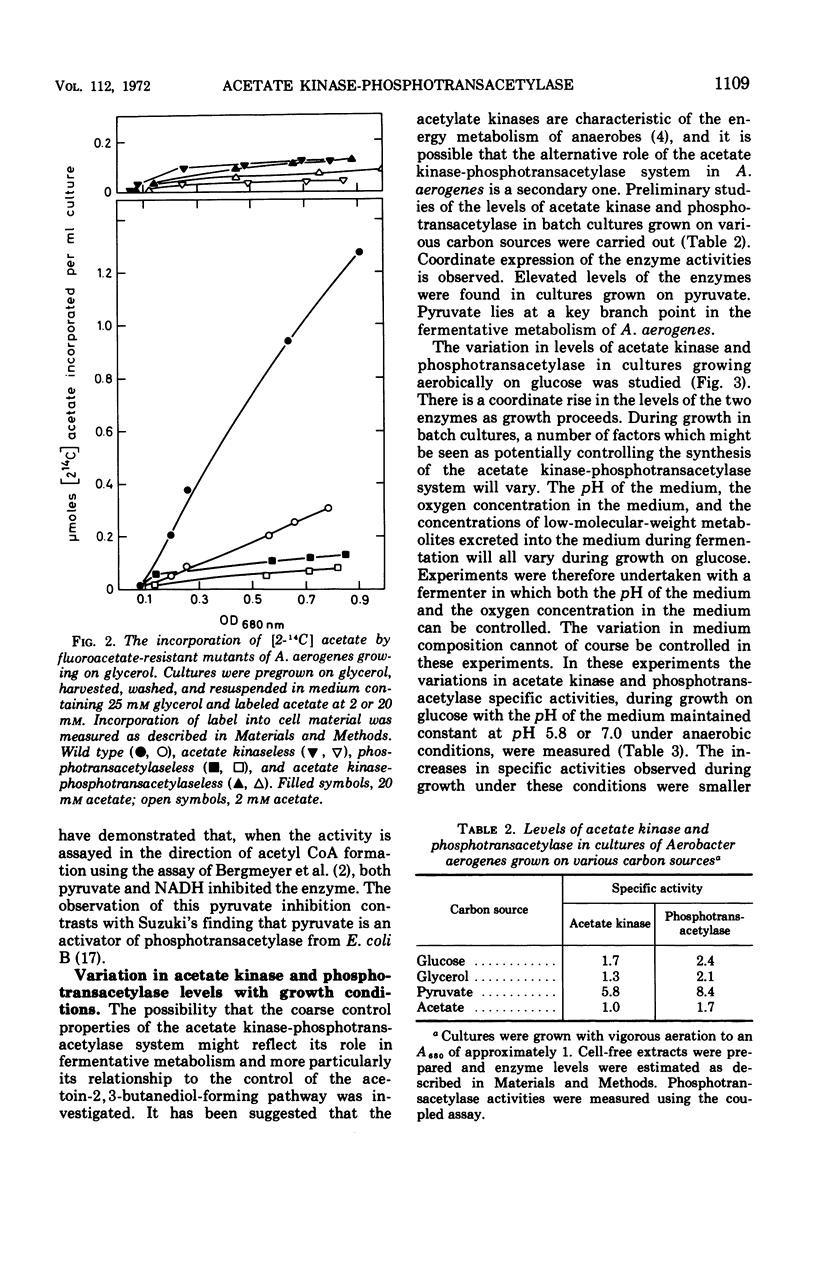
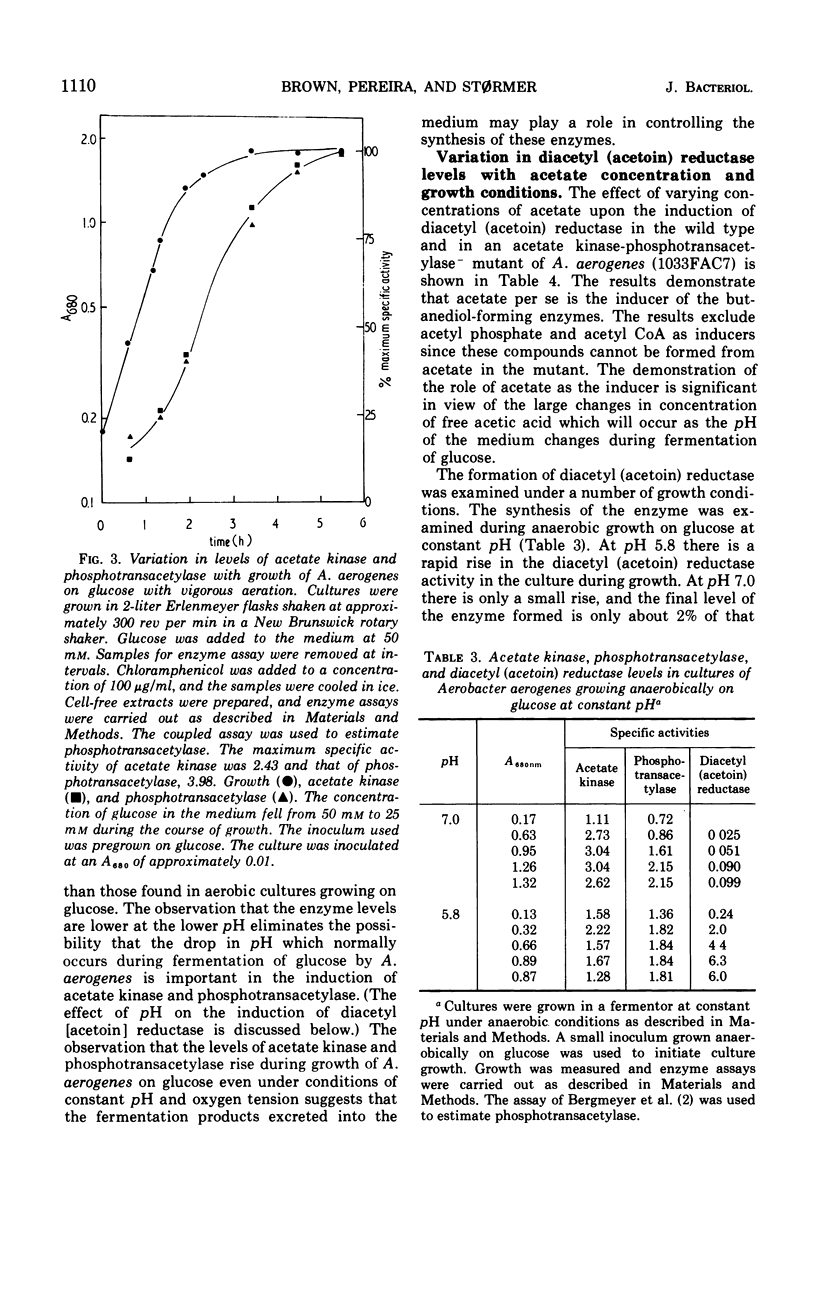
Abstract
Mutants of Aerobacter aerogenes devoid of acetate kinase and phosphotransacetylase activities were isolated by selection for resistance to fluoroacetate on lactate medium. The mutants were used to study the role of the acetate kinase-phosphotransacetylase system in growth on acetate and glucose. Acetate kinase-negative and phosphotransacetylase-negative mutants were unable to grow on acetate minimal medium. Their growth rates on glucose minimal medium were identical with that of the parent strain under aerobic conditions, but lower growth rates were observed in the mutant strains during anaerobic growth on glucose medium. The mutants were unable to incorporate [2-14C]-acetate rapidly while growing on glycerol. Variations in acetate kinase and phosphotransacetylase levels during growth on glucose were studied. The specific activities of the enzymes increased approximately fivefold during aerobic growth on glucose in batch culture. The enzyme levels were also studied during anaerobic growth on glucose at constant pH (pH 5.8 and 7.0). Smaller increases in specific activities were found under these conditions. The role of acetate in the induction of the diacetyl (acetoin) reductase was investigated using a mutant deficient in both acetate kinase and phosphotransacetylase. The effect of pH on the induction of this enzyme during growth on glucose under anaerobic conditions was tested. The data support the idea that free acetic acid is the inducer for the enzymes of the butanediol-forming pathway in A. aerogenes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashworth J. M., Kornberg H. L. The anaplerotic fixation of carbon dioxide by Escherichia coli. Proc R Soc Lond B Biol Sci. 1966 Aug 16;165(999):179–188. doi: 10.1098/rspb.1966.0063. [DOI] [PubMed] [Google Scholar]
- BERGMEYER H. U., HOLZ G., KLOTZSCH H., LANG G. PHOSPHOTRANSACETYLASE AUS CLOSTRIDIUM KLUYVERI. ZUECHTUNG DES BACTERIUMS, ISOLIERUNG, KRISTALLISATION UND EIGENSCHAFTEN DES ENZYMS. Biochem Z. 1963;338:114–121. [PubMed] [Google Scholar]
- Bryn K., Hetland O., Stormer F. C. The reduction of diacetyl and acetoin in Aerobacter aerogenes. Evidence for one enzyme catalyzing both reactions. Eur J Biochem. 1971 Jan 1;18(1):116–119. doi: 10.1111/j.1432-1033.1971.tb01221.x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., GILVARG C. The role of the tricarboxylic acid cycle in acetate oxidation in Escherichia coli. J Biol Chem. 1956 Sep;222(1):307–319. [PubMed] [Google Scholar]
- Decker K., Jungermann K., Thauer R. K. Energy production in anaerobic organisms. Angew Chem Int Ed Engl. 1970 Feb;9(2):138–158. doi: 10.1002/anie.197001381. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Pirt S. J. The influence of dissolved oxygen concentration on the respiration and glucose metabolism of Klebsiella aerogenes during growth. J Gen Microbiol. 1967 Feb;46(2):193–211. doi: 10.1099/00221287-46-2-193. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pelroy R. A., Whiteley H. R. Regulatory properties of acetokinase from Veillonella alcalescens. J Bacteriol. 1971 Jan;105(1):259–267. doi: 10.1128/jb.105.1.259-267.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prival M. J., Magasanik B. Resistance to catabolite repression of histidase and proline oxidase during nitrogen-limited growth of Klebsiella aerogenes. J Biol Chem. 1971 Oct 25;246(20):6288–6296. [PubMed] [Google Scholar]
- ROSE I. A., GRUNBERG-MANAGO M., KOREY S. R., OCHOA S. Enzymatic phosphorylation of acetate. J Biol Chem. 1954 Dec;211(2):737–756. [PubMed] [Google Scholar]
- Sanwal B. D. Allosteric controls of amphilbolic pathways in bacteria. Bacteriol Rev. 1970 Mar;34(1):20–39. doi: 10.1128/br.34.1.20-39.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormer F. C. Evidence for induction of the 2,3-butanediol-forming enzymes in Aerobacter aerogenes. FEBS Lett. 1968 Nov;2(1):36–38. doi: 10.1016/0014-5793(68)80094-8. [DOI] [PubMed] [Google Scholar]
- Störmer F. C. The pH 6 acetolactate-forming enzyme from Aerobacter aerogenes. I. Kinetic studies. J Biol Chem. 1968 Jul 10;243(13):3735–3739. [PubMed] [Google Scholar]
- Suzuki T. Phosphotransacetylase of Escherichia coli B, activation by pyruvate and inhibition by NADH and certain nucleotides. Biochim Biophys Acta. 1969;191(3):559–569. doi: 10.1016/0005-2744(69)90349-0. [DOI] [PubMed] [Google Scholar]
- Tvett B., Störmer F. C. The pH 6 acetolactate-forming enzyme from Aerobacter aerogenes. Determination and function of thiol groups. Eur J Biochem. 1969 Sep;10(2):249–250. doi: 10.1111/j.1432-1033.1969.tb00681.x. [DOI] [PubMed] [Google Scholar]


