitute a family of small, monomeric GTPases that are essential components of virtually all membrane trafficking pathways (Nuoffer and Balch, 1994). The complexity of vesicular transport in eukaryotic cells is emphasized by the fact that more than 40 members of the Rab family have been identified to date in mammals, and many of these have been implicated in the regulation of specific stages of either exocytic or endocytic transport. A surprising number of Rabs have been associated with compartments of the endocytic pathway, suggesting a higher degree of complexity than had originally been appreciated.
Accumulating evidence indicates that early endosomes can be functionally subdivided into two distinct subcompartments. Sorting endosomes, which are located in the peripheral cytoplasm, are the site at which fluid-phase cargo and ligands dissociated from their receptors are segregated for transport to lysosomes. Sorting endosomes are characterized by the presence of Rab5, which is required for the import of endocytic material from the cell surface (Bucci et al., 1992). Many membrane proteins, such as transferrin and low-density lipoprotein receptors, undergo a recycling process that involves their transport to pericentriolar “recycling endosomes,” a tubulovesicular compartment where they are repackaged for transport back to the cell surface (Hopkins et al., 1994; Ghosh and Maxfield, 1995; Green et al., 1997). In nonpolarized cells, Rab11a has been localized to the membranes of recycling endosomes (Ullrich et al., 1996; Green et al., 1997), and transport from sorting endosomes to the recycling compartment has been shown to require functional Rab11a (Ullrich et al., 1996).
An additional level of complexity exists in epithelial cells, which maintain distinct apical and basolateral plasma membrane domains (for reviews, see Mostov et al., 1992b; Wollner and Nelson, 1992; Drubin and Nelson, 1996). Endocytic pathways originate from both poles of the cell, and transcytotic pathways allow the transport of endocytosed material from one pole of the cell to the other. Although some mechanistic differences have been noted between apical and basolateral endocytosis, much of the postendocytic machinery appears to be shared. In Madin–Darby Canine Kidney (MDCK) cells, studies with fluid-phase markers have demonstrated the existence of distinct populations of apical and basolateral early sorting endosomes whose contents become mixed upon reaching the late endosomal compartment (Bomsel et al., 1989; Parton et al., 1989). Rab5a has been localized to both apical and basolateral endosomes in MDCK cells, and its overexpression leads to increased endocytic rates at both poles of the cell (Bucci et al., 1994), again indicating that some aspects of the endosomal machinery are shared.
Several lines of evidence suggest that recycling endosomes are also shared between apical and basolateral endocytic pathways and that this compartment may represent a major hub of postendocytic membrane traffic in polarized cells. First, both the polymeric immunoglobulin (Ig) receptor (pIgR), which mediates basolateral-to-apical transcytosis of IgA and IgM, and the transferrin receptor (TfR), which efficiently recycles to the basolateral plasma membrane, appear to enter a population of apical, pericentriolar endosomes with similar kinetics (Apodaca et al., 1994). This compartment is also accessible to membrane-bound, but not fluid-phase markers internalized from the apical pole (Apodaca et al., 1994; Barroso and Sztul, 1994; Odorizzi et al., 1996), further suggesting that it represents a shared recycling endosome. The existence of a similar compartment has been demonstrated in the enterocyte-like cell line Caco-2 (Hughson and Hopkins, 1990; Knight et al., 1995). We have shown previously that antibodies to Rab11a, which label the recycling endosomal compartment in nonpolarized cells (Ullrich et al., 1996), also stain a population of subapical vesicles in various epithelial tissues, including the recycling H/K-ATPase-containing tubulovesicles of gastric parietal cells (Goldenring et al., 1996), suggesting that Rab11a also associates with recycling endosomes in polarized cells. Most of the known Rabs, including Rab5 and Rab11a, are ubiquitously expressed, consistent with their function in vesicular transport pathways common to all cells; however, a subset of Rabs, including Rab17 (Lutke et al., 1993) and Rab25 (Goldenring et al., 1993), is expressed exclusively in epithelial cells, suggesting that they regulate aspects of vesicular transport that are unique to epithelia. In this regard, Rab17 has recently been reported to modulate transcytosis in both a mouse mammary epithelial cell line (Zacchi et al., 1998) and MDCK cells (Hunziker and Peters, 1998).
We report here that in polarized MDCK cells, Rab11a antibodies label a subapical, pericentriolar compartment whose integrity is dependent on intact microtubules and that can be loaded with IgA internalized from either the apical or basolateral cell surface, confirming the identity of this compartment as a shared recycling endosome. We further demonstrate that Rab25, which is closely related to Rab11a, also distributes to the apical recycling compartment in MDCK cells. Finally, in MDCK cells expressing the pIgR, we show that overexpression of wild-type Rab25 markedly slows the rate of IgA transcytosis as well as apical recycling, whereas basolateral recycling remains unaffected. These findings are consistent with a role for Rab25 in the regulation of transport through the apical recycling endosomes in epithelial cells.
MATERIALS AND METHODS
Materials
Monoclonal antibodies against Rab11a (8H10) and Rab25 (12C3) were prepared as described previously (Goldenring et al., 1996; Calhoun and Goldenring, 1997). Rabbit polyclonal antibodies against Rab11a were purchased from Zymed (South San Francisco, CA). Monoclonal antibodies against γ-adaptin and γ-tubulin were obtained from Sigma (St. Louis, MO). Polyclonal antibodies against the mannose-6-phosphate receptor were a gift from Dr. Lisa Matovcik (Yale University School of Medicine). Polyclonal antibodies against Rab7 and Rab9 were a gift of Marino Zerial (EMBL, Heidelberg, Germany). Secondary antibodies conjugated to FITC, Cy2, Cy3, and Cy5 were purchased from Jackson Immunochemicals (West Grove, PA). Prolong antifade mounting medium was obtained from Molecular Probes (Eugene, OR). All culture media were purchased from Life Technologies (Gaithersburg, MD). G418 and hygromycin were purchased from Calbiochem (La Jolla, CA). Human dimeric IgA was purified from the serum of a patient with excessive IgA production, as described previously (Hansen and Casanova, 1994).
Constructs
Rabbit Rab11a and rabbit Rab25 were cloned into the eukaryotic expression vectors pCB6 (neomycin resistant) and pCB7 (hygromycin resistant). For production of the Rab11aQ70L mutant, the Rab11a cDNA sequence in both pCB7 and pET19b was mutated using a two complementary oligonucleotide strategy with Pfu polymerase (Stratagene, La Jolla, CA) by the method of Costa et al. (1996). Similarly, for production of Rab25T26N, the two complementary oligonucleotide method was used to alter the rabbit Rab25 sequence in pCB7. For prokaryotic expression of Rab25, the rabbit Rab25 sequence was recloned into pGEX-2T to construct a glutathione-S-transferase (GST) fusion protein. All sequences were confirmed for orientation, the presence of the appropriate mutations, and the absence of other mutations using automated DNA sequencing.
MDCK Transfection and Propagation
MDCK cells (strain II) were maintained in DMEM supplemented with 10% fetal bovine serum and antibiotics. All stable transfections of MDCK cells were performed with a standard calcium phosphate precipitation/glycerol shock method using 20 μg of supercoiled plasmid. Clones in pCB6 were selected by culture in the presence of G418, whereas clones in pCB7 were selected with hygromycin. Expression was confirmed by both Western blot and immunofluorescence in cells cultured in chamber slides. Cell lines exhibiting moderate levels of expression that maintained normal morphology were selected for study. For examination of polarized cells, 150,000 cells were plated at confluence on 23-mm Transwell filter inserts (Costar, Cambridge, MA), and media was changed each day. For immunofluorescence examination, cells were fixed in 4% paraformaldehyde for 30 min at 4°C.
GTP-binding Assay
His-tagged Rab11a and His-tagged Rab11aQ70L were expressed in BL21(DE3)pLysS bacteria as described previously (Goldenring et al., 1996). GST-Rab25 was expressed in JM109 bacteria by incubation for 2 h at 30°C in the presence of 0.5 mM isopropyl thio-galactose. GST-Rab25 was then purified from bacterial lysates by glutathione–Sepharose (Pharmacia, Piscataway, NJ) chromatography. Recombinant Rab11a, Rab11aQ70L, and Rab25 proteins (1.0 μg) were incubated in a reaction mixture containing 50 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 10 mM EDTA, 1 mM DTT, 1 mg/ml BSA, and 2 μM [α-32P]GTP at 30°C (Albright et al., 1993; Reynet and Kahn, 1993). For the determination of nonspecific binding, 1.0 mM excess cold GTP was included in the incubation. Over a time course, 50 μl of the reaction mixture were removed, and the reaction was terminated with 450 μl of ice cold buffer containing 50 mM Tris-HCl (pH 7.5), 20 mM MgCl2, 1 mM DTT, and 1 mg/ml BSA. Terminated reaction mixtures were filtered through nitrocellulose (BA85, Schleicher & Schuell, Keene, NH) and washed with 15 ml of the same solution, and the residual radioactivity on the filters was determined by Cerenkov counting. All recombinant protein constructs showed similar specific binding of [α-32P]GTP with saturable kinetics maximized at 15 min (our unpublished results).
GTPase Assay
Recombinant Rab11a, Rab11aQ70L, or Rab25 (20 pM each) was incubated under binding conditions as above with [α-32P]GTP for 15 min at 30°C. GTPase activity was initiated by adding 10 mM MgCl2 (final concentration) with or without 4 μg of cytosol (rabbit gastric mucosal 100,000 × g supernatant, dialyzed against reaction buffer) (Zahraoui et al., 1989). [α-32P]GTP–Rab protein (2 pM) was used per time point. Samples of 10 μl were withdrawn from the incubation mixture over a time course, mixed with 10 μl of 0.5 M EDTA, and immediately frozen on dry ice. Aliquots (1 μl) were spotted on polyethyleneimine–cellulose thin-layer chromatography plates and developed in 1 M LiCl. The dried plates were exposed to Phosphorimaging screens (Molecular Dynamics, Sunnyvale, CA), and conversion to GDP was quantified as a fraction of total [32P]guanine nucleotide.
Immunocytochemistry
Cells fixed on permeable filters were stained in the culture-well inserts. Cells were permeabilized and blocked with 5% goat serum, 0.3% Triton X-100 in PBS for 30 min and then incubated with antibodies as appropriate for 2 h at room temperature. Primary antibodies were used at the following dilutions: murine monoclonal Rab25 ascites (12C3), 1:50; rabbit polyclonal anti-Rab11a, 1:200; murine monoclonal anti-γ-tubulin, 1:100; rat monoclonal anti-ZO-1, 1:300; rabbit polyclonal anti-Rab7 and anti-Rab9, 1:300; rabbit polyclonal antimannose-6-phosphate receptor, 1:1000; murine monoclonal anti-Rab11a (8H10), 1:100. After washing in PBS, cells were then incubated with fluorochrome-conjugated secondary antibodies for 30 min. In cells loaded with dimeric IgA, primary anti-IgA antibodies directly conjugated with FITC were used at 1:100 dilution. For double- and triple-labeling studies, all primary and secondary antibodies were incubated together with the cells. Following final washing, cells were mounted in Prolong Antifade solution (Molecular Probes) and examined with scanning confocal fluorescence microscopy (Molecular Dynamics). For triple-labeling studies, section series (40 optical sections, 0.3 μm each) were performed twice with dual imaging with 488/647 nm excitation laser lines to visualize Cy2/FITC and Cy5 fluorochromes followed by reimaging with 568 nm laser excitation to visualize Cy3. Section series were rendered in maximum intensity projections using Imagespace software (Molecular Dynamics) on a Silicon Graphics Indy workstation.
For localization of IgA, MDCK cells expressing pIgR alone (Mostov and Deitcher, 1986), or coexpressing the pIgR and Rab25, were incubated with purified human dimeric IgA (10 μg/ml) in either the apical or basolateral medium for 30 min at 37°C in Hank’s buffered saline containing calcium, magnesium, and 0.2% BSA, washed three times quickly in the same medium, and fixed for confocal microscopy as described above.
Production and Use of Recombinant Adenovirus
cDNAs encoding wild-type Rab25 and Rab25T26N were subcloned into the shuttle vector pADtet, a tetracycline-regulatable derivative of the previously described vector pAdlox (Hardy et al., 1997). The shuttle plasmid was then inserted into Psi5 adenoviral DNA by cre/lox recombination (as described by Hardy et al., 1997), a recombinant virus amplified and purified by CsCl gradient centrifugation. Recombinant adenovirus was then used to infect MDCK cells stably expressing both the pIgR and the tetracycline-repressible transactivator (clone T23) (Barth et al., 1997). Briefly, cells cultured on 12-mm Transwell filters were infected from the apical side with 5–10 pfu/cell for 16–18 h. In some cases, 20 ng/ml doxycycline was added during the infection period to repress Rab25 synthesis. To document induction and repression of protein expression, Western blots of cell lysates were probed with monoclonal antibodies against Rab25. Transcytosis and recycling of [125I]-dimeric IgA were assayed as described previously (Hansen et al., 1995). Basolateral recycling of [125I]transferrin was quantified as described previously by Apodaca et al. (1994).
RESULTS
Localization of Endogenous Rab11a in MDCK Cells
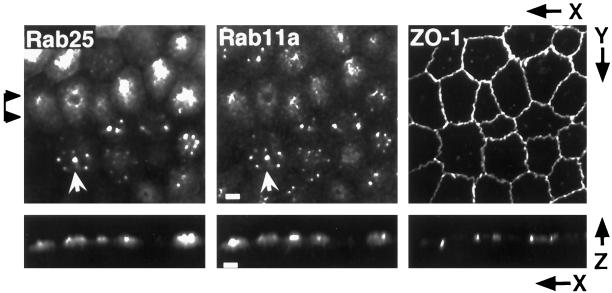
Previous investigations in nonpolarized cells indicated that Rab11a was concentrated on a population of endosomes in close proximity to the centrosome (Ullrich et al., 1996; Green et al., 1997). To determine the distribution of Rab11a-positive endosomes in polarized cells, we used a polyclonal antibody raised against the C-terminal region of human Rab11a to localize the endogenous protein in MDCK cells maintained on permeable Transwell filters. Under these conditions, MDCK cells form a uniform, polarized monolayer that maintains a transepithelial resistance and is impermeable to most macromolecules. As shown in Figure 1 (center), Rab11a immunoreactivity in these cells was observed in a punctate vesicular pattern, concentrated in most cells to a single focus in the center of the cell, immediately beneath the apical plasma membrane and in the same focal plane as the tight junctions (as shown by colabeling of the tight junction marker ZO-1 in Figure 1, right panel). Simultaneous labeling with antibodies to the centrosomal marker γ-tubulin (Figure 1, left panel) revealed that the Rab11a-positive membranes were tightly clustered around the centrosome. A finer, more diffuse staining pattern was often observed radiating from the central Rab11a focus toward the apical and lateral membranes. A similar distribution has been reported for Rab17 in both mouse Eph4 (Zacchi et al., 1998) and MDCK cells (Hunziker and Peters, 1998). These findings indicate that in MDCK cells, Rab11a-positive endosomes are highly polarized in their localization and reminiscent of the apical recycling endosomal compartment (Apodaca et al., 1994; Barroso and Sztul, 1994).
Figure 1.
Localization of Rab11a in MDCK cells. MDCK cells were grown on permeable supports at confluence for 3 d and then fixed in 4% paraformaldehyde. (A) Polarized MDCK cells were triple-stained for γ-tubulin, Rab11a, and ZO-1; specific labeling was identified with secondary antibodies conjugated with Cy5, Cy2, and Cy3, respectively. Top panels show X–Y projections of 40 optical confocal fluorescence sections (0.3 μm each). Arrowheads at right indicate the region used to construct the X–Z projections shown in the bottom panels. Rab11a antibodies labeled vesicular aggregates located adjacent to the centrosome (arrows). Bar, 1 μm.
Expression and Localization of Rab25 in MDCK Cells
Rab25 is closely related to Rab11a (68% amino acid identity) (Goldenring et al., 1993); however, like Rab17, expression of Rab25 appears to be restricted to epithelial tissues, including the gastrointestinal tract, kidney, and lung (Goldenring et al., 1993). We have recently produced monoclonal antibodies that are specific for rabbit and human Rab25 and do not cross-react with Rab11a (Calhoun and Goldenring, 1997). These antibodies, however, did not detect endogenous canine Rab25 by either Western blot or immunocytochemistry (our unpublished results). We found by Northern blot, however, that Rab25 mRNA is expressed endogenously in MDCK cells (our unpublished results), suggesting that either our antibody is species specific or that the endogenous protein is expressed at levels too low to be detected by immunological methods. A similar situation has been reported for endogenous canine Rab17, which is not detectable by antibodies raised against the mouse protein (Hunziker and Peters, 1998).
To investigate the function of Rab25 in MDCK cells we generated stably transfected MDCK lines constitutively expressing rabbit Rab25. Multiple clonal lines were developed, and several in which Rab25 was homogeneously expressed were selected for further study. To examine the subcellular distribution of Rab25, cells were cultured on permeable supports and triple-labeled with mouse monoclonal anti-Rab25, polyclonal anti-Rab11a, and rat monoclonal anti-ZO-1 (Figure 2). As shown in Figure 2, Rab25 is also apical in distribution, concentrated in the same focal plane as ZO-1. Moreover, Rab25 showed near-complete colocalization with Rab11a in the transfected cells; however, the pattern of Rab11a immunostaining was altered, relative to its appearance in nontransfected cells. In many cells, both Rab11a and Rab25 were present in multiple, apparently condensed, subapical structures that were no longer clustered around the centrosome (Figure 2). Although the actual number of these structures varied among cells, Rab11a and Rab25 always colocalized (arrows). Although similar in size, these structures are not late endosomes because no significant overlap of Rab25 staining with the late endosomal markers Rab7 and Rab9 or mannose-6-phosphate receptor was observed (our unpublished results). In addition, Rab25 overexpression did not alter the perinuclear position of the centrosomes as assessed by γ-tubulin staining (our unpublished results).
Figure 2.
Rab25 and Rab11a colocalize in Rab25-transfected cells. MDCK cells stably expressing Rab25 were cultured on permeable supports. Fixed cells were triple-stained with antibodies against Rab11a, Rab25, and ZO-1 with detection by secondary antibodies conjugated with Cy5, Cy2, and Cy3, respectively. Maximum intensity projections were constructed from 40 confocal optical sections (0.3 μm each). Rab25 staining overlapped with Rab11a staining in most cells (arrows). In many cells, instead of a single nidus of immunoreactivity for Rab11a/Rab25, several brightly staining foci were observed in the apical region of the cell. Bar, 4 μm.
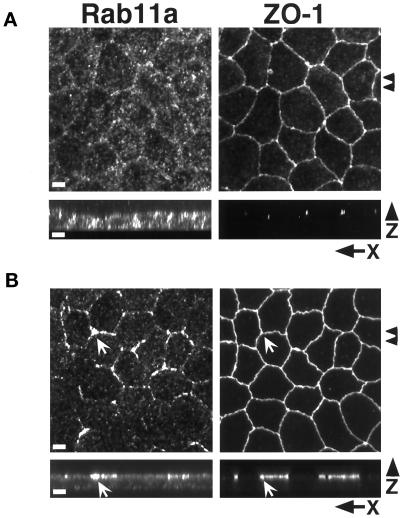
Influence of the Microtubule-based Cytoskeleton on the Distribution of Rab11a and Rab25
In MDCK cells, the integrity of the apical recycling system is dependent on the microtubule-based cytoskeleton (Mostov et al., 1992a; Apodaca et al., 1994). We therefore investigated the influence of microtubules on the distribution of both Rab11a and Rab25. Figure 3A demonstrates that treatment of cells with the microtubule-depolymerizing drug nocodazole led to a dispersal of Rab11a-positive vesicles throughout the cytoplasm, with some concentration at the lateral margins of the cells (arrows). This redistribution was completely reversed 2–4 h after removal of nocodazole (our unpublished results). These results are similar to those observed by Apodaca et al. (1994) for apical recycling endosomes in MDCK cells expressing the pIgR receptor and support the hypothesis that Rab11a is associated with the apical recycling system in MDCK cells.
Figure 3.
Effects of microtubule integrity on Rab11a distribution. (A) MDCK cells cultured on permeable supports were treated with 33 μM nocodazole for 2 h and then fixed in 4% paraformaldehyde. Cells were dual-stained with antibodies against Rab11a and ZO-1 with detection by secondary antibodies conjugated with Cy5 and Cy2, respectively. The top two panels show confocal projections of 40 X–Y optical sections (0.3 μm each) from the apical regions of the cells. Arrowheads at the right mark the region used to construct the X–Z projections shown in the bottom two panels. Punctate Rab11a immunoreactivity is dispersed throughout the cell with some concentration at the lateral margins. (B) Polarized MDCK cells cultured on permeable supports were treated with 5 μM taxol for 4 h at 37°C. Cells were dual-stained with antibodies against Rab11a and ZO-1. Top two panels show maximum intensity projection reconstructions of 40 confocal X–Y optical sections (0.3 μm each). Arrowheads at left indicate the region used to construct X–Z projections shown in bottom panels. Taxol caused the redistribution of Rab11a immunoreactivity to the region of the tight junctions (arrows) as well as a diffuse subapical staining pattern. Bar, 4 μm.
In light of the prominent effect of nocodazole on Rab11a distribution, we also sought to investigate the effects of the microtubule stabilizing agent taxol. Cells were incubated with 5 μM taxol for 4 h, and then the distributions of Rab11a and ZO-1 were examined. As shown in Figure 3B, taxol also caused a redistribution of Rab11a; however, in contrast to the diffuse distribution in nocodazole-treated cells, taxol-treated cells exhibited a prominent accumulation of Rab11a in close apposition to the tight junctions, as determined by ZO-1 staining. A fine punctate staining was also diffusely present below the apical membrane. These results confirm that the subcellular distribution of Rab11a is dependent on the microtubule-based cytoskeleton in MDCK cells.
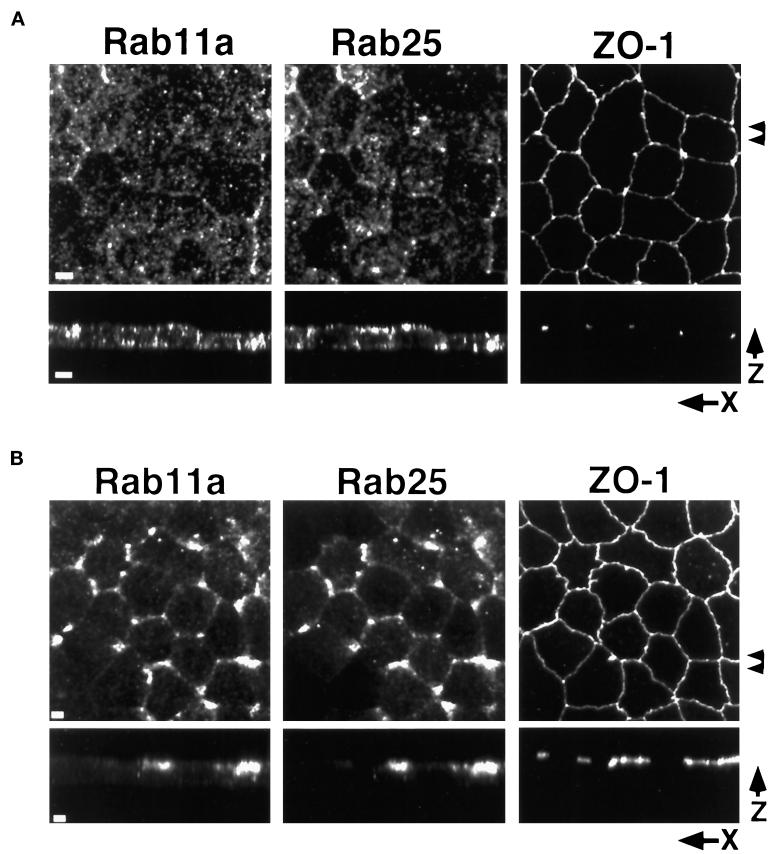
Because of the effects of Rab25 expression on the distribution of endogenous Rab11a, we also determined the effects of nocodazole and taxol treatment on Rab25 localization, relative to endogenous Rab11a. As shown in Figure 4A, nocodazole treatment resulted in the dispersal of both Rab11a- and Rab25-positive membranes; however, close inspection reveals that there are clear areas of nonoverlapping staining under these conditions, suggesting that, to at least some extent, the two markers are present in distinct membrane populations. Interestingly, in taxol-treated cells, virtually complete overlap was observed between Rab11a and Rab25 in the vicinity of the tight junctions (Figure 4B). We presently cannot rule out that Rab11a and Rab25 may be present on separate but related vesicle populations closely apposed near the junctional complexes. Nevertheless, these results demonstrate that the distribution of Rab25 is also dependent on the microtubule-based cytoskeleton.
Figure 4.
Effects of microtubule integrity on Rab11a and Rab25 distribution in Rab25-overexpressing MDCK cells. MDCK cells stably overexpressing Rab25 were grown on permeable filters and treated with either (A) 33 μM nocodazole for 2 h at 4°C or (B) 5 μM taxol for 4 h at 37°C. Cells were fixed in 4% paraformaldehyde and triple-stained with antibodies against Rab11a, Rab25, and ZO-1. Top panels in both A and B show maximum intensity projection reconstructions of 40 (0.3 μm each) confocal X–Y optical sections. Arrowheads at right indicate the region used to construct X–Z projections shown in the bottom panels. In A, nocodazole treatment dispersed both the Rab11a and Rab25 staining vesicles throughout the cytoplasm with some concentration at the lateral and apical surfaces. In B, taxol caused the redistribution of both Rab11a and Rab25 immunoreactivity to the region of the tight junctions. Rab11a and Rab25 immunoreactivity codistributed in taxol-treated cells. Bar, 4 μm.
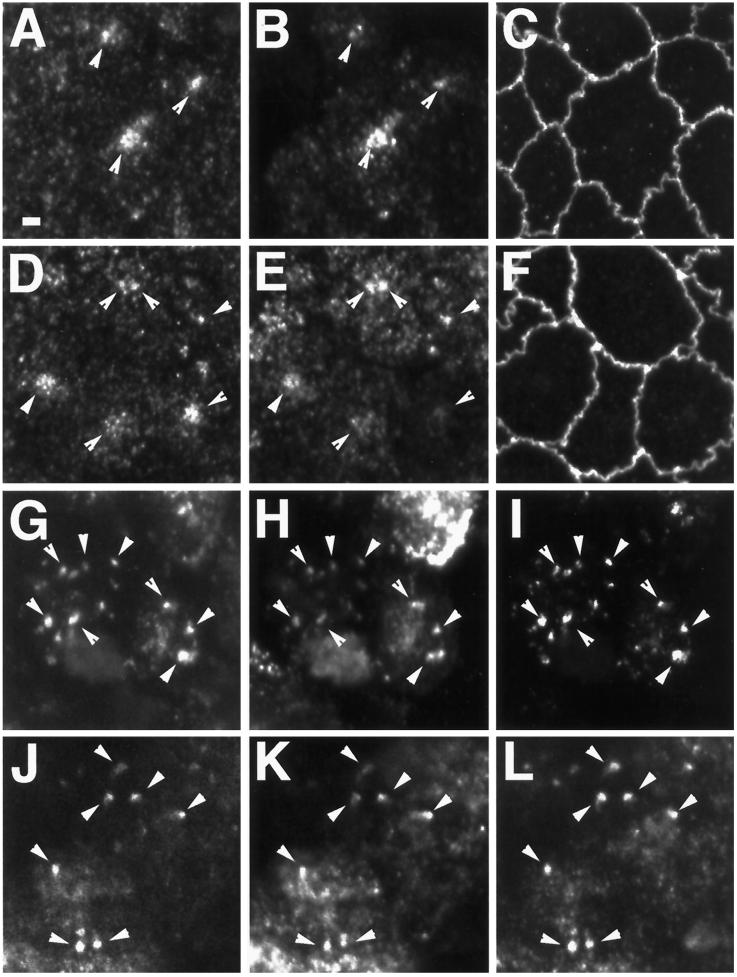
IgA Enters Rab11a- and Rab25-positive Endosomes after Internalization from Either the Apical or Basolateral Plasma Membrane
Previous work has defined apical recycling endosomes as accessible to membrane-bound ligands internalized from either the apical or basolateral pole of the cell (Apodaca et al., 1994; Barroso and Sztul, 1994; Hunziker and Peters, 1998; Zacchi et al., 1998). Apodaca et al. (1994) demonstrated that in MDCK cells expressing the pIgR, IgA internalized from the basolateral pole colocalized with anti-pIgR antibodies internalized from the apical cell surface. We therefore sought to determine whether IgA internalized from either pole of the cell would be transported into the Rab11a-containing endosomal compartment. MDCK cells stably expressing the pIgR were allowed to internalize dimeric IgA from either the apical (Figure 5A–C) or basolateral media (Figure 5G–I) for 30 min at 37°C and then fixed and processed for triple-label confocal immunofluorescence microscopy. As shown in Figure 5A–C, IgA internalized from the apical cell surface was concentrated in a dense cluster of vesicles that also labeled for Rab11a. Negligible immunolabeling for either Rab11a or IgA was observed in optical sections taken deeper in the cell, midway through the nucleus (our unpublished results). Similarly, in cells loaded with IgA from the basolateral pole, both IgA (Figure 5E) and Rab11a (Figure 5D) could be found in the same population of apical endosomes (arrows); however, in basolaterally loaded cells, IgA was also observed in vesicular structures underlying the lateral membranes, presumably representing basolateral early endosomes, which were Rab11a negative (our unpublished results). These results further confirm that Rab11a is associated with the apical recycling system.
Figure 5.
Trafficking of dimeric IgA into the Rab11a- and Rab25-positive endosomal compartment. MDCK cells stably expressing the pIgR alone (A–F) or stably expressing both pIgR and Rab25 (G–L) were cultured on permeable supports and loaded with dimeric IgA for 30 min at 37°C either from the apical (A–C, G–I) or basal media (D–F, J–L). Cells were-triple labeled with anti-Rab11a (A, D, G, J), FITC-conjugated anti-IgA (B, E, H, K) and either anti-ZO-1 (C, F) or anti-Rab25 (I, L). Ten (0.3 μm) X–Y confocal optical sections from the apical regions were used to construct maximum intensity projections. In cells expressing only pIgR (A–F), under both loading conditions, IgA was observed in Rab11a-positive endosomal populations (arrowheads) in the apical region of the cells. In cells expressing both pIgR and Rab25 (G–L), IgA internalized from either pole of the cell could be observed in endosomes that stained for both Rab11a and Rab25 (arrowheads) in the apical region of the cells. Bar, 2 μm.
Given the change in distribution of Rab11a in Rab25-transfected cells, we sought to determine whether Rab25 overexpression would affect the ability of IgA to enter these Rab11a/Rab25-immunoreactive structures. To this end, we established clonal cell lines stably expressing both Rab25 and the pIgR (Rab25/pIgR). In these cells, Rab25 was distributed in a pattern similar to that observed in singly transfected Rab25-overexpressing cells (Figure 2). These double transfectants, cultured on filter supports, were then loaded with dimeric IgA from either the apical or basolateral cell surface. In these cells, IgA internalized from the apical pole (Figure 5H) was observed in apical endosomes that were also labeled by antibodies to both Rab11a (Figure 5G) and Rab25 (Figure 5I). As described above, no apically internalized IgA was observed in focal planes bisecting the nucleus. Similarly, IgA internalized from the basolateral surface was also observed in apical structures (Figure 5K) that were positive for both Rab11a (Figure 5J) and Rab25 (Figure 5L). Thus, although overexpression of Rab25 altered the morphology of the recycling system, these endosomes remained capable of receiving input from both apical and basolateral endocytic pathways.
Rab25 Is an Active GTPase
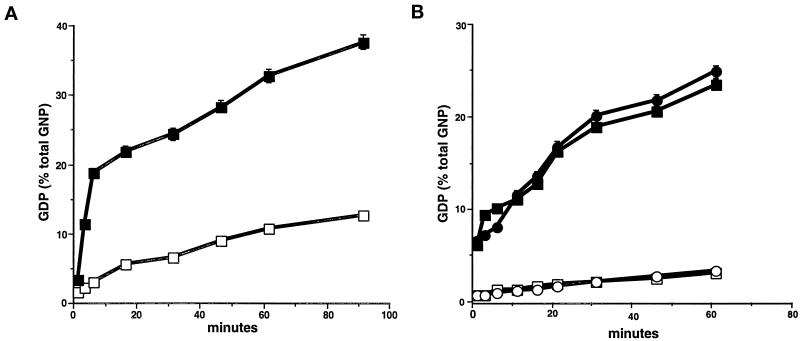
Rab25 is unique among currently known members of the Rab family in that the sequence of its P3 GTP-binding domain is WDTAGLE compared with the consensus WDTAGQE. The substitution of leucine for glutamine within this domain (position 71 in Rab25) is one that is commonly introduced into small GTPases to reduce their GTPase activity, resulting in a dominant, constitutively active protein (Adari et al., 1988). We therefore have investigated the possibility that Rab25 exists in vivo as a constitutively active Rab. To this end, recombinant Rab25 was produced in bacteria as a GST-fusion protein. As shown in Figure 6A, recombinant Rab25 demonstrated an active endogenous GTPase activity that was augmented threefold by the addition of gastric cytosol (as a source of GTPase-activating protein [GAP] activity). No significant GTP hydrolysis was observed in the presence of cytosol alone without recombinant Rab proteins. These results indicate that Rab25 is a functional GTPase, despite the presence of leucine at position 71.
Figure 6.
GTPase activity in Rab11 family members. The GTPase activities of (A) recombinant Rab25 and (B) recombinant Rab11a and Rab11aQ70L were assessed in vitro. (A) GTPase activity was assessed as described in MATERIALS AND METHODS in 1 μg of recombinant GST-Rab25 in the absence (□) or presence (▪) of gastric cytosol as a GAP donor. (B) GTPase activity was assessed in recombinant His-tagged Rab11a (circles) and His-tagged Rab11aQ70L (squares) in the absence (open symbols) or presence (closed symbols) of gastric cytosol as a GAP donor. All results represent the mean + SEM of three separate experiments each with duplicate samples. The results indicate that Rab25, Rab11a, and Rab11aQ70L are all active GTPases.
Previous investigations had suggested that introduction of a Q70L substitution into wild-type Rab11a altered the distribution of the recycling endosomal system but did not detectably affect the ultrastructure of the endosomes themselves (Ullrich et al., 1996). We therefore also compared the GTPase activity of recombinant His-tagged Rab11a and the mutant His-tagged Rab11aQ70L. Figure 6B demonstrates that both wild-type Rab11a and Rab11aQ70L exhibit low intrinsic GTPase activities compared with Rab25; however, both proteins showed a similar tenfold stimulation of GTPase activity by gastric cytosol. Thus, the Rab11aQ70L mutant is also unlikely to be constitutively active in vitro.
Rab25 Overexpression Alters Transcytosis and Apical Recycling
Given the changes in the morphology of the recycling system, we sought to quantitatively assess the effects of Rab25 overexpression on the trafficking of IgA, using adenovirus-mediated expression under the control of a tetracycline-regulatable promotor. A stable line of MDCK cells expressing both the pIgR and the tetracycline-repressible transactivator [clone T23, (Barth et al., 1997)] was cultured on permeable filter supports to establish a polarized monolayer and then infected with recombinant adenovirus encoding Rab25. To control for possible effects of adenovirus infection on monolayer integrity or cellular function, parallel monolayers were infected with identical recombinant virus in the presence of 20 ng/ml doxycycline to repress Rab25 synthesis. Examination of infected monolayers by immunofluorescence microscopy revealed that in the absence of doxycycline, virtually all cells expressed Rab25, with localization similar to that observed in the stably transfected cells (our unpublished results). No immunologically detectable Rab25 was observed in cells infected in the presence of doxycycline.
To measure transcytosis, virally transfected cells cultured on filter supports were allowed to internalize [125I]-dimeric IgA from the basolateral pole for 10 min at 37°C, as described in MATERIALS AND METHODS. At various time points, IgA release into the apical or basolateral chamber was assayed by harvesting the media. Ligand remaining within the cells after the final time point was determined by cutting the filters from their holders, and the data were expressed as a fraction of total internalized ligand. As has been observed previously in pIgR-expressing MDCK cells (Apodaca et al., 1994; Song et al., 1994; Hansen et al., 1995), in mock-infected cells, nearly 70% of the basolaterally internalized ligand was recovered from the apical medium within 60 min (Figure 7A, ▴). Interestingly, transcytosis of [125I]-dimeric IgA was reduced by 50% in cells overexpressing Rab25 (Figure 7A, •). This decrease in transcytosis was not due to an increase in recycling to the basolateral surface, because the fraction of ligand appearing in the basolateral medium remained unchanged (Figure 7A, ○). Rather, a larger fraction of the internalized ligand was retained within the cells (Figure 7B).
Figure 7.
Rab25 inhibits transcytosis and apical recycling in adenovirus-transfected MDCK cells. (A) MDCK cells stably expressing the pIgR and the tetracycline-regulated transactivator were either mock-infected (triangles) or infected with recombinant adenovirus (squares and circles) encoding either wild-type Rab25 (A–C) or the Rab25T26N (D–F) for 18 h at 37°C. Expression of the recombinant proteins was confirmed by Western blot (B and E, insets). Infections were performed either in the absence of doxycycline (circles) or the presence of 20 ng/ml doxycycline (squares) to repress Rab25 synthesis. Cells were loaded with [125I]-dimeric IgA from the basolateral medium for 10 min at 37°C. Filters were then washed, and fresh medium was added to apical and basolateral chambers and incubated for the times indicated. Apical media (containing transcytosed ligand, filled symbols) and basolateral media (recycled ligand, open symbols) were harvested at each time point, and cells were collected after the last time point. Data are the average of two determinations and are expressed as a percentage of total internalized cpm. Triangles indicate mock-infected; circles, − doxycycline; squares, + doxycycline. (B and E) Fraction of internalized IgA remaining inside the cells at the end of the experiment shown in A and D, respectively. (C and F) Apical recycling. Ligand was internalized from the apical surface for 30 min at 37°C. Cells were then cooled to 4°C and washed, and surface-bound ligand was removed by incubation with trypsin (10 μg/ml) for 1 h at 4°C. Trypsinization was stopped by washing with cold medium containing soybean trypsin inhibitor (50 μg/ml). Filters were then placed in warm medium, and ligand was recovered from the apical (recycled) or basolateral (transcytosed) medium at the times indicated. Apical recycling was assayed in both (C) Rab25-infected and (E) Rab25T26N-infected cells. Filled symbols indicate apical recycling; open symbols, apical-to-basolateral transcytosis: triangles, mock-infected; circles, − doxycycline; squares, + doxycycline. All data represent trichloroacetic acid-precipitable counts (typically 97% of internalized ligand was trichloroacetic acid-precipitable) and are representative of at least three separate experiments.
In contrast, cells in which Rab25 synthesis was repressed by doxycycline transcytosed ligand at a rate that was indistinguishable from noninfected cells (Figure 7A, ▪), indicating that there were no adverse effects of adenovirus infection over the time course of the experiment. The effects of Rab25 expression appeared limited to the transcytotic pathway, because neither apical nor basolateral secretion of metabolically labeled proteins was inhibited (our unpublished results). Similarly, no significant effect of Rab25 expression was observed on either the rate of IgA internalization or the number of basolateral IgA binding sites (our unpublished results), indicating that Rab25 acts at a postendocytic step in the transcytotic pathway. This inhibition of IgA transcytosis by Rab25 is similar to that reported for wild-type Rab17 by Hunziker and Peters (1998); however, in that study, Rab17 expression induced an increase in basolateral recycling that we did not observe here for Rab25. Rather, the internalized ligand appears to be retained intracellularly.
Previous studies have shown that apically internalized IgA is recycled back to the apical plasma membrane with high efficiency (Apodaca et al., 1994; Hansen and Casanova, 1994), and it has been hypothesized that transcytosing IgA may reach the apical surface from the apical recycling compartment via an apical recycling pathway. To evaluate the effects of Rab25 expression on apical recycling, cells were loaded with [125I]-dimeric IgA from the apical cell surface for 30 min, as described previously (Hansen et al., 1995), and the reappearance of ligand in the apical and basolateral media was analyzed as described above. In contrast to basolateral recycling, which was unaffected by Rab25 expression, apical recycling was reduced by 30% relative to control (Figure 7C, •). No transcytosis to the basolateral pole was detected, even in the presence of Rab25, indicating that sorting of the internalized ligand was not affected. Similarly, inhibition of apical recycling was also observed in cell lines stably expressing Rab25 (our unpublished results). These data suggest that the observed effects of Rab25 overexpression on the kinetics of IgA transport are due to functional effects at a single site, most likely the apical recycling endosome.
Previous investigations have used mutant Rab proteins defective in either GTP hydrolysis (dominant active) or GTP binding (dominant negative) to selectively alter the function of individual Rabs in intact cells (Ullrich et al., 1996; Zacchi et al., 1998). In some cases these mutants have had profound effects on membrane trafficking (e.g., Rab5 [Bucci et al., 1992]), but in others the effects were more subtle (e.g., Rab11a [Ullrich et al., 1996; Ren et al., 1998]). To determine the effect of Rab25 mutant expression on transcytosis and apical recycling, cells were infected with recombinant adenovirus containing a mutant Rab25 predicted to be defective in GTP binding, Rab25T26N. Immunocytochemical examination of transfected cells showed a diffuse cytosolic staining pattern (our unpublished results), consistent with the known inability of Rabs to associate with membranes in the GDP-bound state. Western blot analysis demonstrated that in the absence of doxycycline, the Rab25 and Rab25T26N proteins were expressed to similar levels (Figure 7, insets). In contrast to the effect of wild-type Rab25, the distribution of endogenous Rab11a was not affected by the expression of Rab25T26N (our unpublished results). Similarly, Rab25T26N expression had no significant effect on either transcytosis (Figure 7D) or apical recycling (Figure 7F) of dimeric IgA, nor did expression of Rab25T26N significantly change the levels of [125I]-dimeric IgA retained within the cells (Figure 7E). These results suggest that Rab25–GDP does not inhibit trafficking through the recycling system.
Finally, because the low fraction of basolaterally recycling IgA did not allow reliable quantitation of the effects of Rab25 expression on basolateral recycling, we examined the recycling of [125I]transferrin, essentially as described by Apodaca et al., (1994). [125I]-labeled canine transferrin was internalized from the basolateral surface for 30 min at 37°C. After extensive washing, cells were placed in ligand-free medium, and the appearance of [125I]transferrin in the basolateral media was quantitated as described above for dimeric IgA. We found that expression of neither wild-type Rab25 nor Rab25T26N had any detectable effect on transferrin recycling. Nearly 80% of the internalized ligand was recycled to the basolateral surface within 60 min (Figure 8A), and the amount of transferrin remaining within the cells at the end of the time course was essentially unchanged (Figure 8B). These data indicate that the effects of Rab25 expression on trafficking are limited to the transcytotic and/or apical recycling pathways.
Figure 8.
Rab25 does not alter basolateral recycling in MDCK cells. (A) Cells were loaded with [125I]-labeled canine transferrin for 30 min at 37°C, washed, and then placed in fresh medium for the times indicated. Apical and basolateral media were harvested at each time point, and (B) the fraction of ligand remaining inside the cells was determined after the last time point. Recycling was measured in mock-infected cells (○) and in cells infected with Rab25 in the presence of doxycycline (□), with Rab25 in the absence of doxycycline (▵), and with Rab25T26N in the absence of doxycycline (⋄). All data are representative of three separate experiments.
DISCUSSION
An accumulation of data in recent years has supported the existence of a distinct endosomal compartment in which membrane proteins and lipids are packaged for recycling to the cell surface. This compartment, termed the recycling endosome, has been characterized morphologically by its pericentriolar location, its tubulovesicular morphology, and its dependence on intact microtubules for its localization. It has also been characterized functionally by the presence of known recycling proteins (e.g., transferrin receptors), exclusion of fluid-phase markers, and the absence of markers of the sorting endosome such as Rab4 and Rab5 (Daro et al., 1996). Recently, investigations have demonstrated that the small GTPase Rab11a is a reliable marker of the recycling endosome compartment in nonpolarized cells (Ullrich et al., 1996; Green et al., 1997). Ullrich et al. (1996) also found that a mutant of Rab11a defective in GTP binding (Rab11aS25N) impaired recycling of internalized transferrin, apparently by inhibiting transport from sorting endosomes to the recycling endosome.
An equivalent of the recycling endosome has also been identified functionally and morphologically in epithelial cells (Apodaca et al., 1994; Barroso and Sztul, 1994; Odorizzi et al., 1996). Like its counterpart in nonpolarized cells, this compartment is clustered around the centrosome, is tubulovesicular in morphology, and requires intact microtubules for its integrity; however, in addition the apical recycling endosomal compartment appears to serve a sorting function that is unique to polarized cells. Because it is accessed by endocytic pathways originating from both poles of the cell, apical and basolateral membrane components (including lipids) become mixed within the recycling endosome and must be resorted before transport back to the appropriate membrane domain. In addition, a subset of internalized proteins is transcytosed in epithelial cells. These transcytosing proteins must be separated from recycling proteins and packaged for transport to the opposite cell surface. Clearly, the function of the recycling endosome in polarized cells is more complex and is likely to require a specialized machinery that does not exist in nonpolarized cells.
In this study, we demonstrate the association of Rab11a with the apical recycling system in polarized (MDCK) cells. This association is based on our findings that 1) antibodies specific for Rab11a label a population of vesicles in close proximity to the centrosome; 2) this localization is dependent on intact microtubules; and 3) IgA internalized from either the apical or basolateral plasma membrane can enter Rab11a-immunoreactive membranes. We also demonstrate that Rab25, a close relative of Rab11a that is expressed exclusively in epithelia, colocalizes with Rab11a in MDCK cells, suggesting that it regulates an epithelial-specific function associated with this endosomal system. In support of this hypothesis, we found that overexpression of Rab25 in MDCK cells coexpressing the pIgR resulted in a decreased rate of receptor transcytosis and of apical, but not basolateral, recycling of internalized ligand. Moreover, Rab25 expression had no detectable effect on basolateral recycling of internalized transferrin, further suggesting a selective role for this GTPase in apically directed postendocytic trafficking.
Although nocodazole caused dispersion of the apical recycling endosomes, taxol, a compound that stabilizes microtubules, induced a distinct concentration of both Rab11a and Rab25 immunostaining in the region of the tight junctions. The reasons for this distribution are not yet clear; however, an association between at least one Rab protein (Rab6) and a novel isoform of kinesin (rabkinesin-6) has been reported recently (Echard et al., 1998) and was found to play an important role in Golgi membrane dynamics. This raises the intriguing possibility that Rab11a and/or Rab25 may also interact with microtubule motors to regulate the movement of vesicles or endosomes along microtubules.
As described above, mammalian Rab11a and Rab25 are closely related and in fact comprise a subfamily of Rab proteins along with mammalian Rab11b (Lai et al., 1994), the Ypt3 protein of Schizosaccharomyces pombe (Drivas et al., 1991), and Ypt31p and Ypt32p of Saccharomyces cerevisiae (Benli et al., 1996; Jedd et al., 1997). The putative effector domains of Rab11a and Rab25 are very similar in sequence (90% conserved, versus 53% between Rab25 and Rab5, and 50% with Rab17), suggesting that they interact with similar or identical targets to achieve their function. Because Rab11a appears to regulate transport of transferrin from sorting endosomes to the recycling endosome, it is tempting to speculate that Rab25 may play a similar role in the transport of apically internalized membrane from apical sorting (Rab5-positive) endosomes to the common recycling endosome in epithelia. Certainly, the selective inhibition of apical but not basolateral recycling of internalized IgA (or basolateral recycling of transferrin) is consistent with such a model, but how could the effects of Rab25 overexpression on basolateral-to-apical transcytosis be explained? Both transferrin receptor and the pIgR are transported from the basolateral cell surface to the apical recycling compartment with similar kinetics (Apodaca et al., 1994); however, transferrin receptors are recycled to the basolateral plasma membrane with high efficiency, whereas the pIgR is thought to enter the apical recycling pathway to reach the apical surface (Apodaca et al., 1994). If this is indeed the case, a decrease in the rate of membrane flow through the apical recycling pathway would be expected to have similar effects on both apical recycling and basolateral-to-apical transcytosis, precisely what we have observed in Rab25-expressing MDCK cells.
Recent investigations have demonstrated the association of another epithelial-specific Rab protein, Rab17, with the apical recycling system in both a mouse mammary epithelial cell line, Eph4 (Zacchi et al., 1998), and MDCK cells (Hunziker and Peters, 1998); however, the functional consequences of Rab17 overexpression in these two studies are contradictory and therefore difficult to interpret. In Eph4 cells, expression of wild-type Rab17 had no effect on transcytosis; however, both a constitutively active (Q77L) and dominant-negative (N132I) mutant stimulated transcytosis as well as apical recycling (Zacchi et al., 1998). In contrast, in MDCK cells, expression of wild-type Rab17 had a significant inhibitory effect on transcytosis of IgA and increased basolateral recycling (Hunziker and Peters, 1998). Neither activating nor inhibitory mutants were tested in this latter study. The inhibition of transcytosis by Rab17 in MDCK cells is similar to that observed here for Rab25, except that we did not detect a stimulation of basolateral recycling. Rather, overexpression of Rab25 led to an intracellular accumulation of internalized ligand.
We also found that expression of a Rab25 mutant predicted to be deficient in GTP binding (Rab25T26N) had no effect on either transcytosis or apical recycling. This was somewhat surprising, given that a similar mutation in Rab11a (S25N) significantly impaired transferrin receptor recycling in baby hamster kidney cells (Ullrich et al., 1996); however, the apparent lack of a Rab25T26N phenotype can be interpreted in several ways. The simplest explanation is that the mutant protein is aberrantly folded and therefore completely nonfunctional; however, the level of expression of Rab25T26N was similar to that of the wild-type protein as determined by immunoblotting (Figure 7E, inset), suggesting that it was not subject to an increased rate of turnover. Alternatively, it is possible that the mutant protein is poorly prenylated. A nonprenylated mutant of Rab17 was similarly without effect when expressed in MDCK cells (Hunziker and Peters, 1998). Still, perhaps the most likely explanation is that because the effects of overexpressed Rab25 are inhibitory, the T26N mutation yields a GTPase that is no longer functionally inhibitory. Clearly further work will be required to distinguish among these possibilities.
The sequence of the GTP-binding site of Rab25 is unique among Rab proteins, in that the P-3 domain consensus sequence WDTAGQE contains a leucine residue in place of the conserved glutamine. An equivalent substitution in Ras creates a dominant-active GTPase (Haubruck and McCormick, 1991). Although the effects of this mutation on Ras GTPase activity have been well characterized, the phenotype of Rab proteins with similar mutations is not as well established. For example, although the Q to L substitution in Rab5 results in a dominant-active Rab–GTP state (Hoffenberg et al., 1995), the same mutation in SEC4 has no effect on GTPase activity (Walworth et al., 1989). The results presented here demonstrate that despite the presence of a leucine residue at position 71, Rab25 is not a constitutively active GTPase. Indeed, recombinant Rab25 exhibited higher intrinsic GTPase activity than recombinant Rab11a in vitro. Furthermore, we found that Rab11aQ70L is also an active GTPase, with cytosol (GAP)-stimulated activity that is indistinguishable from that of wild-type Rab11a. These findings demonstrate that in members of the Rab11 subfamily, substitution of the conserved glutamine in the P-3 GTP-binding domain with leucine does not significantly alter GTPase activity and may explain the unexpectedly minor effects of Rab11aQ70L expression on trafficking through recycling endosomes in nonpolarized cells (Ullrich et al., 1996). We have found that overexpressed wild-type Rab11a and Rab11aQ70L both are localized in a centrosomally clustered distribution that is similar to that observed for endogenous Rab11a in nontransfected MDCK cells (Goldenring and Casanova, our unpublished results). All of these results emphasize the need to document the GTPase activities of P-3 mutations among Rab proteins.
Rab11a was initially characterized as a 24-kDa GTP-binding protein from brain membranes (Kikuchi et al., 1988) and was subsequently cloned from a number of sources, including MDCK (Chavrier et al., 1990), mouse kidney (Chavrier et al., 1992), and rabbit gastric parietal cells (Goldenring et al., 1994). In epithelial tissues, prominent immunostaining for Rab11a was observed in the subapical region of a number of cell types, including surface mucous cells of the gastric mucosa, intestinal enterocytes and colonocytes, kidney collecting duct, pancreatic acinar cells and pancreatic ducts, and hepatocytes and hepatic ducts, as well as the medial layers of the squamous mucosa of the skin and esophagus (Goldenring et al., 1996). Although our initial investigations showed the highest expression in epithelial cells, recent studies have documented that Rab11a is also present in nonpolarized cells, including fibroblasts (Ullrich et al., 1996) and erythroleukemia cells (Green et al., 1997). In brain, Rab11a is associated with cell bodies and dendrites in various nerve cells (Sheehan et al., 1996). Interestingly, Rab11a is highly enriched in rabbit gastric parietal cells, where it is associated with intracellular tubulovesicles containing the H/K-ATPase (Goldenring et al., 1994, 1996; Calhoun and Goldenring, 1997). These tubulovesicles comprise a specialized regulated recycling organelle that sequesters the H/K-ATPase intracellularly and releases it to the apical plasma membrane in response to acid secretagogues. We have also observed that Rab25 colocalizes with Rab11a on parietal cell tubulovesicles (Calhoun and Goldenring, 1997) as well as in other gastrointestinal cells (Nwokeji et al., 1998), suggesting that the two proteins act together to regulate membrane recycling in various epithelial cell types.
In summary, we have demonstrated that Rab11a and Rab25 associate with the apical recycling system in polarized MDCK cells. The inhibition of transit through the apical recycling system by overexpression of Rab25 indicates that members of the Rab11 family may coregulate the rate and efficiency of processing through plasma membrane recycling systems. The presence of at least three different Rabs (Rab11a, Rab25, and Rab17) on recycling endosomes in polarized cells is indicative of the complexity of the recycling system, and it will be of interest to determine the precise role of each in the recycling process.
ACKNOWLEDGMENTS
We thank Lorraine Aron of the University of Georgia Monoclonal Facility for the production of monoclonal antibody ascites, and Drs. Lisa Matovcik and Marino Zerial for the gifts of polyclonal antibodies. We thank Jennetta Smith, Sungjean Chai, and Jessica Hatfield for outstanding technical assistance. This work was supported by National Institutes of Health grants DK-48370 and DK-43405 and a Veterans Administration Merit Award to J.R.G., a National Research Service Award postdoctoral fellowship to X.W., and grants from National Institutes of Health (AI-32991, DK-33506) and the Good Samaritan Foundation to J.E.C.
REFERENCES
- Adari H, Lowy DR, Willumsen BM, Der CJ, McCormick F. Guanosine triphosphatase activating protein (GAP) interacts with the p21 ras effector binding domain. Science. 1988;240:518–521. doi: 10.1126/science.2833817. [DOI] [PubMed] [Google Scholar]
- Albright CF, Giddings BW, Liu J, Vita M, Weinberg RA. Characterization of a guanine nucleotide dissociation stimulator for a Ras related GTPase. EMBO J. 1993;12:339–347. doi: 10.1002/j.1460-2075.1993.tb05662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso M, Sztul E. Basolateral to apical transcytosis in polarized cells is indirect and involves BFA and trimeric G protein sensitive passage through the apical endosome. J Cell Biol. 1994;124:83–100. doi: 10.1083/jcb.124.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AI, Pollack AL, Altschuler Y, Mostov KE, Nelson WJ. NH2-terminal deletion of beta-catenin results in stable colocalization of mutant beta-catenin with adenomatous polyposis coli protein and altered MDCK adhesion. J Cell Biol. 1997;136:693–706. doi: 10.1083/jcb.136.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benli M, Doring F, Robinson DG, Yang X, Gallwitz D. Two GTPase isoforms, Ypt31p and Ypt32p, are essential for Golgi function in yeast. EMBO J. 1996;15:6460–6475. [PMC free article] [PubMed] [Google Scholar]
- Bomsel M, Prydz K, Parton RG, Gruenberg J, Simons K. Endocytosis in filter-grown Madin–Darby canine kidney cells. J Cell Biol. 1989;109:3243–3258. doi: 10.1083/jcb.109.6.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Bucci C, Wandinger-Ness A, Lutcke A, Chiariello M, Bruni CB, Zerial M. Rab5a is a common component of the basolateral and apical endocytic machinery in polarized epithelial cells. Proc Natl Acad Sci USA. 1994;91:5061–5065. doi: 10.1073/pnas.91.11.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun BC, Goldenring JR. Two Rab proteins, VAMP-2, and SCAMPs are present on immunoisolated gastric tubulovesicles. Biochem J. 1997;325:559–564. doi: 10.1042/bj3250559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P, Simons K, Zerial M. The complexity of the rab and rho GTP-binding protein subfamilies revealed by a PCR cloning approach. Gene. 1992;112:261–264. doi: 10.1016/0378-1119(92)90387-5. [DOI] [PubMed] [Google Scholar]
- Chavrier P, Vingron M, Sander C, Simons K, Zerial M. Molecular cloning of YPT1/SEC4-related cDNAs from an epithelial cell line. Mol Cell Biol. 1990;10:6578–6585. doi: 10.1128/mcb.10.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa GL, Bauer JC, McGowan B, Angert M, Weiner MP. Site-directed mutagenesis using a rapid PCR-based method. Methods Mol Cell Biol. 1996;57:239–248. doi: 10.1385/0-89603-332-5:239. [DOI] [PubMed] [Google Scholar]
- Daro E, Van Der Sluijs P, Galli T, Mellman I. Rab4 and cellubrevin define different early endosome populations on the pathway of transferrin receptor recycling. Proc Natl Acad Sci USA. 1996;93:9559–9564. doi: 10.1073/pnas.93.18.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drivas GT, Shih A, Coutevas EE, D’Eustachio P, Rush MG. Identification and characterization of a human homolog of the Schizosaccharomyces pombe ras-like gene YPT-3. Oncogene. 1991;6:3–9. [PubMed] [Google Scholar]
- Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Echard A, Jollivet F, Martinez O, Lacapere J, Rousselet A, Janoueix-Lerosey I, Goud B. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 1998;279:580–585. doi: 10.1126/science.279.5350.580. [DOI] [PubMed] [Google Scholar]
- Ghosh RN, Maxfield FR. Evidence for nonvectorial, retrograde transferrin trafficking in the early endosomes of HEP2 cells. J Cell Biol. 1995;128:549–561. doi: 10.1083/jcb.128.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenring JR, Shen KR, Vaughan HD, Modlin IM. Identification of a small GTP-binding protein, Rab25, expressed in the gastrointestinal mucosa, kidney and lung. J Biol Chem. 1993;268:18419–18422. [PubMed] [Google Scholar]
- Goldenring JR, Smith J, Vaughan HD, Cameron P, Hawkins W, Navarre J. Rab11 is an apically located small GTP-binding protein in epithelial tissues. Am J Physiol. 1996;270:G515–G525. doi: 10.1152/ajpgi.1996.270.3.G515. [DOI] [PubMed] [Google Scholar]
- Goldenring JR, Soroka CJ, Shen KR, Tang LH, Rodriguez W, Vaughan HD, Stoch SA, Modlin IM. Enrichment of rab11, a small GTP-binding protein, in gastric parietal cells. Am J Physiol. 1994;267:G187–G194. doi: 10.1152/ajpgi.1994.267.2.G187. [DOI] [PubMed] [Google Scholar]
- Green EG, Ramm E, Riley NM, Spiro DJ, Goldenring JR, Wessling-Resnick M. Rab11 is associated with transferrin-containing recycling compartments in K562 cells. Biochem Biophys Res Commun. 1997;239:612–616. doi: 10.1006/bbrc.1997.7520. [DOI] [PubMed] [Google Scholar]
- Hansen SH, Casanova JE. Gsα stimulates transcytosis and apical secretion in MDCK cells through cAMP and protein kinase A. J Cell Biol. 1994;126:677–687. doi: 10.1083/jcb.126.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SH, Olsson A, Casanova JE. Wortmannin, an inhibitor of phosphoinositide 3-kinase, inhibits transcytosis in polarized epithelial cells. J Biol Chem. 1995;270:28425–28432. doi: 10.1074/jbc.270.47.28425. [DOI] [PubMed] [Google Scholar]
- Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps ML. Construction of adenovirus vectors through Cre-lox recombination. J Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubruck H, McCormick F. Ras p21: effects and regulation. Biochem Biophys Acta. 1991;1072:215–229. doi: 10.1016/0304-419x(91)90015-d. [DOI] [PubMed] [Google Scholar]
- Hoffenberg S, Sanford JC, Liu S, Daniel DS, Tuvin M, Knoll BJ, Wessling-Resnick M, Dickey BF. Biochemical and functional characterization of a recombinant GTPase, Rab5, and two of its mutants. J Biol Chem. 1995;270:5048–5056. doi: 10.1074/jbc.270.10.5048. [DOI] [PubMed] [Google Scholar]
- Hopkins CR, Gibson A, Shipman M, Strickland DK, Trowbridge IS. In migrating fibroblasts, recycling receptors are concentrated in narrow tubules in the pericentriolar area, and then routed to the plasma membrane of the leading lamella. J Cell Biol. 1994;125:1265–1274. doi: 10.1083/jcb.125.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughson EJ, Hopkins C. Endocytic pathways in polarized Caco-2 cells: identification of an endosomal compartment accessible from both apical and basolateral surfaces. J Cell Biol. 1990;110:337–348. doi: 10.1083/jcb.110.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Peters PJ. Rab17 localizes to recycling endosomes and regulates receptor-mediated transcytosis in epithelial cells. J Biol Chem. 1998;273:15734–15741. doi: 10.1074/jbc.273.25.15734. [DOI] [PubMed] [Google Scholar]
- Jedd G, Mulholland J, Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J Cell Biol. 1997;137:563–580. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A, Yamashita T, Kawata M, Yamamoto K, Ikeda K, Tanimoto T, Takai Y. Purification and characterization of a novel GTP-binding protein with a molecular weight of 24,000 from bovine brain membranes. J Biol Chem. 1988;263:2897–2904. [PubMed] [Google Scholar]
- Knight A, Hughson E, Hopkins CR, Cutler DF. Membrane protein trafficking through the common apical endosome compartment of polarized Caco-2 cells. Mol Biol Cell. 1995;6:597–610. doi: 10.1091/mbc.6.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Stubbs L, Artzt K. Molecular analysis of mouse Rab11b: a new type of mammalian YPT/Rab protein. Genomics. 1994;22:610–616. doi: 10.1006/geno.1994.1434. [DOI] [PubMed] [Google Scholar]
- Lutke A, Jansson S, Parton RG, Chavrier P, Valencia A, Huber LA, Lehtonen E, Zerial M. Rab17, a novel small GTPase, is specific for epithelial cells and is induced during cell proliferation. J Cell Biol. 1993;121:553–564. doi: 10.1083/jcb.121.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K, Apodaca G, Areoti B, Okamoto C. Plasma membrane protein sorting in polarized epithelial cells. J Cell Biol. 1992a;116:577–583. doi: 10.1083/jcb.116.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K, Apodaca G, Aroeti B, Okamoto C. Plasma membrane protein sorting in polarized epithelial cells. J Cell Biol. 1992b;116:577–583. doi: 10.1083/jcb.116.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov KE, Deitcher DL. Polymeric immunoglobulin receptor expressed in MDCK cells transcytoses IgA. Cell. 1986;46:613–621. doi: 10.1016/0092-8674(86)90887-1. [DOI] [PubMed] [Google Scholar]
- Nuoffer C, Balch WE. GTPases: multifunctional molecular switches regulating vesicular traffic. Annu Rev Biochem. 1994;63:949–990. doi: 10.1146/annurev.bi.63.070194.004505. [DOI] [PubMed] [Google Scholar]
- Nwokeji K, Smith J, Goldenring JR. Localization of Rab25 and Rab11a in epithelia of the gastrointestinal tract. Gastroenterology. 1998;114:A1170. [Google Scholar]
- Odorizzi G, Pearse A, Domingo D, Trowbridge IS, Hopkins CR. Apical and basolateral nedosomes of MDCK cells are interconnected and contain a polarized sorting mechanism. J Cell Biol. 1996;135:139–152. doi: 10.1083/jcb.135.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Prydz K, Bomsel M, Simons K, Griffiths G. Meeting of the apical and basolateral endocytic pathways of the Madin–Darby canine kidney cell in late endosomes. J Cell Biol. 1989;109:3259–3272. doi: 10.1083/jcb.109.6.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Xu G, Zeng J, De Lemos-Chiarandini C, Adesnik M, Sabatini DD. Hydrolysis of GTP on rab11 is required for direct delivery of transferrin from the preicentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc Natl Acad Sci USA. 1998;95:6187–6192. doi: 10.1073/pnas.95.11.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynet C, Kahn CR. Rad: a member of the Ras family overexpressed in muscle of type II diabetic humans. Science. 1993;262:1441–1444. doi: 10.1126/science.8248782. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Ray GS, Calhoun BC, Goldenring JR. A somatodendritic distribution of Rab11 in rabbit brain neurons. NeuroReport. 1996;7:1297–1300. doi: 10.1097/00001756-199605170-00016. [DOI] [PubMed] [Google Scholar]
- Song W, Apodaca G, Mostov K. Transcytosis of the polymeric immunoglobulin receptor is regulated in multiple intracellular compartments. J Biol Chem. 1994;269:29474–29480. [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth NC, Goud B, Kabcenell AK, Novick P. Mutational analysis of SEC4 suggests a cyclical mechanism for the regulation of vesicular traffic. EMBO J. 1989;8:1685–1693. doi: 10.1002/j.1460-2075.1989.tb03560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollner DA, Nelson WJ. Establishing and maintaining epithelial cell polarity. Roles of protein sorting, delivery and retention. J Cell Sci. 1992;102:185–190. doi: 10.1242/jcs.102.2.185. [DOI] [PubMed] [Google Scholar]
- Zacchi P, Stenmark H, Parton RG, Orioli D, Lim F, Giner A, Mellman I, Zerial M, Murphy C. Rab17 regulates membrane trafficking through apical recycling endosomes in polarized epithelial cells. J Cell Biol. 1998;140:1039–1053. doi: 10.1083/jcb.140.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahraoui A, Touchot N, Chardin P, Tavitian A. The human rab genes encode a family of GTP-binding proteins related to yeast YPT1 and SEC4 products involved in secretion. J Biol Chem. 1989;264:12394–12401. [PubMed] [Google Scholar]