Abstract
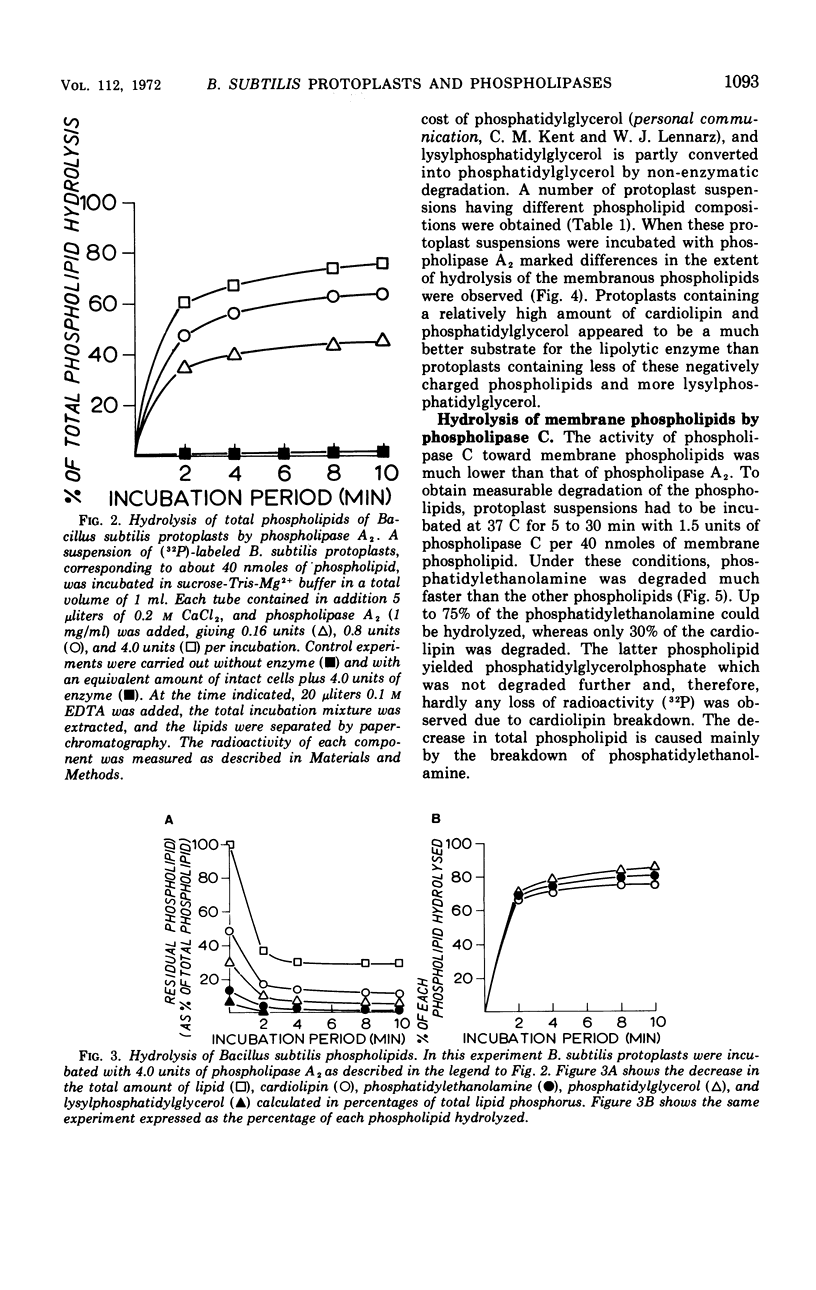
Protoplasts prepared from Bacillus subtilis by lysozyme digestion lysed in the presence of pure pancreatic phospholipase A2. The phospholipids cardiolipin, phosphatidylethanolamine, phosphatidylglycerol and lysylphosphatidylglycerol, which are present in the membrane, are degraded by phospholipase A2 only after removal of the cell wall, giving free fatty acids and lyso derivatives. The four phospholipids are hydrolyzed equally well at a given enzyme concentration. Differences in the phospholipid composition of the protoplasts were obtained by variations in the growth medium, time of harvesting, and preincubation time with lysozyme. The extent of hydrolysis appeared to depend on the initial phospholipid composition. A relative increase in acidic phospholipids in the membrane facilitated the action of phospholipase A2, whereas the rate of hydrolysis was diminished when protoplasts were tested which contained a relatively high amount of positively charged phospholipid. Pure phospholipase C from B. cereus preferentially hydrolyzed phosphatidyl-ethanolamine in the B. subtilis membrane. More than 80% of this phospholipid was converted into diglyceride, whereas only 30% of the cardiolipin was hydrolyzed. Such a loss of phospholipids, however, was not followed by lysis of the protoplasts. Liposomes were prepared from the lipid extracts of B. subtilis and incubated with both phospholipases. The hydrolysis pattern of the phospholipids in these model membrane systems was identical to the hydrolysis pattern of the phospholipids in the protoplast membrane. Phospholipase A2 hydrolyzed all the phospholipids in the liposomes equally well, whereas phospholipase C preferentially degraded phosphatidylethanolamine.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beebe J. L. Isolation and characterization of a phosphatidylethanolamine-deficient mutnt of Bacillus subtilis. J Bacteriol. 1971 Sep;107(3):704–711. doi: 10.1128/jb.107.3.704-711.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch L. L., Bonsen P. P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XIV. Phospholipids in Bacillus megaterium. J Bacteriol. 1969 Apr;98(1):75–81. doi: 10.1128/jb.98.1.75-81.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R., Finean J. B., Knutton S., Limbrick A. R. A structural study of the modification of erythrocyte ghosts by phospholipase C. Biochim Biophys Acta. 1970;219(1):81–92. doi: 10.1016/0005-2736(70)90063-5. [DOI] [PubMed] [Google Scholar]
- Glaser M., Simpkins H., Singer S. J., Sheetz M., Chan S. I. On the interactions of lipids and proteins in the red blood cell membrane. Proc Natl Acad Sci U S A. 1970 Mar;65(3):721–728. doi: 10.1073/pnas.65.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H. H., Kemper S. Endo-N-acetyl-glucosaminidase from Clostridium perfringens, lytic for cell wall murein of gram-negative bacteria. J Bacteriol. 1970 May;102(2):347–350. doi: 10.1128/jb.102.2.347-350.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain D. L., Rapport M. M. The effects of phospholipase C (Clostridium perfringens) on purified myelin. Biochim Biophys Acta. 1971 Jun 8;239(1):71–80. doi: 10.1016/0005-2760(71)90194-9. [DOI] [PubMed] [Google Scholar]
- Minnikin D. E., Abdolrahimzadeh H., Baddiley J. The interrelation of phosphatidylethanolamine and glycosyl diglycerides in bacterial membranes. Biochem J. 1971 Sep;124(2):447–448. doi: 10.1042/bj1240447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanninga N. Preservation of the ultrastructure of Bacillus subtilis by chemical fixation as verified by freeze-etching. J Cell Biol. 1969 Sep;42(3):733–744. doi: 10.1083/jcb.42.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonovski J., Wald R., Paysant M., Rampini C., Barbu E. Métabolisme du phosphatidylglycérol et du cardiolipide chez Staphylococcus aureus. Ann Inst Pasteur (Paris) 1971 May;120(5):589–598. [PubMed] [Google Scholar]
- Roelofsen B., Zwaal R. F., Comfurius P., Woodward C. B., van Deenen L. L. Action of pure phospholipase A 2 and phospholipase C on human erythrocytes and ghosts. Biochim Biophys Acta. 1971 Sep 14;241(3):925–929. doi: 10.1016/0005-2736(71)90024-1. [DOI] [PubMed] [Google Scholar]
- Simpkins H., Panko E., Tay S. Structural changes in the phospholipid regions of the axonal membrane produced by phospholipase C action. Biochemistry. 1971 Oct 12;10(21):3851–3855. doi: 10.1021/bi00797a008. [DOI] [PubMed] [Google Scholar]
- Slein M. W., Logan G. F., Jr Lysis of Escherichia coli by ethylenediaminetetraacetate and phospholipases as measured by beta-galactosidase activity. J Bacteriol. 1967 Oct;94(4):934–941. doi: 10.1128/jb.94.4.934-941.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbaum G., Rich R., Fischman D. A. Enzyme-induced formation of spheres from cells and envelopes of Escherichia coli. J Bacteriol. 1967 May;93(5):1693–1698. doi: 10.1128/jb.93.5.1693-1698.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside T. L., Corpe W. A. Effect of enzymes on the composition and structure of Chromobacterium violaceum cell envelopes. J Bacteriol. 1969 Mar;97(3):1449–1459. doi: 10.1128/jb.97.3.1449-1459.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaal R. F., Roelofsen B., Comfurius P., van Deenen L. L. Complete purification and some properties of phospholipase C from Bacillus cereus. Biochim Biophys Acta. 1971 Apr 13;233(2):474–479. doi: 10.1016/0005-2736(71)90347-6. [DOI] [PubMed] [Google Scholar]
- de Haas G. H., Bonsen P. P., van Deenen L. L. Studies on cardiolipin. 3. Structural identity of ox-heart cardiolipin and synthetic diphosphatidyl glycerol. Biochim Biophys Acta. 1966 Feb 1;116(1):114–124. doi: 10.1016/0005-2760(66)90097-x. [DOI] [PubMed] [Google Scholar]
- de Haas G. H., Postema N. M., Nieuwenhuizen W., van Deenen L. L. Purification and properties of phospholipase A from porcine pancreas. Biochim Biophys Acta. 1968 Apr 24;159(1):103–117. doi: 10.1016/0005-2744(68)90248-9. [DOI] [PubMed] [Google Scholar]
- den Kamp JA O. P., van Iterson W., van Deenen L. L. Studies of the phospholipids and morphology of protoplasts of Bacillus megaterium. Biochim Biophys Acta. 1967;135(5):862–884. doi: 10.1016/0005-2736(67)90056-9. [DOI] [PubMed] [Google Scholar]
- den Kamp J. A., Redai I., van Deenen L. L. Phospholipid composition of Bacillus subtilis. J Bacteriol. 1969 Jul;99(1):298–303. doi: 10.1128/jb.99.1.298-303.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



