Abstract
We have cloned and characterized four Itpk genes from soybean. All four recombinant Itpk proteins showed canonical Ins(1,3,4)P3 5/6-kinase activity, but a kinetic analysis raised questions about its biological significance. Instead, we provide evidence that one alternative biological role for soybean Itpks is to interconvert the Cl− channel inhibitor, Ins(3,4,5,6)P4, and its metabolic precursor, Ins(1,3,4,5,6)P5, within a substrate cycle. The soybean Itpks also phosphorylated Ins(3,4,6)P3 to Ins(1,3,4,6)P4 which was further phosphorylated to Ins(1,3,4,5,6)P5 by soybean Ipk2. Thus, soybean Itpks may participate in an inositol lipid-independent pathway of InsP6 synthesis.
1. INTRODUCTION
InsP6, the most abundant inositol phosphate in plants, has several cell-signaling functions (1), and is also a structural cofactor for the auxin receptor (2). Additionally, InsP6 is a phosphate storage compound, so it accumulates to considerable levels in plant seeds (1). This has a number of nutritional, agricultural and environmental consequences. For example, the inability of humans and other monogastrics to digest dietary InsP6 has anti-nutrient effects due to its chelation of mineral cations such as calcium, iron, zinc, and potassium (1). Another important problem is that phytate-rich soybeans, grains and other plant seeds are widely used as the primary protein source in feed for monogastric farm animals (3). Undigested InsP6 phosphorus is excreted in manure, which is applied to pastures and croplands. Runoff from agricultural fields can result in environmental phosphorus pollution, eutrophication and impaired water quality (4;5). Therefore, there is considerable interest in using molecular genetics to generate crops in which InsP6 synthesis during seed development is disrupted (1). However, it is first necessary to identify the enzymes in this biosynthetic pathway. There is general agreement that the final metabolic step in the pathway of InsP6 synthesis in plants (and other organisms) is the phosphorylation of Ins(1,3,4,5,6)P5 by a 2-kinase (6;7). Thus, a major challenge for this field is to identify the pathway(s) by which Ins(1,3,4,5,6)P5 is synthesized, and to characterize the enzymes that are involved.
Proposed routes of Ins(1,3,4,5,6)P5 synthesis in plants involve “lipid-dependent” and “lipid-independent” pathways. The former involves the phosphorylation of the Ins(1,4,5)P3 that is formed by PLC-mediated hydrolysis of the inositol phospholipid, PtdIns(4,5)P2 (1). In this case, Ins(1,4,5)P3 is phosphorylated to Ins(1,3,4,5,6)P5 in a two-step reaction catalyzed by a single enzyme, Ipk2 (7). With regards to the PtdIns(4,5)P2-independent pathway, there is continuing debate concerning the nature of both the metabolic intermediates and the enzymes involved (6;8). A [32P]-labeling strategy led to the following proposed pathway in duckweed: Ins3P -> Ins(3,4)P2 -> Ins(3,4,6)P3 -> Ins(1,3,4,6)P4 -> Ins(1,3,4,5,6)P5 (9). However, this experimental approach can give misleading information if there is substrate cycling between metabolic intermediates (10).
A low InsP6 phenotype has been observed in a line of maize with a mutation in an Itpk gene (11). This was one of the reasons that we focused on Itpk in the current study, using soybean as a model system because of its agronomic significance. Several Itpk proteins have previously been identified in Arabidopsis (12–14) and rice (13). This enzyme family is best known for its Ins(1,3,4)P3 5/6-kinase and Ins(3,4,5,6)P4 1-kinase activities (12;14), but it has been unclear how these might be relevant to putative “lipid-independent” pathways of InsP6 synthesis (1;7;9). An examination of the kinetic parameters of these two metabolic reactions should help determine possible biological significance; this information is mostly absent from previous studies in this field and so is a particular focus of the current study. The role of the Itpk family in regulating Ins(3,4,5,6)P4 metabolism in plants is also useful to understand because this polyphosphate inhibits transmembrane chloride flux, thereby regulating pollen growth (15).
2. MATERIALS AND METHODS
2.1 Gene Identification and Recombinant Protein Expression
We performed a BLAST database search of the soybean genome using as queries the Itpk sequences previously identified in Arabidopsis thaliana (AF080173) and Zea mays (AY172635). Putative EST clones of soybean Itpk were obtained from Biogenetic Services (Brookings, South Dakota) and were sequenced. Complete coding regions for four Itpk genes (which we named type 1 through type 4) were amplified using the following primer pairs (restriction sites underlined): GmItpk1, 5′-GATCCCCGGGAATGGCGGAGAAGAGATTCG-3′ and 3′-CCTACTCGAGTCAAGCTTGAAGAGATTCCTCTT-5′; GmItpk2, 5′-GATCGAATTCATGTCCGAGTCGGAAGTAGCA-3′ and 3′-CACACTCGAGCTACGCAGTCTTGGAGCGTA-5′; GmItpk3, 3′-GATCGAATTCATGAGGTTGAGGGAGGAGGTAG-5′ and 5′-GATCCTCGAGCTATTTTTTCTTGTACTTCCCCTGC-3′; GmItpk4, 5′-GATCCCCGGGAATGAGGCTAAACGGTGAAATCTC-3′ and 3′-GGCCCTCGAGTTAGGCAGCAAGTTTCTTATTA-5′. The genes were cloned into the protein expression vector pGEX4T-1 (GE Healthcare, Waukesha, WI), and the proteins were expressed in E. coli and purified using GST-sepharose beads (GE Healthcare, Waukesha, WI) as previously described (16). The soybean Ipk2 gene (accession EU033957) was identified based on a search of the soybean database using known Arabidopsis sequences (17). GmIpk2 was amplified and cloned into pGEX4T-1 using the following primer pair: 5’-GACTGGATCCATGCTCAAGATCCCGGAGC-3′ and 5’-ATGATCGCGGCCGCTCAGTCACTTGTGAAGACATGCTAC-3′. Recombinant protein was prepared as described above, and showed canonical Ins(1,3,4,5)P4 6-kinase activity (data not shown).
2.2 Enzyme Assays
[3H]-Ins(1,3,4)P3 was synthesized by incubating [3H]-Ins(1,3,4,5)P4 (Perkin Elmer, Wellesley, MA) with recombinant Ins(1,3,4,5)P4 5-phosphatase (provided by Dr. Gillaspy at Virginia Tech). [3H]-Ins(3,4,5,6)P4 and [3H]-Ins(1,3,4,5,6)P5 were prepared as previously described (16). Non-radioactive Ins(1,3,4)P3, Ins(3,4,6)P3 and Ins(3,4,5,6)P4 were purchased from CellSignals (Columbus, OH). [32P]-ATP was purchased from Perkin Elmer (Perkin Elmer, Wellesley, MA).
To study the metabolism of Ins(1,3,4)P3 and Ins(3,4,5,6)P4, each GmITPK isoform was incubated with radiolabeled substrate (1000 to 3000 D.P.M), plus the appropriate concentration of non-radiolabled substrate. Incubations were performed for various times (<1 hour) at 37°C in 100 µl assay buffer containing 20 mM HEPES pH 7.2, 100 mM KCl, 6 mM MgSO4, 5 mM ATP, 10 mM phosphocreatine, 2 units/ml creatine-phosphokinase, 0.3mg/ml BSA. For Ins(1,3,4,5,6)P5 metabolism, ATP was replaced by ADP. For Ins(3,4,6)P3 metabolism, the reaction volume was reduced to 20 ul and the non-radiolabeled ATP was replaced with 100,000 D.P.M. [32P]-ATP.
Reactions were quenched and neutralized as previously described and analyzed by either gravity-fed ion-exchange columns, or by HPLC (using either a 4.6 × 125 mm Partisphere SAX column, or a 250 × 4.6 mm Q100 column (Thompson Instruments, Clear Brook, VA) (16). The assays containing [32P] were analyzed using an on-line scintillation counter.
2.3 Real-Time PCR Expression Analysis
Soybean seeds were sorted into four different stages of development according to seed size (0–4 mm, 5–6 mm, 7–8 mm, 9–10 mm). Two biological replicates were from the soybean cultivar "Jack" and the third from Virginia experimental line V71-370. RNA was extracted using Tri-Reagent according to manufacturer’s instructions (MRCgene, Cincinnati, OH) and then treated with DNase I using a Turbo DNA-free kit per manufacturer’s instructions (Ambion, Austin, TX). cDNA first strand synthesis was generated using oligo dT primers (12–18 bp) (Invitrogen, Carlsbad, CA) and the Omniscript cDNA kit (Qiagen, Valencia, CA). The absence of residual contaminating DNA was verified prior to cDNA synthesis using RNA as the template for the PCR reaction. The following primer pairs were designed for the 3’ untranslated region: GmItpk1, 5"-CTGCGAAGTAATGCTCAAGA-3" and 3"-GCAACTCGTGCCAACC-5"; GmItpk2, 5"-TGAGGACGCTGAAATGCC-3" and 3"-AGACAACAGTGTAAATGTGTAATAACATC-5" GmItpk3, 5"-CCTACTGTTGCTGAGCTTC-3" and 3"-GGGCTTACGTCATGTGGG-5" GmItpk4, 5"-ATGCCAGGCTATGAGCAC-3" and 3"-ACAGACCCTATTTCCACCTT-5". To check for equivalent synthesis of cDNA we used primers (5"-AGCGTGGTTATGTTGCCTCAAACT-3" and 3"-CTTGATGACTCCCACAGCAACAGT-5") for a soybean housekeeping gene, elongation factor 1A (18;19). Amplification and analysis were conducted using an ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA).
3. RESULTS AND DISCUSSION
3.1 Identification of Four Soybean Itpk Genes
We sequenced four complete GmItpk genes (types 1–4; Fig. 1A) which can be grouped into two pairs (Fig. 1B). GmItpk1 and GmItpk2 share 46% identity. GmItpk3 and GmItpk4 are 67% identical. Alignments of the soybean sequences with homologues from other organisms reveal several highly conserved regions, including 16 residues previously shown to be catalytically important in HsITPK (Fig. 1A). A phylogenetic tree indicates that the soybean enzymes belong to two major branches, along with all but one member of the Arabidopsis and rice gene families (Fig. 1B).
Figure 1. Soybean Itpks: Amino-acid sequences and phylogenetic relationships.

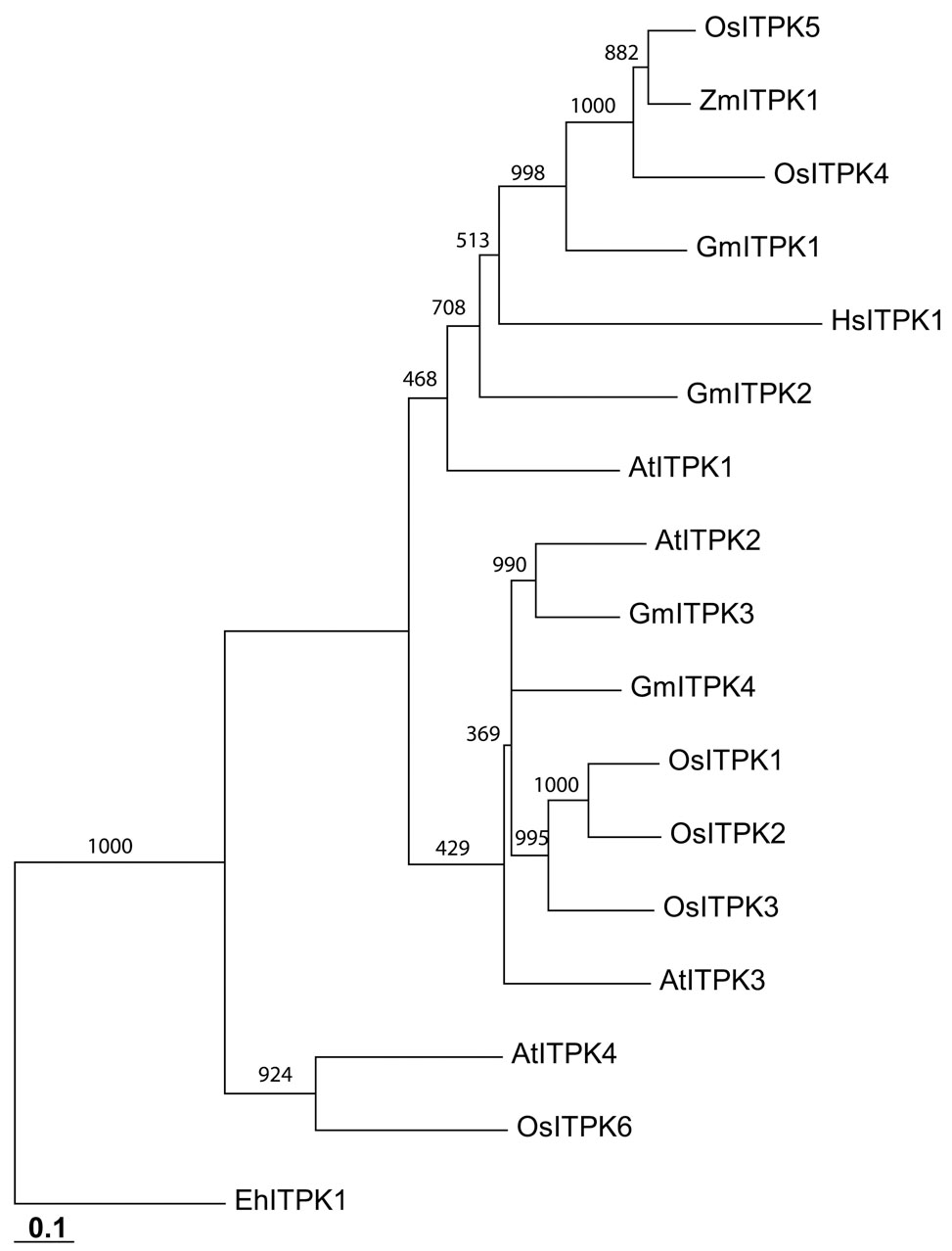
Panel A: Alignment (using ClustalW) of Itpks from the following species (Genbank accession numbers in parentheses): Entamoeba histolytica (Eh; AF118848), Homo sapiens (Hs; NP_055031), Soybean (Gm; type 1, EU033958, type 2, EU033959, type 3, EU033960, type 4, EU033961), Arabidopsis thaliana (At; type 1, Q9SBA5) and Zea mays (Zm; type 1, Q84Y01). The recent publication of the Arabidopsis Itpk gene family (14) led us to renumber the previously designated soybean GmItpk4 sequence (16) as GmItpk2. A small number of C-terminal residues (which show no sequence similarity) are omitted for clarity. Highlighted residues are conserved in at least four of the aligned proteins. Asterisks denote residues in HsITPK which, when mutated, decrease catalytic activity at least 20% (24;25). Panel B shows a phylogram generated with Treeview (26) from a 1000 iteration bootstrap analysis using the sequences described in Panel A, plus additional sequences from Arabidopsis (14) and rice (8). Scale bar indicates nucleotide substitutions per site.
Using real-time PCR (Fig. 2) and Northern analysis (data not shown) we quantified the expression of the four soybean Itpk genes in seeds at several developmental stages. GmItpk3 was much more abundantly expressed, especially during the early stages of seed development.
Figure 2. Expression of soybean Itpk genes in developing seeds.
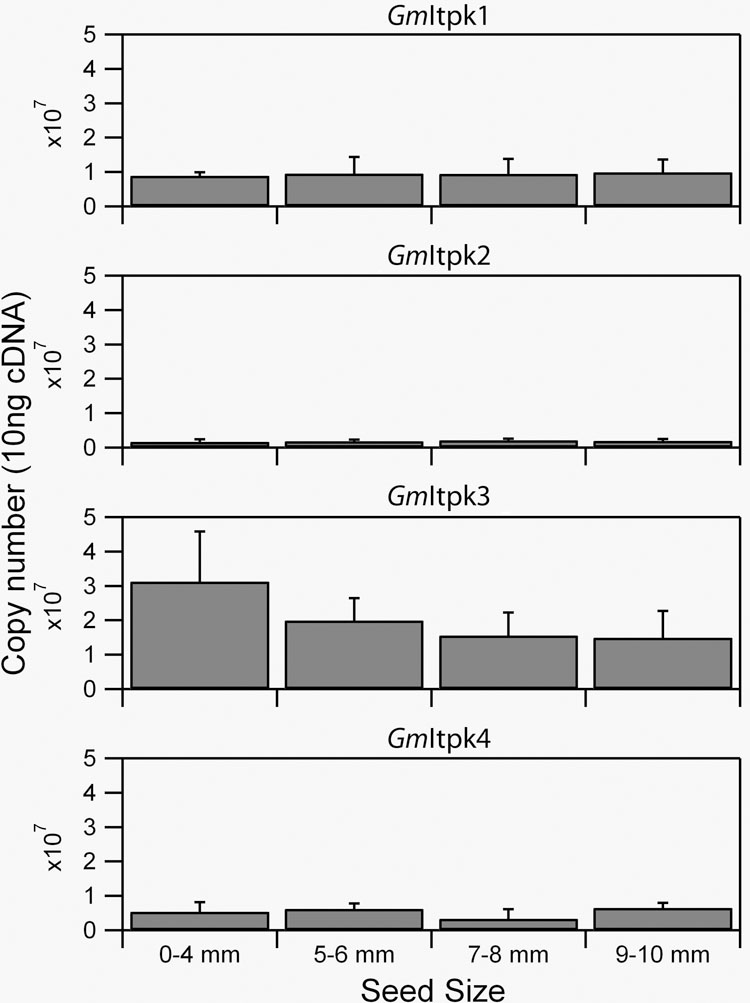
The expression of the GmItpk genes was determined by real-time PCR as described in the Methods Section.
3.2 Phosphorylation of Ins(1,3,4)P3 and Ins(3,4,5,6)P4 by Soybean Itpks
The canonical catalytic activity of all Itpk proteins is the phosphorylation of Ins(1,3,4)P3 to both Ins(1,3,4,5)P4 and Ins(1,3,4,6)P4 (12;14;20;21). We found that all four soybean Itpks phosphorylated Ins(1,3,4)P3 (Fig. 3A; Table 1). The two InsP4 products were initially identified by HPLC co-elution with standards (Fig. 3B). Additionally, we noted that Arabidopsis Ipk2 has previously been shown to convert Ins(1,3,4,5)P4 and Ins(1,3,4,6)P4 to Ins(1,3,4,5,6)P5 (17). Therefore, to further confirm the nature of the two InsP4 products made by the soybean Itpks, the InsP4s were incubated with recombinant GmIpk2 and were both found to be completely phosphorylated to Ins(1,3,4,5,6)P5 (data not shown).
Figure 3. Ins(1,3,4)P3 phosphorylation by soybean Itpk.
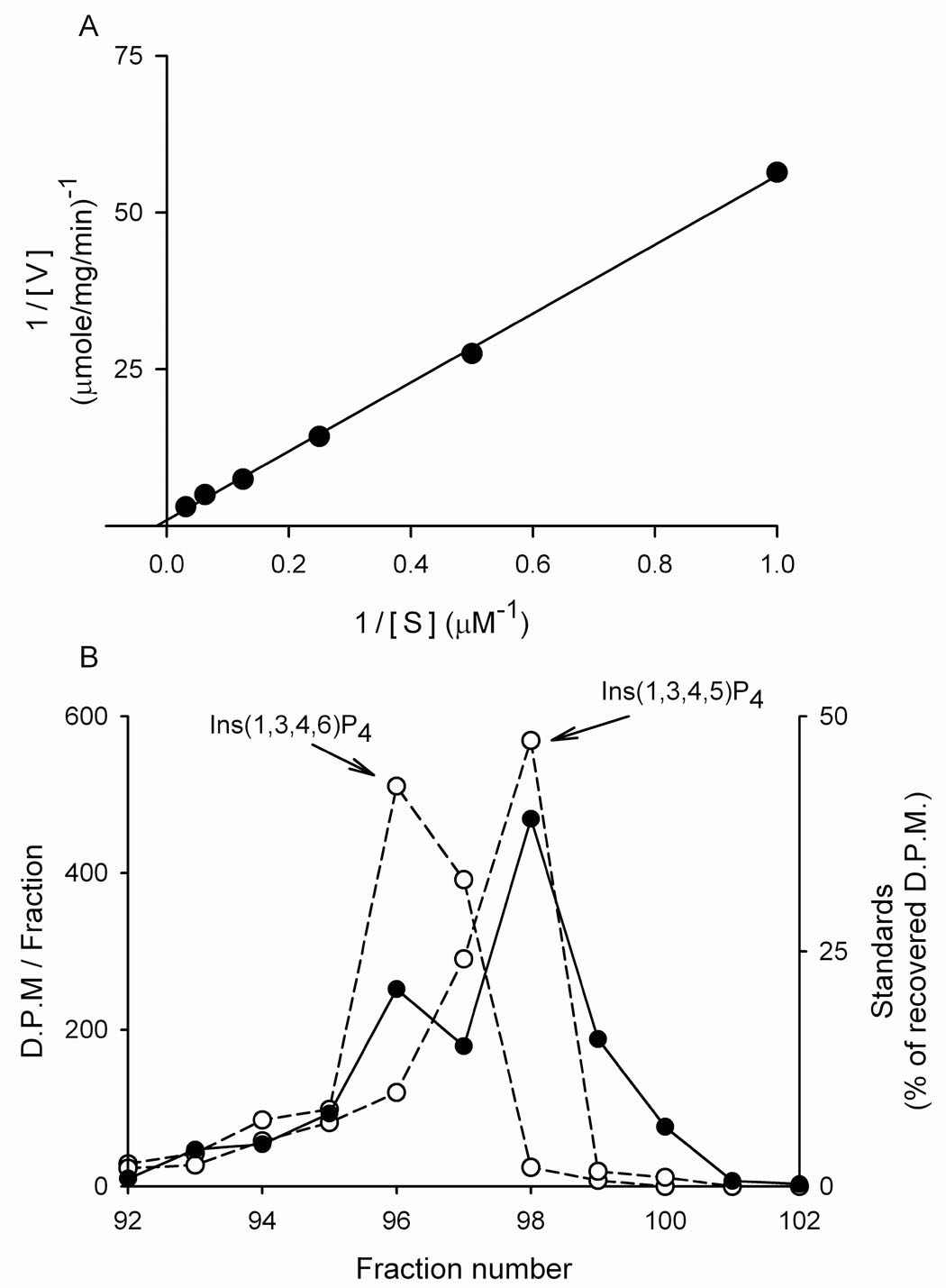
Panel A shows a representative kinetic plot for the phosphorylation of [3H]-Ins(1,3,4)P3 by GmItpk3 (see also Table 1). Panel B describes the analysis of the products of [3H]-Ins(1,3,4)P3 phosphorylation by 2 µg GmItpk2 (filled circles) using Q100 HPLC chromatography as described in Methods. The open circles denote the elution of [14C]-labeled standards of Ins(1,3,4,5)P4 (added in the same HPLC run) and Ins(1,3,4,6)P4 (added in a parallel run). Similar data were obtained in two additional experiments.
Table 1.
Kinetic parameters of soybean Itpks
| Km (µM) | Vmax (µmol/mg/min) | Vmax/Km | ||||
|---|---|---|---|---|---|---|
| Ins(1,3,4)P3 | Ins(3,4,5,6)P4 | Ins(1,3,4)P3 | Ins(3,4,5,6)P4 | Ins(1,3,4)P3 | Ins(3,4,5,6)P4 | |
| GmItpk1 | 3.7 ± 0.4 | 0.28 ± 0.004 | 0.5 ± 0.1 | 0.083 ± 0.02 | 0.135 | 0.3 |
| GmItpk2 | 46.2 ± 6.1 | 0.78 ± 0.04 | 1.97 ± 0.3 | 0.76 ± 0.06 | 0.043 | 0.97 |
| GmItpk3 | 43.4 ± 8.3 | 1.05 ± 0.16 | 0.86 ± 0.1 | 0.22 ± 0.1 | 0.02 | 0.21 |
| GmItpk4 | 4.4 ± 0.6 | 0.7 ± 0.03 | 0.007±0.001 | 0.004±0.0003 | 0.002 | 0.006 |
Data are means and standard errors from 3–4 experiments.
Itpks typically have Km values for their substrates that are around 1 µM or less (21). Two of the soybean proteins (types 2 and 3) showed much lower affinities for Ins(1,3,4)P3 (Table 1), which raises questions concerning the physiological relevance of Ins(1,3,4)P3 phosphorylation by these enzymes in vivo. We therefore turned our attention to an alternate substrate, namely, Ins(3,4,5,6)P4. We found that Ins(3,4,5,6)P4 was phosphorylated by all four soybean Itpks with Km values of 0.3 – 1 µM (Fig 4;Table 1). HPLC analysis revealed that the product of Ins(3,4,5,6)P4 phosphorylation co-eluted with a standard of [3H]-Ins(1,3,4,5,6)P5 and not standards of either D/L-[3H]-Ins(2,3,4,5,6)P5 or [3H]-InsP6 (Fig. 4). These data verify that soybean Itpks specifically phosphorylate the 1-position of Ins(3,4,5,6)P4.
Figure 4. Ins(3,4,5,6)P4 phosphorylation by soybean Itpk.
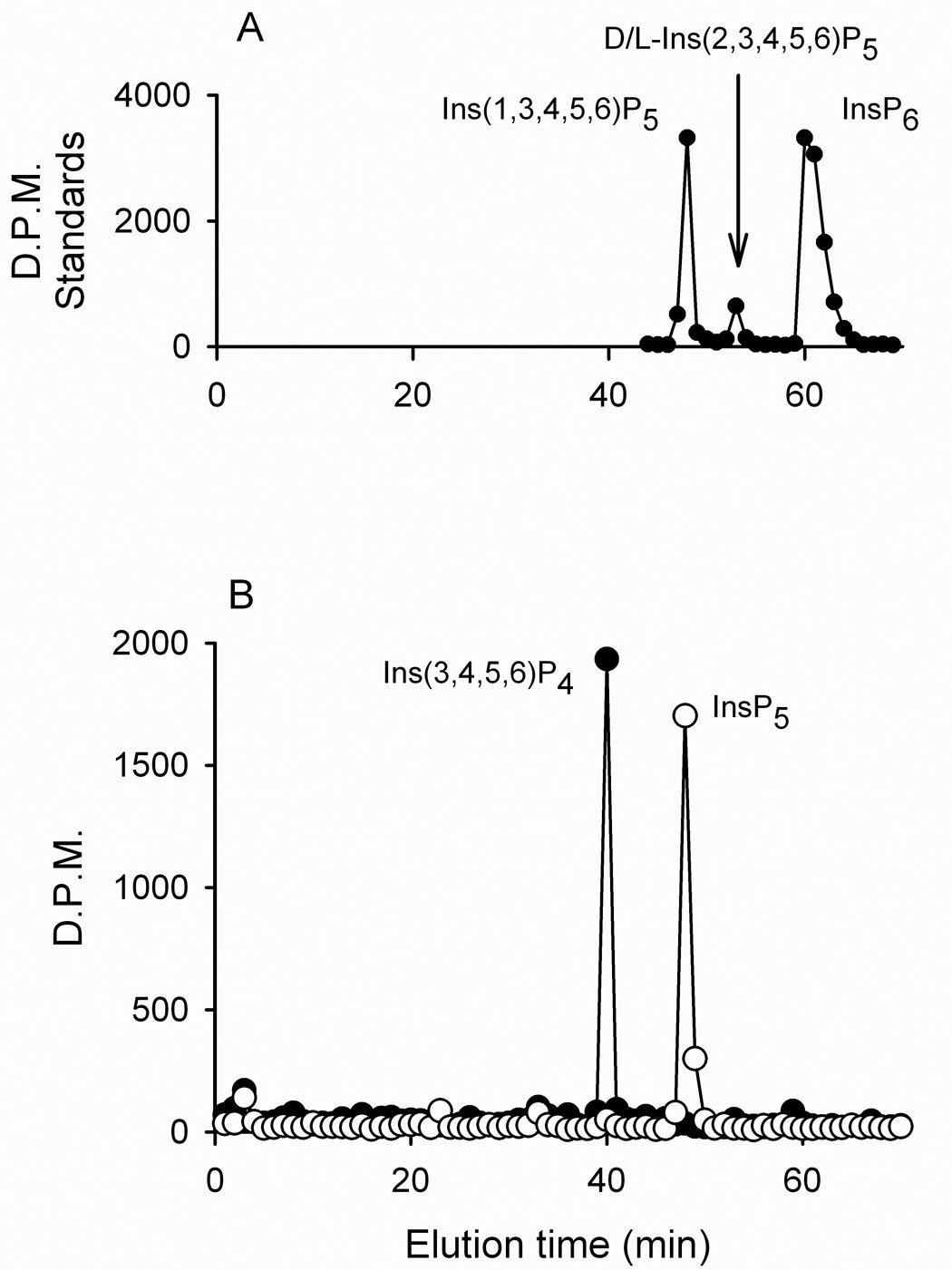
Panel A: A Partisphere SAX HPLC column was calibrated with a mixture of [3H]-labeled standards of Ins(1,3,4,5,6)P5, D/L-Ins(2,3,4,5,6)P5 and InsP6. Panel B: [3H]-Ins(3,4,5,6)P4 was incubated with (closed circles) and without (open circles) 2 µg of GmItpk2 for 20 min (see Methods) and the products were analyzed by Partisphere SAX HPLC. Similar data were obtained with the other three GmItpk isoforms.
A comparison of the catalytic efficiencies (Vmax/Km) of the Ins(1,3,4)P3 and Ins(3,4,5,6)P4 substrates revealed that the latter is the preferred substrate of all four isoforms (Table 1). The type 4 enzyme was the least catalytically efficient, by a factor of 35 to 100 (Table 1).
3.3 Synthesis of Ins(3,4,5,6)P4 by Soybean Itpks
As well as exhibiting Ins(3,4,5,6)P4 1-kinase activity, mammalian Itpk1 has been shown to dephosphorylate Ins(1,3,4,5,6)P5 to Ins(3,4,5,6)P4 (16;22). We recently demonstrated that GmItpk2 can also dephosphorylate Ins(1,3,4,5,6)P5 to Ins(3,4,5,6)P4 (2932 ± 50 µmol/mg protein/min) (16). We now report that the three other GmItpk isoforms also show this activity: type 1 = 403 ± 4, type 3= 487 ± 47, type 4 = 88 ± 16 (µmol/mg protein/min), albeit at rates 6 to 33-fold lower than that of the type 2 enzyme. Substrate cycling between Ins(3,4,5,6)P4 and Ins(1,3,4,5,6)P5 may serve a signaling role in plants, in which Ins(3,4,5,6)P4 has been shown to regulate Cl− channel conductance (15), similar to the role of Ins(3,4,5,6)P4 in animal cells (22).
3.4 Phosphorylation of Ins(3,4,6)P3 by Soybean Itpks
It has been speculated that Ins(3,4,6)P3, a known constituent of plants (23), participates in a “phospholipid-independent” pathway of InsP6 synthesis, with both Ins(1,3,4,6)P4 and Ins(3,4,5,6)P4 being offered as candidate products of Ins(3,4,6)P3 phosphorylation (6;8). Little work has been done to identify enzyme(s) that might catalyze these reactions, with the exception of a recent report showing that a racemic mixture of Ins(3,4,6)P3/Ins(1,4,6)P3 was phosphorylated by the recombinant Itpk from Arabidopsis (14). The use of a substrate mixture prevents us from knowing which of the two were phosphorylated. In the current study we used pure Ins(3,4,6)P3 as a substrate. We did not have radiolabeled Ins(3,4,6)P3, so we instead added [32P]-ATP to our assays and measured [32P]-InsP4 formation. All four isoforms phosphorylated Ins(3,4,6)P3 (Fig. 5). The [32P]-InsP4 that was formed was separated from a standard of [3H]-Ins(3,4,5,6)P4 (Fig. 5). This leaves just two alternative structures for the [32P]-InsP4 that was formed, namely, Ins(2,3,4,6)P4 or Ins(1,3,4,6)P4. The Ins(2,3,4,6)P4 can be excluded, because only one class of enzymes can phosphorylate the 2-position of inositol (the Ins(1,3,4,5,6)P5 2-kinases; (7)). We therefore conclude that soybean Itpks can phosphorylate Ins(3,4,6)P3 to Ins(1,3,4,6)P4. Since the latter can be further phosphorylated to Ins(1,3,4,5,6)P5 by GmIpk2 (Section 3.2), our data indicate that soybean have the enzymatic machinery for the “phospholipid-independent” pathway shown in Fig. 5C. The significance of this information lies in it defining a role for Itpk in InsP6 synthesis in plants. Our data also indicate that it would now be profitable for this field of research to determine the nature of the enzymes that synthesize Ins(3,4,6)P3.
Figure 5. Ins(3,4,6)P3 phosphorylation by soybean Itpk and its contribution to InsP6 synthesis.
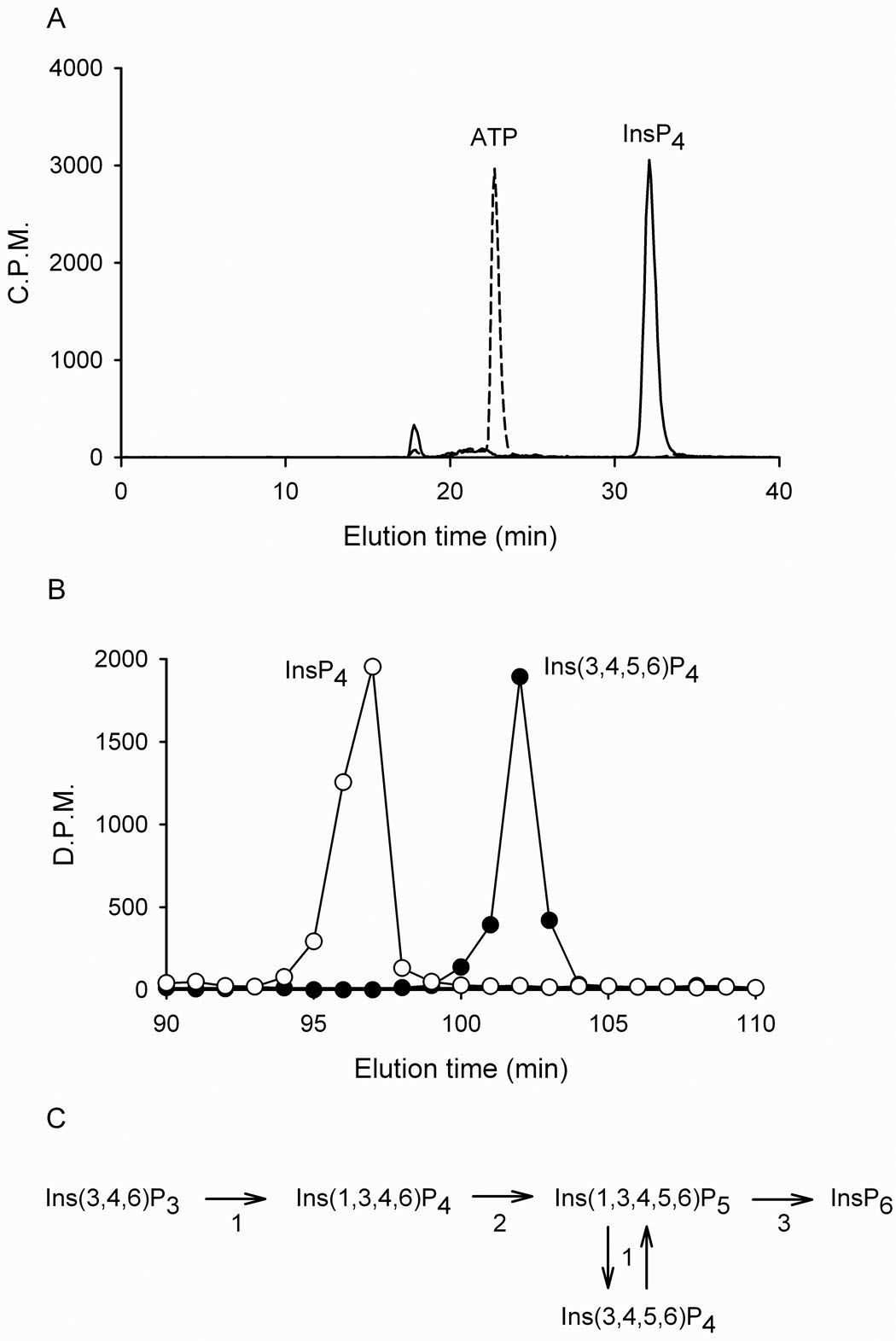
Panel A: [32P]-ATP and 50 µM Ins(3,4,6)P3 were incubated with (solid line) and without (broken line) 2 µg GmItpk1 for 30 min and the products were resolved by Q100 HPLC. Panel B: An aliquot of the [32P]-InsP4 product described in panel A (open circles) was chromatographed with an [3H]-Ins(3,4,5,6)P4 standard (closed circles) using a Partisphere SAX HPLC. Similar data were obtained with the other three GmItpk isoforms. Panel C: Proposed metabolic pathway from Ins(3,4,6)P3 to InsP6 in soybean. Numbers adjacent to the arrows represent the enzymes that are responsible for each reaction: 1 = GmItpk1-4 (for evidence of Ins(3,4,6)P3 phosphorylation see Figs 5A,B and Section 3.4; for Ins(3,4,5,6)P4 / Ins(1,3,4,5,6)P5 interconversion see Section 3.2 and Section 3.3), 2 = GmIpk2 (see Section 3.2), 3 = Ins(1,3,4,5,6)P5 2-kinase (see ref. (7)).
Footnotes
This work was supported by the United States Department of Agriculture National Research Initiative Competitive Grants Program, the United Soybean Board, and the Intramural Research Program of the NIH / National Institute of Environmental Health Sciences.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Raboy V. Seeds for a better future: 'low phytate' grains help to overcome malnutrition and reduce pollution. Trends Plant Sci. 2001;6:458–462. doi: 10.1016/s1360-1385(01)02104-5. [DOI] [PubMed] [Google Scholar]
- 2.Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640–645. doi: 10.1038/nature05731. [DOI] [PubMed] [Google Scholar]
- 3.Mullaney EJ, Daly CB, Ullah AHJ. Advances in phytase research. Advances in Applied Microbiology. 2000;47:157–199. doi: 10.1016/s0065-2164(00)47004-8. [DOI] [PubMed] [Google Scholar]
- 4.Sharley A, Chapra S, Wedepohl R, Sims J, Daniel T, Reddy K. Managing Agricultural Phosphorus for Protection of Surface Waters - Issues and Options. J. Environ. Qual. 2008;23:437–451. [Google Scholar]
- 5.Abelson PH. A potential phosphate crisis. Science. 1999;283:2015. doi: 10.1126/science.283.5410.2015. [DOI] [PubMed] [Google Scholar]
- 6.Raboy V, Bowen D. Genetics of inositol polyphosphates. Subcell. Biochem. 2006;39:71–101. doi: 10.1007/0-387-27600-9_4. [DOI] [PubMed] [Google Scholar]
- 7.Stevenson-Paulik J, Bastidas RJ, Chiou ST, Frye RA, York JD. Generation of phytate-free seeds in Arabidopsis through disruption of inositol polyphosphate kinases. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12612–12617. doi: 10.1073/pnas.0504172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki M, Tanaka K, Kuwano M, Yoshida KT. Expression pattern of inositol phosphate-related enzymes in rice (Oryza sativa L.): implications for the phytic acid biosynthetic pathway. Gene. 2007;405:55–64. doi: 10.1016/j.gene.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Brearley CA, Hanke DE. Metabolic evidence for the order of addition of individual phosphate esters to the myo-inositol moiety of inositol hexakisphosphate in the duckweed Spirodela polrhiza L. Biochem. J. 1996;314:227–233. doi: 10.1042/bj3140227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephens LR, Downes CP. Product-recursor relationships amongst inositol polyphosphates. Incorporation of [32P]Pi into myo-inositol 1,3,4,6-tetrakisphosphate, myo-inositol 1,3,4,5-tetrakisphosphate, myo-inositol 3,4,5,6-tetrakisphosphate and myo-inositol 1,3,4,5,6-pentakisphosphate in intact avian erythrocytes. Biochem. J. 1990;265:435–452. doi: 10.1042/bj2650435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi J, Wang H, Wu Y, Hazebroek J, Meeley RB, Ertl DS. The maize low-phytic acid mutant Ipa2 is caused by mutation in an inositol phosphate kinase gene. Plant Physiol. 2003;131:507–515. doi: 10.1104/pp.014258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson MP, Majerus PW. Characterization of a cDNA encoding Arabidopsis thaliana inositol 1,3,4-trisphosphate 5/6-kinase. Biochem. Biophys. Res. Commun. 1997;232:678–681. doi: 10.1006/bbrc.1997.6355. [DOI] [PubMed] [Google Scholar]
- 13.Josefsen L, Bohn L, Sorensen MB, Rasmussen SK. Characterization of a multifunctional inositol phosphate kinase from rice and barley belonging to the ATP-grasp superfamily. Gene. 2007;397:114–125. doi: 10.1016/j.gene.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Sweetman D, Stavridou I, Johnson S, Green P, Caddick SE, Brearley CA. Arabidopsis thaliana inositol 1,3,4-trisphosphate 5/6-kinase 4 (AtITPK4) is an outlier to a family of ATP-grasp fold proteins from Arabidopsis. FEBS Lett. 2007;581:4165–4171. doi: 10.1016/j.febslet.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 15.Zonia L, Cordeiro S, Tupý J, Feijó JA. Oscillatory Chloride Efflux at the Pollen Tube Apex Has a Role in Growth and Cell Volume Regulation and Is Targeted by Inositol 3,4,5,6-Tetrakisphosphate. Plant Cell. 2002;14:2233–2249. [PubMed] [Google Scholar]
- 16.Chamberlain PP, Qian X, Stiles AR, Cho J, Jones DH, Lesley SA, Grabau EA, Shears SB, Spraggon G. Integration of inositol phosphate signaling pathways via human ITPK1. J. Biol. Chem. 2007;282:28117–28125. doi: 10.1074/jbc.M703121200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevenson-Paulik J, Odom AR, York JD. Molecular and biochemical characterization of two plant inositol polyphosphate 6-/3-/5-kinases. J. Biol. Chem. 2002;277:42711–42718. doi: 10.1074/jbc.M209112200. [DOI] [PubMed] [Google Scholar]
- 18.Nicot N, Hausman JF, Hoffmann L, Evers D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005;56:2907–2914. doi: 10.1093/jxb/eri285. [DOI] [PubMed] [Google Scholar]
- 19.Nunes AC, Vianna GR, Cuneo F, maya-Farfan J, de CG, Rech EL, Aragao FJ. RNAi-mediated silencing of the myo-inositol-1-phosphate synthase gene (GmMIPS1) in transgenic soybean inhibited seed development and reduced phytate content. Planta. 2006;224:125–132. doi: 10.1007/s00425-005-0201-0. [DOI] [PubMed] [Google Scholar]
- 20.Wilson MP, Majerus PW. Isolation of inositol 1,3,4-trisphosphate 5/6-kinase, cDNA cloning, and expression of recombinant enzyme. J. Biol. Chem. 1996;271:11904–11910. doi: 10.1074/jbc.271.20.11904. [DOI] [PubMed] [Google Scholar]
- 21.Yang X, Shears SB. Multitasking in Signal Transduction by a Promiscuous Human Ins(3,4,5,6)P4 1-Kinase/Ins(1,3,4)P3 5/6-Kinase. Biochem. J. 2000;351:551–555. [PMC free article] [PubMed] [Google Scholar]
- 22.Ho MWY, Yang X, Carew MA, Zhang T, Hua L, Kwon Y-U, Chung S-K, Adelt S, Vogel G, Riley AM, et al. Regulation of Ins(3456)P4 signaling by a reversible kinase/phosphatase. Current Biology. 2002;12:477–482. doi: 10.1016/s0960-9822(02)00713-3. [DOI] [PubMed] [Google Scholar]
- 23.Brearley CA, Hanke DE. Inositol phosphates in the duckweed Spirodela polrhiza L. Biochem. J. 1996;314:215–225. doi: 10.1042/bj3140215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller GJ, Wilson MP, Majerus PW, Hurley JH. Specificity determinants in inositol polyphosphate synthesis: crystal structure of inositol 1,3,4-trisphosphate 5/6-kinase. Mol Cell. 2005;18:201–212. doi: 10.1016/j.molcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Qian X, Mitchell J, Wei SJ, Williams J, Petrovich RM, Shears SB. The Ins(1,3,4)P3 5/6-kinase / Ins(3,4,5,6)P 4 1-kinase is not a protein kinase. Biochem J. 2005;389:389–395. doi: 10.1042/BJ20050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page RD. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]


