Abstract
It has been conclusively demonstrated in juvenile rodents that the inhibitory neurons of the nucleus reticularis thalami (NRT) communicate with each other via connexin 36 (Cx36)-based electrical synapses. However, whether functional electrical synapses persist into adulthood is not fully known. Here we show that in the presence of the metabotropic glutamate receptor (mGluR) agonists, trans-ACPD (100 μM) or DHPG (100 μM), 15% of neurons in slices of the adult cat NRT maintained in vitro exhibit stereotypical spikelets with several properties that indicate that they reflect action potentials that have been communicated through an electrical synapse. In particular, these spikelets, i) display a conserved all-or-nothing waveform with a pronounced after-hyperpolarization (AHP), ii) exhibit an amplitude and time to peak that are unaffected by changes in membrane potential, iii) always occur rhythmically with the precise frequency increasing with depolarization, and iv) are resistant to blockers of conventional, fast chemical synaptic transmission. Thus, these results indicate that functional electrical synapses in the NRT persist into adulthood where they are likely to serve as an effective synchronizing mechanism for the wide variety of physiological and pathological rhythmic activities displayed by this nucleus.
Keywords: Gap junctions, connexin36, mGluRs, EEG rhythms, sleep spindles
INTRODUCTION
The nucleus reticularis thalami (NRT) is a thin sheet of GABAergic neurons which surrounds the dorsal thalamus. This nucleus densely innervates neighbouring thalamic relay nuclei (Uhlrich et al., 1991) and has long been recognised as a key component in bringing about neuronal synchrony during both normal sleep rhythms (Steriade et al., 1985; Steriade et al., 1987; Steriade et al., 1993; von Krosigk et al., 1993; Bal et al., 1995; Crunelli et al., 2005; Fuentealba and Steriade, 2005; Steriade, 2005; Blethyn et al., 2006) and some types of epileptic discharges (von Krosigk et al., 1993; Bal et al., 1995; Steriade and Contreras, 1995; Huntsman et al., 1999; Sohal et al., 2000; Slaght et al., 2002). Recently, it has been shown in juvenile rodents that NRT neurons communicate with each other via electrical synapses (Landisman et al., 2002; Long et al., 2004; Landisman and Connors, 2005; Deleuze and Huguenard, 2006; Lam et al., 2006). These electrical synapses provide an effective means for closely situated cells to synchronize their firing (Landisman et al., 2002; Long et al., 2004; Landisman and Connors, 2005). In turn, this is postulated as a key mechanism by which the NRT can provide coherent rhythmic output to thalamocortical (TC) relay neurons (Long et al., 2004; Hughes and Crunelli, 2005; Deleuze and Huguenard, 2006).
Electrical synapses in the NRT are based on gap junctions (GJs) derived from connexin 36 (Cx36) (Landisman et al., 2002). As both in situ hybridization and immunolabelling studies indicate that Cx36 declines sharply after the second week of postnatal life (Belluardo et al., 2000; Condorelli et al., 2000; Liu and Jones, 2003), and because electrical synapses in the NRT have so far only be demonstrated in immature rodents (Landisman et al., 2002; Long et al., 2004; Landisman and Connors, 2005; Deleuze and Huguenard, 2006; Lam et al., 2006), an important issue is determining whether functional electrical synapses are present in the mature NRT. In vivo data from Mircea Steriade's laboratory obtained in adult cats strongly suggest that this is the case because intracellular recordings from NRT neurons in these animals commonly exhibit so-called spikelets (Amzica et al., 1992; Contreras et al., 1992, , 1993; Steriade et al., 1993; Fuentealba et al., 2004). Spikelets are small, stereotypical subthreshold depolarizing potentials that differ considerably to conventional excitatory postsynaptic potentials (EPSPs) and which, in many neuronal types, including the immature NRT (Landisman et al., 2002; Long et al., 2004), have been shown to represent action potentials that have been transmitted through an electrical synapse (Logan et al., 1996; Galarreta and Hestrin, 1999; Gibson et al., 1999; Venance et al., 2000).
In this paper, we report the presence of spikelets in neurons of the adult cat NRT maintained in vitro. These spikelets become apparent in a subset of neurons following activation of mGluRs with either trans-ACPD or DHPG and possess a number of properties which strongly indicate that they represent attenuated action potentials from a distinct, electrically coupled NRT neuron. Most notably, we show that spikelets, i) are insensitive to blockers of fast chemical synaptic transmission, ii) possess a conserved all-or-nothing waveform that is followed by a marked after-hyperpolarization (AHP) and which is identical to that which is generated by the passage of action potentials through electrical synapses in the NRT of young rats (Landisman et al., 2002; Long et al., 2004), iii) exhibit an amplitude and time to peak that are not affected by changes in membrane polarization, and iv) always occur rhythmically with the inter-spikelet frequency increasing with depolarization. Thus, these results indicate that electrical synapses are integral component of the mature NRT where they are likely to operate as a key synchronizing mechanism for a range of both normal (Steriade et al., 1993; Steriade, 2005; Blethyn et al., 2006) and pathological (Steriade and Contreras, 1995; Slaght et al., 2002; Gareri et al., 2005; Steriade, 2005; Proulx et al., 2006) rhythms.
OBJECTIVE
To examine and discuss the properties of spikelets in intracellular recordings of neurons from the adult cat NRT in vitro.
METHODS
Experiments were carried out in accordance with local ethical committee guidelines and the U.K. Animals (Scientific Procedure) Act, 1986. All efforts were made to minimize the suffering and number of animals used in each experiment.
In vitro slice preparation and maintenance
Young adult cats (1-1.5 kg) were deeply anaesthetized with a mixture of O2 and NO2 (2:1) and 2.5-5% halothane, a wide craniotomy performed and the brain removed. Sagittal slices (450-500 μm) of the thalamus containing either the lateral geniculate nucleus (LGN), ventrolateral (VL) nucleus or ventrobasal (VB) nuclei and the associated sectors of the NRT, i.e. perigeniculate nucleus (PGN), peri-VL sector or peri-VB sector respectively, were prepared and maintained as described previously (Blethyn et al., 2006). For recording, slices were perfused with a warmed (35±1 °C) continuously oxygenated (95% O2, 5% CO2) artificial cerebrospinal fluid (ACSF) containing (mM): NaCl (134); KCl (2); KH2PO4 (1.25); MgSO4 (1); CaCl2 (2); NaHCO3 (16); glucose (10). All drugs were dissolved directly in ACSF. The following drugs were all obtained from Tocris-Cookson (UK): DL-2-amino-5-phosphonovaleric acid (APV), [S-(R*,R*)]-[3-[[1-(3,4-dichlorophenyl)ethyl]amino]-2-hydroxypropyl](cyclohexylmethyl) phosphinic acid (CGP54626), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), (S)-3,5-dihydroxyphenylglycine (DHPG), 6-Imino-3-(4-methoxyphenyl)-1(6H)-pyridazine butanoic acid hydrobromide (SR95531), (+/−)-1-aminocyclopentane-trans-1,3-dicarboxylic acid (trans-ACPD),
In vitro electrophysiology
Intracellular recordings, using the current clamp technique, were performed with standard or thin wall glass microelectrodes filled with 1M potassium acetate (resistance: 80-120 MΩ and 30-60 MΩ respectively), and in some cases 2% biocytin or neurobiotin, and connected to an Axoclamp-2A amplifier (Axon Instruments, Foster City, USA) operating in bridge mode. Impaled cells were identified as NRT neurons using established electrophysiological and morphological criteria (Steriade et al., 1985; Mulle et al., 1986; Uhlrich et al., 1991; Bal and McCormick, 1993; Contreras et al., 1993; Blethyn et al., 2006). In slices where neurons had been filled with biocytin or neurobiotin, visualization of the dye was performed as described previously (Hughes et al., 2002; Hughes et al., 2004). Voltage and current records were stored on a Biologic DAT recorder (IntraCel, Royston, UK) and later analyzed using Clampfit (Axon Instruments). The properties of individual spikelets were assessed according to the details given in Figure 1. The numbers given in parentheses refer to the number of neurons. Typically the properties of 5-10 spikelets were assessed in each neuron. Statistical significance was determined using Student's t-test. All quantitative results are reported in the text as mean ± s.e.m.
Figure 1. Explanation of spikelet parameters.
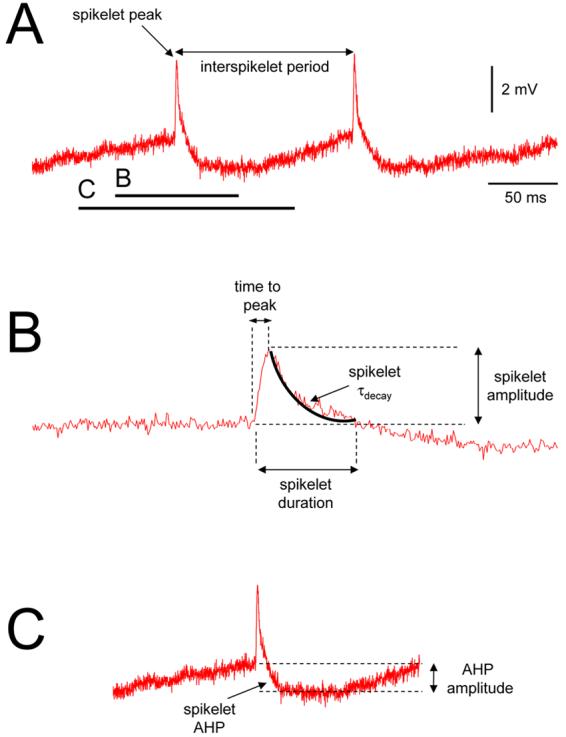
A. Example of spikelets recorded intracellularly from the adult cat NRT. The frequency of spikelets is taken as the reciprocal of the interspikelet period, i.e. the time between successive events. The sections marked B and C are enlarged below and illustrate the parameters used for assessing the fast depolarizing phase of the spikelet and the spikelet AHP, respectively.
RESULTS
A subset of neurons in slices of the adult cat NRT exhibit stereotypical spikelets
Following the application of either the non-specific Group I/II metabotropic glutamate receptor (mGluR) agonist, trans-ACPD (100 μM), or the Group I mGluR agonist, DHPG (100 μM), a subset of NRT neurons (trans-ACPD: n=7 of 47, 15%; DHPG: n=1 of 6; 17%; overall: n=8 of 53, 15%) recorded intracellularly in vitro exhibited stereotypical, short duration depolarizing events or spikelets (duration: 3.18 ± 0.22 ms; n=8) (Fig. 2A). All NRT neurons that displayed spikelets possessed electrophysiological properties that were otherwise typical for this cell type including a robust, low-threshold calcium potential (LTCP)-mediated rebound burst comprising a characteristic accelerating-decelerating action potential pattern (Fig. 2A4). Both within the same NRT neuron and between different NRT neurons, spikelets exhibited a highly conserved, all-or-nothing waveform that was characterised by a fast time to peak (time to peak: 0.78 ± 0.03 ms; amplitude: 1.9 ± 0.2 mV n=8) that was followed by a slower decay (τdecay: 1.30 ± 0.15 ms; n=8) and then a pronounced afterhyperpolarization (AHP) (amplitude: 2.1 ± 0.2 mV; n=8) (Fig. 2B1). Spikelets were resistant to a combination of blockers of fast chemical synaptic transmission (20 μM CNQX, AMPA/kainate receptor antagonist; 100 μM APV, NMDA receptor antagonist; 10 μM SR95531, GABAA receptor antagonists; 20 μM CGP54626, GABAB receptor antagonist; n=4) (Fig. 2) indicating that they do not represent conventional EPSPs or a combination of EPSPs and IPSPs.
Figure 2. Basic manifestation of spikelets in NRT neurons.
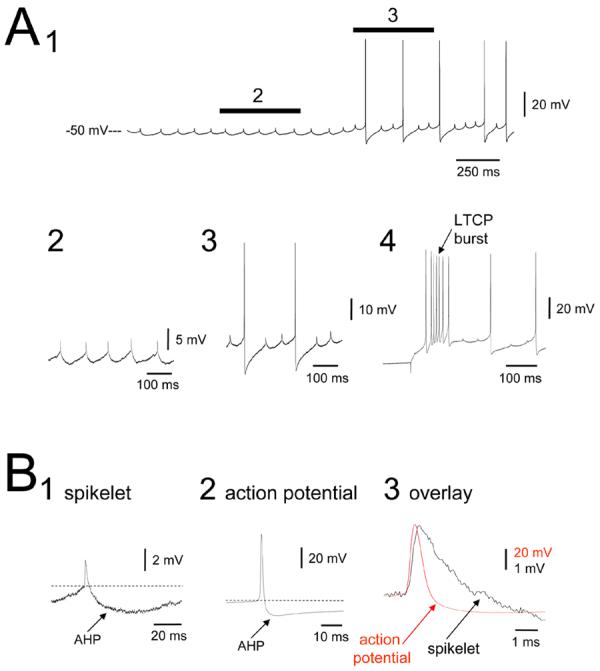
A. Intracellular recording from the adult cat NRT in vitro exhibiting spikelets (1). The sections marked 2 and 3 are expanded below and illustrate the conserved, rhythmic and stereotypical nature of spikelets. The trace on the far right shows the presence of a robust LTCP-mediated burst of action potentials following release from hyperpolarization. B. Enlarged spikelet (1) and action potential (2). Note the pronounced AHP that follows both the spikelet and action potential. The overlay of the spikelet and action potential on the far right (3) reveals that the spikelet has a slightly longer time to peak and a substantially slower decay time. CNQX, APV, SR95531 and CGP54626 were present in the recording medium during this experiment (concentrations given in the results).
Voltage-dependence of spikelets in NRT neurons and entrainment of action potentials
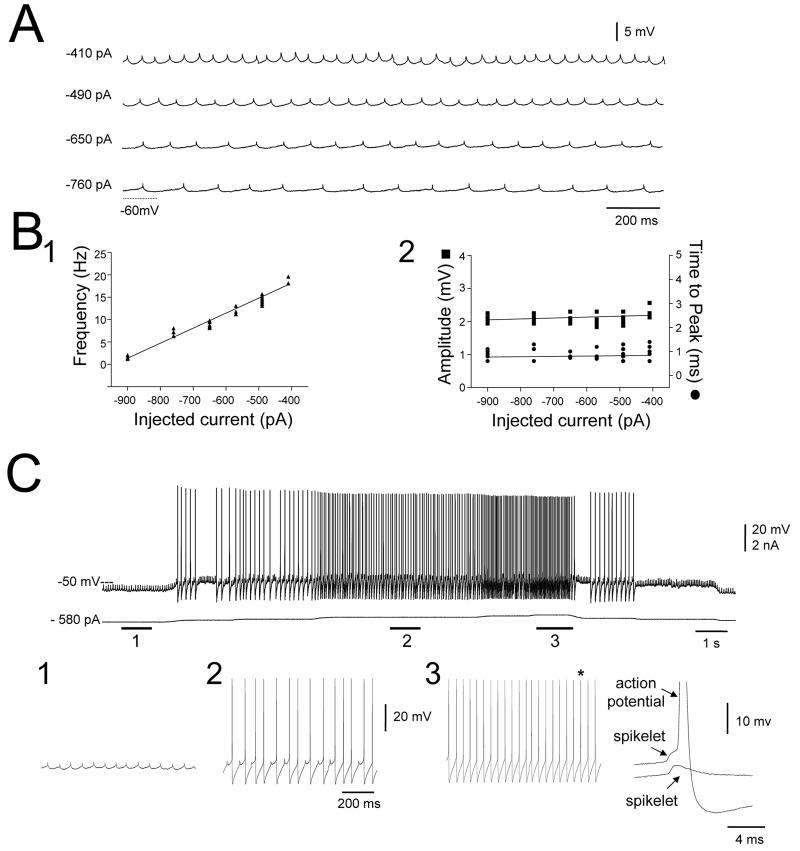
In all NRT neurons, spikelets occurred in a rhythmic manner (Figs. 2A and 3) with the precise interspikelet frequency increasing linearly with depolarization (Fig. 3B1). At certain levels of steady depolarization spikelets appeared to entrain action potentials (Fig. 3C2), whereas upon additional depolarization the two types of events seemed to coalesce (Fig. 3C3). However, in the latter case, close scrutiny revealed that an independent spikelet and action potential could still be discriminated with the spikelet tending to occur just before the action potential (Fig. 3C3). Spikelets were always abolished by sufficiently large hyperpolarization (not illustrated). Neither the time to peak nor the amplitude of spikelets was affected by changes in membrane polarization (Fig. 3A, 3B2).
Figure 3. Dependence of spikelet properties on membrane polarization and the interaction of spikelets with action potentials.
A. Effect of varying membrane potential through the injection of steady d.c. current on the generation of spikelets in an NRT neuron. B. Plots showing the variation in the frequency of spikelets with respect to injected steady current (1) and the relationships between spikelet amplitude and injected current and between the time to peak of spikelets and injected current (2). C. Recording of an NRT neuron at various levels of injected steady current showing the presence of both spikelets and action potentials. The sections marked 1, 2 and 3 are expanded below. At sufficiently hyperpolarized membrane potentials, spikelets occur in isolation (1), whereas upon depolarization spikelets can trigger full blown action potentials (2). After further depolarization the two events seem to coalesce (3). However, enlargement of one of the action potentials (indicated by the asterisk) from this period (far right) reveals the presence of both the action potential and the spikelet. An isolated spikelet from the same neuron is shown below for comparative purposes.
Lack of dye coupling in the NRT and comparison of spikelets between NRT and TC neurons
We have previously shown that similar stereotypical spikelets to those described here are also present following mGluR activation in a subset (∼20%) of thalamocortical (TC) neurons of the cat lateral geniculate nucleus (LGN) (Fig. 4A) (Hughes et al., 2002; Hughes et al., 2004). In LGN TC neurons, spikelets are often accompanied by dye coupling indicating that they represent action potentials which have been transmitted from distinct cells via an electrical synapse (Fig. 4B). Interestingly, dye coupling was never observed for NRT neurons regardless of whether cells exhibited spikelets or not (n=30) (Fig. 4B). There were also clear differences in the properties of spikelets between the two cell types. NRT neurons exhibited a significantly shorter time to peak (p<0.001), duration (p<0.001) and decay time constant (i.e. τdecay) (p<0.05) and a significantly larger AHP amplitude (p<0.001) (Fig. 4C and Table 1; see also Fig. 1). With respect to this it was interesting to note that the duration of action potentials in NRT neurons was also significantly shorter than in TC neurons (p<0.001) and that NRT neurons exhibited a considerably larger and slower action potential AHP (p<0.01) (Fig. 4C and Table 1).
Figure 4. Comparison of spikelets in TC and NRT neurons.
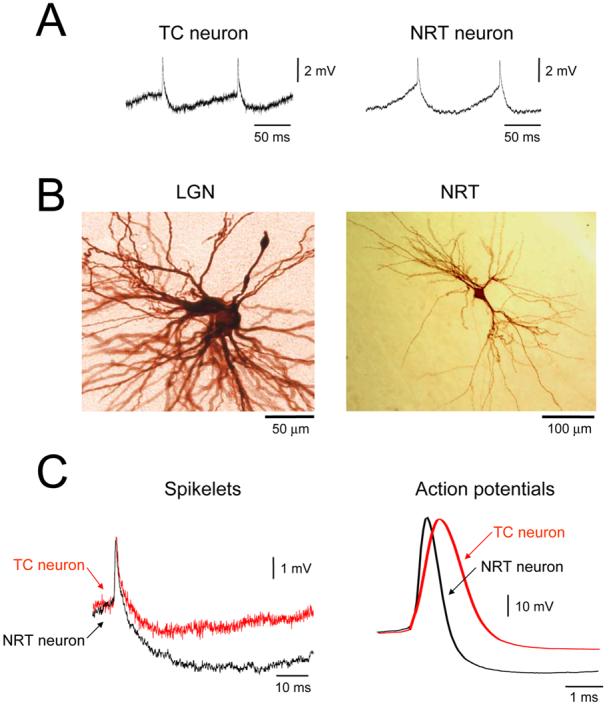
A. Spikelets from an LGN TC neuron (left) and NRT neuron (right). B. TC neurons in the LGN commonly exhibit dye coupling (example shown on the left) whereas NRT neurons never do (see right). C, left. Overlay of a typical spikelet from a TC neuron (red trace) with a characteristic spikelet from an NRT neuron (black trace). Note the slower decay of the TC neuron spikelet and the larger AHP following the NRT neuron spikelet (see Table 1). These properties are consistent with the action potentials displayed by these two cell types (right). Action potentials from NRT neurons (black trace) are faster than those from TC neurons (red trace) and exhibit a substantially larger AHP (see Table 1).
Table 1.
Comparison of the properties of spikelets and action potentials in TC and NRT neurons (n=number of cells).
| Spikelets | Action Potentials | ||||||
|---|---|---|---|---|---|---|---|
| Amplitude (mV) |
Time to peak (ms) |
Duration (ms) |
τdecay (ms) |
AHP amplitude (mV) |
Duration (ms) |
AHP amplitude (mV) |
|
| TC neurons |
2.5± 0.1 n=18 |
1.34±0.12 n=18 |
7.22±0.74 n=18 |
3.04±0.22 n=18 |
0.9±0.1 n=18 |
1.66±0.03 n=10 |
4.4±0.3 n=10 |
| NRT neurons |
1.9±0.2 n=8 |
0.78±0.03 n=8 |
3.18±0.22 n=8 |
1.30±0.15 n=8 |
2.1±0.2 n=8 |
0.80±0.11 n=10 |
15.3±2.4 n=10 |
| p value | p = 0.22 | p < 0.001 | p < 0.001 | p < 0.05 | p < 0.001 | p < 0.001 | p < 0.01 |
CONCLUSION
This study shows that in the presence of either of the mGluR agonists, trans-ACPD or DHPG, 15% of neurons in the adult cat NRT maintained in vitro exhibit stereotypical, all-or-nothing depolarizing events or spikelets.
DISCUSSION
Several lines of evidence suggest that the spikelets observed in this study result from the passage of action potentials from distinct cells through an electrical synapse. First, they exhibit a highly conserved waveform which is identical to that which unambiguously results from action potential transfer through electrical synapses in the NRT of young rodents (Landisman et al., 2002; Long et al., 2004). Second, this waveform possesses a pronounced AHP which probably reflects the transmission of the large AHP that typically occurs following action potentials in NRT neurons. The relatively large amplitude of this event is likely to be due to the facilitated transfer of slower potentials by the low-pass filtering characteristics of electrical synapses. Third, spikelets always occurred rhythmically which parallels the manner in which NRT neurons spontaneously generate action potentials following mGluR activation (Blethyn et al., 2006). Fourth, spikelets in NRT neurons were resistant to a combination of antagonists of conventional synaptic transmission indicating that they are neither EPSPs nor a mixture or EPSPs and IPSPs. Finally, because the time to peak and amplitude of spikelets is not significantly affected by membrane polarization it is unlikely that they are generated by intrinsic subthreshold voltage-dependent conductances (e.g. high-voltage-activated Ca2+ channels) as these would be expected to generate events with kinetics and amplitude that show clear voltage-dependence.
Prevalence and extent of electrical coupling in the NRT
Spikelets were only observed in this study in the presence of mGluR agonists. This is almost certainly because in vitro these agonists usually depolarize NRT neurons from a state of hyperpolarized quiescence to one where they exhibit spontaneous tonic firing (Blethyn et al., 2006). Consequently, this spontaneous firing is then evident in electrically coupled cells as spikelets. According to this scheme, electrical synapses in our study will only have been detected if the cell from which we are recording was coupled to one which is firing spontaneously. Because spontaneous firing may not be present in all NRT neurons following mGluR activation (Cox and Sherman, 1999), it is likely that many more than the 15% of neurons in which we observed spikelets expressed electrical synapses. Indeed, previous investigations indicate that electrical coupling prevalence in the NRT is considerably higher (between 17% and 47%) (Landisman et al., 2002; Long et al., 2004; Deleuze and Huguenard, 2006; Lam et al., 2006). If we further consider that activation of mGluRs in NRT neurons can lead to a long-term reduction in electrical coupling strength (Landisman and Connors, 2005), it is possible that our results may also under-represent the strength of electrical synapses in this nucleus. Of course, it should be noted that previous estimates of coupling prevalence and the demonstration of mGluR-induced suppression of coupling strength derive from experiments in the immature NRT. Nevertheless, it is still likely that more than 15% of adult neurons express electrical synapses and it is important to recognise that these electrical synapses may also be dynamic entities in mature animals (Hughes and Crunelli, 2005)
Since it was possible in this study to affect the frequency of spikelets by varying the amount of injected steady current it appears that, in similarity to TC neurons (Hughes et al., 2002; Hughes et al., 2004) but in contrast to many other brain areas (Llinas and Yarom, 1981), NRT neurons are not densely interconnected by electrical synapses. This is consistent with that found by Long et al. (2004) who used paired whole cell recordings to show that NRT neurons tend to be coupled in small clusters. One way in which the extent of electrical coupling can be gauged independently of electrophysiological properties is by assessing the prevalence and extent of dye coupling. For example, a considerable proportion TC neurons are dye coupled, usually to between 1 and 3 other cells, with this proportion substantially exceeding the quantity that exhibit spikelets (37% vs 19%) (Hughes et al., 2002; Hughes et al., 2004). However, none of the NRT neurons examined in this study exhibited dye coupling. Whilst this lack of dye coupling may seem surprising, a similar situation was also noted for the immature NRT (Landisman et al., 2002) and it seems to be a curious but common finding for other inhibitory neuron networks which are coupled by Cx36-based electrical synapses (Galarreta and Hestrin, 1999; Gibson et al., 1999; Galarreta and Hestrin, 2001).
Functional consequences of electrical synapses in the NRT
Stereotypical subthreshold depolarizing events resembling spikelets have also been observed in the NRT of adults cats recorded intracellularly in vivo (Amzica et al., 1992; Contreras et al., 1992, , 1993; Steriade et al., 1993; Fuentealba et al., 2004). Given the similarity of these events to those observed in vitro, and because they are strongly suppressed by halothane (Fuentealba et al., 2004), a putative GJ blocker, it has been suggested that they also reflect operational electrical synapses. Within this context, electrical synapses have been suggested to play an important role in bringing about neuronal synchronization during 7-14 Hz spindle oscillations (Fuentealba et al., 2004). This proposal is consistent with in vitro studies showing that the combination of intrinsic properties of NRT neurons and electrical coupling leads to synchronized oscillations at ∼10 Hz (Long et al., 2004). It is also conceivable that electrical coupling may promote synchrony for other types of oscillations in the NRT, most notably the slow (<1 Hz) sleep oscillation (Crunelli et al., 2005; Blethyn et al., 2006). In terms of pathological rhythmic activity, recent in vivo work has indicated that electrical synapses in the adult NRT, as well as other thalamic regions, might also be involved in the generation of both typical (Slaght et al., 2002; Gareri et al., 2005) and atypical absence paroxysms (Proulx et al., 2006).
Concluding remarks
Several recent investigations have demonstrated the presence of electrical synapses in NRT neurons from immature rodents (up to 21 days) (Landisman et al., 2002; Long et al., 2004; Landisman and Connors, 2005; Deleuze and Huguenard, 2006; Lam et al., 2006) which are formed by Cx36 (Landisman et al., 2002). However, in situ hybridization and immunolabelling studies indicate that Cx36 peaks during the first two weeks of postnatal life and then declines sharply (Belluardo et al., 2000; Condorelli et al., 2000; Liu and Jones, 2003). These points clearly raise questions regarding whether functional electrical synapses persist into adulthood. Here we have shown the presence of stereotypical spikelets in neurons from the mature cat NRT recorded in vitro which are identical to those that result from electrical synapses in young rodents. Thus, electrical synapses appear to be a species-independent component of the mature NRT where they are likely to play a central role in both physiological and pathological neuronal synchronization.
ACKNOWLEDGEMENTS
This paper is dedicated to the memory of Mircea Steriade. We wish to thank Mr. T.M. Gould for technical assistance. This work was supported by the Wellcome Trust (grants 71436, 78403 and 78311). Additional information regarding this and other published work from the Crunelli lab is available at http://www.thalamus.org.uk.
REFERENCES
- Amzica F, Nunez A, Steriade M. Delta frequency (1-4 Hz) oscillations of perigeniculate thalamic neurons and their modulation by light. Neuroscience. 1992;51:285–294. doi: 10.1016/0306-4522(92)90315-s. [DOI] [PubMed] [Google Scholar]
- Bal T, McCormick DA. Mechanisms of oscillatory activity in guinea-pig nucleus reticularis thalami in vitro: a mammalian pacemaker. J. Physiol. (Lond.) 1993;468:669–691. doi: 10.1113/jphysiol.1993.sp019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T, von Krosigk M, McCormick DA. Role of the ferret perigeniculate nucleus in the generation of synchronized oscillations in vitro. J. Physiol. (Lond.) 1995;483:665–685. doi: 10.1113/jphysiol.1995.sp020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluardo N, Mudo G, Trovato-Salinaro A, Le Gurun S, Charollais A, Serre-Beinier V, Amato G, Haefliger JA, Meda P, Condorelli DF. Expression of connexin36 in the adult and developing rat brain. Brain Res. 2000;865:121–138. doi: 10.1016/s0006-8993(00)02300-3. [DOI] [PubMed] [Google Scholar]
- Blethyn KL, Hughes SW, Tóth TI, Cope DW, Crunelli V. Neuronal basis of the slow (<1 Hz) oscillation in neurons of the nucleus reticularis thalami in vitro. J. Neurosci. 2006;26:2474–2486. doi: 10.1523/JNEUROSCI.3607-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli DF, Belluardo N, Trovato-Salinaro A, Mudo G. Expression of Cx36 in mammalian neurons. Brain Res. Brain Res. Rev. 2000;32:72–85. doi: 10.1016/s0165-0173(99)00068-5. [DOI] [PubMed] [Google Scholar]
- Contreras D, Curro Dossi R, Steriade M. Bursting and tonic discharges in two classes of reticular thalamic neurons. J. Neurophysiol. 1992;68:973–977. doi: 10.1152/jn.1992.68.3.973. [DOI] [PubMed] [Google Scholar]
- Contreras D, Curro Dossi R, Steriade M. Electrophysiological properties of cat reticular thalamic neurones in vivo. J. Physiol. (Lond.) 1993;470:273–294. doi: 10.1113/jphysiol.1993.sp019858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Sherman SM. Glutamate inhibits thalamic reticular neurons. J. Neurosci. 1999;19:6694–6699. doi: 10.1523/JNEUROSCI.19-15-06694.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Tóth TI, Cope DW, Blethyn K, Hughes SW. The ‘window’ T-type calcium current in brain dynamics of different behavioural states. J. Physiol. 2005;562:121–129. doi: 10.1113/jphysiol.2004.076273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleuze C, Huguenard JR. Distinct electrical and chemical connectivity maps in the thalamic reticular nucleus: potential roles in synchronization and sensation. J. Neurosci. 2006;26:8633–8645. doi: 10.1523/JNEUROSCI.2333-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba P, Crochet S, Timofeev I, Bazhenov M, Sejnowski TJ, Steriade M. Experimental evidence and modeling studies support a synchronizing role for electrical coupling in the cat thalamic reticular neurons in vivo. Eur. J. Neurosci. 2004;20:111–119. doi: 10.1111/j.1460-9568.2004.03462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba P, Steriade M. The reticular nucleus revisited: intrinsic and network properties of a thalamic pacemaker. Prog. Neurobiol. 2005;75:125–141. doi: 10.1016/j.pneurobio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Electrical synapses between GABA-releasing interneurons. Nat. Rev. Neurosci. 2001;2:425–433. doi: 10.1038/35077566. [DOI] [PubMed] [Google Scholar]
- Gareri P, Condorelli D, Belluardo N, Citraro R, Barresi V, Trovato-Salinato A, Mudo G, Ibbadu GF, Russo E, De Sarro G. Antiabsence effects of carbenoxolone in two genetic animal models of absence epilepsy (WAG/Rij rats and lh/lh mice) Neuropharmacology. 2005;49:551–563. doi: 10.1016/j.neuropharm.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Blethyn KL, Cope DW, Crunelli V. Properties and origin of spikelets in thalamocortical neurones in vitro. Neuroscience. 2002;110:395–401. doi: 10.1016/s0306-4522(01)00577-2. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Crunelli V. Hardwiring goes soft: long-term modulation of electrical synapses in the mammalian brain. Cellscience. 2005;2:1–9. [PMC free article] [PubMed] [Google Scholar]
- Hughes SW, Lörincz M, Cope DW, Blethyn KL, Kekesi KA, Parri HR, Juhasz G, Crunelli V. Synchronized oscillations at alpha and theta frequencies in the lateral geniculate nucleus. Neuron. 2004;42:253–268. doi: 10.1016/s0896-6273(04)00191-6. [DOI] [PubMed] [Google Scholar]
- Huntsman MM, Porcello DM, Homanics GE, DeLorey TM, Huguenard JR. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science. 1999;283:541–543. doi: 10.1126/science.283.5401.541. [DOI] [PubMed] [Google Scholar]
- Lam YW, Nelson CS, Sherman SM. Mapping of the Functional Interconnections between Reticular Neurons Using Photostimulation. J. Neurophysiol. 2006 doi: 10.1152/jn.00555.2006. In press; doi:10.1152/jn.00555.2006. [DOI] [PubMed] [Google Scholar]
- Landisman CE, Connors BW. Long-term modulation of electrical synapses in the mammalian thalamus. Science. 2005;310:1809–1813. doi: 10.1126/science.1114655. [DOI] [PubMed] [Google Scholar]
- Landisman CE, Long MA, Beierlein M, Deans MR, Paul DL, Connors BW. Electrical synapses in the thalamic reticular nucleus. J. Neurosci. 2002;22:1002–1009. doi: 10.1523/JNEUROSCI.22-03-01002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XB, Jones EG. Fine structural localization of connexin-36 immunoreactivity in mouse cerebral cortex and thalamus. J. Comp. Neurol. 2003;466:457–467. doi: 10.1002/cne.10901. [DOI] [PubMed] [Google Scholar]
- Llinas R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J. Physiol. (Lond.) 1981;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan SD, Pickering AE, Gibson IC, Nolan MF, Spanswick D. Electrotonic coupling between rat sympathetic preganglionic neurones in vitro. J. Physiol. (Lond.) 1996;495:491–502. doi: 10.1113/jphysiol.1996.sp021609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Landisman CE, Connors BW. Small clusters of electrically coupled neurons generate synchronous rhythms in the thalamic reticular nucleus. J. Neurosci. 2004;24:341–349. doi: 10.1523/JNEUROSCI.3358-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle C, Madariaga A, Deschenes M. Morphology and electrophysiological properties of reticularis thalami neurons in cat: in vivo study of a thalamic pacemaker. J. Neurosci. 1986;6:2134–2145. doi: 10.1523/JNEUROSCI.06-08-02134.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx E, Leshchenko Y, Kokarovtseva L, Khokhotva V, El-Beheiry M, Snead OC, 3rd, Perez Velazquez JL. Functional contribution of specific brain areas to absence seizures: role of thalamic gap-junctional coupling. Eur. J. Neurosci. 2006;23:489–496. doi: 10.1111/j.1460-9568.2005.04558.x. [DOI] [PubMed] [Google Scholar]
- Slaght SJ, Leresche N, Deniau JM, Crunelli V, Charpier S. Activity of thalamic reticular neurons during spontaneous genetically determined spike and wave discharges. J. Neurosci. 2002;22:2323–2334. doi: 10.1523/JNEUROSCI.22-06-02323.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Huntsman MM, Huguenard JR. Reciprocal inhibitory connections regulate the spatiotemporal properties of intrathalamic oscillations. J. Neurosci. 2000;20:1735–1745. doi: 10.1523/JNEUROSCI.20-05-01735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends Neurosci. 2005;28:317–324. doi: 10.1016/j.tins.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D. Relations between cortical and thalamic cellular events during transition from sleep patterns to paroxysmal activity. J. Neurosci. 1995;15:623–642. doi: 10.1523/JNEUROSCI.15-01-00623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Curro Dossi R, Nunez A. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J. Neurosci. 1993;13:3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Deschenes M, Domich L, Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J. Neurophysiol. 1985;54:1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- Steriade M, Domich L, Oakson G, Deschenes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J. Neurophysiol. 1987;57:260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- Uhlrich DJ, Cucchiaro JB, Humphrey AL, Sherman SM. Morphology and axonal projection patterns of individual neurons in the cat perigeniculate nucleus. J. Neurophysiol. 1991;65:1528–1541. doi: 10.1152/jn.1991.65.6.1528. [DOI] [PubMed] [Google Scholar]
- Venance L, Rozov A, Blatow M, Burnashev N, Feldmeyer D, Monyer H. Connexin expression in electrically coupled postnatal rat brain neurons. Proc. Natl. Acad. Sci. U.S.A. 2000;97:10260–10265. doi: 10.1073/pnas.160037097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261:361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]