Abstract
β3-adrenergic receptor (β3-AR) activation produces a negative inotropic effect in human ventricles. Here we explored the role of β3-AR in the human atrium. Unexpectedly, β3-AR activation increased human atrial tissue contractility and stimulated the L-type Ca2+ channel current (ICa,L) in isolated human atrial myocytes (HAMs). Right atrial tissue specimens were obtained from 57 patients undergoing heart surgery for congenital defects, coronary artery diseases, valve replacement, or heart transplantation. The ICa,L and isometric contraction were recorded using a whole-cell patch-clamp technique and a mechanoelectrical force transducer. Two selective β3-AR agonists, SR58611 and BRL37344, and a β3-AR partial agonist, CGP12177, stimulated ICa,L in HAMs with nanomolar potency and a 60%–90% efficacy compared with isoprenaline. The β3-AR agonists also increased contractility but with a much lower efficacy (~10%) than isoprenaline. The β3-AR antagonist L-748,337, β1-/β2-AR antagonist nadolol, and β1-/β2-/β3-AR antagonist bupranolol were used to confirm the involvement of β3-ARs (and not β1-/β2-ARs) in these effects. The β3-AR effects involved the cAMP/PKA pathway, since the PKA inhibitor H89 blocked ICa,L stimulation and the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) strongly increased the positive inotropic effect. Therefore, unlike in ventricular tissue, β3-ARs are positively coupled to L-type Ca2+ channels and contractility in human atrial tissues through a cAMP-dependent pathway.
Introduction
In cardiac myocytes, Ca2+ current through the L-type Ca2+ channels (ICa,L) provides Ca2+ for the activation of the contractile apparatus and is a crucial determinant of cardiac contractile activity (1). Among several regulatory pathways that control cardiac Ca2+ channel activity, the best described is the β-adrenergic stimulation of ICa,L, which contributes to the positive inotropic and part of the positive chronotropic effects of catecholamines (1, 2). Although to date, 3 types of β-adrenergic receptors (β-ARs) have been cloned, β1-, β2-, and β3-ARs, respectively, the effect of catecholamines in human heart is generally attributed to β1- and β2-ARs, with opposite effects on hypertrophy (3, 4) and apoptosis (5, 6) and with a respective contribution of each receptor subtype that varies significantly depending on the cardiac tissue, the pathophysiological state, the age, or the developmental stage (7). However, expression of a β3-AR in human myocardium was also demonstrated both at the mRNA (8, 9) and protein level (9–12).
Like β1- and β2-ARs, β3-ARs are positively coupled to adenylyl cyclase in fat and colon as well as in CHO cells transfected with the cloned receptor (13–15). Therefore, activation of β3-ARs in human heart would be expected to stimulate ICa,L and increase contractility. However, β3-AR agonists were shown to inhibit contractility in human endomyocardial biopsies from transplanted hearts (8, 16) and in left ventricular samples from failing and nonfailing explanted hearts (11), an effect mediated by Gi/o proteins and NO production via activation of endothelial type-3 NO synthase (8, 16, 17). This effect was accompanied by a shortening of the action potential (8, 18). A similar finding was obtained with BRL37344, a β3-AR agonist, in Langendorff-perfused isolated guinea pig hearts (19) and was accompanied by a decrease in Ca2+ transients. The NO dependency of β3-AR action was also found in mice, using animals with a homozygous deletion mutation of the β3-AR (ADRB3–/–) (20). While NOS3 inhibitors (arginine analogues) potentiated the positive inotropic effect of isoprenaline (ISO) in wild-type mice, this effect was absent in either ADRB3–/– or NOS3–/– mice (20). In another study, ISO applied to a culture of neonatal cardiomyocytes isolated from mice with a double homozygous deletion mutation of the β1-AR and β2-AR (ADRB1–/–/ADRB2–/–) was found to slightly decrease spontaneous beating frequency, a phenomenon mimicked by CL316243, a β3-AR agonist (21). Moreover, this negative chronotropic effect of ISO turned into a positive one after treatment of the myocytes with Bordetella pertussis toxin (21), confirming the original finding that β3-AR signaling involves activation of Gi/o proteins (8).
Although the case seems rather strong for a negative inotropic and chronotropic effect of β3-ARs, a number of studies are inconsistent with such hypothesis. For instance, several reports demonstrate the absence of a functional β3-AR in mouse heart (22–24). Cardiac overexpression of human β3-ARs in mice results in Gs activation of adenylyl cyclase and positive inotropy on stimulation with a β3-AR agonist (25). In human atrium and failing ventricle, several β3-AR agonists were found to be devoid of any cardiodepressant effect (26–28). These latter findings are of importance, since the amount of β3-AR proteins was shown to increase up to 3-fold in different models of heart failure (11, 29, 30).
In this study, our aim was to get further insights into the role of β3-ARs on human cardiac function at the single-cell level. For this, we examined the effects of several β3-AR ligands on ICa,L recorded in isolated human atrial myocytes (HAMs): SR58611 (31) and BRL37344 (32), which are 2 β3-AR agonists; CGP12177, which is a partial β3-AR agonist (33) as well as a β2-AR antagonist and a partial agonist at β1-AR through a low-affinity site (34, 35); and L-748,337, which is a β3-AR antagonist (36). These effects were compared with those obtained on the contractility of human atrial strips.
Results
β3-AR agonists stimulate ICa,L in HAMs.
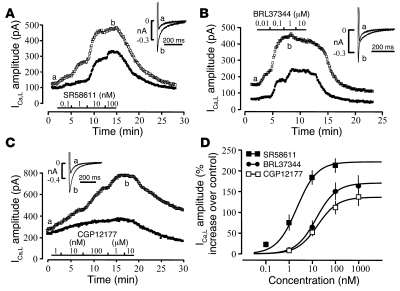
To examine the regulation of ICa,L by β3-ARs in HAMs, we used 2 β3-AR agonists, SR58611 (31) and BRL37344 (32), as well as CGP12177, which is a β3-AR partial agonist (33), a β2-AR antagonist, and a partial agonist at β1-AR through a low-affinity site (34, 35). Typical experiments are shown in Figure 1. In each example, 2 cells were simultaneously exposed to increasing concentrations of SR58611 (Figure 1A), BRL37344 (Figure 1B), and CGP12177 (Figure 1C). Such double-cell experiments provided a simple way to evaluate cell-to-cell variability, since all external parameters (temperature, perfusion, time after isolation, etc.) were strictly identical during the recordings obtained in the 2 cells. As can be seen in Figure 1, all compounds produced a dose-dependent and reversible increase in ICa,L. The cumulative dose-response curves shown in Figure 1D were fitted to the Michaelis equation to derive the maximal stimulation (Emax) of ICa,L and the concentration required for half-maximal effect (EC50) for each agonist. Thus, Emax and EC50 values, respectively, were as follows: 221% ± 20% and 2.5 ± 0.8 nM (n = 6) for SR58611; 172% ± 26% and 15.2 ± 0.6 nM (n = 10) for BRL37344; and 136% ± 21% and 17.1 ± 3.0 nM (n = 8) for CGP12177 (Figure 1D). The efficacy of SR58611 and BRL37344 on ICa,L was comparable to that of ISO (241% ± 47% at 1 μM ISO; n = 5), while that of CGP12177 was somewhat smaller. None of the 3 β3-AR agonists tested had any stimulatory effect in the nanomolar range of concentrations on ICa,L measured in ventricular myocytes isolated from frog and rat hearts (Supplemental Results; supplemental material available online with this article; doi: 10.1172/JCI32519DS1).
Figure 1. β3-AR stimulation of ICa,L in HAMs.
Each experiment shows the time course of ICa,L amplitude recorded in 2 HAMs that were simultaneously exposed to increasing concentrations of SR58611 (A), BRL37344 (B), and CGP12177 (C). Each symbol indicates a net amplitude of ICa,L measured every 8 seconds at 0 mV membrane potential. The individual current traces were obtained in each experimental condition at the times indicated by the corresponding letters in the main graph. (D) Concentration-response curve for the effects of the 3 agonists on ICa,L. The points show the mean ± SEM of 6 (SR58611, filled square), 10 (BRL37344, filled circle), or 8 (CGP12177, open square) cells.
β3-AR agonists increase ICa,L in HAMs via β3-AR activation.
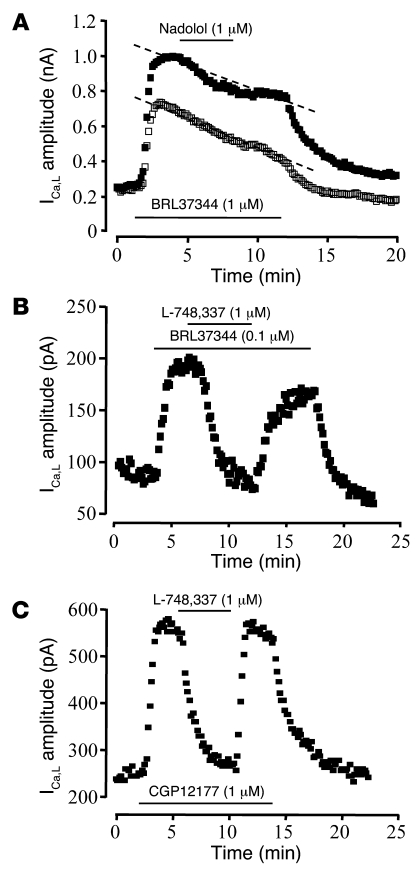
The subtype selectivity of β-AR ligands is always questionable. For instance, SR58611 and BRL37344 were shown to also activate β1- and β2-ARs in the micromolar range of concentrations (13), and CGP12177 was shown to be an agonist of β1-AR at a low-affinity site (34, 35). Since human cardiomyocytes also possess β1- and β2-ARs, we examined the possibility that the stimulatory effects of the 3 agonists on ICa,L in HAMs were caused by activation of β1- and/or β2-ARs. To do this, we examined whether the β1-AR/β2-AR antagonist nadolol was able to antagonize the stimulatory effect of SR58611 and BRL37344. Because the Kd for nadolol on the human β3-AR is more than 600 nM as compared with 14–40 nM on the β1- and β2-ARs (13), most experiments were performed using 200 nM nadolol. At this concentration, nadolol antagonized some of the stimulatory effect of SR58611 (1 μM; data not shown) on ICa,L, indicating that the effect of this agonist was at least partly mediated by activation of β1- and/or β2-ARs. However, even a 5-fold higher concentration of nadolol (1 μM) had no effect on BRL37344-induced (1 μM) stimulation of ICa,L (5.4% ± 3.9%; n = 4; P > 0.05; Figure 2A). In contrast, the effect of BRL37344 was almost completely blocked (by 88.5% ± 5.5%; n = 5; P < 0.001) by 1 μM of the β3-AR antagonist L-748,337 (36, 37) (Figure 2B). Interestingly, L-748,337 also almost completely antagonized the stimulatory effect of CGP12177 on ICa,L (by 87.9% ± 4.4%; n = 4; P < 0.01; Figure 2C), which excludes the possibility that CGP12177 was acting via the low-affinity site of the β1-AR (34, 35). As seen in Figure 2, B and C, the effect of L-748,337 was fully reversible. In another series of experiments, bupranolol (1 μM), which combines β1-/β2-/β3-AR antagonistic properties (38), was found to strongly antagonize the stimulatory effect of 1 μM CGP12177 (by 79.3% ± 1.6%; n = 5; P < 0.05). Altogether, these results indicate that at the concentrations used the stimulatory effects of BRL37344, CGP12177, and, to a large extent, SR58611 on ICa,L in HAMs result from activation of β3-ARs.
Figure 2. Effects of β3-AR and β1-/β2-AR antagonists on β3-AR stimulation of ICa,L in HAMs.
In each experiment, ICa,L was recorded in 1 (B and C) or 2 (A) HAMs, which were simultaneously exposed to BRL37344 (1 μM, A; 0.1 μM, B) or CGP12177 (1 μM, C) and then to the β1-/β2-AR antagonist nadolol (1 μM, A) or to the β3-AR antagonist L-748,337 (1 μM, B and C). The dotted lines in A indicates spontaneous rundown.
NO pathway is not involved in the β3-AR regulation of ICa,L.
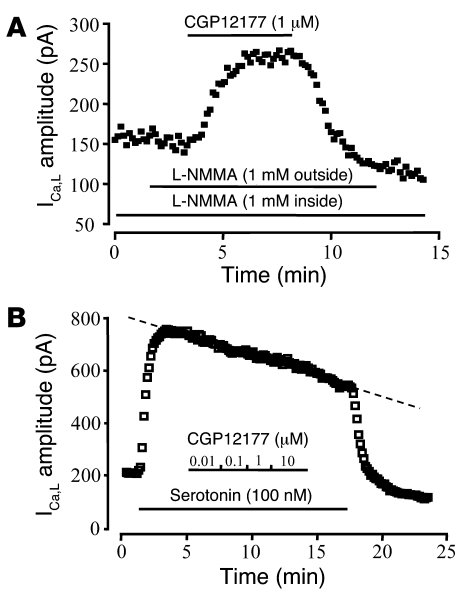
In human ventricle, β3-AR agonists were shown to produce a negative inotropic effect as a consequence of NOS3 activation (17). Through an activation of the soluble guanylyl cyclase and accumulation of intracellular cGMP, NO can lead to opposite effects on human atrial ICa,L: a stimulation at low concentrations due to inhibition of the cGMP-inhibited cAMP-phosphodiesterase (PDE3) (39, 40) and an inhibition at higher concentrations due to activation of the cGMP-stimulated phosphodiesterase (PDE2) (39, 40). To evaluate the contribution of the NO/cGMP pathway in the regulation of ICa,L by β3-AR agonists, we examined the effect of CGP12177 in the presence of the NOS inhibitor NG-monomethyl-l-arginine (L-NMMA). As shown in Figure 3A, the presence of L-NMMA (1 mM) in both the extracellular and pipette solutions did not modify the stimulatory effect of CGP12177 (1 μM) on ICa,L (with L-NMMA, 65% ± 11% over control, n = 4; without L-NMMA, 58% ± 4% over control, n = 4). This indicates that the NO/cGMP pathway is not required for the β3-AR stimulation of ICa,L. To examine whether β3-AR stimulation can lead to inhibitory effects via this pathway, HAMs were first exposed to serotonin (100 nM), which strongly increased ICa,L (by 154% ± 64%; n = 3; Figure 3B) via activation of the 5-HT4 receptor (41), and then to CGP12177 in the presence of serotonin. As shown in Figure 3B, CGP12177, at concentrations ranging from 0.01 to 10 μM, had no inhibitory effect on prestimulated ICa,L (101% ± 1% of serotonin level; n = 3). These experiments indicate that the NO/cGMP pathway is not involved in the activation of ICa,L by β3-AR agonists, and that β3-AR stimulation does not activate a parallel inhibitory pathway regulating ICa,L.
Figure 3. NO pathway is not involved in the stimulation of ICa,L by β3-AR agonists.
In each experiment, ICa,L was recorded in a single HAM exposed to CGP12177 in the presence of either 1 mM L-NMMA in intracellular and extracellular solutions (A) or serotonin (100 nM, B). The dotted line in B indicates spontaneous rundown.
β3-AR activation of ICa,L involves cAMP-dependent protein kinase.
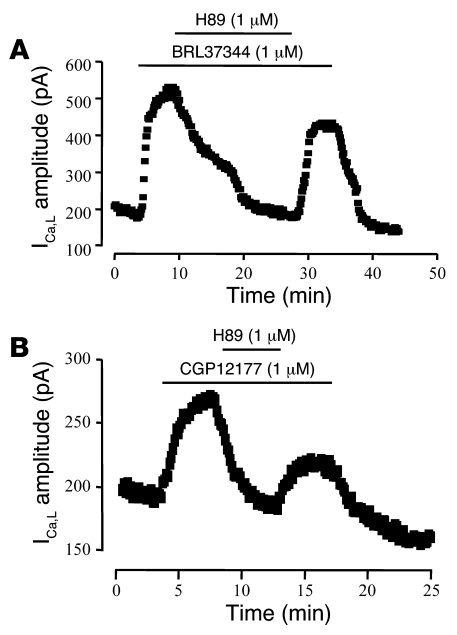
Because cAMP-dependent PKA is a classic activator of L-type Ca2+ channels and accounts for the stimulatory effects of β1- and β2-AR agonists on ICa,L, we examined whether PKA was also involved in the β3-AR activation of ICa,L. Figure 4 shows 2 individual experiments in which the cell permeant PKA inhibitor H89 (42) was applied to HAMs on top of BRL37344 (1 μM; Figure 4A) or CGP12177 (1 μM; Figure 4B). In both cases, H89 (1 μM) completely inhibited the stimulatory effect of the β3-AR agonist. The inhibitory effect of H89 was mostly reversible upon washout. On average, BRL37344 and CGP12177 increased ICa,L by 297% ± 84% (n = 4) and 45% ± 6% (n = 4), respectively, in the absence of H89, and the current amplitude differed from basal level by only 3% ± 6% (n = 4) and –7% ± 3% (n = 4) in the presence of the PKA inhibitor. These experiments indicate that β3-AR agonists increase ICa,L in HAMs through activation of the cAMP/PKA pathway.
Figure 4. PKA mediates the stimulation of ICa,L by β3-AR agonists.
ICa,L was recorded in a single HAM exposed to either 1 μM BRL3744 (A) or 1 μM CGP12177 (B). When the current was at its maximal stimulation, the PKA inhibitor H89 (1 μM) was added to the extracellular solutions in the presence of the β3-AR agonists, and the current returned to basal levels.
β3-AR agonists increase force of contraction in human atrial trabeculae.
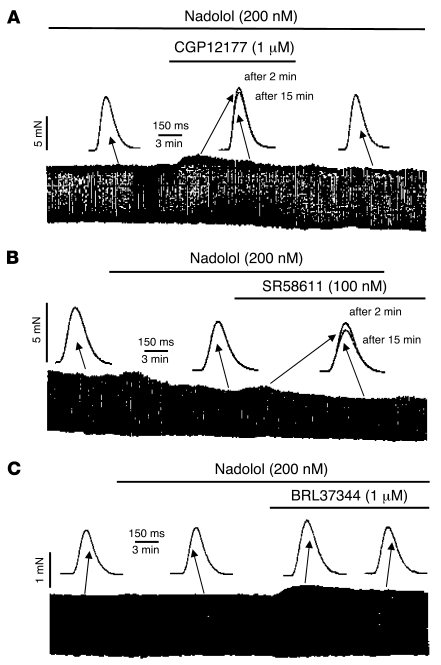
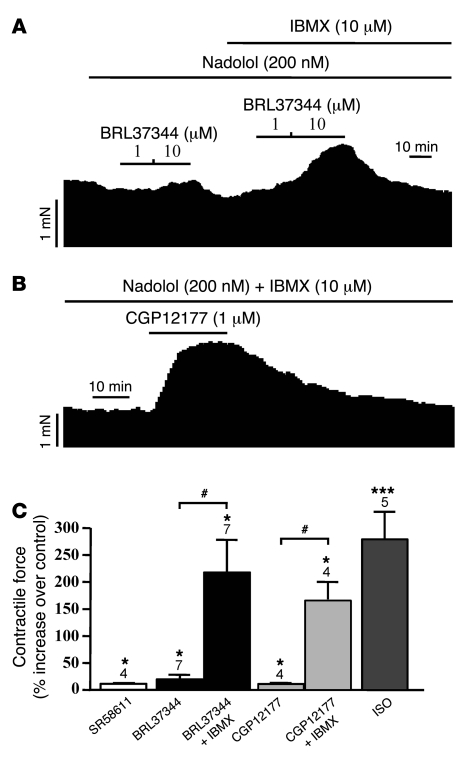
In cardiac myocytes, ICa,L provides Ca2+ for the activation of the contractile proteins and is a crucial determinant of the cardiac contractile activity. Therefore, any modulation of ICa,L amplitude might be expected to induce parallel changes in contractile amplitude. Since our results so far indicate that β3-ARs are positively coupled to ICa,L in HAMs, additional experiments were performed to evaluate the effects of β3-AR agonists on the force of contraction in trabeculae isolated from human atrial tissues. To eliminate any possible interaction of the agonists with β1- and/or β2-ARs (43), the experiments were performed in the presence of nadolol (200 nM), which alone reduced atrial basal contractile amplitude to 82% ± 9% (n = 8) of control level. The individual experiments shown in Figure 5 indicate that CGP12177 (1 μM; Figure 5A), SR58611 (100 nM; Figure 5B), and BRL37344 (1 μM; Figure 5C) induced a modest and transient positive inotropic effect in human atrial muscle. A 10%–20% peak stimulation was observed with all 3 compounds at approximately 2 minutes after drug application, and a steady state was reached at approximately 15 minutes. The summary data in Figure 6C indicate that all 3 β3-AR agonists induced a significant increase in contractile amplitude at 2 minutes in human atrium, but only BRL37344 maintained a significant positive inotropic effect at 15 minutes (11.7% ± 1.7%; n = 6; P < 0.05). In contrast, the effect of ISO (1 μM) was more prominent (Figure 6C). Because the β3-AR stimulation of ICa,L was mediated by PKA, and because different Gs-coupled receptors generate heterogeneous cAMP signals due to specific functional coupling of phosphodiesterase (PDE) isoforms (44, 45), we questioned whether the modest effect on contractility was due to a limited diffusion of cAMP that might result from a high PDE activity. If this is the case, then PDE inhibition should potentiate the contractile effects of β3-AR agonists. We tested this hypothesis by reevaluating the contractile effects of β3-AR agonists in the presence of 10 μM 3-isobutyl-1-methylxanthine (IBMX), a wide-spectrum PDE inhibitor. As shown in Figure 6, the effects of BRL37344 (Figure 6A) and CGP12177 (Figure 6B) were greatly increased by IBMX. While IBMX alone had no significant effect on contraction force (n = 9), it strongly increased the effects of BRL37344 (1 and 10 μM) and CGP12177 (1 μM). Figure 6C shows that in the presence of IBMX, the effects of the 2 β3-AR agonists were comparable with those produced by ISO (1 μM).
Figure 5. Effect of β3-AR ligands on contractility in human atrial trabeculae.
CGP12177 (A), SR58611 (B), or BRL37344 (C) were applied to human atrial preparations at the concentrations indicated, after 15 minutes of perfusion with 200 nM nadolol followed by the continuous presence of nadolol. The individual contractile traces were obtained in each experimental condition at the times indicated by the corresponding arrows. All 3 compounds significantly (P < 0.05) increased force of contraction.
Figure 6. PDE inhibition potentiates the effects of β3-AR agonists on contractility in human atrial trabeculae.
BRL37344 (A) or CGP12177 (B) were applied to human atrial preparations at the concentrations indicated, in the presence of 200 nM nadolol and in the absence (A) or presence (A and B) of the PDE inhibitor IBMX (10 μM). (C) Summary of the effects of the β3-AR agonists on contractility. The effects of SR58611 (100 nM), BRL37344 (10 μM), and CGP12177 (1 μM), with or without IBMX (10 μM), are compared with that of ISO (1 μM). The bars show the mean ± SEM of the number of experiments indicated above the bars. *P < 0.05; ***P < 0.005 versus control. ANOVA followed by post-hoc Bonferroni’s test was used to compare the effects of the β3-AR agonists in the absence or presence of IBMX. #P < 0.05.
Discussion
Adenylyl cyclase stimulation assays performed in CHO cells stably transfected with either the human β1-, β2-, or β3-ARs demonstrated that BRL37344, SR58611, and CGP12177 displayed a high β3-AR selectivity, with EC50 values ranging from 15 to 140 nM (13, 46). In the present study, all 3 ligands increased ICa,L in HAMs with similar low EC50 values (3–17 nM; Figure 1). Even though human heart contains more spare β1- and β2-AR receptors in the atrial than in the ventricular chambers (47–49), activation of 10%–20% of the total amount of β-ARs would be needed to produce the observed large increase in ICa,L (49), which is very unlikely at the nanomolar concentrations of β3-AR agonists used here. Besides, the effects of SR58611, BRL37344, and CGP12177 on ICa,L resembled their effects in β3-AR adenylyl cyclase assays (13), with SR58611 and BRL37344 behaving as full agonists (by comparison with ISO) and CGP12177 only as partial agonist (Figure 1D) (13, 33). With respect to CGP12177, some caution may be required because the drug is not only a β3-AR agonist and a β2-AR antagonist, but is also a partial agonist at β1-AR through a low-affinity site (34, 35). However, the stimulatory effect of CGP12177 on ICa,L (like that of BRL37344) was blocked by L-748,337, a highly potent and selective β3-AR antagonist (36), at a concentration that had no effect on the stimulation of the current by ISO (data not shown). Moreover, the effect of CGP12177 on ICa,L was inhibited by the β1-/β2-/β3-AR antagonist bupranolol but not by the β1-/β2-AR antagonist nadolol. Therefore, we are confident that the effects of the agonists tested involved β3-AR stimulation rather than nonselective β1- or β2-AR stimulation and conclude that β3-AR activation leads to stimulation of the ICa,L and contractile activity in human atrium.
Although a number of studies have demonstrated the expression of β3-ARs in human ventricular (9–12) and atrial tissue (10), little is known about their function in human heart at the single-cell level. A single abstract reports on the lack of β3-AR modulation of contractile function in human ventricular myocytes (28). Reports on the effects of β3-AR agonists in isolated cardiomyocytes from other species are limited to 6 studies (29, 50–54). In 3 of these, the effect of a β3-AR activation was investigated on ICa,L in ventricular myocytes isolated from dog (29), rat (53), and guinea pig hearts (50) using BRL37344 as a β3-AR agonist. In these studies, the drug induced a small, approximately 20%, maximal reduction of basal ICa,L, and the effect was increased to approximately 30% when the myocytes were isolated from failing hearts (29, 53). Surprisingly, the effects of BRL37344 persisted after washout of the drug, which is a concern when considering that ICa,L is subjected to substantial spontaneous rundown in mammalian cardiomyocytes (55, 56). However, the inhibitory effects of BRL37344 were attenuated by preincubation of the cells with the NOS inhibitor NG-nitro-l-arginine methyl ester (L-NAME) (50, 53) or after treatment of the myocytes with Bordetella pertussis toxin (53), which is consistent with earlier findings that a β3-AR signaling cascade involved Gi/o protein and NOS3 activation (8, 16, 17). In our experiments, after rundown subtraction, neither BRL37344, SR58611, or CGP12177, in the range of concentrations at which these ligands act selectively via β3-ARs (13), had any significant effect on basal ICa,L in rat ventricular myocytes (Supplemental Figure 2), while they all stimulated the current in HAMs.
The β3-AR stimulatory effect on ICa,L was not due to NOS activation, because the effect persisted in the presence of L-NMMA. The effect was not mediated by Gi/o proteins, since none of the β3-AR agonists tested had any effect on ICa,L prestimulated by either serotonin or forskolin, while Gi/o protein activation via muscarinic M2 receptors inhibits the current under similar conditions (57). On the contrary, the β3-AR stimulatory effect on ICa,L involved PKA activation, since it was blocked by H89, a cell-permeant PKA inhibitor (42). Therefore, when combining our results in human, rat, and frog cardiomyocytes (Supplemental Figure 1) with those from earlier studies in rat, guinea pig, and dog heart, β3-AR regulation of ICa,L appears clearly different in human heart from any other species explored so far.
Our results raise an interesting possibility that the β3-AR can change its signaling pathway from a stimulatory, Gs-mediated cAMP/PKA mechanism to an inhibitory, Gi/o-mediated one, depending on the cellular context. Some support for this hypothesis comes from heterologous β3-AR expression studies. For instance, in CHO cells (14, 58) and murine 3T3-F442A preadipocytes (59) transfected with rat β3-ARs, the receptors are coupled to Gs proteins, leading to an increase in intracellular cAMP level. However, in rat and mice adipocytes, in addition to their coupling to Gs, β3-ARs couple to Gi/o proteins and inhibit adenylyl cyclase (60, 61). In the human A549 lung epithelial cell line and in the human colonic carcinoma cell line T84, β3-ARs appear to be exclusively linked to Gi/o proteins (18, 62). Functional response to β3-AR activation may also depend on the amount of expressed receptors. Indeed, cardiac overexpression of human β3-ARs in mice results in Gs activation of adenylyl cyclase and positive inotropy on stimulation with a β3-AR agonist (25). Thus, if the human atrium expresses a higher amount of β3-ARs than the ventricle (which would go in line with the higher amount of spare β1-/β2-ARs in the atrium; ref. 49), it might favor a Gs- over Gi/o-mediated signaling pathway. Finally, depending on its cellular environment, the β3-AR might either activate or inhibit the same ion channel, such as the L-type Ca2+ channel. Such a situation has been reported for the β3-AR regulation of the slow component of the delayed rectifier potassium current (IKs), a current which like ICa,L is activated by cAMP/PKA cascade (63). Indeed, in isolated guinea pig ventricular myocytes, β3-AR activation by BRL37344 inhibits IKs by 20%–40% (51). However, when KvLQT1/MinK channels, which underline IKs current, are heterologously coexpressed with the human β3-AR in Xenopus oocytes, activation of the receptor increases IKs (64).
Although β3-AR activation increased ICa,L in HAMs, the positive inotropic effects in human atrium were modest as compared with β1-/β2-AR activation with ISO. Because contractility of a trabeculum is a more integrated response than ICa,L amplitude in a single myocyte, many other parameters will participate in the inotropic response to β3-AR agonists. One of these is the action potential duration, which is affected not only by β3-AR regulation of ICa,L but also by the effect of the receptor on other ion channels, such as IKs (51), the rapid component of the delayed rectifier potassium current (IKr) (52), the hyperpolarization-activated pacemaker current If (54), and the cystic fibrosis transmembrane conductance regulator (CFTR) Cl– current (18). Other downstream factors include intracellular Ca2+ transient and sarcoplasmic reticulum function and the phosphorylation status of key proteins such as phospholamban, ryanodine receptor, and contractile proteins. Finally, human cardiac trabeculae are multicellular preparations composed of myocytes and nonmyocytes such as endothelial cells, which also express β3-ARs (65, 66). Of note, β3-ARs in endothelial cells activate NO production that may reduce cardiac contractility (67, 68) and counterbalance a stimulatory effect of β3-ARs in myocytes. We tested this hypothesis in 4 human atrial trabeculae exposed to CGP12177 (100 nM) in the presence or absence of L-NMMA and found that NOS inhibition increased approximately 2-fold the positive inotropic effect of the agonist in atrium (P < 0.05; data not shown). Therefore, the overall effect of a β3-AR activation on the contractility of human atrium may vary from a stimulation to an inhibition, depending on the chamber, the amount of nonmyocyte endothelial and endocardial cells, and the activity of NOS enzymes. Another level of complexity may result from the ability of the myocyte to distinguish among different stimuli acting on a common signaling cascade. Indeed, even though β1-, β2-, and β3-ARs may all activate the cAMP/PKA pathway, the signaling cascade generated by each receptor may be confined to distinct intracellular compartments, which may result in different functional responses (45). A number of studies, including our own (44, 69), have underlined the importance of cAMP PDEs in generating and maintaining intracellular cAMP domains (45). Our findings that PDE inhibition with IBMX transformed an unimpressively small contractile response to β3-AR agonists to a large, positive inotropic effect similar to a β1-AR response strongly suggest that not all cAMP/PKA effectors are normally activated by β3-AR activation. A similar observation was recently reported for the serotonin 5-HT4 receptors in failing human ventricular muscle, where these receptors increased contractility only when PDE activity was blocked (70).
In summary, while β3-AR activation produces negative inotropic effects in human endomyocardial biopsies from transplanted hearts (8, 16) and in left ventricular samples from failing and nonfailing explanted hearts (11, 16) (see also refs. 26–28), our study demonstrates that β3-AR activation increases contractility in human atrial tissue. This is reminiscent of the contractile effects of the serotonin 5-HT4 receptors (71), which are also coupled to increases in force of contraction (72) or ICa,L (41) in atria but not in healthy ventricles. In human ventricle, the negative inotropic effect of β3-AR activation was proposed to spare the heart of excessive oxygen demand and may therefore play a role as a “safety-valve” during intense adrenergic stimulation (16). The identification of an opposite effect of β3-ARs in human atrium is therefore of importance, particularly in the scope of the development of new therapeutic agents acting on these receptors for the control of the adrenergic system in cardiovascular diseases. Finally, the positive coupling of β3-ARs to ICa,L in human atrium, with relatively modest consequences on force of contraction, might confer to these receptors a role in the control of beat frequency. During heart failure, the expression level of β3-ARs increases (11, 29, 30), and this might change their beneficial effect into a deleterious one, with a persistent negative inotropic effect enhancing myocardial depression. It remains to be determined whether the level of β3-ARs and their functional role are also modified in human atrial pathologies such as atrial dilation, arrhythmias, or fibrillation. This is particularly relevant, since congestive heart failure and atrial fibrillation are commonly encountered together.
Methods
Our investigations conform with the local ethics committee (Comité Régional d’Ethique en matière d’Expérimentation Animale [CREEA], Ile-de-France, Sud, France) guidelines and French decree no. 87-848 of October 19, 1987 (Journal Officiel De La Republique Francaise, pp. 12245–12248, 20 October 1987). All protocols for obtaining human cardiac tissue were approved by the ethics committee of our institutions (Groupe de Réflexion Ethique Biomédicale de Bicêtre [GREBB], Hôpital de Bicêtre, Université de Paris-Sud and Kaunas University Hospital); informed consent was obtained before cardiac surgery. Whole-cell patch-clamp experiments performed in ventricular myocytes isolated from frog and rat hearts are detailed in the Supplemental Methods.
HAMs.
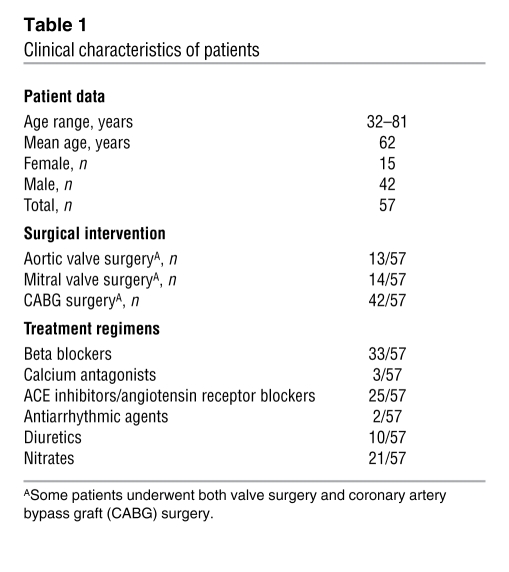
Specimens of right atrial trabeculae were obtained from 57 patients (42 males aged 61 ± 2 years and 15 females aged 66 ± 3 years) undergoing heart surgery for congenital defects, coronary artery diseases, valve replacement, or heart transplantation at the Hôpital Marie-Lannelongue, Le Plessis-Robinson, France or at the Department of Cardiosurgery of Kaunas University Hospital, Kaunas, Lithuania. Most patients received a pharmacological pretreatment (Ca2+-channel blockers, digitalis, β-AR antagonists, diuretics, ACE inhibitors, NO donors, and/or antiarrhythmic drugs) that was stopped 24 hours before surgery. In addition, all patients received sedatives, anesthesia, and antibiotics. Details regarding the clinical characteristics of the patients and their drug regimens are shown in Table 1.
Table 1 .
Clinical characteristics of patients
Dissociation of the cells was performed immediately after surgery as described previously (39, 73). The cell suspension was filtered, centrifuged, and the pellet resuspended in Dulbecco minimal essential medium supplemented with 10% fetal calf serum, nonessential amino acids, 1 nM insulin, and antibiotics (100 IU/ml penicillin and 0.1 μg/ml streptomycin).
Solutions.
For electrophysiology, the control external solution contained (in mM): 107 NaCl, 10 HEPES, 40 CsCl, 4 NaHCO3, 0.8 NaH2PO4, 1.8 MgCl2, 1.8 CaCl2, 5 d-glucose, 5 sodium pyruvate, 6 × 10–3 tetrodotoxin, pH 7.4 adjusted with NaOH. Patch electrodes (0.6–1.5 Mohms) were filled with control internal solution, which contained (in mM): 119.8 CsCl; 5 EGTA (acid form); 4 MgCl2; 5 creatine phosphate disodium salt; 3.1 Na2ATP; 0.42 Na2GTP; 0.062 CaCl2 (pCa 8.5); 10 HEPES; pH 7.3 adjusted with CsOH. Collagenase type IV and protease type XXIV were purchased from Sigma-Aldrich. DMEM was obtained from Gibco-BRL. Tetrodotoxin was from Latoxan. SR58611 was a generous gift from Sanofi Recherche. BRL37344 was a generous gift from SmithKline & Beecham. CGP12177 was a generous gift from Novartis Pharmaceutical. L-748,337 was a generous gift from Merck & Co. Inc. All other drugs were from Sigma-Aldrich. All drugs tested in patch-clamp experiments were solubilized in experimental solutions just before application onto the cell studied, i.e., only fresh solutions were tested.
Whole-cell current recording.
The whole-cell configuration of the patch-clamp technique was used to record ICa,L in Ca2+-tolerant HAMs as described previously (39, 57, 74). In the routine protocols, the cells were depolarized every 8 seconds from a holding potential of –80 mV by a short prepulse (50 milliseconds) to –50 mV and then to 0 mV for 200 or 400 milliseconds. The prepulse and the application of tetrodotoxin were used to eliminate fast sodium currents. K+ currents were blocked by replacing all K+ ions with intracellular and extracellular Cs+. Voltage-clamp pulses were generated and currents recorded using either a single (VP-500) or 2 separate patch-clamp amplifiers (RK400; Bio-Logic). In the latter case, when possible, 2 different, nearby cells in the same dish were successively attached to 2 patch pipettes, whole-cell configuration was applied to both, and 2 different ICa,L currents were simultaneously recorded. Visual-Patch version 1.30 (Bio-Logic) or computer software that was generated in-house were used to control all experimental parameters, cell stimulation, and current recording. Recordings were low-pass filtered at 2 kHz and stored on the hard disc of an IBM-compatible computer. Control and drug-containing solutions were applied to the exterior of the cell by placing the cell at the opening of 300-μm inner diameter capillary tubes that flow at a rate of about 50 μl/min. Changes in extracellular solutions were automatically achieved using a rapid solution changer (RSC-200; Bio-Logic). All experiments were done at room temperature (19°C–25°C), and the temperature did not vary by more than 1°C in a given experiment.
Mechanoelectrical measurements.
Experiments were performed on human trabeculae isolated from the right atrium. During transport from hospital to the laboratory, atrial tissues were placed in a cold (10°C) St. Thomas cardioplegic solution composed of (in mM) 110 NaCl, 16 KCl, 1.2 CaCl2, 16 MgCl2, 5 glucose, 10 HEPES, pH 7.4 adjusted with NaOH. Trabeculae were then placed in an experimental chamber and superfused at a flow rate of 6 ml/min with oxygenated (100% O2) Tyrode solution (pO2 580–600 mmHg) composed of (in mM) 137 NaCl, 5.4 KCl, 1.8 CaCl2, 0.9 MgCl2, 5 glucose, 10 HEPES at 36.0°C ± 0.5°C, pH 7.4. Trabeculae were subjected to field stimulation with the following characteristics: stimulus pulse width was 5 milliseconds, stimulation rate was 1.0 Hz, and amplitude was twice the diastolic threshold. Isometric contraction was recorded using a mechanoelectrical force transducer. The β3-AR agonists and other drugs were applied after steady-state conditions were established, i.e. after 40–50 minutes perfusion with control Tyrode solution. All experiments were done at 37°C.
Statistics.
The maximal amplitude of whole-cell ICa,L was measured as previously described (39, 74). Currents were not compensated for capacitive and leak currents. On-line analysis of the recordings was done for each membrane depolarization to determine peak and steady-state current values. The changes in contractile force were expressed as percentage variation versus control. The results are expressed as mean ± SEM. For statistical evaluation the paired and unpaired 1-tailed Student’s t test were used, and a difference was considered statistically significant when P was < 0.05. ANOVA followed by post-hoc Bonferroni’s test was used for contractile experiments when comparing the effects of the β3-AR agonists in the absence or presence of IBMX.
Supplementary Material
Acknowledgments
This work was supported by a grant from INSERM Programme National de Recherche sur les Maladies Cardiovasculaires, the Fondation Leducq for the Transatlantic Network of Excellence 06 CVD 02 (to R. Fischmeister), and by European Union contract LSHM-CT-2005-018833/EUGeneHeart (to R. Fischmeister). We thank Patrick Lechêne and Antanas Navalinskas for skillful technical assistance, Florence Lefebvre for preparation of the cells, and Bertrand Crozatier for critical reading of the manuscript. V.A. Skeberdis was supported by a fellowship from INSERM (Poste Vert).
Footnotes
Nonstandard abbreviations used: β-AR, β-adrenergic receptor; HAM, human atrial myocyte; IBMX, 3-isobutyl-1-methylxanthine; ICa,L, L-type Ca2+ channel current; IKs, delayed rectifier potassium current; ISO, isoprenaline; L-NMMA, NG-monomethyl-l-arginine; PDE, phosphodiesterase.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:3219–3227 (2008). doi:10.1172/JCI32519
References
- 1.Bers D.M. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 2.Brette F., Leroy J., Le Guennec J.Y., Sallé L. Ca2+ currents in cardiac myocytes: old story, new insights. . Prog. Biophys. Mol. Biol. 2006;91:1–82. doi: 10.1016/j.pbiomolbio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Morisco C., Zebrowski D.C., Vatner D.E., Vatner S.F., Sadoshima J. β-Adrenergic cardiac hypertrophy is mediated primarily by the β1-subtype in the rat heart. . J. Mol. Cell. Cardiol. 2001;33:561–573. doi: 10.1006/jmcc.2000.1332. [DOI] [PubMed] [Google Scholar]
- 4.Ahmet I., et al. Beneficial effects of chronic pharmacological manipulation of β-adrenoreceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation. 2004;110:1083–1090. doi: 10.1161/01.CIR.0000139844.15045.F9. [DOI] [PubMed] [Google Scholar]
- 5.Communal C., Singh K., Sawyer D.B., Colucci W.S. Opposing effects of β1- and β2-adrenergic receptors on cardiac myocyte apoptosis - Role of a pertussis toxin-sensitive G proteins. . Circulation. 1999;100:2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 6.Zaugg M., et al. β-Adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation. 2000;102:344–350. doi: 10.1161/01.cir.102.3.344. [DOI] [PubMed] [Google Scholar]
- 7.Brodde O.E., Bruck H., Leineweber K. Cardiac adrenoceptors: physiological and pathophysiological relevance. J. Pharmacol. Sci. 2006;100:323–337. doi: 10.1254/jphs.CRJ06001X. [DOI] [PubMed] [Google Scholar]
- 8.Gauthier C., Tavernier G., Charpentier F., Langin D., Lemarec H. Functional β3-adrenoceptor in the human heart. . J. Clin. Invest. 1996;98:556–562. doi: 10.1172/JCI118823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moniotte S., et al. Real-time RT-PCR for the detection of beta-adrenoceptor messenger RNAs in small human endomyocardial biopsies. J. Mol. Cell. Cardiol. 2001;33:2121–2133. doi: 10.1006/jmcc.2001.1475. [DOI] [PubMed] [Google Scholar]
- 10.Chamberlain P.D., et al. The tissue distribution of the human β3-adrenoceptor studied using a monoclonal antibody: direct evidence of the β3-adrenoceptor in human adipose tissue, atrium and skeletal muscle. . Int. J. Obes. Relat. Metab. Disord. 1999;23:1057–1065. doi: 10.1038/sj.ijo.0801039. [DOI] [PubMed] [Google Scholar]
- 11.Moniotte S., et al. Upregulation of β3-adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. . Circulation. 2001;103:1649–1655. doi: 10.1161/01.cir.103.12.1649. [DOI] [PubMed] [Google Scholar]
- 12.De Matteis R., et al. Immunohistochemical identification of the β3-adrenoceptor in intact human adipocytes and ventricular myocardium: effect of obesity and treatment with ephedrine and caffeine. . Int. J. Obes. Relat. Metab. Disord. 2002;26:1442–1450. doi: 10.1038/sj.ijo.0802148. [DOI] [PubMed] [Google Scholar]
- 13.Blin N., Camoin L., Maigret B., Strosberg D. Structural and conformational features determining selective signal transduction in the β3-adrenergic receptor. Mol. Pharmacol. 1993;44:1094–1104. [PubMed] [Google Scholar]
- 14.Emorine L.J., et al. Molecular characterization of the human β3-adrenergic receptor. . Science. 1989;245:1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- 15.Strosberg A.D. Structure and function of the β3-adrenergic receptor. . Annu. Rev. Pharmacol. Toxicol. 1997;37:421–450. doi: 10.1146/annurev.pharmtox.37.1.421. [DOI] [PubMed] [Google Scholar]
- 16.Rozec B., Gauthier C. β3-Adrenoceptors in the cardiovascular system: Putative roles in human pathologies. . Pharmacol. Ther. 2006;111:652–673. doi: 10.1016/j.pharmthera.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Gauthier C., et al. The negative inotropic effect of β3-adrenoreceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. . J. Clin. Invest. 1998;102:1377–1384. doi: 10.1172/JCI2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leblais V., et al. β3-adrenoceptor control the cystic fibrosis transmembrane conductance regulator through a cAMP/Protein kinase A-independent pathway. . J. Biol. Chem. 1999;274:6107–6113. doi: 10.1074/jbc.274.10.6107. [DOI] [PubMed] [Google Scholar]
- 19.Kitamura T., et al. The negative inotropic effect of β3-adrenoceptor stimulation in the beating guinea pig heart. . J. Cardiovasc. Pharmacol. 2000;35:786–790. doi: 10.1097/00005344-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Varghese P., et al. β3-adrenoceptor deficiency blocks nitric oxide-dependent inhibition of myocardial contractility. . J. Clin. Invest. 2000;106:697–703. doi: 10.1172/JCI9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devic E., Xiang Y., Gould D., Kobilka B. β-adrenergic receptor subtype-specific signaling in cardiac myocytes from β1 and β2 adrenoceptor knockout mice. . Mol. Pharmacol. 2001;60:577–583. [PubMed] [Google Scholar]
- 22.Kaumann A.J., et al. (-)-CGP 12177 causes cardiostimulation and binds to cardiac putative β4-adrenoceptors in both wild-type and β3-adrenoceptor knockout mice. . Mol. Pharmacol. 1998;53:670–675. doi: 10.1124/mol.53.4.670. [DOI] [PubMed] [Google Scholar]
- 23.Oostendorp J., Kaumann A.J. Pertussis toxin suppresses carbachol-evoked cardiodepression but does not modify cardiostimulation mediated through β1- and putative β4-adrenoceptors in mouse left atria: no evidence for β2- and β3-adrenoceptor function. . Naunyn Schmiedebergs Arch. Pharmacol. 2000;361:134–145. doi: 10.1007/s002109900156. [DOI] [PubMed] [Google Scholar]
- 24.Heubach U.F., Rau T., Eschenhagen T., Ravens U., Kaumann A.J. Physiological antagonism between ventricular β1-adrenoceptors and α1-adrenoceptors but no evidence for β2- and β3-adrenoceptor function in murine heart. . Br. J. Pharmacol. 2002;136:217–229. doi: 10.1038/sj.bjp.0704700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohout T.A., et al. Augmentation of cardiac contractility mediated by the human β3-adrenergic receptor overexpressed in the hearts of transgenic mice. . Circulation. 2001;104:2485–2491. doi: 10.1161/hc4501.098933. [DOI] [PubMed] [Google Scholar]
- 26.Kaumann A.J., Molenaar P. Modulation of human cardiac function through 4 β-adrenoceptor populations. Naunyn Schmiedebergs Arch. Pharmacol. 1997;355:667–681. doi: 10.1007/PL00004999. [DOI] [PubMed] [Google Scholar]
- 27.Molenaar P., Sarsero D., Kaumann A.J. Proposal for the interaction of non-conventional partial agonists and catecholamines with the ‘putative beta 4-adrenoceptor’ in mammalian heart. Clin. Exp. Pharmacol. Physiol. 1997;24:647–656. doi: 10.1111/j.1440-1681.1997.tb02107.x. [DOI] [PubMed] [Google Scholar]
- 28.Harding S.E. Lack of evidence for β3-adrenoceptor modulation of contractile function in human ventricular myocytes [abstract]. . Circulation. 1997;96(Suppl. S):284. [Google Scholar]
- 29.Cheng H.J., et al. Upregulation of functional β3-adrenergic receptor in the failing canine myocardium. . Circ. Res. 2001;89:599–606. doi: 10.1161/hh1901.098042. [DOI] [PubMed] [Google Scholar]
- 30.Morimoto A., Hasegawa H., Cheng H.J., Little W.C., Cheng C.P. Endogenous β3-adrenoreceptor activation contributes to left ventricular and cardiomyocyte dysfunction in heart failure. . Am. J. Physiol. Heart Circ. Physiol. 2004;286:H2425–H2433. doi: 10.1152/ajpheart.01045.2003. [DOI] [PubMed] [Google Scholar]
- 31.Simiand J., et al. Antidepressant profile in rodents of SR 58611A, a new selective agonist for atypical β-adrenoceptors. Eur. J. Pharmacol. 1992;219:193–201. doi: 10.1016/0014-2999(92)90296-G. [DOI] [PubMed] [Google Scholar]
- 32.Muzzin P., Seydoux J., Giacobino J.P., Venter J.C., Fraser C. Discrepancies between the affinities of binding and action of the novel β-adrenergic agonist BRL 37344 in rat brown adipose tissue. Biochem. Biophys. Res. Commun. 1988;156:375–382. doi: 10.1016/S0006-291X(88)80851-9. [DOI] [PubMed] [Google Scholar]
- 33.Staehelin M., Simons P. Rapid and reversible disappearance of β-adrenergic cell surface receptors. EMBO. J. 1982;1:187–190. doi: 10.1002/j.1460-2075.1982.tb01145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granneman J.G. The putative beta4-adrenergic receptor is a novel state of the beta1-adrenergic receptor. Am. J. Physiol. Endocrinol. Met. 2001;280:E199–E202. doi: 10.1152/ajpendo.2001.280.2.E199. [DOI] [PubMed] [Google Scholar]
- 35.Joseph S.S., Lynham J.A., Colledge W.H., Kaumann A.J. Binding of (-)-[3H]- CGP12177 at two sites in recombinant human β1-adrenoceptors and interaction with β-blockers. . Naunyn Schmiedebergs Arch. Pharmacol. 2004;369:525–532. doi: 10.1007/s00210-004-0884-y. [DOI] [PubMed] [Google Scholar]
- 36.Candelore M.R., et al. Potent and selective human β3-adrenergic receptor antagonists. . J. Pharmacol. Exp. Ther. 1999;290:649–655. [PubMed] [Google Scholar]
- 37.Kozlovski V.I., Chlopicki S., Gryglewski R.J. Effects of two β3-agonists, CGP 12177A and BRL 37344, on coronary flow and contractility in isolated guinea pig heart. . J. Cardiovasc. Pharmacol. 2003;41:706–713. doi: 10.1097/00005344-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Galitzky J., et al. Coexistence of β1-, β2-, and β3-adrenoceptors in dog fat cells and their differential activation by catecholamines. . Am. J. Physiol. 1993;264:E403–E412. doi: 10.1152/ajpendo.1993.264.3.E403. [DOI] [PubMed] [Google Scholar]
- 39.Kirstein M., et al. Nitric oxide regulates the calcium current in isolated human atrial myocytes. J. Clin. Invest. 1995;95:794–802. doi: 10.1172/JCI117729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vandecasteele G., Verde I., Rucker-Martin C., Donzeau-Gouge P., Fischmeister R. Cyclic GMP regulation of the L-type Ca2+ channel current in human atrial myocytes. . J. Physiol. 2001;533:329–340. doi: 10.1111/j.1469-7793.2001.0329a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouadid H., Seguin J., Dumuis A., Bockaert J., Nargeot J. Serotonin increases calcium current in human atrial myocytes via the newly described 5-hydroxytryptamine4 receptors. . Mol. Pharmacol. 1992;41:346–351. [PubMed] [Google Scholar]
- 42.Chijiwa T., et al. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5- isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J. Biol. Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- 43.Pott C., et al. The preferential β3-adrenoceptor agonist BRL 37344 increases force via β1-/β2-adrenoceptors and induces endothelial nitric oxide synthase via β3-adrenoceptors in human atrial myocardium. . Br. J. Pharmacol. 2003;138:521–529. doi: 10.1038/sj.bjp.0705065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rochais F., et al. A specific pattern of phosphodiesterases controls the cAMP signals generated by different Gs-coupled receptors in adult rat ventricular myocytes. . Circ. Res. 2006;98:1081–1088. doi: 10.1161/01.RES.0000218493.09370.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fischmeister R., et al. Compartmentation of cyclic nucleotide signaling in the heart: The role of cyclic nucleotide phosphodiesterases. Circ. Res. 2006;99:816–828. doi: 10.1161/01.RES.0000246118.98832.04. [DOI] [PubMed] [Google Scholar]
- 46.Howe R. β3-adrenergic agonists. . Drugs Future. 1993;18:529–549. [Google Scholar]
- 47.Kaumann A.J., Lemoine H., Morris T.H., Schwederski U. An initial characterization of human heart beta-adrenoceptors and their mediation of the positive inotropic effects of catecholamines. Naunyn Schmiedebergs Arch. Pharmacol. 1982;319:216–221. doi: 10.1007/BF00495868. [DOI] [PubMed] [Google Scholar]
- 48.Schwinger R.H., Bohm M., Erdmann E. Evidence against spare or uncoupled beta-adrenoceptors in the human heart. Am. Heart J. 1990;119:899–904. doi: 10.1016/S0002-8703(05)80329-1. [DOI] [PubMed] [Google Scholar]
- 49.Brown L., et al. Spare receptors for beta-adrenoceptor-mediated positive inotropic effects of catecholamines in the human heart. J. Cardiovasc. Pharmacol. 1992;19:222–232. doi: 10.1097/00005344-199202000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Au A.L., Kwan Y.W. Modulation of L-type Ca2+ channels by β3-adrenoceptor activation and the involvement of nitric oxide. . J. Card. Surg. 2002;17:465–469. doi: 10.1111/j.1540-8191.2001.tb01179.x. [DOI] [PubMed] [Google Scholar]
- 51.Bosch R.F., et al. β3-Adrenergic regulation of an ion channel in the heart-inhibition of the slow delayed rectifier potassium current IKs in guinea pig ventricular myocytes. . Cardiovasc. Res. 2002;56:393–403. doi: 10.1016/S0008-6363(02)00601-6. [DOI] [PubMed] [Google Scholar]
- 52.Karle C.A., et al. Rapid component IKr of the guinea-pig cardiac delayed rectifier K+ current is inhibited by β1-adrenoreceptor activation, via cAMP/protein kinase A-dependent pathways. . Cardiovasc. Res. 2002;53:355–362. doi: 10.1016/S0008-6363(01)00509-0. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Z.S., et al. Enhanced inhibition of L-type Ca2+ current by β3-adrenergic stimulation in failing rat heart. . J. Pharmacol. Exp. Ther. 2005;315:1203–1211. doi: 10.1124/jpet.105.089672. [DOI] [PubMed] [Google Scholar]
- 54.Sartiani L., et al. Functional remodeling in post-myocardial infarcted rats: focus on beta-adrenoceptor subtypes. J. Mol. Cell. Cardiol. 2006;40:258–266. doi: 10.1016/j.yjmcc.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 55.Belles B., Malécot C.O., Hescheler J., Trautwein W. ‘Run-down’ of the Ca current during long whole-cell recordings in guinea pig heart cells: role of phosphorylation and intracellular calcium. Pflügers Arch. 1988;411:353–360. doi: 10.1007/BF00587713. [DOI] [PubMed] [Google Scholar]
- 56.Kepplinger K.J.F., et al. Molecular determinant for run-down of L-type Ca2+ channels localized in the carboxyl terminus of the a1C subunit. . J. Physiol. 2000;529:119–130. doi: 10.1111/j.1469-7793.2000.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vandecasteele G., Eschenhagen T., Fischmeister R. Role of the NO-cGMP pathway in the muscarinic regulation of the L-type Ca2+ current in human atrial myocytes. . J. Physiol. 1998;506:653–663. doi: 10.1111/j.1469-7793.1998.653bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Granneman J.G., Lahners K.N., Chaudhry A. Molecular cloning and expression of the rat β3-adrenergic receptor. . Mol. Pharmacol. 1991;40:895–899. [PubMed] [Google Scholar]
- 59.Feve B., et al. Atypical β-adrenergic receptor in 3T3-F442A adipocytes. J. Biol. Chem. 1991;266:20329–20336. [PubMed] [Google Scholar]
- 60.Chaudhry A., MacKenzie R.G., Georgic L.M., Granneman J.G. Differential interaction of β1- and β3-adrenergic receptors with Gi in rat adipocytes. . Cell. Signal. 1994;6:457–465. doi: 10.1016/0898-6568(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 61.Begin-Heick N. β3 -adrenergic activation of adenylyl cyclase in mouse white adipocytes: modulation by GTP and effect of obesity. . J. Cell. Biochem. 1995;58:464–473. doi: 10.1002/jcb.240580409. [DOI] [PubMed] [Google Scholar]
- 62.Robay A., Toumaniantz G., Leblais V., Gauthier C. Transfected β3- but not β2-adrenergic receptors regulate cystic fibrosis transmembrane conductance regulator activity via a new pathway involving the mitogen-activated protein kinases extracellular signal-regulated kinases. . Mol. Pharmacol. 2005;67:648–54. doi: 10.1124/mol.104.002097. [DOI] [PubMed] [Google Scholar]
- 63.Kurokavva J., Chen L., Kass R.S. Requirement of subunit expression for cAMP-mediated regulation of a heart potassium channel. Proc. Natl. Acad. Sci. U. S. A. 2003;100:2122–2127. doi: 10.1073/pnas.0434935100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kathöfer S., et al. Functional coupling of human β3-adrenoreceptors to the KvLQT1/MinK potassium channel. . J. Biol. Chem. 2000;275:26743–26747. doi: 10.1074/jbc.M003331200. [DOI] [PubMed] [Google Scholar]
- 65.Dessy C., et al. Endothelial β3-adrenoreceptors mediate nitric oxide-dependent vasorelaxation of coronary microvessels in response to the third-generation β-blocker nebivolol. . Circulation. 2005;112:1198–205. doi: 10.1161/CIRCULATIONAHA.104.532960. [DOI] [PubMed] [Google Scholar]
- 66.Dessy C., et al. Endothelial β3-adrenoceptors mediate vasorelaxation of human coronary microarteries through nitric oxide and endothelium-dependent hyperpolarization. . Circulation. 2004;110:948–954. doi: 10.1161/01.CIR.0000139331.85766.AF. [DOI] [PubMed] [Google Scholar]
- 67.Massion P.B., Feron O., Dessy C., Balligand J.L. Nitric oxide and cardiac function — Ten years after, and continuing. Circ. Res. 2003;93:388–398. doi: 10.1161/01.RES.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- 68.Fischmeister R., Castro L., Abi-Gerges A., Rochais F., Vandecasteele G. Species- and tissue-dependent effects of NO and cyclic GMP on cardiac ion channels. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2005;142:136–143. doi: 10.1016/j.cbpb.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 69.Jurevicius J., Skeberdis V.A., Fischmeister R. Role of cyclic nucleotide phosphodiesterase isoforms in cAMP compartmentation following β2- adrenergic stimulation of ICa,L in frog ventricular myocytes. . J. Physiol. 2003;551:239–252. doi: 10.1113/jphysiol.2003.045211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brattelid T., et al. Functional serotonin 5-HT4 receptors in porcine and human ventricular myocardium with increased 5-HT4 mRNA in heart failure. . Naunyn Schmiedebergs Arch. Pharmacol. 2004;370:157–166. doi: 10.1007/s00210-004-0963-0. [DOI] [PubMed] [Google Scholar]
- 71.Schoemaker R.G., Du X.Y., Bax W.A., Bos E., Saxena P.R. 5-Hydroxytryptamine stimulates human isolated atrium but not ventricle. Eur. J. Pharmacol. 1993;230:103–105. doi: 10.1016/0014-2999(93)90417-G. [DOI] [PubMed] [Google Scholar]
- 72.Jahnel U., Rupp J., Ertl R., Nawrath H. Positive inotropic response to 5-HT in human atrial but not in ventricular heart muscle. Naunyn Schmiedebergs Arch. Pharmacol. 1992;346:482–485. doi: 10.1007/BF00169000. [DOI] [PubMed] [Google Scholar]
- 73.Rucker-Martin C., et al. Behaviour of human atrial myocytes in culture is donor age dependent. Neuromusc. Disord. 1993;3:385–390. doi: 10.1016/0960-8966(93)90082-U. [DOI] [PubMed] [Google Scholar]
- 74.Rivet-Bastide M., et al. cGMP-stimulated cyclic nucleotide phosphodiesterase regulates the basal calcium current in human atrial myocytes. . J. Clin. Invest. 1997;99:2710–2718. doi: 10.1172/JCI119460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.