Abstract
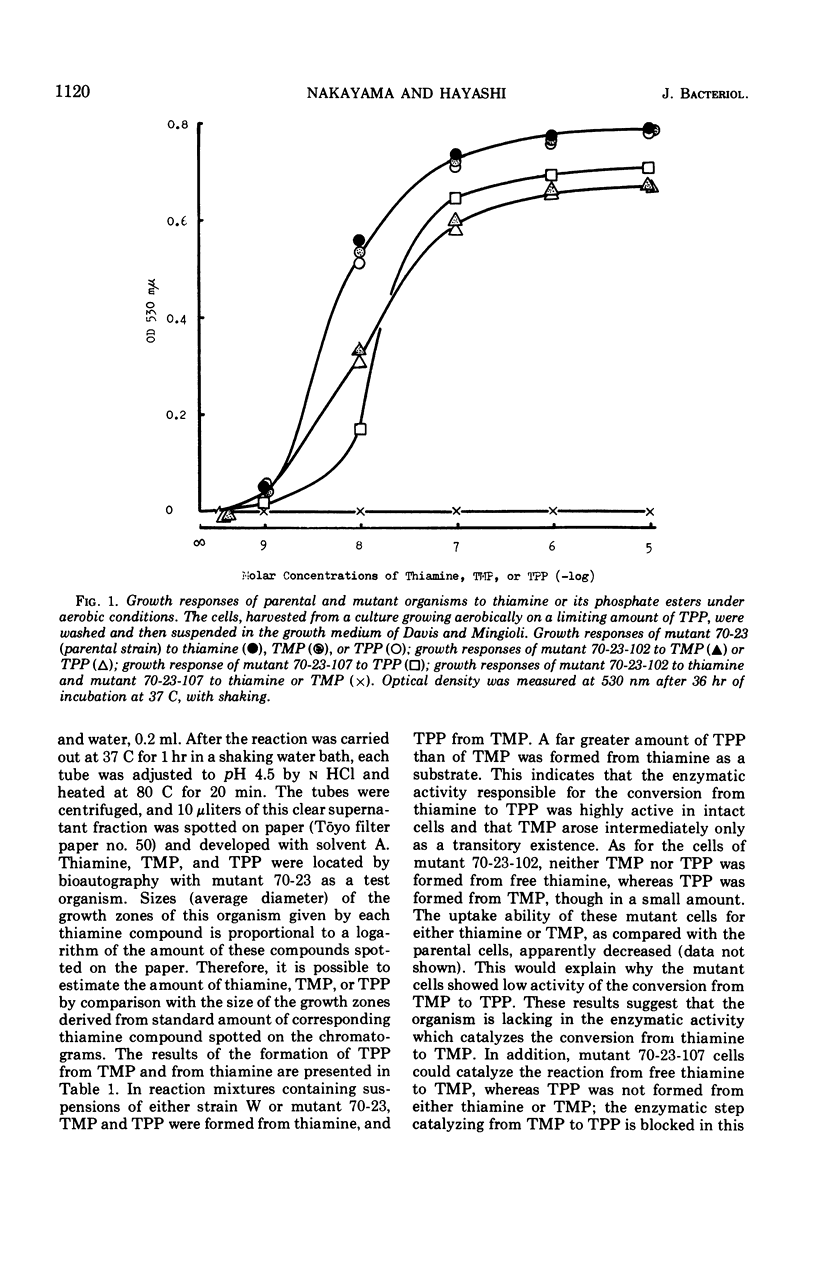
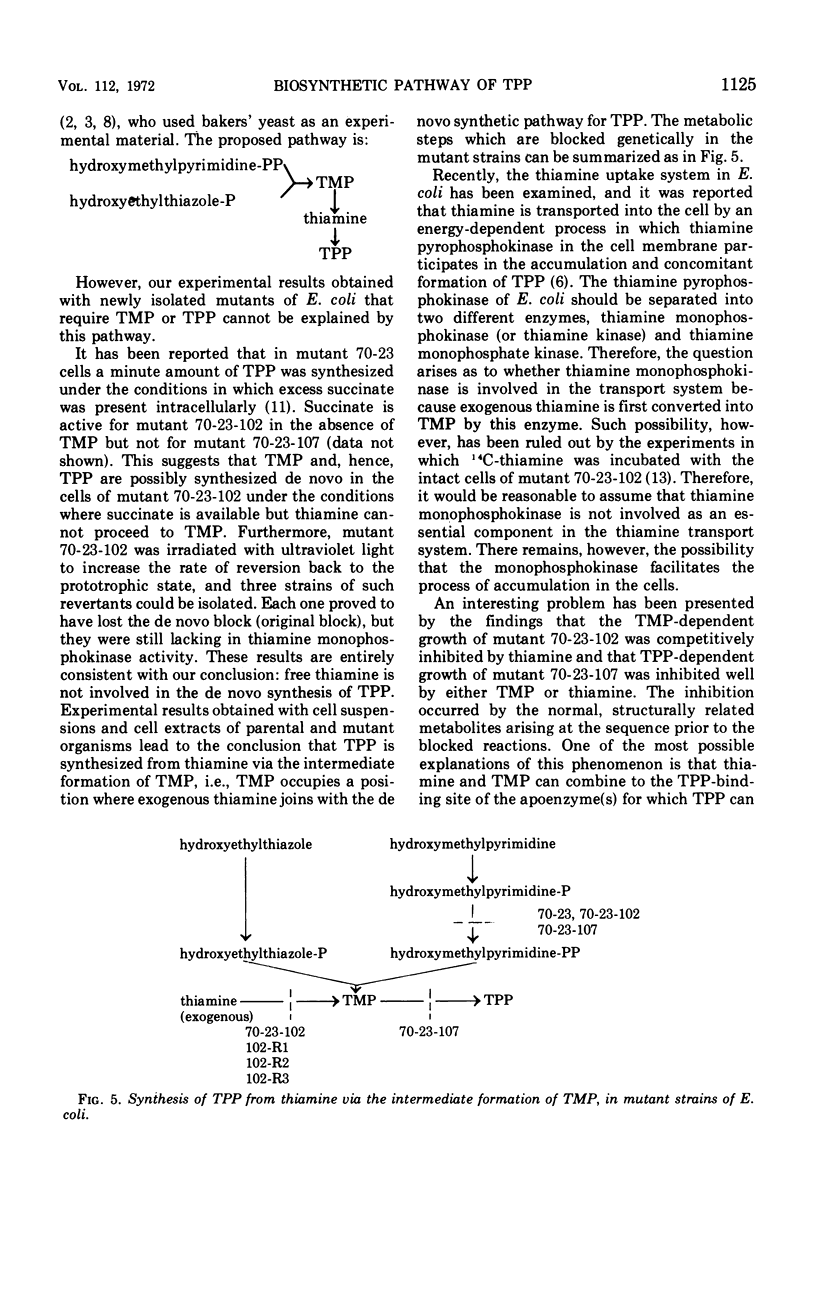
Two types of mutants of Escherichia coli were isolated, one of which (mutant 70-23-107) responded to thiamine pyrophosphate, and the other (mutant 70-23-102) to thiamine monophosphate and thiamine pyrophosphate. They were produced by further mutation of a thiamine auxotroph of E. coli 70-23 with N-methyl-N′-nitro-N-nitrosoguanidine. The parent organism required thiamine because phosphohydroxymethylpyrimidine kinase activity was lacking in this organism, and hydroxymethylpyrimidine pyrophosphate was not permeable through the cell membrane of E. coli. Thiamine, thiamine monophosphate, and thiamine pyrophosphate were all equally active for the parent, whereas mutants 70-23-102 and 70-23-107 lost their ability to grow on thiamine. Both mutants differed only in the growth response to thiamine monophosphate: the former could grow on thiamine monophosphate, whereas the latter could not. Experimental results with the newly isolated mutants indicate that in E. coli the free form of thiamine is not involved in de novo synthesis of thiamine pyrophosphate, but thiamine monophosphate, an exclusive product formed by the reaction between hydroxymethylpyrimidine pyrophosphate and hydroxyethylthiazole monophosphate, is directly phosphorylated to form thiamine pyrophosphate. Exogenous thiamine, on the other hand, is converted to thiamine pyrophosphate via the intermediate formation of thiamine monophosphate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAMIENER G. W., BROWN G. M. The biosynthesis of thiamine. 1. Enzymatic formation of thiamine and phosphate esters of the pyrimidine moiety of thiamine. J Biol Chem. 1960 Aug;235:2404–2410. [PubMed] [Google Scholar]
- CAMIENER G. W., BROWN G. M. The biosynthesis of thiamine. 2. Fractionation of enzyme system and identification of thiazole monophosphate and thiamine monophosphate as intermediates. J Biol Chem. 1960 Aug;235:2411–2417. [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata I., Kawasaki T., Nose Y. Thiamine kinase in the membrane fraction. Biochem Biophys Res Commun. 1967 Jun 23;27(6):601–606. doi: 10.1016/s0006-291x(67)80076-7. [DOI] [PubMed] [Google Scholar]
- NOSE Y., TOKUDA Y., HIRABAYASHI M., IWASHIMA A. THIAMINE BIOSYNTHESIS FROM HYDROXYMETHYLPYRIMIDINE AND THIAZOLE BY WASHED CELLS AND CELL EXTRACTS OF ESCHERICHIA COLI AND ITS MUTANTS. J Vitaminol (Kyoto) 1964 Jun 10;10:105–110. doi: 10.5925/jnsv1954.10.105. [DOI] [PubMed] [Google Scholar]
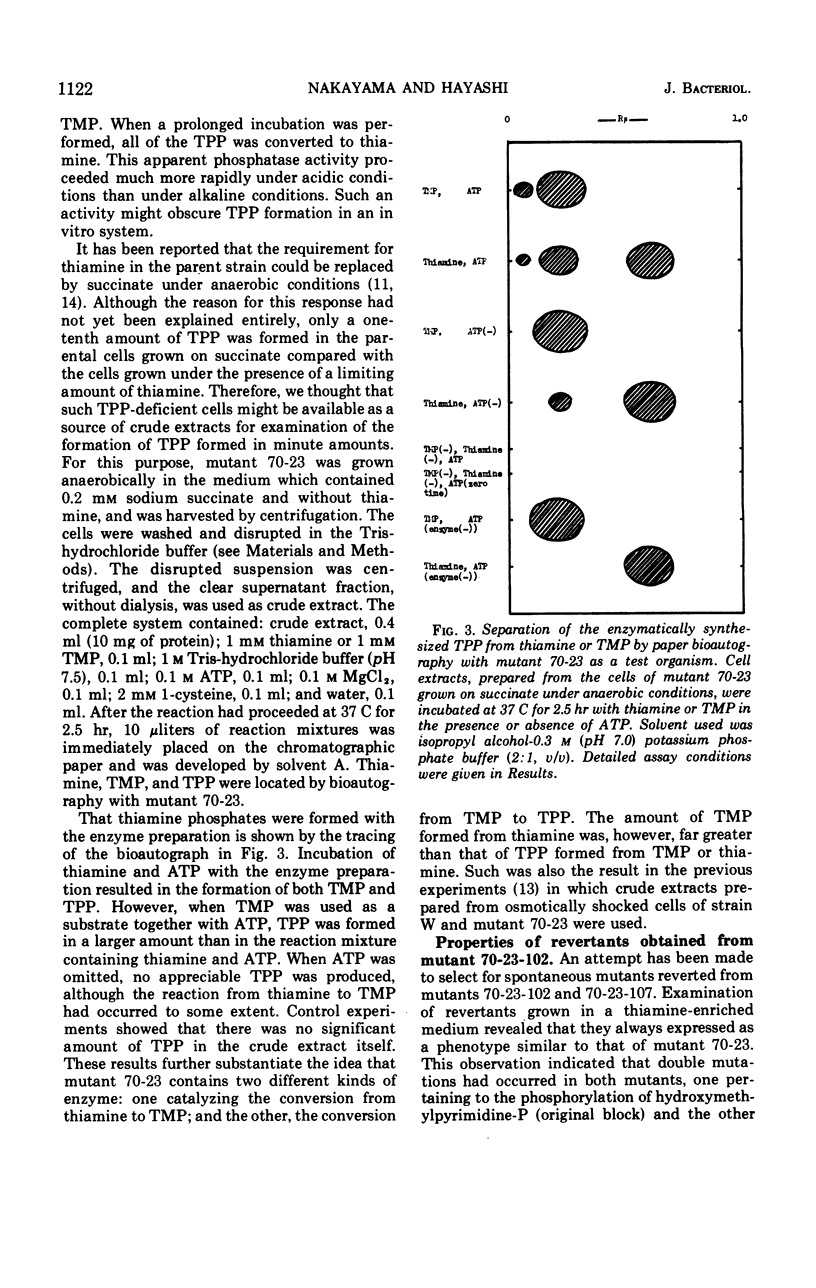
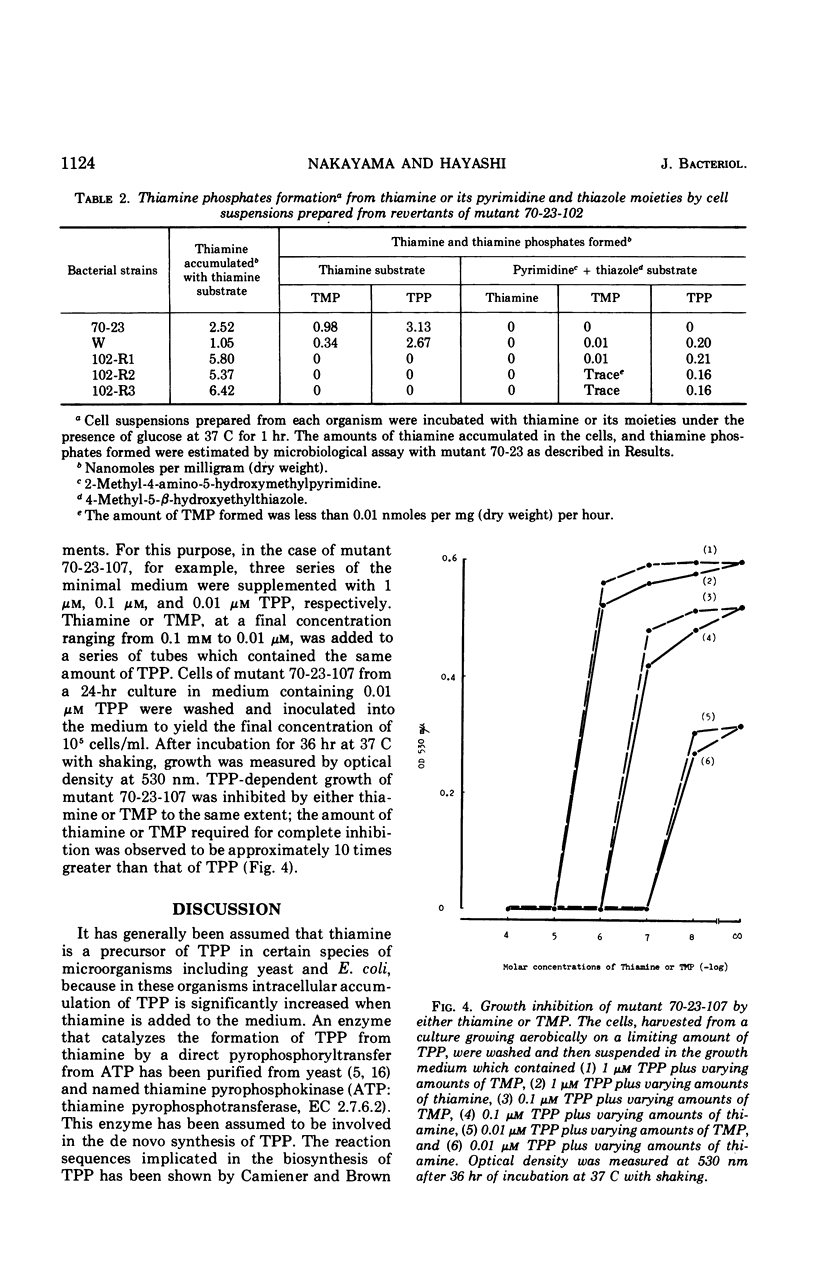
- Nakayama H., Hayashi R. Biosynthesis of thiamine pyrophosphate in Escherichia coli. J Bacteriol. 1972 Feb;109(2):936–938. doi: 10.1128/jb.109.2.936-938.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H., Hayashi R. Utilization of hydroxymethylpyrimidine phosphates by a mutant strain of Escherichia coli. J Vitaminol (Kyoto) 1970 Jun 10;17(2):64–72. doi: 10.5925/jnsv1954.17.64. [DOI] [PubMed] [Google Scholar]