Abstract
The yeast transport GTPase Ypt6p is dispensable for cell growth and secretion, but its lack results in temperature sensitivity and missorting of vacuolar carboxypeptidase Y. We previously identified four yeast genes (SYS1, 2, 3, and 5) that on high expression suppressed these phenotypic alterations. SYS3 encodes a 105-kDa protein with a predicted high α-helical content. It is related to a variety of mammalian Golgi-associated proteins and to the yeast Uso1p, an essential protein involved in docking of endoplasmic reticulum–derived vesicles to the cis-Golgi. Like Uso1p, Sys3p is predominatly cytosolic. According to gel chromatographic, two-hybrid, and chemical cross-linking analyses, Sys3p forms dimers and larger protein complexes. Its loss of function results in partial missorting of carboxypeptidase Y. Double disruptions of SYS3 and YPT6 lead to a significant growth inhibition of the mutant cells, to a massive accumulation of 40- to 50-nm vesicles, to an aggravation of vacuolar protein missorting, and to a defect in α-pheromone processing apparently attributable to a perturbation of protease Kex2p cycling between the Golgi and a post-Golgi compartment. The results of this study suggest that Sys3p, like Ypt6p, acts in vesicular transport (presumably at a vesicle-docking stage) between an endosomal compartment and the most distal Golgi compartment.
INTRODUCTION
Vesicular transport of proteins and lipids is mediated by a number of soluble and membrane-associated proteins that are required for vesicle formation and their docking to and fusion with specific target membranes. Of these proteins, small GTPases of the Ypt/Rab family act as major regulators most likely in the vesicle-docking process. At steady state, they are mostly localized to the surface of distinct subcompartments of the exocytotic and endocytotic pathways, and they cycle between a GTP-bound, active and GDP-bound, inactive conformation (Lazar et al., 1997; Novick and Zerial, 1997; Schimmöller et al., 1998).
One of the small GTP-binding proteins, Ypt6p of Saccharomyces cerevisiae, a homologue of the mammalian Rab6p, is supposed to have a Golgi-associated function. In contrast to the Ypt/Rab GTPases acting at various steps of the secretory pathway (Ypt1p, Ypt31/32p, and Sec4p), Ypt6p is not essential for yeast cell viability. However, the deletion of the YPT6 gene or a specific truncation of the C-terminal two-thirds of Ypt6p resulted in temperature sensitivity of the respective mutant cells. From studies with different ypt6 mutants and multicopy suppressors of their temperature-sensitive phenotypes, it has been concluded that Ypt6p has a function in secretion (Li and Warner, 1996) or in transport between the late Golgi and a prevacuolar, endosome-like compartment (Tsukada and Gallwitz, 1996).
We previously reported on the isolation of four yeast genes, termed SYS1, SYS2, SYS3, and SYS5, that on high expression rescued ypt6 null mutants from temperature sensitivity and from the partial missorting of the vacuolar carboxypeptidase Y (CPY). We had also observed that double deletions of YPT6 and either SYS1 or SYS2 led to an aggravation of the missorting of the vacuolar hydrolase and an inhibition of α-pheromone maturation. In an attempt to further elucidate the function of the Ypt6 GTPase and the multicopy suppressors of the ypt6 loss-of-function mutants, we initiated a study of the SYS3 gene and the protein it encodes. This gene was also found in a suppressor screen by Li and Warner (1996) and named IMH1 because of the structural similarity of its protein product with integrins and myosins. The results of our study strengthen the arguments for a role of Ypt6p and Sys3p in a transport step between the Golgi and an endocytotic compartment through which the CPY receptor and the Kex2 protease involved in α-factor maturation cycle.
MATERIALS AND METHODS
Strains, Growth Conditions, and Genetic Methods
The genotypes of the yeast strains used in this study are listed in Table 1. Yeast cells were grown in standard medium (YEPD) containing 1% yeast extract (Life Technologies, Gaithersburg, MD), 2% peptone 140 (Life Technologies), 2% glucose or in synthetic glucose medium (SD) supplemented with appropriate amino acids (Rose et al., 1990). Solid media were prepared by adding 2% agar (Life Technologies). Standard genetic methods, such as crossing, sporulation of diploids, and tetrad dissection, and auxotrophic selections of diploids were performed as described by Rose et al. (1990). Lithium acetate transformation of yeast cells was performed as previously described (Ito et al., 1983).
Table 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| GFUI-5B/9D | MATa/α his3/his3 leu2/leu2 URA3/ura3 | This laboratory |
| 81-2B/3D | MATa/α his3/his3 leu2/leu2 ura3/ura3 SYS3/sys3::HIS3 | This study |
| 81-2A | MATα his3 leu2 | This study |
| MT1 | MATα his3 leu2 ura3 ypt6::HIS3 | This study |
| 81-2D | MATα his3 leu2 ura3 sys3::HIS3 | This study |
| 11A | MATα his3 leu2 ura3 sys3::HIS3 ypt6::URA3 | This study |
| cl3-ABYS-86 | MATα ura3-Δ5 leu2-3, 112 his− pra1-1 prb1-1 prc1-1 cps1-3 canR | D.H. Wolf (University of Stuttgart, Germany) |
| cl3-ABYS-86-6 | cl3-ABYS-86 ypt6::HIS3 | This study |
| cl3-ABYS-86-3 | cls-ABYS-86 sys3::HIS3 | This study |
| cl3-ABYS-86-3/6 | cl3-ABYS-86 sys3::HIS3 ypt6::URA3 | This study |
| RC898 | MATa leu1 trp5 ade2 can1 sst1 sst2 | H. Riezman (University of Basel, Switzerland) |
| SEY6210 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | S.D. Emr (University of California, San Diego, CA) |
| DKX6210 | SEY6210 kex2::TRP1 | This laboratory |
Gene Disruptions
Single step disruptions of chromosomal genes were done with linear DNA fragments. Part of the protein-coding region of the gene was removed and replaced by a yeast marker gene (HIS3 or URA3). The YPT6 gene was disrupted as previously described (Tsukada and Gallwitz, 1996). From the SYS3 gene residing on a 3.8-kb NdeI–BamHI DNA fragment in pBluescriptIIKS+, whose PvuII sites were deleted, codons 5–542 were removed as a PvuII fragment and replaced by the HIS3 gene as described previously (Tsukada and Gallwitz, 1996). To disrupt one copy of SYS3 in the diploid his3/his3 strain GFUI-5B/9D, competent cells were transformed with a 1.9-kb SspI sys3::HIS3 fragment. Correct integration was verified by Southern blot analysis using the ECL system (Amersham, Arlington Heights, IL). For double disruptions, the protein-coding region of YPT6 on pBluescriptIIKS+-YPT6 was disrupted with URA3 as previously described (Tsukada and Gallwitz, 1996). The resulting plasmid was cut with BamHI and DraI, and the 2.2-kb BamHI–DraI fragment was used to replace one copy of YPT6 in the diploid strain 81-2B/3D, which is heterozygous with respect to the disruption of SYS3. Transformants were sporulated and subjected to tetrad analysis, and the double null mutants were identified, guided by the linked marker genes.
Two-Hybrid Analysis
The SYS3 gene was amplified by PCR using the oligonucleotides 5′-GCT GAT TCC ATG GTC AAA CAG CTG TCA C-3′ and 5′-CTT CTG GAT CCA GCT ACA TTT TTA CTT CAG-3′, thus creating an NcoI restriction site at the ATG initiation codon and a BamHI site 3′ to the TAA(ochre) stop codon. The NcoI–BamHI fragment was cloned into the following vectors (kindly provided by S. Elledge, Baylor College of Medicine, Houston, TX): pASI for the fusion to the transcription activation domain of Gal4p (amino acids 1–147) and pACTII for the fusion to the Gal4 DNA-binding domain (amino acids 768–881). To exclude possible PCR errors, two different PCR products were cloned and analyzed independently.
The YPT6(Q69L) mutant gene was created by an overlap extension method (Ho et al., 1989) using the oligonucleotides 5′-GGG ATA CAG CAG GTC TGG AAA GAT TTA G-3′ and 5′-CTA AAT CTT TCC AGA CCT GCT GTA TCC C-3′ for the codon exchange and 5′-CTT CTC ATA TGA GCA GAT CCG GGA AAT CAT T-3′ and 5′-CCT ATG GAT CCG AAA TAT TAG GTG CTA ACA C-3′ as flanking primers bearing an NdeI restriction site at the ATG initiation codon and a BamHI restriction site downstream of the amber stop codon, respectively. The final PCR product was digested with NdeI and BamHI, inserted into the pASI vector, and verified by sequence analysis.
The pACTII-GYP6 plasmid was constructed by blunt-end insertion of the NdeI–BamHI fragment derived from pET11a-GYP6 (Strom et al., 1993) into the NcoI-cut pACTII. The yeast reporter strain Y190 (Harper et al., 1993) was transformed with both the “bait” pASI construct and the “prey” pACTII construct, and double transformants were analyzed for β-galactidosidase activity by the 5-bromo-4-chloro-3-indolyl galactopyranoside filter assay as previously described (Dalton and Treisman, 1992). In parallel, expression of the Gal4-hybrid proteins was controlled by Western blot analysis using hemagglutinin (HA) monoclonal antibody (Boehringer Mannheim, Mannheim, Germany).
Halo Assay
The halo assay to check α-factor activity was performed as described previously (Tsukada and Gallwitz, 1996), except that 2.5 μl of cultures were spotted onto a lawn of the supersensitive strain RC898 (see Table 1) and cultured for 2–3 d at 25°C.
Radiolabeling and Immunoprecipitation
Radiolabeling and immunoprecipitation of vacuolar CPY and α-factor were performed as described previously (Tsukada and Gallwitz, 1996), except that labeling was performed at 25°C.
Preparation of Sys3p-specific Antisera
To generate a polyclonal antibody directed against Sys3p, the 1.3-kb HindIII fragment of the SYS3 gene (amino acids 325–720) was inserted into the HindIII site of the bacterial expression plasmid pQE30 (Qiagen, Hilden, Germany), which provided a stretch of six histidines located at the N terminus of the Sys3p fragment. Induction of Escherichia coli DH5α carrying the resulting plasmid pQE30-SYS3 was achieved by adding isopropyl-1-thio-β-d-galactopyranoside (2 mM) for 4 h at 37°C. After harvesting, the E. coli cells were lysed in buffer (50 mM NaH2PO4, 300 mM NaCl, 8 M urea, adjusted to pH 8.0) by sonication. Sys3 protein was isolated from the cell lysate by binding to nickel-agarose. After washing with buffer (50 mM NaH2PO4, 300 mM NaCl, 8 M urea, adjusted to pH 6.3), the Sys3 protein was obtained from nickel-agarose by eluting with wash buffer containing 30 mM EDTA. A concentrated and purified Sys3 protein was used to inject rabbits for production of anti-Sys3p antibody.
Fluorescence Microscopy
FM4-64 uptake by living yeast cells was analyzed as described previously (Vida and Emr, 1995). The labeling was performed at 25°C for 1 h at a concentration of 30 μM FM4-64 (Molecular Probes, Eugene, OR); the cells were washed three times with cold PBS buffer (pH 7.0) and chased in YPD for up to 2 h. Labeled cells were visualized with a Zeiss (Thornwood, NY) Axiophot using a rhodamine filter.
Electron Microscopy
Logarithmically growing cells were fixed and stained with potassium permanganate and processed for electron microscopy as previously described (Tsukada and Gallwitz, 1996).
Subcellular Fractionation and Identification of Sys3p
Cells of the proteinase-deficient strain cl3-ABYS-86 (see Table 1) were grown to midlog phase in YEPD medium at 30°C. Thirty OD600 units of cells were collected and suspended in ice-cold lysis buffer (0.2 M Tris-Cl, pH 8.0, 6 mM MgCl2, 1 mM EGTA, 1 mM DTT) containing 4 mM Pefabloc (Boehringer Mannheim) and 1/25 vol of protease inhibitor mixture (Complete TM, Boehringer Mannheim). The cells were lysed with glass beads. To remove unbroken cells and cell debris, the lysate (500 μl) was centrifuged at 500 × g for 3 min. The supernatant (S1) was centrifuged at 10,000 × g for 10 min to generate a pellet (P2) and a supernatant (S2) fraction. Finally, the supernatant (S2) was centrifuged at 100,000 × g for 1 h to generate a pellet (P3) and a supernatant (S3) fraction.
Gel Filtration
S3 fraction prepared in the presence of 0.5 M KCl (5 mg of protein in 1 ml of lysis buffer containing 0.5 M KCl) was applied to a Sephacryl 400 column (1.6 × 70 cm; Pharmacia, Piscataway, NJ), and fractionation by fast protein liquid chromatography was performed using the same buffer at a flow rate of 0.5 ml/min. Fractions of 2 ml were collected, and after precipitation with trichloroacetic acid, proteins were analyzed by SDS-PAGE and Western blotting.
Cross-Linking of Sys3p
Cells of the proteinase-deficient strain cl3-ABYS-86 (see Table 1) were grown to midlog phase in YEPD medium at 30°C. Two hundred OD600 units of cells were collected and suspended in 0.1 M sodium phosphate buffer, 0.15 M NaCl, 6 mM MgCl2, 1 mM DTT, 1 mM EGTA, pH 7.2, containing 4 mM Pefabloc and 1/25 vol of protease inhibitor cocktail (Complete TM, Boehringer Mannheim). The cells were lysed with glass beads. The lysate (1 ml) was centrifuged as described before to obtain a soluble fraction (S3). Ninety microliters of the S3 fraction were used in each reaction. Ten microliters of bis-(sulfosuccinimidyl) suberate (BS3; Pierce, Rockford, IL) were dissolved in 0.1 M sodium phosphate buffer, 0.15 M NaCl, pH 7.2, immediately before use and added to final concentrations of 0.1, 0.25, 0.5, 1, 1.5, and 2 mM, respectively. The reaction mixtures were incubated for 30 min at 30°C. The reaction was stopped by adding 10 μl of 0.2 M Tris-Cl, pH 7.5, and incubating for 30 min on ice. Proteins were analyzed by SDS-PAGE and Western blotting.
RESULTS
Sys3p Is a Nonessential Protein
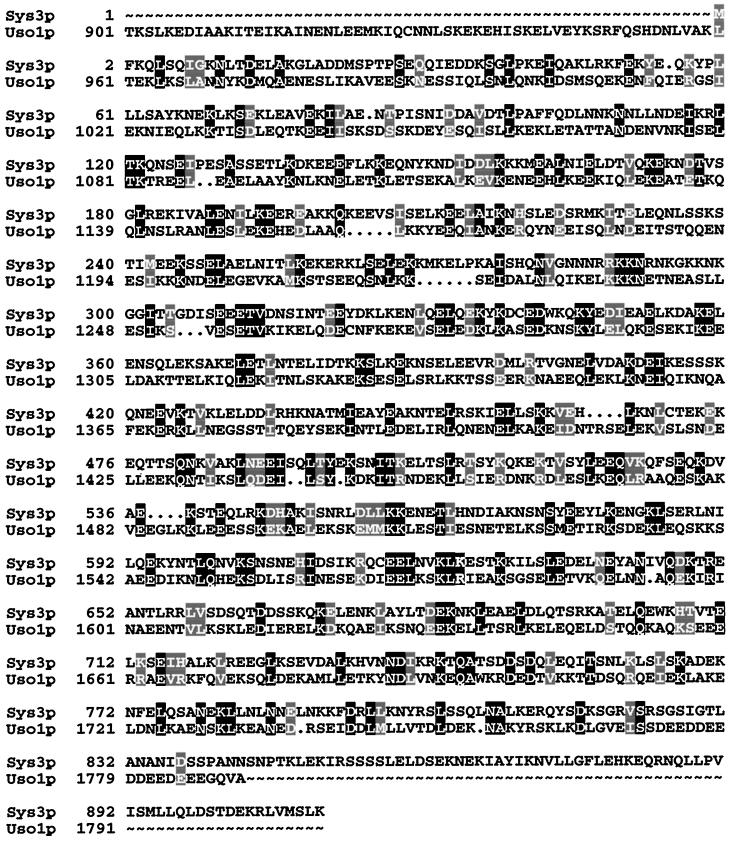
As previously described, SYS3 was identified as a multicopy suppressor for a temperature-sensitive ypt6 null mutant (Tsukada and Gallwitz, 1996) and, in an independent study, as a week single copy suppressor of a yeast mutant strain expressing a C-terminally truncated version of Ypt6p (Li and Warner, 1996). Subcloning of the original suppressor plasmid identified SYS3/IMH1 as ORF YLR309C, which resides on chromosome XII. As deduced from the DNA sequence, Sys3/Imh1p is a 105.2-kDa protein of hydrophilic nature and, as already pointed out (Li and Warner, 1996), is structurally related to myosins and to Uso1p, the latter being an essential component in yeast endoplasmic reticulum (ER)-to-Golgi trafficking (Nakajima et al., 1991; Sapperstein et al., 1996). Uso1p is in fact the yeast protein that is most highly related to Sys3p. When a sequence alignment allowing a minimum of gaps (3.4%) is performed (Figure 1), the primary sequences of Sys3p and the C-terminal portion of the 1790-amino acid-long Uso1 protein are identical to an extent of ∼20%. According to sequence alignments with BLAST 2.0.2 (Altschul et al., 1995), allowing for gaps in the range of 10%, the two proteins exhibit 24% identity and 46% similarity. The identities are scattered throughout the entire Sys3p. However, Sys3p differs from Uso1p by the absence of the highly acidic C terminus and a globular N-terminal domain, sequence features shared between Uso1p and the mammalian vesicle-docking protein p115 (Sapperstein et al., 1995; Nakamura et al., 1997).
Figure 1.
Comparison of Sys3p and Uso1p amino acid sequences. Identical residues are on a black background, and similar residues are shaded. The alignment was made using the program PILEUP (Genetics Computer Group, Inc., Madison, WI) (Feng and Doolittle, 1987).
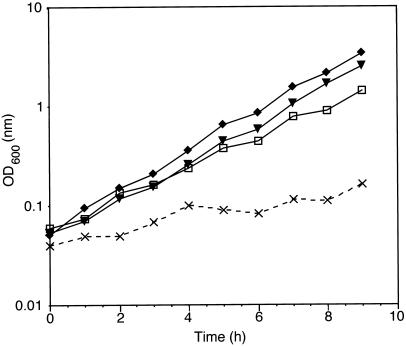
To determine whether SYS3 is an essential gene such as USO1, the chromosomal SYS3 allele was disrupted by the HIS3 marker gene (described in MATERIALS AND METHODS). Disruption of SYS3 did not result in significant growth defects. Similar to the previously described SYS1 and SYS2 deletions, the doubling time in YEPD of strains carrying the disrupted SYS3 allele was indistinguishable from that of the isogenic wild-type strain (Figure 2). Furthermore, sys3 null mutants grew perfectly well at 37 or 13°C.
Figure 2.
Synthetic negative growth phenotype of double deletion mutants. Growth curves of wild-type (▾), Δypt6 (□), Δsys3 (♦), and Δypt6/sys3 (×) strains are shown. Precultures of corresponding strains were grown to stationary phase at 25°C. Cells were diluted into fresh YEPD medium and incubated overnight to an OD600 of 1–3. Cells were again diluted into fresh YEPD medium to an OD600 of ∼0.05 and incubated at 30°C. Cell growth was followed by measuring the optical density at 600 nm.
Double Disruption of YPT6 and SYS3 Has a Synthetic Growth-inhibitory Effect
As we reported earlier, the deletion of SYS1 and/or SYS2 in the ypt6 null mutant enhanced defects in cell growth as well as in vacuolar protein sorting. To check whether the simultaneous deletion of YPT6 and SYS3 exhibits a synthetic growth-inhibitory effect, we constructed a ypt6/sys3 double deletion mutant. A heterozygous diploid SYS3/sys3::HIS3 deletion strain was transformed with a fragment containing the disrupted YPT6 gene (ypt6::URA3). Southern blot analysis of several Ura+ transformants confirmed the integration of the URA3 marker at the YPT6 locus. After sporulation and tetrad dissection, half of the tetrads analyzed produced four large spores, and half of them produced one tiny colony and three large colonies at 25°C. The smaller colonies were always His+ and Ura+ and therefore carried the YPT6/SYS3 double disruption. As shown in Figure 2, the growth of the ypt6/sys3 double null mutant was significantly inhibited at 30°C. All experiments with this mutant were done therefore at 25°C. The doubling time of the ypt6/sys3 null mutant at 25°C (∼225 min) was still significantly prolonged compared with the wild-type strain (∼135 min). The growth defect in the ypt6/sys3 double mutant was more severe than that of the previously described ypt6/sys1 double null mutant. In addition, the ypt6/sys3 double null mutant was not able to grow at 13°C.
Disruption of SYS3 Aggravates the CPY Missorting Defect in ypt6 Null Mutants
In previous studies it was shown that the vacuolar proteinase CPY is partially missorted in a ypt6 null mutant (Hengst, 1992) and that this defect is enhanced by the deletion of SYS1 or SYS2 (Tsukada and Gallwitz, 1996). Transport of newly synthesized CPY can be easily followed by the appearance of three forms, a 67-kDa ER core-glycosylated form (p1CPY), a 69-kDa Golgi-modified form (p2CPY), and the 61-kDa mature form (mCPY) found in the vacuole. In a ypt6 null mutant as well as in many vacuolar protein-sorting (vps)–defective mutants, the 69-kDa Golgi-glycosylated p2CPY is partially missorted and secreted.
The influence of SYS3 deletion on CPY sorting was investigated by a pulse–chase experiment. Cells were spheroplasted, labeled with Tran35S label for 15 min, and chased with an excess of cold methionine and cysteine for 30 min at 25°C. Labeled CPY was then immunoprecipitated from the intracellular and extracellular fractions and analyzed by SDS-PAGE. As described previously (Tsukada and Gallwitz, 1996), a small fraction of the unprocessed Golgi form (p2CPY) was secreted from ypt6 null mutant cells (Figure 3, lane 4). Partial missorting of p2CPY was also observed in the sys3 null mutant (Figure 3, lane 6). In sys/ypt6 double null mutants, the CPY missorting defect was significant and clearly more than additive.
Figure 3.
Sorting of the vacuolar enzyme CPY. Wild-type (wt) cells and strains carrying gene deletions as indicated were grown to exponential phase, spheroplasted, labeled for 15 min at 25°C with Tran35S-label, and chased for 30 min at 25°C. The labeled spheroplasts were separated into pellet (intracellular [I]) and supernatant (extracellular [E]) fractions. The presence of CPY in these fractions was determined by SDS-PAGE of these proteins immunoprecipitated with anti-CPY antibodies.
These results argue for a functional involvement of the Ypt6 GTPase and the Sys3 protein in vacuolar protein sorting rather than in secretion.
Inhibition of α-Factor Maturation in ypt6/sys3 Null Mutants Because of Kex2 Protease Cycling Defect
To assess a possible role of Sys3p in secretion, sys3 null mutants and ypt6/sys3 double deletion mutants were analyzed for α-pheromone formation. To examine the secretion of functional α-factor in the deletion mutants, the halo assay was performed. The secretion of active α-factor from a MATα strain induces G1 cell cycle arrest in cells of the opposite mating type (MATa). The size of the halo correlates well with the amount of the secreted active α-factor (Julius et al., 1983). As shown in Figure 4, the size of the halo generated by the ypt6/sys3 deletion mutant was smaller than those of the isogenic wild-type strain and the single deletion mutants.
Figure 4.
Secretion of active α-factor from various deletion strains. MATα strains carrying gene deletions as indicated were grown to stationary phase, and after appropriate dilution, 2.5-μl cultures were spotted onto a lawn of the MATa supersensitive strain. The diameter of the growth-inhibitory zone (halo) is proportional to the amount of α-factor secreted.
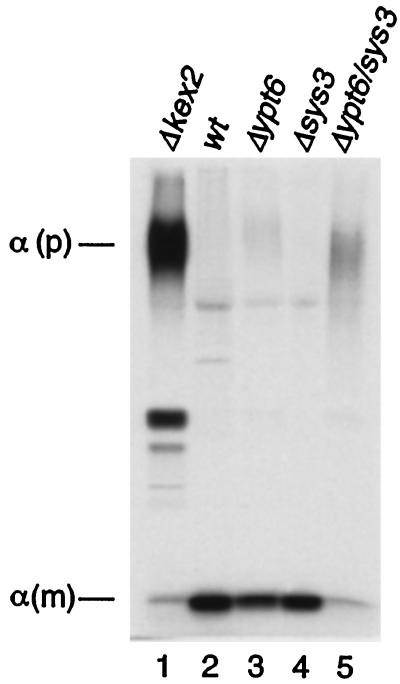
To distinguish between a defect in secretion and the processing of the α-pheromone, which is formed as a highly glycosylated precursor of ∼125 kDa and proteolytically processed by Kex2p in the late Golgi, cells were radioactively labeled, and the fate of newly synthesized pheromone secreted into the growth medium was followed. As shown in Figure 5, wild-type as well as sys3 single deletion strains secreted nearly exclusively the mature α-factor of 3.5 kDa, whereas cells of a kex2 null mutant secreted the glycosylated precursor molecules. As we reported previously (Tsukada and Gallwitz, 1996), the deletion of YPT6 resulted in a weak inhibition of α-factor maturation seen by the secretion of a small amount of highly glycosylated precursor (Figure 5, lane 3). Importantly, most of the immunoreactive species detected in the culture medium of ypt6/sys3 null mutants comigrated with highly glycosylated α-factor precursor. This result showed that the deletion of the SYS3 gene enhanced the inhibition of α-factor precursor maturation in the ypt6 single null mutant, as is the case with the deletion of the SYS1 gene (Tsukada and Gallwitz, 1996). It also explains the smaller halo generated by the ypt6/sys3 deletion mutant compared with the one generated by the isogenic wild-type strain (Figure 4). The amounts of the labeled immunoreactive α-factor species detected in the medium of ypt6/sys3 null mutants were less than those of wild-type strains most likely because of the growth retardation of the mutant cells. It was, however, not due to a defect in secretion from the ypt6/sys3 null mutant cells, because other experiments showed that secreted invertase was not accumulated in this mutant.
Figure 5.
Secretion of highly glycosylated α-factor precursor in ypt6/sys3 double deletion mutants. MATα strains carrying gene deletions as indicated were grown to exponential phase and labeled for 30 min at 25°C with Tran35S-label. Culture media were collected, and α-factor was immunoprecipitated with anti-α-factor antiserum and analyzed by SDS-PAGE on 15% gels. α (p), highly glycosylated α-factor precursor; α (m), mature α-factor.
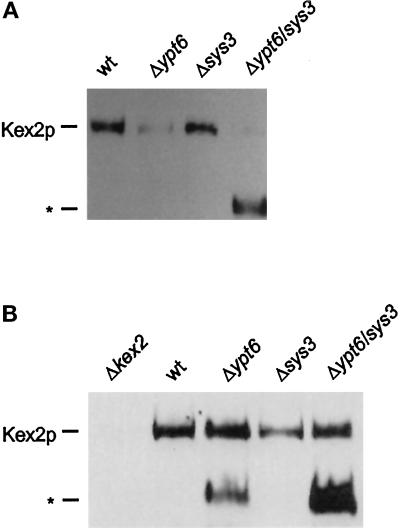
As shown previously, the secretion of highly glycosylated α-factor precursor from ypt6 and ypt6/sys1 null mutants was due to an apparent reduction of the intracellular levels of the Kex2 protease (Tsukada and Gallwitz, 1996), which is known to cycle between the late Golgi and an endocytic compartment (Wilsbach and Payne, 1993). These experiments were performed with cells expressing C-terminally HA epitope–tagged Kex2p, and HA antibodies were used to detect the protease. In the present study, the steady-state levels of Kex2p were also measured in ypt6/sys3 null mutants. Cellular proteins were prepared, and Kex2p was detected by immunoblotting using polyclonal anti-Kex2p antibodies. As shown in Figure 6A, levels of Kex2p were strikingly reduced in both ypt6 and ypt6/sys3 null mutants. Furthermore, a faster running protein species (Figure 6A, *), which was not observed in the previous study using antibodies specific for HA, was always detected with anti-Kex2p antibodies in the ypt6/sys3 double null mutant.
Figure 6.
Kex2p steady-state levels in ypt6/sys3 null mutants. (A) Cell extracts were prepared from cultures of wild-type (wt), Δypt6, Δsys3, and Δypt6/sys3 strains, and levels of Kex2p were analyzed by SDS-PAGE (6% polyacrylamide) and immunoblotting using a polyclonal antiKex2p antibody. (*) Position of a possible Kex2p degradation product. (B) Analysis of Kex2p levels were determined as in A, except that cell extracts were prepared from cultures of pra1-1/prb1-1/prc1-1 versions of each strain, and a Δkex2 strain was included in the analysis.
To see whether the reduction of cellular Kex2 protease resulted from a missorting of the Golgi-localized enzyme to the vacuole, ypt6 and sys3 deletions in a vacuolar proteinase-deficient strain were also analyzed for their effects on Kex2p. As shown in Figure 6B, the steady-state levels of Kex2p were restored to the wild-type level both in ypt6 and ypt6/sys3 null mutants, and the amount of the additional protein cross-reacting with the Kex2p antibody increased in the ypt6/sys3 double mutant. This apparent Kex2p degradation product was also detected in the ypt6 deletion mutant but was absent from wild-type and kex2 null mutant cells.
These results indicate that the Ypt6 GTPase and the Sys3 protein may act together or in parallel in protein transport between the Golgi and a post-Golgi, endosome-like compartment.
ypt6/sys3 Double Deletion Mutants Have Fragmented Vacuoles and Accumulate 40- to 50-nm Vesicles
Our previous studies (Tsukada and Gallwitz, 1996) had revealed that ypt6 null mutant cells contained 40- to 50-nm vesicles well in excess over wild-type cells and exhibited moderately fragmented vacuoles. Cells of a ypt6/sys1 double deletion strain accumulated in addition spherical structures lacking a clearly discernible unit membrane and filled with electron-dense material.
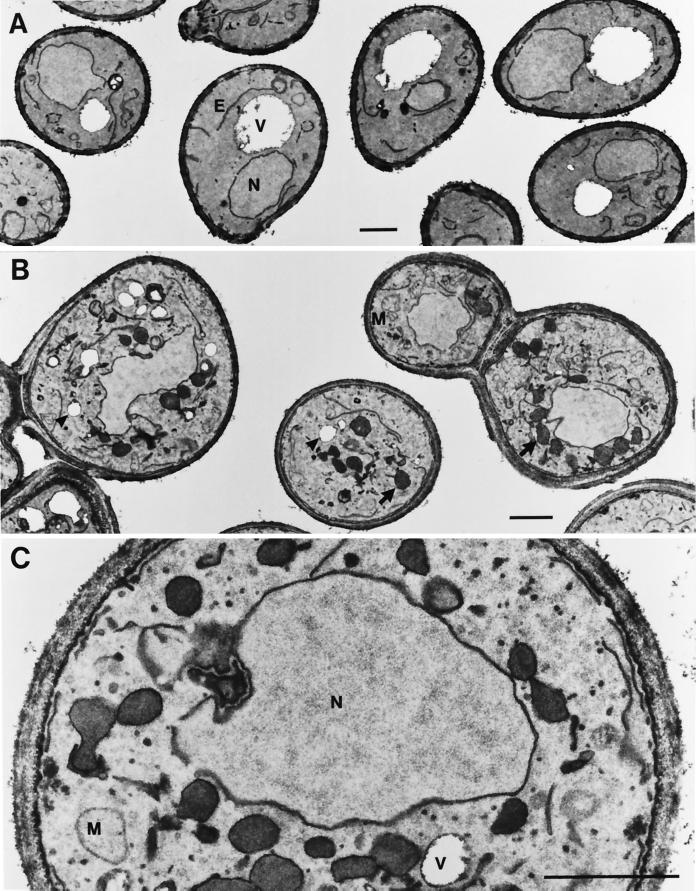
To investigate possible morphological alterations, we also subjected cells of a sys3 null and a ypt6/sys3 double deletion strain to an electron microscopic analysis using potassium permanganate fixation and staining. Whereas sys3 null mutant cells did not show any significant morphological alteration compared with wild-type cells (Figure 7A), cells of the double mutant strain massively accumulated small vesicles ∼40–50 nm in diameter and the spherical structures, often surrounding the nucleus, that were seen also in the ypt6/sys1 null mutants. As can be seen in Figure 7, B and C, these structures were delimited by a thin, electron-dense layer different from unit membranes that could often be visualized to surround the fragmented vacuoles (empty structures of similar size; Figure 7, B and C).
Figure 7.
Morphological alterations in ypt6/sys3 deletion mutant cells. Logarithmically growing cells were fixed with potassium permanganate to highlight membrane structures. Neighboring cells of one section are shown to document the specificity of the alterations. (A) Single sys3 disruptants shown here and wild-type cells were indistinguishable. (B) Cells of a ypt6/sys3 null mutant strain. Arrowheads point to clearly identifiable vacuoles; arrows point to the spherical structures. (C) Higher magnification of a double mutant cell showing the accumulation of 40- to 50-nm vesicles. V, vacuole; E, endoplasmic reticulum; N, nucleus; M, mitochondria. Bars, 1 μm.
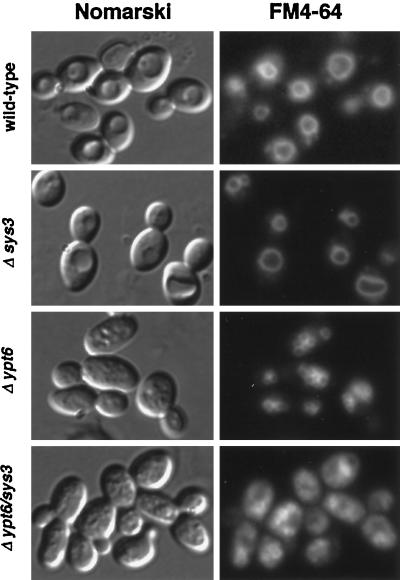
The appearance of the apparently fragmented vacuoles, a feature of several yeast mutants defective in endocytic trafficking (Raymond et al., 1992), prompted us to investigate whether the endocytic marker FM4-64 (Vida and Emr, 1995) was properly delivered to the vacuolar compartments in ypt6 and in ypt6/sys3 double knock-out strains. As can be seen in Figure 8, cells of a sys3 deletion strain and the isogenic wild-type strain exhibited membrane fluorescence of the large vacuolar compartment by the styryl dye. Fluorescence of smaller ring-like structures corresponding to the fragmented vacuoles was also observed in cells of ypt6 and ypt6/sys3 deletion strains. Careful microscopic inspection revealed that the seemingly higher background fluorescence in cells of the ypt6/sys3 double knock-out strain was most likely due to the smaller vacuolar compartments in these cells.
Figure 8.
FM4-64 staining of vacuolar membranes in ypt6/sys3 null mutants. Wild-type, Δsys3, Δypt6, and Δypt6/sys3 cells were grown at 25°C, labeled for 1 h with 30 μM FM4-64, washed three times with cold PBS buffer, and chased in YPD for 2 h. Then cells were examined by Nomarski and fluorescence microscopy for FM4-64 fluorescence. Essentially the same result was obtained after a 30-min chase time.
Taken together, these results might indicate that in ypt6/sys3 deletion cells a vesicular transport step is severely disturbed without a significant alteration of endocytic trafficking.
Identification and Subcellular Localization of the Sys3 Protein
To characterize the SYS3 gene product, we prepared a polyclonal antiserum against a fragment of the Sys3 protein (amino acids 325–720), which was produced in E. coli as an N-terminally (His)6-tagged fusion. Among total cellular proteins, the antibody recognized a polypeptide of ∼105 kDa, the expected size for Sys3p. This protein was not detected in a Δsys3 strain or by the preimmune serum.
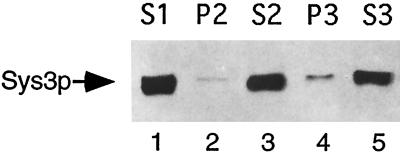
After breaking cells with glass beads, a differential centrifugation of the lysate was performed, and the presence of the Sys3 protein in membrane fractions sedimenting either at 10,000 × g (P2) or 100,000 × g (P3) and in the 100,000 × g supernatant (S3) was investigated. It was found (Figure 9) that most of Sys3p was in the cytoplasmic fraction and only a small amount was reproducibly pelletable at 100,000 × g. Interestingly, this subcellular distribution of Sys3p is similar to that of the related Uso1 protein (Nakajima et al., 1991; Seog et al., 1994). Indirect immunofluorescence using the anti-Sys3p antibody did not reveal any particular labeling of cellular structures.
Figure 9.
Subcellular localization of the Sys3 protein. Yeast cells lacking vacuolar proteinases (strain cl3-ABYS-86) were grown to exponential phase at 30°C in YEPD medium and disrupted with glass beads. Unbroken cells were removed by centrifugation at 500 × g. The soluble fraction (S1) was separated into pellet (P2) and supernatant (S2) fractions by centrifugation for 10 min at 10,000 × g. The S2 fraction was then centrifuged for 1 h at 100,000 × g to generate soluble (S3) and pellet (P3) fractions. Equal portions of the different fractions were analyzed by SDS-PAGE (6% polyacrylamide) and immunoblotting using anti-Sys3p antibodies.
The Sys3 protein Is Present in Soluble High-Molecular-Mass Complexes and Forms Oligomers
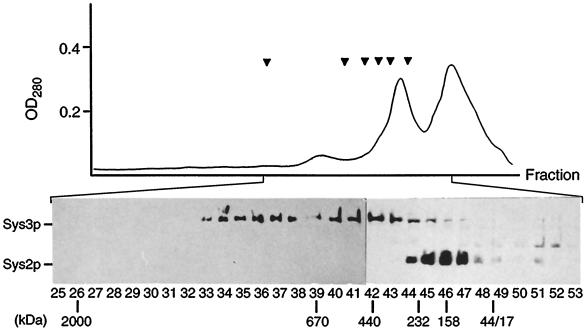
The primary sequence of Sys3p predicts a soluble, predominantly α-helical protein with extended regions containing heptad repeats with apolar amino acids in the first and fourth positions (Figure 1). Several secondary structure prediction methods (Levin et al., 1986; Deleage and Roux, 1987; Gibrat et al., 1987; Geourjon and Deleage, 1995) revealed that 80% of the entire Sys3 protein might be helical. These structural features indicate that Sys3p, like the C-terminal half of Uso1p, has a high potential to form coiled coils and to have a rod-like structure. In fact, on fractionation of the 100,000 × g supernatant proteins by Sephacryl 400 chromatography, the Sys3 protein eluted in two major peaks with molecular masses of 700–900 kDa and of ∼400 kDa when compared with globular proteins (Figure 10). These complexes did not cofractionate with either Sys2p, a 51-kDa protein likewise able to suppress growth and transport defects of ypt6 mutants (Tsukada and Gallwitz, 1996), or with the Ypt6-GTPase.
Figure 10.
Gel filtration of Sys3 protein. Soluble proteins (S3 fraction prepared in the presence of 0.5 M KCl) were chromatographed on Sephacryl 400. The absorbtion profile of the eluted fractions is shown. Arrowheads (from left to right) show the positions of marker proteins (thyroglobulin [670 kDa], ferritin [440 kDa], catalase [232 kDa], γ-globulin [158 kDa], ovalbumin [44 kDa], and myoglobin [17 kDa]) and dextran 2000. Eluted fractions were analyzed for Sys3p and Sys2p by SDS-PAGE and Western blotting. The positions of molecular size standards used to calibrate the column are shown below the fraction numbers.
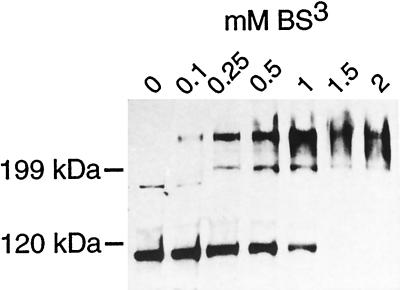
To see whether Sys3p oligomerizes in vivo, soluble proteins were prepared from a protease-deficient yeast strain and treated with different concentrations of the homobifunctional cross-linking reagent BS3, which reacts with primary amines to form covalent amide bonds. Immunoblotting of soluble proteins (without BS3 treatment) with an anti-Sys3p antibody revealed a single protein band with the expected size of 105 kDa, together with trace amounts of a larger band. Chemical cross-linking resulted in an increased yield of an apparent dimer of ∼240 kDa (Figure 11). Further evidence for oligomerization of Sys3p comes from a two-hybrid analysis. The entire Sys3p was fused to the Gal4p DNA-binding domain as well as to the Gal4p transcription activation domain in the two-hybrid vectors pACTII and pAS1, respectively. After transforming a yeast strain harboring a Gal4p-regulated lacZ gene with the recombinant vectors, the production of the fusion proteins was verified by Western blot analysis, and protein–protein interactions were identified by the generation of β-galactosidase activity. As shown in Figure 12, Sys3p did not have self-activating activity but clearly interacted with itself. In contrast, neither Ypt6 wild-type protein nor a GTPase-deficient, activated Ypt6 mutant protein (Q69L substitution) interacted with Sys3p. The GTPases, on the other hand, strongly interacted with its GTPase-activating protein Gyp6p (Strom et al., 1993).
Figure 11.
Oligomerization of Sys3p. A cytosolic S3 fraction (9 mg/ml) was incubated with the cross-linker BS3 at the concentrations indicated. After SDS-PAGE, immunoblot analysis was performed using anti-Sys3p antibodies. The positions of molecular mass markers are given to the left.
Figure 12.
Sys3 protein interactions in the two-hybrid system. The Y190 reporter strain was transformed with either pASI-SYS3, pASI-YPT6(Q69L), or pASI-YPT6 (wild-type) as baits in combination with either pACTII-SYS3 or pACTII-GYP6 as prey. β-Galactosidase activity was detected by the 5-bromo-4-chloro-3-indolyl galactopyranoside filter assay. The lack of transcription activation by the baits alone is shown for Sys3p and Ypt6(Q69L)p. The functionality of the Gal4–Ypt6 fusion proteins is confirmed by their strong interaction with the GTPase-activating protein Gyp6p (Strom et al., 1993).
In summary, several lines of evidence indicate that Sys3p forms homooligomers in vivo, but Sys3p does not appear to physically interact with Ypt6p.
DISCUSSION
In a previous study, we identified several yeast genes that on high expression suppressed the temperature sensitivity of mutants lacking the transport GTPase Ypt6 (Tsukada and Gallwitz, 1996). In this report, we show that one of these multicopy suppressors, SYS3, encodes a protein that like Ypt6p is not essential for cell viability. However, its loss of function in ypt6 deletion strains results in a severe growth inhibition and in protein sorting and maturation defects at a late Golgi compartment. The results on a functional interplay of Sys3p and Ypt6p are very similar to those that we previously obtained with two other multicopy suppressors of ypt6 deletions, the Sys1 and Sys2 proteins. The results of our present study strengthen the view that Ypt6p and the Sys proteins are most likely involved in protein transport between the Golgi and an endosomal compartment. A straightforward explanation for CPY missorting and the inhibition of α-pheromone maturation in the ypt6/sys3 double mutant cells would be the perturbation of transport from an endocytic organelle back to the trans-Golgi compartment. It has been shown that the α-factor–processing Kex2 protease as well as the CPY sorting receptor Vps10p cycle between these organelles (Wilsbach and Payne, 1993; Nothwehr and Stevens, 1994; Cereghino et al., 1995; Cooper and Stevens, 1996). The interference with retrograde transport to the Golgi would thus lead to an escape of the two Golgi-resident proteins to the vacuole. This is in fact what we have observed in the present study for Kex2p, whose intracellular level decreased dramatically in ypt6/sys3 double deletion mutants obviously because of a missorting of the enzyme to and its degradation in the vacuole.
As Li and Warner (1996) concluded from their studies that Ypt6p might have a function in protein transport to and through the Golgi, we stress again that we could not find any evidence for a role of the Ypt6 GTPase in secretion. One explanation for this discrepancy is that all of our studies were performed at permissive temperatures of the deletion mutants used. In contrast, Li and Warner (1996) investigated their ypt6 null mutants after a shift to nonpermissive conditions. Clearly, if Ypt6p would serve a function in the secretory pathway, one would expect cells deprived of this GTPase to display a defect in secretion also at a permissive growth temperature. Under such conditions, however, secretion defects of Ypt6p-lacking cells were evident neither from the investigations of Li and Warner (1996) nor from our own (Tsukada and Gallwitz, 1996). In addition, it is our experience that a slight inhibition of invertase secretion from ypt6 deletion cells even at nonpermissive temperature is strain-dependent. In this context, it is also interesting to note that SYS3, independently isolated as a suppressor for the temperature-sensitive phenotype of a ypt6 truncation mutant (and termed IMH1, for integrin myosin homologue), did not rescue the mutant cells from the apparent secretory transport defect (Li and Warner, 1996). In contrast, our studies clearly demonstrate that the functional loss of Ypt6p and that of Sys3p have comparable consequences with respect to a post-Golgi transport reaction.
The combined functional loss of Ypt6p and Sys3p resulted in a severe missorting of vacuolar CPY that was more than additive with respect to the single mutations. Likewise, the massive accumulation of small vesicles, presumably transport intermediates, was more pronounced in the double deletion strain than in single ypt6 mutant cells or in vesicular transport-defective mutants lacking other Ypt-GTPases, for example, Ypt1p (Becker et al., 1991) or Ypt51p (Horazdovsky et al., 1994; Singer-Krüger et al., 1994). Whether the appearance of the spherical bodies conspiciously surrounding the nucleus is a direct or secondary consequence of the transport defect attributable to the lack of Ypt6p and Sys3p is not known and difficult to clarify using deletion mutants. As we previously discussed (Tsukada and Gallwitz, 1996), these structures, also seen in ypt6/sys1 disruptants, might be related to the vacuolar compartment, which is highly fragmented in these mutants. A defect in retrograde transport from an endosomal compartment to the Golgi apparatus that we presently favor as the most likely explanation for the phenotypic alterations of these mutants could also impede the biosynthesis and functioning of the endosomal–vacuolar membrane system. Importantly, as judged from the proper delivery of the styryl dye FM4-64 to the apparently unperturbed vacuoles in sys3 deletion strains and to the fragmented vacuoles in ypt6 and in ypt6/sys3 knock-out strains, it appears that the endocytic pathway is still functional in the absence of Ypt6p and Sys3p.
Secondary structure predictions show that the Sys3 protein has a high α-helical content over its entire length (Levin et al., 1986; Gibrat et al., 1987; Rost and Sander, 1994; Geourjon and Deleage, 1995) and a high potential to form coiled coils. The apparent dimerization of Sys3p after treatment of soluble cellular proteins with a bifunctional cross-linker indicates that the protein interacts with itself in vivo. Sys3–Sys3 protein interaction could also be shown in a two-hybrid analysis. However, according to gel filtration chromatography, the dimers might form higher-order structures with themselves or complexes with other proteins. Interestingly, Sys2p, a hydrophilic protein (Tsukada and Gallwitz, 1996) which is nearly exclusively membrane associated but salt extractable (our unpublished observations), does not appear to be complexed with Sys3p and neither is Ypt6p.
In comparing the primary sequence of Sys3p with those of mammalian proteins made public in several data banks, we have noted that among the related proteins with the highest scores are the trans-Golgi p230 (Erlich et al., 1996), giantin (Linstedt and Hauri, 1993; Seelig et al., 1994), and the human endosome-associated EEA1 protein (Mu et al., 1995). Those α-helical proteins share with Sys3p the extraordinarily high number of heptad repeats and the potential to form coiled coils. The mammalian proteins are peripherally membrane associated and have been implicated to participate in vesicular protein transport through the Golgi complex and the endocytic pathway, respectively. EEA1 has recently been shown to act as an effector of the Rab5 GTPase (Simonsen et al., 1998). EEA1 interacts with the GTP-bound form of Rab5p via a small N-terminal domain containing a zinc finger and through a C-terminal fragment adjacent to the so-called FYVE finger. In contrast, Sys3p, which shares with EEA1 the extended α-helical regions but does not contain sequences related to its Rab5p-interacting regions, does not interact with Ypt6p or its GTPase-deficient mutant Ypt6(Q69L)p. Giantin, a 376-kDa, rod-shaped protein bound to the cytoplasmic surface of Golgi membranes (Linstedt et al., 1995), has been reported recently to have a role in COPI vesicle docking (Sönnichsen et al., 1998). Likewise, the Sys3p-related Uso1 protein, which like Sys3p is only partially membrane associated, is needed for the docking of ER-derived vesicles to the cis-Golgi compartment (Sapperstein et al., 1996; Cao et al., 1998). Although a physical interaction of Uso1p and the GTPase Ypt1p has not been shown, the two proteins appear to act together in the same vesicle-docking event before v-SNARE pairing (Cao et al., 1998).
An attractive working hypothesis is that rod-shaped, dimerized proteins, including p115, giantin, and possibly Sys3p, bridge the membranes to be fused (Warren and Malhotra, 1998). Giantin and Uso1p are two examples for which a role in vesicle docking to the target membrane is in fact well founded. Although it has to be elucidated to which membrane(s) Sys3p can bind, its functional interplay with Ypt6p makes it a candidate for having a “bridging” function in the docking of endosome-derived vesicles to the trans-Golgi compartment. The fact that high intracellular levels of Sys3p suppress the transport and growth defects of Ypt6p-lacking cells could be explained by mass action, and it seems to be comparable to the suppressing activity of highly expressed v-SNAREs (Sec22p and Bet1p) in ER-to-Golgi transport-defective ypt1 deletion mutants (Dascher et al., 1991; Ossig et al., 1991). The massive accumulation of vesicles observed in the mutant cells lacking Sys3p and Ypt6p strongly argues for a role of the two proteins in vesicle docking.
ACKNOWLEDGMENTS
We are indebted to H.-H. Trepte for performing the electron microscopic analysis. We thank U. Welscher-Altschäffel and H. Behr for technical assistance, H. D. Schmitt for providing yeast strains, and I. Balshüsemann for secretarial assistance. This work was supported by the Max-Planck Society and by grants to D.G. from the Deutsche Forschungsgemeinschaft and Fonds der Chemischen Industrie.
REFERENCES
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zangh Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein data base search programs. Nucleic Acids Res. 1995;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Tan TJ, Trepte H-H, Gallwitz D. Mutational analysis of the putative effector domain of the GTP-binding Ypt1 protein in yeast suggests specific regulation by a novel GAP activity. EMBO J. 1991;10:785–792. doi: 10.1002/j.1460-2075.1991.tb08010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Ballew N, Barlowe C. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J. 1998;17:2156–2165. doi: 10.1093/emboj/17.8.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghino JL, Marcusson EG, Emr SD. The cytoplasmic tail domain of the vacuolar protein sorting receptor Vps10p and subset of VPS gene products regulate receptor stability, function, and localization. Mol Biol Cell. 1995;6:1089–1102. doi: 10.1091/mbc.6.9.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AA, Stevens TH. Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J Cell Biol. 1996;133:529–541. doi: 10.1083/jcb.133.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton S, Treisman R. Characterization of SAP-l, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992;68:597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- Dascher C, Ossig R, Gallwitz D, Schmitt HD. Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the ras-superfamily. Mol Cell Biol. 1991;11:872–885. doi: 10.1128/mcb.11.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleage G, Roux B. An algorithm for protein secondary structure prediction based on class prediction. Protein Eng. 1987;1:289–294. doi: 10.1093/protein/1.4.289. [DOI] [PubMed] [Google Scholar]
- Erlich R, Gleeson PA, Campbell P, Dietzsch E, Toh B-H. Molecular characterization of trans-Golgi p230. J Biol Chem. 1996;271:8328–8337. doi: 10.1074/jbc.271.14.8328. [DOI] [PubMed] [Google Scholar]
- Feng DF, Doolittle RF. Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol. 1987;25:351–360. doi: 10.1007/BF02603120. [DOI] [PubMed] [Google Scholar]
- Geourjon C, Deleage G. SOPMA: significant improvements in protein secondary structure prediction by prediction from multiple alignments. Comput Appl Biosci. 1995;11:681–684. doi: 10.1093/bioinformatics/11.6.681. [DOI] [PubMed] [Google Scholar]
- Gibrat J-F, Garnier J, Robson B. Further developments of protein secondary structure prediction using information theory. New parameters and consideration of residue pairs. J Mol Biol. 1987;198:425–443. doi: 10.1016/0022-2836(87)90292-0. [DOI] [PubMed] [Google Scholar]
- Harper JW, Adami GR, Wei N, Keyomarski K, Elledge SJ. The p21 Cdk-interacting protein Cipl is a potential inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Hengst L. Struktur und Funktion der GTP-bindenden, ras-verwandten Ryh1-Proteine aus Hefen. Dissertation zur Erlangung des Doktorgrades der Naturwissenschaft. Ph.D. Thesis. Marburg/Lahn: Philipps-Universität; 1992. [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the PCR. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Horazdovsky BF, Busch GR, Emr SD. Vps21 encodes a rab5-like GTP-binding protein that is required for the sorting of yeast vacuolar proteins. EMBO J. 1994;13:1297–1309. doi: 10.1002/j.1460-2075.1994.tb06382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Blair L, Brake A, Sprague G, Thorner J. Yeast α factor is processed from a larger precursor polypeptide: the essential role of a membrane-bound dipeptidyl aminopeptidase. Cell. 1983;32:839–852. doi: 10.1016/0092-8674(83)90070-3. [DOI] [PubMed] [Google Scholar]
- Lazar T, Götte M, Gallwitz D. Vesicular transport: how many Ypt/Rab-GTPases make a eukaryotic cell? Trends Biochem Sci. 1997;22:468–472. doi: 10.1016/s0968-0004(97)01150-x. [DOI] [PubMed] [Google Scholar]
- Levin JM, Robson B, Garnier J. An algorithm for secondary structure determination in proteins based on sequence similarity. FEBS Lett. 1986;205:303–308. doi: 10.1016/0014-5793(86)80917-6. [DOI] [PubMed] [Google Scholar]
- Li B, Warner JR. Mutation of the Rab6 homologue of Saccharomyces cerevisiae, YPT6, inhibits both early Golgi function and ribosome biosynthesis. J Biol Chem. 1996;271:16813–16819. doi: 10.1074/jbc.271.28.16813. [DOI] [PubMed] [Google Scholar]
- Linstedt AD, Foguet M, Renz M, Seelig HP, Glick BS, Hauri H-P. A C-terminally-anchored Golgi protein is inserted into the endoplasmic reticulum and then transported to the Golgi apparatus. Proc Natl Acad Sci USA. 1995;92:5102–5105. doi: 10.1073/pnas.92.11.5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstedt AD, Hauri H-P. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350-kDa. Mol Biol Cell. 1993;4:679–693. doi: 10.1091/mbc.4.7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu F-T, Callaghan JM, Steele-Mortimer O, Stenmark H, Parton RG, Campbell PL, McCluskey J, Yeo J-P, Tock EPC, Toh B-H. EEA1, an early endosome-associated protein. J Biol Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Hirata A, Ogawa Y, Yonehara T, Yoda K, Yamasaki M. A cytoskeleton-related gene, USO1, is required for intracellular protein transport in Saccharomyces cerevisiae. J Cell Biol. 1991;113:245–260. doi: 10.1083/jcb.113.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Lowe M, Levine TP, Rabouille C, Warren G. The vesicle docking protein p115 binds gm130, a cis-Golgi matrix protein, in a mitotically regulated manner. Cell. 1997;89:445–455. doi: 10.1016/s0092-8674(00)80225-1. [DOI] [PubMed] [Google Scholar]
- Nothwehr SF, Stevens TH. Sorting of membrane proteins in the yeast secretory pathway. J Biol Chem. 1994;269:10185–10188. [PubMed] [Google Scholar]
- Novick P, Zerial M. The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- Ossig R, Dascher C, Trepte H-H, Schmitt HD, Gallwitz D. The yeast SLY gene-products, suppressors of defects in the essential GTP-binding Ypt1 protein, may act in endoplasmic reticulum-to-Golgi transport. Mol Cell Biol. 1991;11:2980–2993. doi: 10.1128/mcb.11.6.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CK, Howald-Stevenson I, Vater CA, Stevens TH. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol Biol Cell. 1992;3:1389–1402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MD, Winston F, Hieter P. In: Methods in Yeast Genetics: A Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1990. [Google Scholar]
- Rost B, Sander C. Combining evolutionary information and neural networks to predict protein secondary structure. Proteins. 1994;19:55–72. doi: 10.1002/prot.340190108. [DOI] [PubMed] [Google Scholar]
- Sapperstein SK, Lupashin VV, Schmitt HD, Waters MG. Assembly of the ER to Golgi SNARE complex required Uso1p. J Cell Biol. 1996;132:755–767. doi: 10.1083/jcb.132.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapperstein SK, Walter DM, Grosvenor AR, Heuser JE, Waters MG. p115 is a general vesicular transport factor related to the yeast ER-Golgi transport factor Uso1p. Proc Natl Acad Sci USA. 1995;92:522–526. doi: 10.1073/pnas.92.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmöller F, Simon I, Pfeffer SR. Rab GTPases, directors of vesicle docking. J Biol Chem. 1998;273:22161–22164. doi: 10.1074/jbc.273.35.22161. [DOI] [PubMed] [Google Scholar]
- Seelig HP, Schranz P, Schröter H, Wiemann C, Griffiths G, Renz M. Molecular genetic analyses of a 376-kilodalton Golgi complex membrane protein (giantin) Mol Cell Biol. 1994;14:2564–2576. doi: 10.1128/mcb.14.4.2564. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Seog D-H, Kito M, Igarashi K, Yoda K, Yamasaki M. Molecular characterization of the USO1 gene product which is essential for vesicular transport in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1994;200:647–653. doi: 10.1006/bbrc.1994.1497. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links P1(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Singer-Krüger B, Stenmark H, Düsterhöft A, Philippsen P, Yoo S-J, Gallwitz D, Zerial M. Role of three rab-like GTPases, Ypt51p, Ypt52p and Ypt53p, in the endocytic and vacuolar protein sorting pathway in yeast. J Cell Biol. 1994;125:283–298. doi: 10.1083/jcb.125.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönnichsen B, Lowe M, Levine T, Jämsä E, Dirac-Svejstrup B, Warren G. A role for giantin in docking COPI vesicles to Golgi membranes. J Cell Biol. 1998;140:1013–1021. doi: 10.1083/jcb.140.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom M, Vollmer P, Tan TJ, Gallwitz D. A yeast GTPase-activating protein that interacts specifically with a member of the Ypt/Rab family. Nature. 1993;361:736–739. doi: 10.1038/361736a0. [DOI] [PubMed] [Google Scholar]
- Tsukada M, Gallwitz D. Isolation and characterization of SYS genes from yeast, multicopy suppressors of the functional loss of the transport GTPase Ypt6p. J Cell Sci. 1996;109:2471–2481. doi: 10.1242/jcs.109.10.2471. [DOI] [PubMed] [Google Scholar]
- Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G, Malhotra V. The organization of the Golgi apparatus. Curr Opin Cell Biol. 1998;10:493–498. doi: 10.1016/s0955-0674(98)80064-1. [DOI] [PubMed] [Google Scholar]
- Wilsbach K, Payne GS. Dynamic retention of TGN membrane proteins in Saccharomyces cerevisiae. Trends Cell Biol. 1993;3:426–432. doi: 10.1016/0962-8924(93)90031-u. [DOI] [PubMed] [Google Scholar]