Abstract
Catecholaminergic polymorphic ventricular tachycardia (VT) is a lethal familial disease characterized by bidirectional VT, polymorphic VT, and ventricular fibrillation. Catecholaminergic polymorphic VT is caused by enhanced Ca2+ release through defective ryanodine receptor (RyR2) channels. We used epicardial and endocardial optical mapping, chemical subendocardial ablation with Lugol’s solution, and patch clamping in a knockin (RyR2/RyR2R4496C) mouse model to investigate the arrhythmogenic mechanisms in catecholaminergic polymorphic VT. In isolated hearts, spontaneous ventricular arrhythmias occurred in 54% of 13 RyR2/RyR2R4496C and in 9% of 11 wild-type (P=0.03) littermates perfused with Ca2+ and isoproterenol; 66% of 12 RyR2/RyR2R4496C and 20% of 10 wild-type hearts perfused with caffeine and epinephrine showed arrhythmias (P=0.04). Epicardial mapping showed that monomorphic VT, bidirectional VT, and polymorphic VT manifested as concentric epicardial breakthrough patterns, suggesting a focal origin in the His–Purkinje networks of either or both ventricles. Monomorphic VT was clearly unifocal, whereas bidirectional VT was bifocal. Polymorphic VT was initially multifocal but eventually became reentrant and degenerated into ventricular fibrillation. Endocardial mapping confirmed the Purkinje fiber origin of the focal arrhythmias. Chemical ablation of the right ventricular endocardial cavity with Lugol’s solution induced complete right bundle branch block and converted the bidirectional VT into monomorphic VT in 4 anesthetized RyR2/RyR2R4496C mice. Under current clamp, single Purkinje cells from RyR2/RyR2R4496C mouse hearts generated delayed afterdepolarization–induced triggered activity at lower frequencies and level of adrenergic stimulation than wild-type. Overall, the data demonstrate that the His–Purkinje system is an important source of focal arrhythmias in catecholaminergic polymorphic VT.
Keywords: ryanodine receptor, CPVT, transgenic mice, bidirectional ventricular tachycardia, sudden cardiac death
Catecholaminergic polymorphic ventricular tachycardia (CPVT) (Online Mendelian Inheritance in Man no.604772) is an inherited disease leading to arrhythmias and sudden cardiac death.1 The autosomal dominant form has been linked to ryanodine receptor gene (RyR2) mutations, leading to increased spontaneous Ca2+ release from the sarcoplasmic reticulum.2 Typical arrhythmias are bidirectional ventricular tachycardia (BVT) and polymorphic ventricular tachycardia (PVT) that can degenerate into ventricular fibrillation (VF) and thus sudden cardiac death.3 BVT is infrequent, characterized by beat-to-beat 180° alternation of the QRS of the ECG and occurs in CPVT, as well as in digitalis toxicity; thus, it has been inferred that arrhythmogenesis in CPVT is mediated by delayed afterdepolarization (DAD)-induced triggered activity (TA).
Mice heterozygous for the R4496C mutation (RyR2/RyR2R4496C) recapitulate the human phenotype of CPVT by developing BVT, PVT, and/or VF under adrenergic stimulation.4 Recently, Liu et al5 have demonstrated DADs in RyR2/RyR2R4496C mouse ventricular myocytes both in control and in the presence of isoproterenol. However, it remains to be demonstrated whether the arrhythmia originates in the 3D myocardium or in the cable-like Purkinje fiber network. In addition, it is unknown whether the transition from BVT into PVT and VF involves triggered or reentrant mechanisms.
Here we used the RyR2/RyR2R4496C mouse to investigate arrhythmia mechanisms associated with elevated extracellular calcium ([Ca2+]o) and/or catecholaminergic stimuli. Our main objective was to test the idea that arrhythmias in this mouse model, and by inference in CPVT patients, are triggered by DADs occurring in Purkinje fibers on the right and left branches of the specialized ventricular conducting system.
Materials and Methods
Animals
This study conformed to the Guide for the Care and Use of Laboratory Animals (NIH Publication no. 85–23, revised 1996). Details of the production of knockin RyR2/RyR2R4496C mice have been published.4
Optical Mapping Experiments
Thirty heterozygous RyR2/RyR2R4496C (16 males; 4.2±1 month) and 21 wild-type (WT) (10 males, 4.5±0.9 months) littermates were used. Epicardial and endocardial optical mapping was performed in isolated, Langendorff-perfused hearts. Volume-conducted ECG was recorded; activation and phase maps were generated (see the online data supplement at http://circres.ahajournals.org).6–10
Two different protocols were used to induce ventricular arrhythmias. First, for epicardial mapping, 13 RyR2/RyR2R4496C and 11 WT hearts were perfused with Tyrode’s solution containing 2.7 to 3.6 mmol/L Ca2+ and 100 to 200 nmol/L isoproterenol. Five additional RyR2/RyR2R4496C hearts were perfused with the above-mentioned drug-containing solution for endocardial mapping. Second, 12 RyR2/RyR2R4496C and 10 WT hearts were perfused with Tyrode’s solution containing caffeine (1 to 5 mmol/L) and epinephrine (0.1 to 1.6 μmol/L).
Chemical Subendocardial Ablation
Mice were anesthetized with Avertin (Sigma) and ventilated through a tracheostomy. Lead I ECG was recorded. IP caffeine (120 mg/kg) and epinephrine (2 mg/kg) were injected as described previously4; several minutes were allowed for arrhythmia initiation.5 Subsequently, a bolus of Lugol’s solution (5 to 7 μL; Humco) or Tyrode’s solution was carefully injected directly into the right ventricular (RV) cavity using a Hamilton syringe inserted through the diaphragm from a minimal abdominal incision. Six RyR2/RyR2R4496C mice (4 females; 5.5±3.8 months) were used in these experiments. For control, we injected 5 to 7 μL of Lugol’s solution in 3 WT (2 females; 4.3±0.5 months) mice and 5 to 7 μL of normal Tyrode’s solution in another set of 3 WT mice (2 females; 4.3±0.5 months) during sinus rhythm (SR). See the online data supplement for details.
Purkinje Cell Recordings
Adult RyR2/RyR2R4496C and WT mouse Purkinje cells were obtained by enzymatic dissociation.11 Under whole-cell current-clamp conditions, action potentials were elicited by 5-ms stimuli at ≈2× threshold amplitude; resting membrane potential, action potential amplitude, action potential duration (APD50, 70,90), and dV/dtmax were determined. DADs and TA were induced by trains of 20 pulses at 1, 5, 10, and 20 Hz in control and in the presence of 30 nmol/L isoproterenol. See the online data supplement for details.
Statistical Analyses
Student t test was used to compare normally distributed variables. Cross tabulation with Fischer’s exact test was used for categorical variables. The data are presented as means±SD. We used the SPSS (version 15.0) or the Origin (version 7.0) statistical packages.
Results
Normal Sequence of Ventricular Activation
Cerrone et al4 demonstrated that ECG patterns of PVT and BVT may be obtained in RyR2/RyR2R4496C mice under conditions that closely resemble those in CPVT patients. However, understanding arrhythmia mechanisms requires a clear knowledge of the normal sequence of ventricular epicardial activation of the anterior surface of the heart. Figure 1A and 1B shows representative activation maps and volume-conducted ECGs (approximate Lead II) obtained, respectively, from WT and RyR2/RyR2R4496C hearts during SR. C and D in Figure 1 are the mean activation maps of 4 WT (C) and 5 RyR2/RyR2R4496C (D) hearts. These maps show the high reproducibility of the breakthrough patterns, which are nearly identical in the 2 genotypes and similar to those reported for human12 and mouse.6, 7, 9 Epicardial activation starts with 2 quasisimultaneous concentric breakthroughs on the anterior RV and left ventricular (LV) free walls, at sites corresponding to the endocardial insertion of the major Purkinje network branches (see the online data supplement). The wavefronts emanating from such breakthroughs merge at the septum and then propagate upward to activate the rest of the ventricular walls. Thus, in the absence of external stimuli, the isolated RyR2/RyR2R4496C mouse heart shows no abnormalities of ventricular excitation or propagation during SR.
Figure 1.

Ventricular epicardial activation during SR. A, Activation map and ECG from a representative WT heart. B, Map and ECG from a RyR2/RyR2R4496C heart. C, Mean activation map of 4 WT hearts. D, Mean activation map of 5 RyR2/RyR2R4496C hearts. Maps are superimposed on the image of a mouse heart. Asterisks indicate sites of initial breakthrough; AAo, aorta; RA, right atrium; LA, left atrium; PA, pulmonary artery.
Ventricular Arrhythmias
Spontaneous ventricular arrhythmias occurred in 7 of 13 (54%) RyR2/RyR2R4496C and 1 of 11 (9%) WT hearts (P=0.03) perfused with high Ca2+ and isoproterenol. A total of 41 arrhythmic episodes occurred in RyR2/RyR2R4496C hearts (mean duration, 24±76 seconds). These included 1 episode of BVT, 33 episodes of PVT, and 7 episodes of alternating monomorphic VT (MVT) and PVT. Furthermore, 6 mutant hearts showed 11 episodes of ventricular couplets, with a mean duration of 1.68±0.67 seconds.
Only 1 WT heart had ventricular arrhythmias in this protocol. Of a total of 16 episodes in this heart, 12 were PVT, 2 were MVT, and 2 alternated between PVT and MVT. The mean duration of the episodes was 26±60 seconds (P=NS versus mutants). The same heart presented 5 episodes of ventricular couplets (mean duration, 2.72±1.78 seconds).
Of hearts perfused with caffeine and epinephrine, 8 of 12 (66%) RyR2/RyR2R4496C and 2 of 10 (20%) WT (P=0.04) showed spontaneous arrhythmias. Twenty arrhythmic episodes occurred in the RyR2/RyR2R4496C hearts, with a mean duration of 62±156 seconds. Of these, 16 were PVT, 2 were focal MVT, and 2 were VF with a mechanism that was clearly reentrant, as demonstrated by the presence of a stable rotor (see below). Four hearts presented 11 episodes of ventricular couplets, with a mean duration of 15.3±36.8 seconds. Of 7 arrhythmic episodes in WT hearts (mean duration, 13±11 seconds), 1 was MVT and 6 were PVT. One WT heart showed 6 episodes of couplets (mean duration, 45.7±52.3 seconds). BVT was not documented in WT hearts.
As a further confirmation of the site of origin of arrhythmic foci in the RyR2/RyR2R4496C hearts, we performed endocardial optical mapping with direct imaging of the Purkinje network in 5 mutant hearts, perfused with 2.7 mmol/L Ca2+ and 200 nmol/L isoproterenol. MVT, PVT, and ventricular couplets were recorded and mapped in 3 hearts.
MVT Is Attributable to a Focal Source in the Purkinje Network
In Figure 2, we show examples of MVT occurring in hearts perfused with high Ca2+ and 200 nmol/L isoproterenol. The red curves are the upstrokes of the optical single-pixel recording (OSPR) at the earliest activation site during SR and MVT. The black traces are the ECGs, recorded simultaneously. In Figure 2A, the epicardial SR breakthroughs are similar to those in Figure 1. Note that in this experiment, the OSPR corresponding to the asterisk on the RV occurred slightly later than the onset of the QRS. On average (n=4), this signal started 4.3±1 ms before the QRS. In Figure 2B, the ECG is that of an MVT (cycle length≈40 ms), where the P waves are absent. The multiphasic QRS complex is suggestive of a left bundle branch block. On the map, an epicardial activation wave appeared repetitively as a highly localized breakthrough on the anterior wall of the RV. This concentric breakthrough pattern was similar to that emerging from the RV during SR. Here again, the upstroke of the OSPR of the earliest activation site in the breakthrough appeared 1.4±1.5 ms (n=4) before the QRS. Figure 2C was obtained from an experiment in which the RV cavity was opened for endocardial mapping during SR. As expected,6 the initial breakthrough occurred at the His bundle, and the impulse propagated down the right bundle branch (RBB) on the septal wall to reach the anterior papillary muscle within 4 ms. The endocardial OSPR demonstrated that the upper RBB activated appreciably earlier than the appearance of the QRS. On average (n=4), upper RBB activation occurred 29±1.8 ms before the QRS (P<0.001 versus epicardial SR). Figure 2D was from a different open-RV heart, during MVT (cycle length≈50 ms). On the RV endocardial activation map, this tachycardia originates as a focus that fires repetitively from the same location. The upstroke of the OSPR at the asterisk occurred much earlier than the QRS inscription on the lower ECG. On average (n=4) during MVT, endocardial activation occurred 14.5±1.8 ms before the QRS (P<0.001 versus epicardial MVT).
Figure 2.
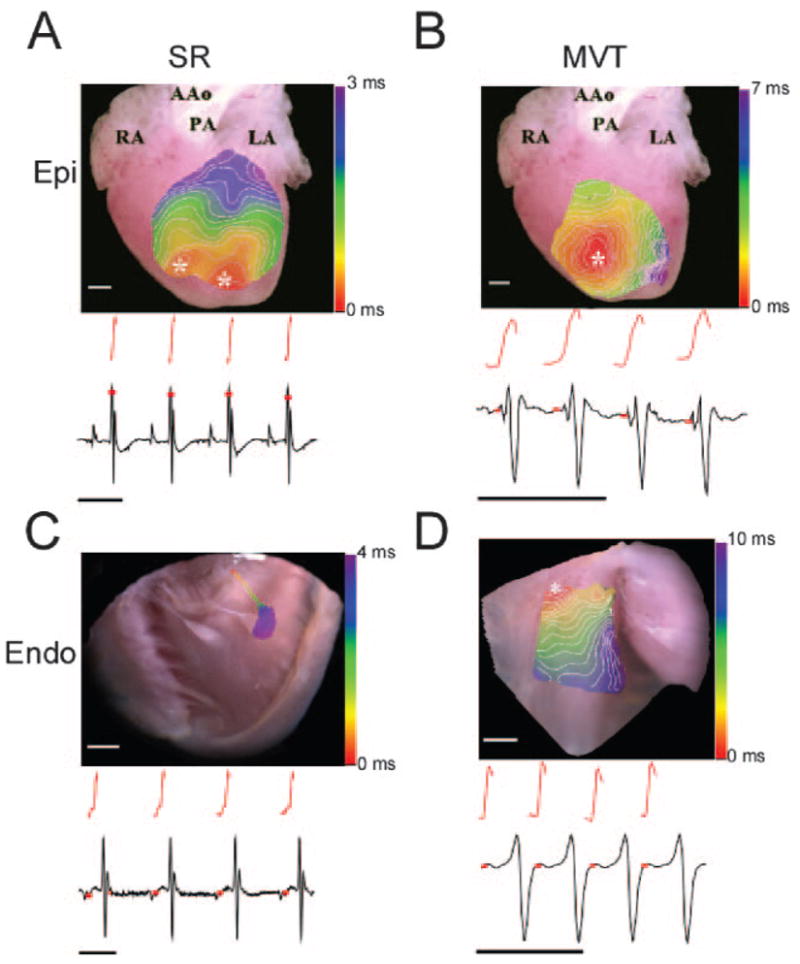
Epicardial (A and B) and endocardial (C and D) activation during SR and MVT in RyR2/RyR2R4496C hearts. A, Epicardial activation map in SR, with corresponding OSPR (red) and ECG. B, Epicardial activation map during MVT, with corresponding OSPR and ECG. C, RV endocardial activation map in SR showing activation sequence in the RBB. OSPR and ECG are shown below. D, RV endocardial activation map during MVT with corresponding OSPR and ECG. White scale bars, 1 mm; black scale bars, 100 ms. Red squares indicate time of OPSR upstroke with respect to ECG; white lines on color maps, 0.5-ms isochrones; Epi, epicardium; Endo, endocardium. See Figure 1, for the remaining abbreviations.
Altogether, the data in Figure 2 demonstrate that the focus responsible for MVT is much closer to the endocardium than the epicardium. The long delay between the endocardial discharge at the OSPR and the onset of the QRS complex likely represents the propagation time from the focus at a distal RV Purkinje fiber, transmurally to the epicardium and to the rest of the ventricular wall.
Bidirectional Tachycardia
One of our objectives was to determine the dynamics of wave propagation that underlie the alternating QRS morphology of BVT in the RyR2/RyR2R4496C mouse. Figure 3 shows results from the experiment in which this was done successfully. Figure 3A is the fluorescent image of the heart. Figure 3B depicts data in SR. The ECG shows 10 sinus beats at a cycle length of 179 ms, and the isochrone map illustrates the normal sequence of ventricular epicardial activation during a single beat. The pattern is characterized by 2 focal breakthroughs (asterisks) that originate almost simultaneously on the free walls of the right and left ventricles. The breakthroughs form wavefronts that fully activate the field of view within 2.5 ms (see also Figures 1 and 2B). As discussed previously, these breakthroughs are the sites of epicardial emergence of sinus impulses from the distal ends of the RBB and left bundle branch across the ventricular walls. Figure 3C shows the same RyR2/RyR2R4496C mouse heart in the presence of 2.7 mmol/L Ca2+ and 100 nmol/L isoproterenol. The ECG shows a classic example of BVT with 180° beat-to-beat alternations in the QRS. As shown by the map obtained during beat 1, upward QRS complexes coincided with repetitive, highly localized epicardial breakthroughs on the lateral wall of the RV, with the wavefront propagating slowly toward the LV. In contrast, downward QRS deflections (2) were associated with LV breakthroughs and slow propagation toward the RV. These results strongly support our hypothesis that BVT in RyR2/RyR2R4496C mice is triggered by DADs occurring alternatively at specific Purkinje fibers on the RBB and left bundle branch.
Figure 3.
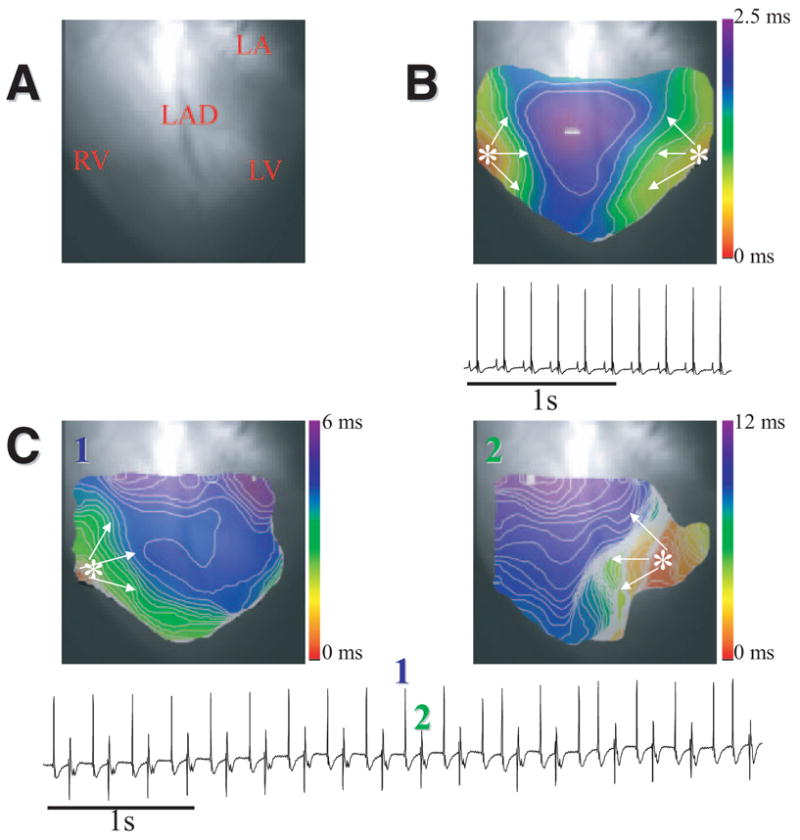
BVT in a RyR2/RyR2R4496C heart. A, Heart image. B, Epicardial activation map and ECG in SR. C, Epicardial activation maps of 2 consecutive ventricular beats with an origin that changed from RV (beat 1) to LV (beat 2). Bottom, ECG in BVT (2.7 mmol/L Ca2+; 100 nmol/L isoproterenol). LAD indicates left anterior descendent coronary; LA, left atrium.
In addition to BVT, in both protocols, RyR2/RyR2R4496C hearts showed ventricular bigeminal couplets, defined as pairs of QRS complexes with alternating morphology followed by a relatively long pause. This arrhythmic pattern is similar to that of BVT, with the exception that, in this case, each couplet is followed by a long pause (see the online data supplement).
Multifocal PVT
In Figure 4, we mapped the endocardial activation of each of 9 consecutive QRS complexes in an episode of nonsustained multifocal PVT (mean cycle length, 35 ms) induced by 2.7 mmol/L Ca2+ and 200 nmol/L isoproterenol in an isolated RyR2/RyR2R4496C mouse heart. In Figure 4A, high-resolution image analysis of the initial site of activation for each QRS complex (bottom) demonstrated 8 different foci on the RV septal wall. Beat 5 originated outside the field of view. The ECG shows absence of P waves and varying QRS morphologies from beat to beat reflecting the multifocal origin of the arrhythmia. Figure 4B shows enlarged views of the two areas boxed in A. The image contrast in these areas was enhanced to visualize the Purkinje fibers. Each colored number shows that the initial site of activation for the respective QRS complexes corresponds to the location of a distal, free-running Purkinje fiber. These results establish that PVT in this model is attributable to focal discharges at multiple locations within the specialized conduction system.
Figure 4.
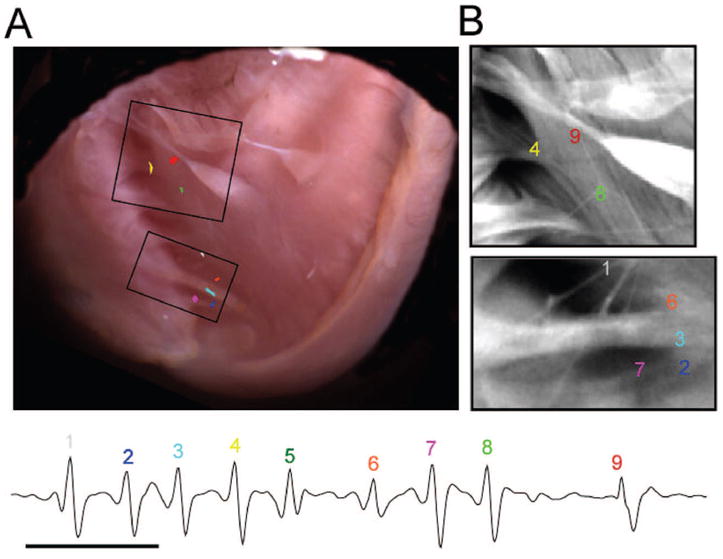
PVT in RyR2/RyR2R4496C heart. A, Location of each of 8 consecutive ectopic discharges during PVT superimposed on a high-resolution image of RV endocardium. Each color corresponds to a different discharge and complex number on ECG. B, Magnification of 2 boxed areas in A highlights Purkinje fibers. Each number and color indicates the origin of each discharge and complex number on ECG. Beat 5 originated outside the field of view and is not represented on the map.
PVT to VF Transition
The cause of sudden cardiac death in CPVT patients is degeneration of BVT or PVT into VF.2,3 In some RyR2/RyR2R4496C mouse hearts, we documented the transition from PVT to VF. In Figure 5, perfusion with Tyrode’s solution (1.8 mmol/L Ca2+) containing 1 mmol/L caffeine and the administration of a bolus of epinephrine (1.6 μmol/L) initiated a run of PVT, which manifested as repetitive multifocal RV epicardial breakthroughs and deteriorated into VF. Figure 5, top left, shows three 10-ms snapshots from the phase map during 1 focal discharge. The middle 3 images show similar maps obtained during the transition from focal to reentrant activity brought about by a wave break formed between the first and second frames. In this episode, the focal activity lasted 1.1 second (frequency, 39 Hz) and the rotor lasted 1.7 second (frequency, 43 Hz). The maps on the bottom demonstrate that VF was maintained by a high-frequency rotor throughout the reminder of the episode (see the recording to the right of the figure).
Figure 5.
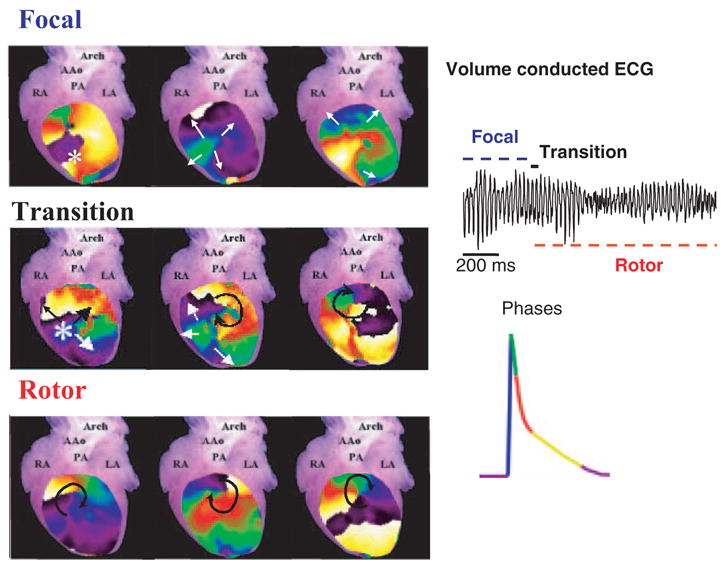
Left, PVT-to-VF transition in RyR2/RyR2R4496C heart (1.8 mmol/L Ca2+; 1 mmol/L caffeine). Left, successive epicardial phase maps (10-ms snapshots). Top, Three maps showing a focal discharge during PVT. Middle, Transition from focal discharge (leftmost) to reentry (rightmost) by formation of a wave break (center). Bottom, Drifting rotor maintained VF. Right, ECG (top) and color code for action potential phases.
Subendocardial Ablation Terminates BVT
At least 4 different groups of investigators13–17 have used selective destruction of the Purkinje network by Lugol as a means to determine the importance of the specialized conducting system in the activation of the ventricular endocardium and epicardium and in the transition to VF. Thus, to establish the role of the Purkinje network in the mechanism of BVT, Lugol’s solution was directly injected into the RV cavity of anesthetized WT and RyR2/RyR2R4496C mice. We used anesthetized mice in consideration of the rare occurrence of BVT in the isolated Langendorff-perfused heart. The mice underwent the same protocol of induction of arrhythmias that was used in the previous studies4–5; ie, caffeine (120 mg/kg) and epinephrine (2 mg/kg) IP.
As shown in Figure 6A, when 5 to 7 μL of Lugol’s solution was injected into the RV of an anesthetized WT mouse during SR, the QRS widened as a result of RBB block and the PR interval prolonged slightly, but the SR pattern and the R-R interval were unchanged. QRS prolongation under these conditions was clearly visualized by comparing the complexes shown on the top of Figure 6C at baseline (left) and after Lugol injection (right). In 3 WT mice, the mean QRS duration was 16.6±1.3 ms at baseline, and it increased to 26.8±3.2 ms after Lugol injection (P<0.001). As an additional control, in yet another group of 3 anesthetized WT mice, we injected 5 to 7 μL of normal Tyrode’s solution into the RV cavity. No changes in QRS width were recorded (baseline: 16.5±1.9 versus 16.8±1.9 ms after Tyrode’s solution; P=NS).
Figure 6.

Effects of chemical ablation of RV endocardium in anesthetized WT and RyR2/RyR2R4496C mice. A, ECG during SR in WT mouse (top) and ECG during SR after Lugol’s solution injection (bottom). Note RBB block pattern. B, ECG during SR in RyR2/RyR2R4496C mouse (top), BVT after administration of 120 mg/kg caffeine and 2 mg/kg epinephrine (middle), and Lugol’s solution injection in RV cavity converts BVT to MVT with RBB block (bottom). C, Morphology and width of the QRS complex in baseline (left) and after Lugol (right) in WT and RyR2/RyR2R4496C mice.
We induced BVT in all 4 anesthetized RyR2/RyR2R4496C mice. Figure 6B shows a representative example. The top ECG was obtained during SR. The middle trace, recorded a few minutes after IP injection of caffeine and epinephrine, shows a classic pattern of BVT with narrow QRS and alternating QRS axis. As clearly illustrated by the bottom trace, in all the cases, after carefully injecting 5 to 7 μL of Lugol’s solution into the RV cavity, BVT was converted to MVT with wide QRS and RBB block (see also bottom traces in Figure 6C). Statistical analysis demonstrated that the QRS widening was highly significant. The mean QRS duration recorded in the RyR2/RyR2R4496C mice in SR was 15.75±1 ms; after Lugol injection, it increased to 33±4.2 ms (P<0.001). As a further control, RV injection of 5 to 7 μL of Tyrode’s solution in 2 RyR2/RyR2R4496C hearts had no effect on the BVT pattern (data not shown).
These experiments further support our contention that BVT originates at the ventricular Purkinje fibers.
Single Purkinje Cells From Mutant and WT Mice
The results thus far, together with previous data in the literature,5, 18, 19 suggest that focal arrhythmias in the RyR2/RyR2R4496C mouse heart are the result of DAD-induced triggered discharges. In this regard, Liu et al5 demonstrated that single ventricular myocytes from RyR2/RyR2R4496C mice undergo DADs and TA when subjected to high-frequency excitation and isoproterenol. On the other hand, mammalian Purkinje fibers are known to be more sensitive to calcium overload than ventricular myocytes.20 We have therefore performed a group of patch-clamp experiments in isolated Purkinje cells from both RyR2/RyR2R4496C and WT mouse hearts to demonstrate their ability to undergo DADs and TA. As presented in the online data supplement, cells were identified by their typical spindle-shaped morphology, lack of T-tubules, baseline electrophysiological characteristics, and ability to undergo pacemaker activity.
DADs and TA in Isolated Purkinje Cells
Nine cells (9 mice) from RyR2/RyR2R4496C and 9 cells (9 mice) from WT hearts were paced with repetitive trains of 20 stimuli at increasing frequencies (1, 5, 10, 20 Hz) in control and in the presence of 30 nmol/L isoproterenol. As illustrated by the representative data in Figure 7A, in WT Purkinje cells at baseline (A1 to A3), high frequency trains generated occasional DADs at very long coupling intervals (CIs) (arrow). TA occurred only at very high frequencies of stimulation in the presence of 30 mol/L isoproterenol (A4 to A6). On the other hand, Purkinje cells from RyR2/RyR2R4496C hearts were extremely vulnerable to DAD-induced TA, as shown in Figure 7B1 and 7B2. In these cells, multiple DADs and TA appeared already at baseline, particularly at 10 Hz. Even relatively slow frequency trains of stimuli (5 Hz) applied to mutant cells at baseline (B3) led to repetitive DADs of varying amplitude and short CIs (arrows). Such DADs readily reached threshold in the presence of isoproterenol (B4) and resulted in long episodes of TA.
Figure 7.

Frequency dependence of DADs and TA. A, WT Purkinje cell driven by trains of 20 pulses at 1, 5, 10 Hz at baseline (A1 to A3) and during 30 nmol/L isoproterenol superfusion (A4 to A6). B, Two different RyR2/RyR2R4496C Purkinje cells (B1 and B2; B3 and B4) subjected to the same protocol. DADs and TA were elicited in RyR2/RyR2R4496C already at baseline (B1 to B3). In the presence of isoproterenol (30 nmol/L), trains of stimuli (5 Hz) generated long episodes of sustained TA.
In Figure 8, we present a comparison of DAD amplitude as a function of CI obtained for trains of 10 Hz in 5 RyR2/RyR2R4496C Purkinje cells under basal conditions and 5 WT Purkinje cells exposed to isoproterenol. A minimum of 2 DADs were taken from each cell. In each case, the CI was measured from the stimulus onset to the peak of the first DAD. Despite the absence of isoproterenol, mutant cells showed a shorter mean CI than WT (0.27±0.07 versus 0.63±0.09 sec, respectively; P<0.0001). The mean DAD amplitude was similar for both genotypes (RyR2/RyR2R4496C, 2.99±1.2; WT, 2.23±1.09 mV; P=0.07).
Figure 8.

DAD amplitude vs CI in Purkinje cells from RyR2/RyR2R4496C (○) (5 mice) and WT (●) (5 mice) at 10 Hz. The RyR2/RyR2R4496C data are in the absence of isoproterenol. The WT data are in the presence of 30 nmol/L isoproterenol.
Discussion
The most important results of this study are as follows. (1) The isolated RyR2/RyR2R4496C mouse heart showed no abnormalities of ventricular excitation or propagation during SR in the absence of stimuli leading to Ca2+ overload. (2) The Ca2+-overloaded or adrenergically stimulated RyR2/RyR2R4496C mouse heart undergoes episodes of MVT, BVT, and PVT, the epicardial breakthrough patterns of which strongly suggest that all these arrhythmias may originate from focal sources in the His–Purkinje networks of the RV and/or LV. (3) Endocardial optical mapping of the RV demonstrates that the arrhythmic foci in this model do originate within the specialized conduction system. In the case of MVT, the arrhythmias are clearly unifocal, whereas in PVT, the arrhythmia is initially multifocal but eventually becomes reentrant upon degeneration into VF. The relation between the onset of the optical signal during arrhythmias and SR and the onset of the QRS complex of the ECG further confirms our proposition. (4) In anesthetized RyR2/RyR2R4496C mice, selective chemical ablation of the RV Purkinje network changed the BVT into MVT with wide QRS, demonstrating that in BVT the focal origin alternates beat-to-beat between Purkinje sources in the RV and LV. (5) Single Purkinje cells from RyR2/RyR2R4496C mouse hearts generated DAD-induced TA at lower frequencies than WT. They did so even in the absence of isoproterenol. WT cells did not. Altogether, the data strongly suggest that the His–Purkinje system is a major source of focal arrhythmias in CPVT.
The RyR2/RyR2R4496C Mouse
The demonstration in various in vitro models that the R4497C mutation results in increased calcium release has led to the speculation that life-threatening arrhythmias may develop in CPVT patients as a consequence of abnormal sarcoplasmic reticulum calcium release.2, 18, 19, 21 However, experimental evidence linking this mutation to the ventricular tachycardias that characterize the disease was still lacking. The recent demonstration that the R4496C mutation in RyR2 predisposes the murine heart to BVT, PVT, and VF upon administration of caffeine and adrenergic agonists opened the possibility of investigating the molecular and cellular mechanisms of arrhythmias in CPVT. In this regard, Cerrone et al4 demonstrated that PVT and BVT may be elicited in the RyR2/RyR2R4496C mice under conditions that closely resemble those in CPVT patients. In fact, not all RyR2/RyR2R4496C mice developed arrhythmias, which is also consistent with the incomplete penetrance of CPVT in humans. Similarly, Cerrone et al4 were unable to find differences in the levels of mutant mRNA when comparing animals that developed arrhythmias with those that remained asymptomatic. The studies presented here naturally extend those observations by providing new insight into the dynamics of epicardial propagation during MVT, BVT, and PVT and demonstrating their focal origin and their close relationship with the epicardial patterns during normal sequence of activation. Furthermore, when selective chemical ablation of 1 of the 2 branches of the Purkinje system was performed, the alternating QRS pattern of BVT converted to a unifocal pattern. These experiments, together with the demonstration that Purkinje cells from the RyR2/RyR2R4496C mouse heart have an abnormally high propensity to undergo DADs and TA, establish that the origin of the arrhythmias lies in the His–Purkinje network.
Mechanism of BVT
BVT is an infrequent arrhythmia that has nevertheless mesmerized electrophysiologists for many years. It is most commonly observed under conditions of digitalis intoxication and in advanced heart disease.22 On ECG, BVT is manifested as an alternation in the polarity of the QRS axis in some of the leads; the remaining leads may demonstrate changes in morphology.22 The tachycardia is often regular, occurs in brief salvoes, and often resolves spontaneously or may degenerate into PVT or VF. The alternating pattern is usually associated with bundle branch block morphology in the precordial leads, with the alternating QRS complexes differing from each other in amplitude and duration. Since its first description in 1922,23 several hypotheses have been postulated for the mechanism of BVT, including enhanced automaticity with the existence of 2 separate ventricular foci22, 24 or even reentry.22 More recently, the demonstration of RyR2 gain-of-function mutations in patients with familial CPVT has led to the hypothesis that BVT results from TA secondary to the disruption of the normal process of release of Ca2+ from the sarcoplasmic reticulum during EC coupling.19, 21
Our experiments extend substantially such a hypothesis by highlighting for the first time the potential role played by the Purkinje fiber network in the mechanism of the focal arrhythmias that characterize both the calcium overloaded and/or adrenergically stimulated RyR2/RyR2R4496C mouse heart and the patient with CPVT. Several pieces of evidence lead us to think that the above hypothesis is correct: (1) the ability of RyR2/RyR2R4496C Purkinje cells to undergo TA at relatively low stimulation frequencies, even in the absence of adrenergic stimuli (see Figure 7); (2) the focal nature of MVT and PVT in the isolated RyR2/RyR2R4496C mouse heart (Figures 2 and 3); (3) the location of the origin of ectopic beats during MVT and PVT in endocardial optical mapping experiments and the relation between the optical signal and the QRS complexes (Figures 2 and 3); (4) the occurrence of alternating RV and LV epicardial breakthroughs accompanying the beat-to-beat changes in QRS axis during bidirectional couplets and BVT (see Figure 3 and the online data supplement); and (5) the conversion of BVT into MVT with RBB block configuration on RV endocardial ablation with Lugol’s solution (see Figure 6).
Several different scenarios have been invoked previously to explain the origin of BVT. Accordingly we have performed computer simulations in a 2D as well as in a geometrically realistic 3D mouse heart model to explore the ability of these different scenarios to result in BVT. In general, our experiments reinforce the idea that the arrhythmias are initiated in the specialized conduction system rather than in the muscle. In addition, the simulations enabled us to provide testable predictions for future experiments and also test the validity of alternative hypothesis regarding the mechanisms of maintenance and initiation of BVT. The hypotheses investigated were (1) 2 alternating sources of epicardial ventricular activity located, respectively, 1 in the RV and 1 in the LV; (2) alternating epicardial and endocardial discharges, as previously proposed25; (3) a single Purkinje focus with alternating bundle branch block. Detailed descriptions and a discussion about these simulations are presented in the online data supplement.
Although BVT is frequent in conscious4 and anesthetized mice, the incidence of the arrhythmia in the isolated heart seems quite low. In fact, we could map only 1 example of BVT in an isolated RyR2/RyR2R4496C heart. As such, a crucial role for the autonomic nervous system in promoting ventricular arrhythmias in the in situ RyR2/RyR2R4496C heart that may be absent or attenuated in the denervated heart cannot be disputed. Resolving this issue, however, is well beyond the reach of the present study.
In addition, it needs to be demonstrated in the whole heart that Purkinje cells are not only capable of forming ectopic foci and generating triggered discharges in situ but also of precisely coordinating their activity to give rise to the peculiar ECG patterns of BVT and bidirectional couplets. Finally, a role of the autonomic nervous system in modulating such a highly coordinated and predictable activity needs to be verified. Although these questions are clearly outside the scope of the present study, they do offer some tantalizing prospects for research leading to a detailed understanding of the mechanisms of CPVT.
Supplementary Material
Acknowledgments
Sources of Funding This work was supported in part by National Heart, Lung, and Blood Institute grants PO1 HL039707, RO1 HL070074, and RO1 HL060843 (to J.J.); American Heart Association Postdoctoral Fellowships (to M.C. and S.V.P.); and Telethon grants GGP06007 and GGP04066, Fondo per gli Investimenti della Ricerca di Base grants RBNE01XMP4_006 and RBLA035A4X_002, and European Union Sixth Framework Programme grant STREP LSHM-CT-2005-018802 (to S.G.P.).
Footnotes
Disclosures None.
References
- 1.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 2.Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 3.Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, DeSimone L, Coltorti F, Bloise R, Keegan R, Cruz Filho FE, Vignati G, Benatar A, DeLogu A. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 4.Cerrone M, Colombi B, Santoro M, di Barletta MR, Scelsi M, Villani L, Napolitano C, Priori SG. Bidirectional ventricular tachycardia and fibrillation elicited in a knockin mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ Res. 2005;96:e77–e82. doi: 10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- 5.Liu N, Colombi B, Memmi M, Zissimopoulos S, Rizzi N, Negri S, Imbriani M, Napolitano C, Lai FA, Priori SG. Arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia. Insights from a RyR2 R4496C knockin mouse model. Circ Res. 2006;99:292–298. doi: 10.1161/01.RES.0000235869.50747.e1. [DOI] [PubMed] [Google Scholar]
- 6.Tamaddon HS, Vaidya D, Simon AM, Paul DL, Jalife J, Morley GE. High-resolution optical mapping of the right bundle branch in connexin40 knockout mice reveals slow conduction in the specialized conduction system. Circ Res. 2000;87:929–936. doi: 10.1161/01.res.87.10.929. [DOI] [PubMed] [Google Scholar]
- 7.Chuck ET, Meyers K, France D, Creazzo TL, Morley GE. Transitions in ventricular activation revealed by two-dimensional optical mapping. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:990–1000. doi: 10.1002/ar.a.20083. [DOI] [PubMed] [Google Scholar]
- 8.Bagwe S, Berenfeld O, Vaidya D, Morley GE, Jalife J. Altered right atrial excitation and propagation in connexin40 knockout mice. Circulation. 2005;112:2245–2253. doi: 10.1161/CIRCULATIONAHA.104.527325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morley GE, Vaidya D, Samie FH, Lo C, Delmar M, Jalife J. Characterization of conduction in the ventricles of normal and heterozygous Cx43 knockout mice using optical mapping. J Cardiovasc Electrophysiol. 1999;10:1361–1375. doi: 10.1111/j.1540-8167.1999.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 10.Samie FH, Berenfeld O, Anumonwo J, Mironov SF, Udassi S, Beaumont J, Taffet S, Pertsov AM, Jalife J. Rectification of the background potassium current: a determinant of rotor dynamics in ventricular fibrillation. Circ Res. 2001;89:1216–1223. doi: 10.1161/hh2401.100818. [DOI] [PubMed] [Google Scholar]
- 11.Tolkacheva EG, Vaidyanathan R, Munoz V, Noujaim S, Anumonwo J. Inward rectifier current properties of isolated murine Purkinje cells. Heart Rhythm. 2006;3:S303 Abstract. [Google Scholar]
- 12.Durrer D, van Dam RT, Freud GE, Janse MJ, Meijler FL, Arzbaecher RC. Total excitation of the isolated human heart. Circulation. 1970;41:899–912. doi: 10.1161/01.cir.41.6.899. [DOI] [PubMed] [Google Scholar]
- 13.Bittencourt D, Long DM, Lee YK, Lillehei CW. Intravital staining of the atrioventricular bundle with iodine compounds during cardiopulmonary bypass. Circ Res. 1959;7:753–758. doi: 10.1161/01.res.7.5.753. [DOI] [PubMed] [Google Scholar]
- 14.Janse MJ. Vulnerability to ventricular fibrillation. Chaos. 1998;8:149–156. doi: 10.1063/1.166295. [DOI] [PubMed] [Google Scholar]
- 15.Chen PS, Wolf PL, Cha YM, Peters BB, Topham SL. Effects of subendocardial ablation on anodal supernormal excitation and ventricular vulnerability in open-chest dogs. Circulation. 1993;87:216–229. doi: 10.1161/01.cir.87.1.216. [DOI] [PubMed] [Google Scholar]
- 16.Cha YM, Uchida T, Wolf PL, Peters BB, Fishbein MC, Karagueuzian HS, Chen PS. Effects of chemical subendocardial ablation on activation rate gradient during ventricular fibrillation. Am J Physiol. 1995;269:H1998–H2009. doi: 10.1152/ajpheart.1995.269.6.H1998. [DOI] [PubMed] [Google Scholar]
- 17.Damiano RJ, Jr, Smith PK, Tripp HF, Jr, Asano T, Small KW, Lowe JE, Ideker RE, Cox JL. The effect of chemical ablation of the endocardium on ventricular fibrillation threshold. Circulation. 1986;74:645–652. doi: 10.1161/01.cir.74.3.645. [DOI] [PubMed] [Google Scholar]
- 18.Jiang D, Xiao B, Zhang L, Chen SR. Enhanced basal activity of a cardiac Ca2+ release channel (ryanodine receptor) mutant associated with ventricular tachycardia and sudden death. Circ Res. 2002;91:218–225. doi: 10.1161/01.res.0000028455.36940.5e. [DOI] [PubMed] [Google Scholar]
- 19.Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, Cheng H, Chen SR. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci U S A. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vassalle M, Lin CI. Calcium overload and cardiac function. J Biomed Sci. 2004;11:542–565. doi: 10.1007/BF02256119. [DOI] [PubMed] [Google Scholar]
- 21.Lehnart SE, Wehrens XH, Kushnir A, Marks AR. Cardiac ryanodine receptor function and regulation in heart disease. Ann N Y Acad Sci. 2004;1015:144–159. doi: 10.1196/annals.1302.012. [DOI] [PubMed] [Google Scholar]
- 22.Levy S, Aliot E. Bidirectional tachycardia: a new look on the mechanism. Pacing Clin Electrophysiol. 1989;12:827–834. doi: 10.1111/j.1540-8159.1989.tb01905.x. [DOI] [PubMed] [Google Scholar]
- 23.Schwensen C. Ventricular Tachycardia as the result of the administration of digitalis. Heart. 1922;9:199–204. [Google Scholar]
- 24.Morris SN, Zipes DP. His bundle electrocardiography during bidirectional tachycardia. Circulation. 1973;48:32–36. doi: 10.1161/01.cir.48.1.32. [DOI] [PubMed] [Google Scholar]
- 25.Nam GB, Burashnikov A, Antzelevitch C. Cellular mechanisms underlying the development of catecholaminergic ventricular tachycardia. Circulation. 2005;111:2727–2733. doi: 10.1161/CIRCULATIONAHA.104.479295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


