Abstract
The gastrointestinal system plays a central role in immune system homeostasis. It is the main route of contact with the external environment and is overloaded every day with external stimuli, sometimes dangerous as pathogens (bacteria, protozoa, fungi, viruses) or toxic substances, in other cases very useful as food or commensal flora. The crucial position of the gastrointestinal system is testified by the huge amount of immune cells that reside within it. Indeed, gut-associated lymphoid tissue (GALT) is the prominent part of mucosal-associated lymphoid tissue (MALT) and represents almost 70% of the entire immune system; moreover, about 80% of plasma cells [mainly immunoglobulin A (IgA)-bearing cells] reside in GALT. GALT interacts strictly with gastrointestinal functions in a dynamic manner; for instance, by increasing intestinal permeability in replay to particular stimulations, or orientating the immune response towards luminal content, allowing either tolerance or elimination/degradation of luminal antigens, or sometimes provoking damage to the intestinal mucosa, such as in coeliac disease or food allergy. The immune mechanisms implicated in these actions are very complex and belong to both innate and adaptive immunity; innate immunity supplies an immediate non-specific response that is indispensable before specific adaptive immunity, which needs 7–10 days to be efficacious, takes place. The results of their interactions depend upon different contexts in which contact with external agents occurs and may change according to different genetic settings of the hosts.
Keywords: gastrointestinal system, gut-associated lymphoid tissue, innate immunity, adaptive immunity, allergy
Introduction
The gastrointestinal system plays a key role in the complex mechanisms of immunoregulation. The common embryonic matrix of entodermic derivation of respiratory and gastrointestinal tissues confers immunological peculiarities regarding the early sensitization and activation of tolerance mechanisms to the digestive system. Usually, the antigens responsible of initial sensitization and thus of allergic reactions are subsequently well tolerated by complex mechanisms, including immunodeviation and immunotolerance [1]. Such processes initiate during fetal life, when allergens and immunoglogulins in their Fab component are ingested by the fetus by swallowing amniotic liquid, and contact with the primordial gastrointestinal immune system activates the antigen-presenting cells (APC) already present in some mucosal intestinal sites [2,3].
The sensitizing or tolerogenic effect of this contact is not completely clear; it is not known what drives sensitization or tolerance once potentially allergenic proteins are absorbed. Studies conducted in the last 20 years suggest that a diet without the major sensitizing proteins (mainly from cow's milk and hen's egg) has no real advantage in terms of protection from allergy in the newborn.
Concerning respiratory allergy, it is known that exposure during the gestational phase to an environment rich in perennial allergens such as house dust mites and cat epithelium is able to stimulate the production of immunoglobulin E (IgE) antibodies, as assessed by detection in cord blood. It is also known that the fetus has an immunological system orientated towards the T helper type 2 (Th2) responses, which protect it from ‘graft-versus-host’-type reactions, and that only following birth can the Th2 phenotype shift to a Th1 phenotype which provides protection from allergic reactions. This is modulated by microbial agents, and particularly by being given lipopolysaccharides (LPS) typical of Gram-negative bacteria. By contrast, poor exposure to such microbes and strict hygiene will orientate towards persistence of the Th2 phenotype, with facilitation of the IgE-mediated allergic response [4–6].
The importance of immune modulation at the gastrointestinal level can be understood easily, considering that approximately 70% of the entire immune system is found in this site and that in the lamina propria there are about 80% of all plasma cells responsible for IgA antibody production [7,8].
Responses to the immune homeostasis to pathogens (bacteria, viruses, fungi and parasites), recognition and tolerance of self-antigens, tolerance to commensal flora and tolerance, but also sensitization and desensitization to foods, take place in the gastrointestinal system. However, the gastrointestinal system may be the target of immune reactions such as allergic reactions of the anaphylactic type and autoimmune reactions, such as occurs in Behçet disease.
Focusing upon particular immunological aspects, innate immunity provides a non-specific but extremely rapid response based on digestion of microbes or foreign antigens by macrophages [9,10].
A sophisticated receptor family, the Toll-like receptors (TLR), has considerable activity towards pathogens and foreign substances, being ready to defend within a few minutes to hours following contact. TLRs are very important in the clearance of pathogens and also orientation of the adaptive response to other antigens (towards sensitization or tolerance). The TLR family (which belongs to pattern recognition receptors, PRR) constitutes one of the major bridges between innate and acquired immunity. Thus far, 13 different TLRs are known, with different specificities to pathogens' categories differing by relatively small molecular characteristics. TLR-4 is responsible for recognition of the bacterial endotoxin LPS and other substances implicated in tolerance. TLRs are expressed as transmembrane proteins (heterodimers) on myelomonocytic, endothelial and epithelial cells, as well as on cells from various organs. Their immediate action is based on the activity of reactive oxygen, anti-microbial peptides, cytokines, chemokines, adhesion molecules and acute-phase proteins [11,12].
Such defence mechanisms are fundamental in the period preceding the induction of immunospecific adaptive responses, which require a relatively long time (7–10 days) [13,14]. TLR signalling of the presence of foreign agents drives a number of events, such as activation of the complement cascade, of interferon and of natural killer cells, and production of cytokines, chemokines and adhesion molecules.
A particular aspect of the immune system in the gastroenteric tract, i.e. the gut-associated lymphoid tissue (GALT), is the induction of immunotolerance mechanisms to food proteins introduced commonly in the diet. Involved in this complex process are the lymphocytes Th1 and T regulatory cells (Tregs). Recently, a pivotal role for Treg CD4+cells has been suggested [15]. These cells comprise two naturally occurring types, CD4+CD25+ and forkhead box P3 (FoxP3), responsible for anergy and immunosuppression phenomena, and T regulatory type 1 (Tr1) cells induced in particular situations characterized by the production of interleukin (IL)-10, transforming growth factor (TGF)-β and interferon (IFN)-γ.
Another cell type implicated in the immune response is the dendritic cell (DC), which has continuous cross-talk with T cells [16]. In particular, DC10 is a unique subset of tolerogenic DCs which secrete high levels of IL-10 and induce Tr1 cells [17]. A number of other factors co-operate in oral tolerance: for instance, vasoactive intestinal polypeptide, which is an important molecule of the neuroendrocrine–immune network and acts as an endogen anti-inflammatory mediator by regulating both cellular and humoral immune responses [18], also having the capacity to stimulate tolerogenic DCs. Additionally, the mechanisms regulating the intestinal barrier, as shown in Fig. 1, are extremely important in determining the type of response to foreign agents.
Fig. 1.
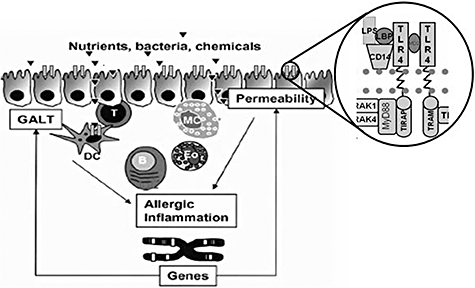
Control mechanisms of gastrointestinal barrier.
Clinical manifestations
The main factor underlying the clinical expression of gastrointestinal allergy is an imbalance of the Treg system and prevalence of the Th2 response with its cytokine pattern (IL-4 and IL-5). At intestinal level, the allergic manifestations are essentially IgE-mediated and more rarely cell-mediated, or caused by immune complexes. Table 1 describes the various disorders and their characteristics [19–22]
Table 1.
Clinical manifestations of gastroenteric allergy.
| Disorder | Mechanism | Symptoms | Diagnosis |
|---|---|---|---|
| Gastrointestinal anaphylaxis | IgE | Rapid onset of nausea, abdominal pain, cramps, vomiting and/or diarrhoea; other target organ responses (i.e. skin, respiratory tract) often involved | Clinical history and positive SPT responses or RAST results; ±oral challenge |
| Allergic eosinophilic oesophagitis | IgE and/or cell-mediated | Gastro-oesophageal reflux or excessive spitting-up or emesis, intermittent dysphagia, abdominal pain, irritability, sleep disturbance, failure to respond to conventional reflux medications | Clinical history, SPTs, endoscopy and biopsy, elimination diet and challenge |
| Allergic eosinophilic gastroenteritis | IgE and/or cell-mediated | Recurrent abdominal pain, irritability, early satiety, intermittent vomiting, FTT and/or weight loss, peripheral blood eosinophilia (in 50%) | Clinical history, SPTs, endoscopy and biopsy, elimination diet and challenge |
| Food protein-induced proctocolitis | Cell-mediated | Gross or occult blood in stool; typically thriving; usually presents in first few months of life | Negative SPT responses; elimination of food protein/clearing of most bleeding in 72 h; 6 endoscopy and biopsy; challenge induces bleeding within 72 h |
| Food protein-induced enterocolitis | Cell-mediated | Protracted vomiting and diarrhoea, (also bloody), not infrequently with dehydration; abdominal distention, FTT; vomiting typically delayed 1–3 h after feeding | Negative SPT responses; elimination of food protein/clearing of symptoms in 24–72 h, challenge/recurrent vomiting within 1–2 h; 15% have hypotension |
| Food protein-induced enteropathy | Cell-mediated | Diarrhoea or steatorrhoea, abdominal distention and flatulence, weight loss or FTT, nausea and vomiting, oral ulcers | Endoscopy and biopsy; elimination diet with resolution of symptoms and food rechallenge |
FTT: failure to thrive; IgE: immunoglobulin E; RAST: radioallergosorbent test; SPT: skin prick test.
Gastrointestinal anaphylaxis
Gastrointestinal anaphylaxis is a very severe reaction caused by the ingestion of foods such as cow's milk, hen's egg, peanut, fish and crustaceans. Its systemic nature is suggested strongly by the development of reactions to foods in subjects receiving liver transplantation from donors suffering from severe hypersensitivity to the same foods [23].
Allergic eosinophilic oesophagitis
Allergic eosinophilic oesophagitis is a disease characterized by swelling of the oesophagus caused by massive infiltration of eosinophils [24]. Symptoms can range from severe heartburn to difficulty swallowing, food impaction in the oesophagus, nausea, vomiting and weight loss. There appear to be some age-related differences in symptoms, with younger children having more symptoms of weight loss and older children and adults having food impaction and difficulty swallowing. It is not precisely clear what causes eosinophilic oesophagitis, although this disease may be related to other allergic diseases, particularly asthma. A personal or family history of other allergic diseases, such as hay fever, food allergy and asthma, is frequently observed. Various studies have shown that patients with eosinophilic oesophagitis have positive allergy tests to foods, and that avoidance of these foods leads to the resolution of symptoms. Culprit foods include milk, egg, peanut, shellfish, pea, beef, chicken, fish, rye, corn, soy, potatoes, oats, tomatoes and wheat. Of these, the most common food triggers are milk, egg, wheat, rye and beef. Environmental allergens such as pollens, moulds, cat, dog and dust mite allergens may also be involved in the development of eosinophilic oesophagitis. Diagnosis is generally made by performing a biopsy of the oesophagus, with evidence of eosinophils infiltrating the oesophageal tissue. Once diagnosed, extensive allergy testing by skin prick tests and or IgE sera detection is recommended.
Food protein enteropathy and food protein enterocolitis/proctitis
Food protein enteropathy and food protein enterocolitis/proctitis is an adverse reaction to foods affecting mainly children, mostly aged under 2–3 years [25]. The major causative allergens are milk and soybean. The immunological mechanism is linked to immune complexes and/or cell-mediated reactions, and clinical symptoms include vomiting, diarrhoea, enteropathy with protein loss and malabsorption and failure to thrive (FTT). Diagnosis is by endoscopy and histology, with identification of intraepithelial lymphocytes and eosinophils and atrophy of villuses (sprue-like). Usually spontaneous remission occurs within 2–3 years. A similar but more severe pathology is proctitis enterocolitis, which presents important ulcerations with consequent bleeding, haemorrhagia and anaemia.
Oral allergy syndrome (OAS)
OAS consists of itching and swelling of the lips, the oral mucosa and the soft palate immediately after eating fruits or vegetables. If the culprit food is ingested despite the local disturbances, gastrointestinal symptoms such as vomiting, abdominal pain and diarrhoea may occur and, more rarely, urticaria and anaphylaxis may also develop. OAS affects approximately 40% of subjects suffering from pollinosis; its pathogenetic mechanism is explained by cross-reacting allergens shared by pollens and vegetable foods [26]. Diagnosis of OAS relies on clinical history, physical examination, appropriate skin and/or in vitro testing. IgE-mediated sensitization is frequently not detected by using allergenic extracts because of the lability of the allergen responsible.
References
- 1.Adlerberth I, Hansson LÅ, Wold AE. The ontogeny of the intestinal flora. In: Sanderson IR, Walker WA, editors. Development of the gastrointestinal tract. Hamilton: BC Decker; 1999. pp. 279–92. [Google Scholar]
- 2.Gillian H, Vance S, Holloway JA. Early life exposure to dietary and inhalant allergens. Pediatr Allergy Immunol. 2002;13(Suppl)(15):14–8. doi: 10.1034/j.1399-3038.13.s.15.5.x. [DOI] [PubMed] [Google Scholar]
- 3.Warner JO, Jones CA, Kilburn SA, et al. Pre-natal sensitization in humans. Pediatr Allergy Immunol. 2000;11(Suppl)(13):6–8. doi: 10.1034/j.1399-3038.2000.00501.x. [DOI] [PubMed] [Google Scholar]
- 4.Wold AE. The hygiene hypothesis revised: is the rising frequency of allergy due to changes in the intestinal flora? Allergy. 1998;53:20–5. doi: 10.1111/j.1398-9995.1998.tb04953.x. [DOI] [PubMed] [Google Scholar]
- 5.Jones CA, Warner JA, Warner JO. Fetal swallowing of IgE [Letter] Lancet. 1998;351:1859. doi: 10.1016/S0140-6736(05)78805-X. [DOI] [PubMed] [Google Scholar]
- 6.Lilja G, Dannaeus A, Falth-Magnusson K, et al. Immune response of the atopic woman and foetus: effects of high- and low-dose food allergen intake during late pregnancy. Clin Allergy. 1988;18:131–4. doi: 10.1111/j.1365-2222.1988.tb02852.x. [DOI] [PubMed] [Google Scholar]
- 7.Weiner HL. Oral tolerance, an active immunologic process mediated by multiple mechanisms. J Clin Invest. 2000;106:935–7. doi: 10.1172/JCI11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–59. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–41. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 10.Cebra JJ, Periwal SB, Lee G, et al. Development and maintenance of the gut-associated lymphoid tissue (GALT): the roles of enteric bacteria and viruses. Dev Immunol. 1998;6:13–18. doi: 10.1155/1998/68382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 12.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Stuart E, Turvey A, Thomas R, Hawn B. Towards subtlety: understanding the role of Toll-like receptor signaling in susceptibility to human infections. Clin Immunol. 2006;120:1–9. doi: 10.1016/j.clim.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Minns LA, Menard LC, Foureau DM, et al. TLR9 is required for the gut-associated lymphoid tissue response following oral infection of Toxoplasma gondii. J Immunol. 2006;176:7589–97. doi: 10.4049/jimmunol.176.12.7589. [DOI] [PubMed] [Google Scholar]
- 15.Qiao M, Thornton AM, Shevach EM. CD4+ CD25+ regulatory T cells render naïve CD4+ CD25– T cells anergic and suppressive. Immunology. 2007;120:447–55. doi: 10.1111/j.1365-2567.2007.02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reschner A, Huberth P, Delvenne P, et al. Innate lymphocyte and dendritic cell cross-talking: a key factor in the regulation of the immune response. Clin Exp Immunol. 2008;152:219–26. doi: 10.1111/j.1365-2249.2008.03624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carbonnel C, Seidi H, Donkova-Petrini V, et al. DC generated in the presence of interferon alpha stimulate allogeneic CD4+ T cells proliferation: modulation by autocrine IL-10, enhanced T cell apoptosis and T regulatory type 1 cells. Int Immunol. 2004;16:1037–52. doi: 10.1093/intimm/dxh106. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Mei Y, Bao S, Xu L. Vasoactive intestinal polypeptide enhances oral tolerance by regulating both cellular and humoral immune responses. Clin Exp Immunol. 2007;148:178–87. doi: 10.1111/j.1365-2249.2007.03322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roehr CC, Edenharterw G, Reimannz S, et al. Food allergy and non-allergic food hypersensitivity in children and adolescents. Clin Exp Allergy. 2004;34:1534–41. doi: 10.1111/j.1365-2222.2004.02080.x. [DOI] [PubMed] [Google Scholar]
- 20.Muraro MA. Diagnosis of food allergy: the oral provocation test. Pediatr Allergy Immunol. 2001;12(Suppl)(14):31–6. doi: 10.1034/j.1399-3038.2001.121407.x. [DOI] [PubMed] [Google Scholar]
- 21.Sampson HA. Food allergy. Part 1. Immunopathogenesis and clinical disorders. J Allergy Clin Immunol. 1999;103:717–28. doi: 10.1016/s0091-6749(99)70411-2. [DOI] [PubMed] [Google Scholar]
- 22.Sampson HA. Food allergy. Part 2. Diagnosis and management. J Allergy Clin Immunol. 1999;103:981–9. doi: 10.1016/s0091-6749(99)70167-3. [DOI] [PubMed] [Google Scholar]
- 23.Boyle RJ, Hardikar W, Tang MLK. The development of food allergy after liver transplantation. Liver Transplant. 2005;11:326–30. doi: 10.1002/lt.20368. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson DD, Foxx-Orenstein AE. Eosinophlic esophagitis: an update. Dis Esophagus. 2007;20:2–8. doi: 10.1111/j.1442-2050.2007.00649.x. [DOI] [PubMed] [Google Scholar]
- 25.Chehade M, Mafid MS, Mofidi S, et al. Allergic eosinophilic gastroenteritis with protein-loss enteropathy: intestinal pathology, clinical course, and long-term follow-up. J Pediatr Gastroenterol Nutr. 2006;42:516–21. doi: 10.1097/01.mpg.0000221903.61157.4e. [DOI] [PubMed] [Google Scholar]
- 26.Pastorello EA, Incorvaia C, Pravettoni V, et al. Crossreactions in food allergy. Clin Rev Allergy Immunol. 1997;15:415–27. doi: 10.1007/BF02737737. [DOI] [PubMed] [Google Scholar]


