Abstract
The eye represents an ideal and frequent site for the allergic reactions. The term ‘allergic conjunctivitis’ refers to a collection of disorders that affect the lid, conjunctiva and/or cornea. Even though the diagnosis is essentially clinical, local tests such as cytology, conjunctival provocation and tear mediator analysis can be performed. The immunoglobulin E (IgE)-mediated mechanism does not explain completely the severity and the clinical course of chronic allergic ocular diseases such as vernal (VKC) and atopic keratoconjunctivitis (AKC), which are probably also related to T cell-mediated responses, massive eosinophil attraction and activation and non-specific hypersensitivity. An altered balance between T helper type 1 (Th1) and Th2 cells and between Th1- and Th2-types of cytokines is thought to be responsible of the development of ocular allergic disorders. New findings suggest that a wide range of cytokines, chemokines, proteases and growth factors are involved by complex interwoven interactions rather than distinct and parallel pathways. In addition, several non-specific enzymatic systems may be activated during acute and chronic allergic inflammation, thus contributing to the complex pathogenesis of the disease. Current drug treatment for ocular allergy targets the key mechanisms involved in the development of clinical disease: mast cells with mast cell stabilizers, histamine with histamine receptor antagonists and inflammation with corticosteroids, severe inflammation with immunomodulators. None of these agents lacks side effects and none abolishes signs and symptoms completely. New therapeutic strategies are still needed to respond to the complex pathogenesis of severe forms of ocular allergy such as VKC and AKC.
Keywords: allergic conjunctivitis, cytokines, treatment of ocular allergy, VKC
Introduction
Allergic conjunctivitis is a localized allergic condition associated frequently with rhinitis but often observed as the only or prevalent allergic sensitization. Ocular symptoms have been estimated to be present in 40–60% of the allergic population [1]. The disease ranges in severity from mild forms, which can still interfere significantly with quality of life, to severe cases characterized by potential impairment of visual function.
The term ‘allergic conjunctivitis’ refers to a collection of hypersensitivity disorders that affect the lid, conjunctiva and/or cornea. Various clinical forms are included in the classification of ocular allergy and may be divided two main groups, the first one including the more frequent, seasonal (SAC) and perennial allergic conjunctivitis (PAC), which may also be called rhinoconjunctivitis or conjunctivorhinitis depending on the main symptoms. These forms are diagnosed and managed by general practitioners, allergists, paediatricians or ophthalmologists, but frequently only by the pharmacists or by the patient himself. The second group includes the less frequent chronic severe forms of ocular allergy, vernal keratoconjunctivitis (VKC) and atopic keratoconjunctivitis (AKC), that may be complicated by the corneal involvement and that need careful management by expert ophthalmologists in association with allergists.
Contact lenses or ocular prosthesis-associated giant papillary conjunctivitis (GPC), even though sharing similarities with VKC, should not be considered as real allergic diseases, but as exclusively ocular, chronic micro-trauma related disorders, which need to be managed by ophthalmologists in association with contact lenses experts. The increasing number of contact bepharitis and drug-induced conjunctivitis or dermato-conjunctivitis suggests that additional entities, partially toxic, partially immune-mediated, may occur to the ocular surface as a result of increased susceptibility and hypersensitivity to haptens, cosmetics, drugs, pollutants or other environmental factors.
Clinical forms
Seasonal and perennial allergic conjunctivitis are caused by a typical imunoglobulin E (IgE)-mediated reaction to environmental airborne allergens, such as grass and tree pollens, mites, mould and animal dander. SAC is usually an acute or subacute manifestation characterized by peaks of self-limiting signs and symptoms that become persistent in repeated allergen stimulations during pollen season. The hallmark signs and symptoms are itching, redness and lid swelling, while patients may also complain of tearing, mucous discharge and burning. In PAC, the non-specific signs and symptoms of redness, burning and chemosis may persist with varying severity for months, accompanied by a low level of itching, making the patient uncomfortable and worried. Ocular symptoms may be associated with seasonal, persistent and chronic rhinitis, and less frequently may present occasionally in asthmatic patients [1]. Skin prick testing may be useful in identifying relevant allergens, but it must be remembered that a significant number of patients presenting with SAC may have negative skin tests.
Vernal keratoconjunctivitis is a relatively rare ocular allergic disease affecting children and young adults living in warm climates and associated, in approximately half of cases, with other allergic manifestations [2–4]. The disease is usually seasonal, lasting from the beginning of spring until autumn. However, perennial cases that are persistent throughout the year are not rare, especially in patients living in subtropical or desert climates. Its predominance during the high pollen season lends credence to the widely accepted hypothesis that VKC is an immunologically mediated hypersensitivity reaction to environmental antigens.
Atopic keratoconjunctivitis is the ocular manifestation of a complex and systemic altered immune response, often associated with atopic dermatitis and with other allergic disorders such as rhinitis and asthma. Ocular clinical expression can involve eyelids, conjunctiva and cornea, and includes different levels of severity, from mild injuries to sight-threatening complications [5].
The eye and allergic inflammation
The ocular allergic response results from exposure of the conjunctiva to an environmental allergen and binding with specific IgE on the conjunctival mast cells. This immediate response lasts clinically for 20–30 min, as demonstrated by the specific conjunctival allergen challenge (CAC) [6]. It induces enhanced tear levels of histamine, tryptase, prostaglandins and leukotrienes [1]. Mast cell degranulation also induces activation of vascular endothelial cells and thus the expression of chemokines and adhesion molecules, such as regulated upon activation normal T cell expressed and secreted (RANTES) chemokines, monocyte chemoattractant protein (MCP), interleukin (IL)-8, eotaxin, macrophage inflammatory protein (MIP)-1α, intercellular adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM) and p-selectin. These factors initiate the recruitment phase of inflammatory cells in the conjunctival mucosa, which leads to the ocular late-phase reaction [1]. The late phase corresponds to the persistent clinical inflammation that characterizes the ocular signs and symptoms in perennial and chronic allergic diseases [1,7], and is characterized by the mucosal infiltration of eosinophils, neutrophils, basophils and T lymphocytes [8]. In addition, conjunctival and corneal epithelial cells and fibroblasts may contribute to mounting the allergic inflammation by expressing and producing cytokines, chemokines, adhesion molecules and factors that maintain local inflammation and lead to tissue remodelling [3,9,10].
Mast cells play a key in the pathogenesis of ocular allergic reaction. The number of mast cells in the human conjunctiva has been calculated to be between 5000 and 6000 cell/mm3[11]. Immunohistochemical phenotyping of mast cells (MC) in the normal human conjunctiva has demonstrated that the MCTC (tryptase and chymase-positive MC) phenotype is predominant, similar to the findings in human derma [12,13]. During the pollen season, the median mast cell numbers in the lamina propria were increased by 61% in SAC patients compared to normal subjects and remained increased in allergic patients out of season [11]. The total number of mast cells is increased in the stroma and epithelium of VKC and AKC patients.
Activated mast cells, besides the gamut of preformed and newly formed mediators, can release several cytokines such as IL-4, IL-6, IL-8, IL-13, tumour necrosis factor (TNF)-α and transforming growth factor (TGF)-β, which have profound effects on the mucosa, including the induction of chemokines and adhesion molecules that contribute to the recruitment of inflammatory cells [14,15].
Vernal keratoconjunctivitis
VKC is a rare and severe form of allergic conjunctivitis. Although no studies provide accurate calculations of VKC prevalence in the general population in European Union (EU), Asia, America or Africa, epidemic characteristics have been described in case series [2–4]. In one epidemiological report from Sweden, prevalence of VKC was clearly augmented by immigration of children with an African and Asian origin [16], suggesting that both genetic and environmental factors are implicated in the incidence of the disease. We calculated that seven new cases of VKC every 100 000 inhabitants were reported each year in children up to 15 years of age, with approximately one new case per 1 600 000 people in the population over 16 years of age (0·06/100 000) [17]. This confirms that VKC is not a rare event in the paediatric population, but is an extremely rare new disease in adults. Interestingly, the new cases of VKC are much more frequent in males compared to females in the young population whereas, over 16 years of age, the incidence of the disease was higher in females than in males.
In our series of 509 VKC patients, 77% of patients are male. Distribution of the three clinical forms, age of onset and duration of the disease and family history of allergy are shown in Table 1. The disease was bilateral in 96·7% of the cases; all unilateral cases involved the tarsal form of VKC. The results of allergy tests, the mean total serum IgE, the mean blood eosinophil count and the mean level of serum eosinophil cationic protein (ECP) are shown in Table 2.
Table 1.
Demographic data from 509 vernal keratoconjunctivitis patients.
| Sex (M/F) | Tarsal form | Limbal form | Mixed form | Age of onset | Duration | Family history of allergy |
|---|---|---|---|---|---|---|
| 393/116 | 29% | 55% | 16% | 7·5 ± 5·5 | 6·7 ± 4 | 45·3% |
Table 2.
Immunological data in vernal keratoconjunctivitis patients: mean ± standard deviation and range.
| Positive prick test (%) | Positive specific serum IgE (%) | Positive CPT (%) | Serum total IgE (KU/l) | Blood eosinophil count (×109/l) | Serum ECP (mg/l) |
|---|---|---|---|---|---|
| 208/509 (40%) | 165/309 (53%) | 62/110 (56%) | 428 ± 1063 (0–12801) | 0·6 ± 0·5 (0–4·8) | 36 ± 354 (0·3–257) |
CPT: conjuctival provocation test; ECP: eosinophil cationic protein; IgE: immunoglobulin E.
The massive infiltration of inflammatory cells, typical of VKC, differentiates this disease from SAC and PAC. The presence of T helper type 2 (Th2) cells [18] and Th2-type cytokines has been proven and confirmed in several studies. However, during the active inflammatory phase of the disease multiple Th1-type and Th2-type cytokines are overexpressed and produced [10,19], including the typical Th1-type cytokine, interferon (IFN)-γ, which probably contribute to increasing ocular inflammation similarly to what has been shown in the animal models [20,21]. When we looked at the tear cytokine profile in the active phase (Fig. 1), the different ocular allergic diseases differ predominantly in the quantity, rather than the quality, of cytokine present [10]. Chemokines such as IL-8, MCP-1, RANTES and eotaxins are secreted actively in VKC and produced by mast cells, macrophages, epithelial cells and fibroblasts [9,10,22]. Chemokine receptors, CCR3 and CXCR3, were found to be greatly up-regulated in conjunctival tissues and T cells [23].
Fig. 1.
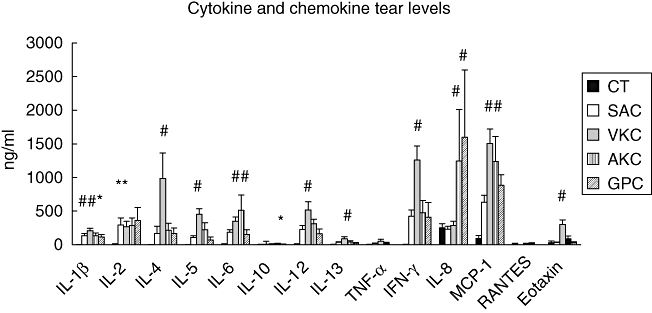
Cytokine and chemokine tear levels measured by multiplex bead immunoassay in normal subjects (CT; n = 14) and in patients with different active ocular allergic diseases: seasonal allergic conjunctivitis (SAC; n = 12), vernal keratoconjunctivitis (VKC; n = 18), atopic keratoconjunctivitis (AKC; n = 6) and contact lens related giant papillary conjunctivitis (GPC; n = 4). Bars represent the median value for each group. #P < 0·01; *≤ 0·05 compared to controls.
The subsequent massive eosinophil infiltration and activation is responsible for the corneal complications associated with the diseases [24]. Corneal involvement has been correlated with conjunctival redness and expression of local inflammation, but not to the size of giant papillae or other clinical signs [25]. Corneal epithelial punctate keratitis may evolve to macroerosion, ulcers and plaques, which are all expressions of epithelial toxicity extricated by epitheliotoxic factors released by activated eosinophils.
Th2-type cytokines IL-4 and IL-13 also have another important role in the pathogenesis of this disease, as they stimulate the migration, proliferation and collagen production from conjunctival fibroblasts [2,9,10]. The altered balance between the expression of matrix metalloproteinases (MMPs) and tissue inhibitors of MMP (TIMP) contribute to the excessive deposition of extracellular matrix and the formation of giant papillae [26]. It has been shown that several growth factors, including vascular endothelial growth factor (VEGF), are overexpressed in VKC tissues and may involve tissue growth and remodelling [27,28]. Other enzymatic systems are involved in the inflammatory and remodelling processes in this disease. Urokinase, an extravascular fibrinolytic system activator, is highly produced in active patients, and expressed by inflammatory and conjunctival cells [29]. The activity of alpha 1 anti-trypsin (AAT), the archetype of the serine protease inhibitor, is reduced locally in VKC creating an imbalance between protease and inhibitors, and facilitating or prolonging conjunctival inflammation [30].
Recently we have shown increased and altered expression of muscarinic and adrenergic receptors and neurotransmitters in VKC tissues [31], explaining the mucus hypersecretion, goblet cells hyperplasia and conjunctival hyperreactivity typical of this disease.
One of the most spectacular events in the tarsal form of VKC is the overgrowth of conjunctival connective tissue, with the formation of large and sessile papillae from which overflow an abundance of collagen fibres. The term ‘tissue remodelling’ defines a gamut of alterations involving structural cells and tissues such as conjunctival thickening, subepithelial fibrosis, mucus metaplasia, neovascularization and scarring. Many elements contribute to this dramatic response, including epithelial changes, connective tissue deposition, oedema, inflammatory cell infiltration and glandular hypertrophy.
Notes of treatment
The primary goal in management of ocular allergies is to identify the causes and to prevent recurrence by eliminating them. If this seems easy in theory, it is often difficult or impossible to achieve in practice. Thus medication is the obligate option, which need to be supported by patient and family education especially in the chronic forms. Current drug treatment for ocular allergy targets the key mechanisms involved in the development of clinical disease: mast cells with mast cell stabilizers, histamine with histamine receptor antagonists and inflammation with corticosteroids, severe inflammation with immunomodulators [32]. None of these agents lacks side effects and none completely abolishes signs and symptoms. New therapeutic strategies are still needed to respond to the complex pathogenesis of severe forms of ocular allergy such as VKC and AKC.
References
- 1.Ono SJ, Abelson MB. Allergic conjunctivitis: update on pathophysiology and prospects for future treatment. J Allergy Clin Immunol. 2005;115:118–22. doi: 10.1016/j.jaci.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 2.Leonardi A. Vernal keratoconjunctivitis: pathogenesis and treatment. Prog Ret Eye Res. 2002;21:319–39. doi: 10.1016/s1350-9462(02)00006-x. [DOI] [PubMed] [Google Scholar]
- 3.Bonini S, Bonini S, Lambiase A, et al. Vernal keratoconjunctivitis revisited. A case series of 195 patients with long-term followup. Ophthalmology. 2000;107:1157–63. doi: 10.1016/s0161-6420(00)00092-0. [DOI] [PubMed] [Google Scholar]
- 4.Pucci N, Novembre E, Lombardi E, et al. Atopy and serum eosinophil cationic protein in 110 white children with vernal keratoconjunctivitis: differences between tarsal and limbal forms. Clin Exp Allergy. 2003;33:325–30. doi: 10.1046/j.1365-2222.2003.01538.x. [DOI] [PubMed] [Google Scholar]
- 5.Foster CS, Calonge M. Atopic keratoconjunctiuvitis. Ophthalmology. 1990;97:992–100. doi: 10.1016/s0161-6420(90)32477-6. [DOI] [PubMed] [Google Scholar]
- 6.Abelson MB, Chambers WA, Smith LM. Conjunctival allergen challenge. A clinical approach to studying allergic conjunctivitis. Arch Ophthalmol. 1990;108:84–8. doi: 10.1001/archopht.1990.01070030090035. [DOI] [PubMed] [Google Scholar]
- 7.Bacon AS, Ahluwalia P, Irani AM, et al. Tear and conjunctival changes during the allergen-induced early- and late-phase responses. J Allergy Clin Immunol. 2000;106:948–54. doi: 10.1067/mai.2000.110930. [DOI] [PubMed] [Google Scholar]
- 8.Bonini S, Bonini S, Vecchione A, et al. Inflammatory changes in conjunctival scrapings after allergen challenge in humans. J Allergy Clin Immunol. 1988;82:462–6. doi: 10.1016/0091-6749(88)90020-6. [DOI] [PubMed] [Google Scholar]
- 9.Kumagai N, Fukuda K, Fujitsu Y, Yamamoto K, Nishida T. Role of structural cells of the cornea and conjunctiva in the pathogenesis of vernal keratoconjunctivitis. Prog Retina Eye Res. 2006;25:165–87. doi: 10.1016/j.preteyeres.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Leonardi A, Curnow SJ, Zhan H, Calder VL. Multiple cytokines in human tear specimens in seasonal and chronic allergic eye disease and in conjunctival fibroblast cultures. Clin Exp Allergy. 2006;36:777–84. doi: 10.1111/j.1365-2222.2006.02499.x. [DOI] [PubMed] [Google Scholar]
- 11.Anderson DF, MacLeod JD, Baddeley SM, et al. Seasonal allergic conjunctivitis is accompanied by increased mast cell numbers in the absence of leukocyte infiltration. Clin Exp Allergy. 1997;27:1060–6. doi: 10.1111/j.1365-2222.1997.tb01258.x. [DOI] [PubMed] [Google Scholar]
- 12.Irani AM, Butrus SI, Tabbara KF, Schwartz LB. Human conjunctival mast cells: distribution of MCT and MCTC in vernal conjunctivitis and giant papillary conjunctivitis. J Allergy Clin Immunol. 1990;86:34–40. doi: 10.1016/s0091-6749(05)80120-4. [DOI] [PubMed] [Google Scholar]
- 13.Miller S, Cook E, Graziano F, Spellman J, Yanni J. Human conjunctival mast cell responses in vitro to various secretagogues. Ocular Immunol Inflamm. 1996;4:39–49. doi: 10.3109/09273949609069126. [DOI] [PubMed] [Google Scholar]
- 14.Anderson DF, Zhang S, Bradding P, et al. The relative contribution of mast cell subsets to conjunctival T(H)2-like cytokines. Invest Ophthalmol Vis Sci. 2001;42:995–1001. [PubMed] [Google Scholar]
- 15.Cook EB, Stahl JL, Miller ST, et al. Isolation of human conjunctival mast cells and epithelial cells: tumor necrosis factor-alpha from mast cells affects intercellular adhesion molecule 1 expression on epithelial cells. Invest Ophthalmol Vis Sci. 1998;39:336–43. [PubMed] [Google Scholar]
- 16.Montan PG, Ekstrom K, Hedlin G, et al. Vernal keratoconjunctivitis in a Stockholm ophthalmic centre: epidemiological, functional, and immunologic investigations. Acta Ophthalmol Scand. 1999;77:559–63. doi: 10.1034/j.1600-0420.1999.770516.x. [DOI] [PubMed] [Google Scholar]
- 17.Leonardi A, Busca F, Motterle L, et al. Case series of 406 vernal keratoconjunctivitis patients: a demographic and epidemiological study. Acta Ophthalmol Scand. 2006;84:406–10. doi: 10.1111/j.1600-0420.2005.00622.x. [DOI] [PubMed] [Google Scholar]
- 18.Calder VL, Jolly G, Hingorani M, et al. Cytokine production and mRNA expression by conjunctival T cell lines in chronic allergic eye diseases. Clin Exp Allergy. 1999;29:1214–22. doi: 10.1046/j.1365-2222.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- 19.Leonardi A, Fregona IA, Plebani M, et al. Th1- and Th2-type cytokines in chronic ocular allergy. Graefe's Arch Clin Exp Ophthalmol. 2006;244:1240–45. doi: 10.1007/s00417-006-0285-7. [DOI] [PubMed] [Google Scholar]
- 20.Stern ME, Siemasko K, Gao J, et al. Role of Interferon-gamma in a mouse model of allergic conjunctivitis. Invest Ophthalmol Vis Sci. 2005;46:3239–46. doi: 10.1167/iovs.05-0138. [DOI] [PubMed] [Google Scholar]
- 21.Fukushima F, Sumi T, Fukuda K, et al. Analysis of the interaction between IFNγ and IFNγR in the effector phase of experimental murine allergic conjunctivitis. Immunol Lett. 2006;107:119–24. doi: 10.1016/j.imlet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Leonardi A, Jose PJ, Zhan H, Calder VL. Tear and mucus eotaxin-1 and eotaxin-2 in allergic keratoconjunctivitis. Ophthalmology. 2003;110:487–92. doi: 10.1016/S0161-6420(02)01767-0. [DOI] [PubMed] [Google Scholar]
- 23.El-Asrar AM, Struyf S, Al-Kharashi SA, Missotten L. Chemokine receptors in vernal keratoconjunctivitis. Br J Ophthalmol. 2001;85:1360–66. doi: 10.1136/bjo.85.11.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ono SJ. Vernal keratoconjunctivitis: evidence for immunoglobulin E-dependent and immunoglobulin E-independent eosinophilia. Clin Exp Allergy. 2003;33:279–81. doi: 10.1046/j.1365-2745.2003.01616.x. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka M, Dogru M, Takano Y, et al. The relation of conjunctival and corneal findings in severe ocular allergies. Cornea. 2004;23:464–7. doi: 10.1097/01.ico.0000114836.13127.45. [DOI] [PubMed] [Google Scholar]
- 26.Leonardi A, Brun P, Abatangelo G, Plebani M, Secchi AG. Tear levels and activity of matrix metalloproteinases (MMP)-1 and MMP-9 in vernal keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2003;44:3052–8. doi: 10.1167/iovs.02-0766. [DOI] [PubMed] [Google Scholar]
- 27.Leonardi A, Tavolato M, Brun P, et al. Growth factors and collagen distribution in vernal keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2000;41:4175–81. [PubMed] [Google Scholar]
- 28.Asano-Kato N, Fukagawa K, Okada N, et al. TGF-beta1, IL-1beta, and Th2 cytokines stimulate vascular endothelial growth factor production from conjunctival fibroblasts. Exp Eye Res. 2005;80:555–60. doi: 10.1016/j.exer.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Leonardi A, Brun P, Sartori MT, et al. Urokinase plasminogen activator, uPA receptor and its inhibitor in veronal keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2005;46:1364–70. doi: 10.1167/iovs.04-1196. [DOI] [PubMed] [Google Scholar]
- 30.Gravami S, Hashemi M, de Serres FJ, et al. Trypsin inhibitory capacity in vernal keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2007;48:264–9. doi: 10.1167/iovs.06-0758. [DOI] [PubMed] [Google Scholar]
- 31.Motterle L, Diebold Y, Enriquez de Salamanca A, et al. Altered expression of neurotrasmitter receptors and neuromediators in vernal keratoconjunctivitis. Arch Ophthalmol. 2006;124:462–8. doi: 10.1001/archopht.124.4.462. [DOI] [PubMed] [Google Scholar]
- 32.Leonardi A. Emerging drugs for ocular allergy. Expert Opin Emerging Drugs. 2005;10:505–20. doi: 10.1517/14728214.10.3.505. [DOI] [PubMed] [Google Scholar]


