Abstract
Patients with rheumatic disorders have an increased risk of cardiovascular disease (CVD). This excess co-morbidity is not fully explained by traditional risk factors. Disease severity is a major risk factor for CVD in patients with rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). Shared disease mechanisms in atherosclerosis and rheumatic disorders include immune dysregulation and inflammatory pathways, which are potential targets for therapy. Lessons from RA and SLE may have implications for future research on the pathogenesis of atherosclerotic vascular disease in general. Recent data indicate that suppression of inflammation reduces the risk of CVD morbidity and mortality in patients with severe RA. The modest, but clinically relevant, efficacy of atorvastatin treatment in RA adds to the evidence for important anti-inflammatory properties for statins. There is increased recognition of the need for structured preventive strategies to reduce the risk of CVD in patients with rheumatic disease. Such strategies should be based on insights into the role of inflammation in CVD, as well as optimal management of life style related risk factors. In this review, the research agenda for understanding and preventing CVD co-morbidity in patients with rheumatic disorders is discussed.
Keywords: rheumatoid arthritis, systemic lupus erythematosus, cardiovascular disease, inflammation
Introduction
Rheumatic diseases lead to chronic disability and reduced quality of life in many patients. Rheumatoid arthritis (RA), which is characterized by inflammatory polyarthritis with progressive joint damage, occurs in about 0.5%–1% of the adult population in most countries (Silman and Hochberg 1993). The spectrum of chronic rheumatic disorders also includes spondyloarthropathies such as ankylosing spondylitis (AS), psoriasis associated arthropathies, and autoimmune syndromes such as systemic lupus erythematosus (SLE), systemic sclerosis and systemic vasculitides. Osteoarthritis (OA) is by far the most common chronic joint disease, and it is increasingly being recognized as a major contributor to the overall health burden in the society (Hootman et al 2006).
In addition to their impact on quality of life, RA and SLE are associated with an increased mortality compared to the general population (Gabriel et al 2003; Doria et al 2006). A major part of the excess mortality has been attributed to cardiovascular disease (CVD) (Jacobsson et al 1993; Manzi et al 1997). Suggested explanations for this pattern of CVD-related morbidity and mortality include a direct impact of chronic inflammation (Sattar and McInnes 2005) and secondary effects of physical inactivity (Minor et al 1996; Turesson et al 2007b) and drugs used in the management of these diseases, including corticosteroids and nonsteroidal anti-inflammatory drugs (NSAIDs) (Turesson et al 2007b). Similarities and differences in patterns of vascular morbidity between patients with RA and SLE may reflect important concepts in the pathophysiology of atherosclerosis and autoimmune features in this process (Chogle and Chakravarty 2007). There is need for research on optimal preventive strategies for CVD in such patients (Chogle and Chakravarty 2007). In this review, we examine the evidence of risk for CVD in patients with rheumatic diseases and the suggested underlying mechanisms, and discuss potential strategies for the prevention of CVD in such patients.
Epidemiology of CVD in patients with rheumatic diseases
SLE is associated with a substantially increased risk of coronary artery disease, in particular in premenopausal women (Manzi et al 1997). There is also an increased risk of stroke in patients with SLE (Esdaile et al 2001), partly explained by co-existing antiphospholipid antibody syndrome in a subset of the patients. Patients with RA also have an increased incidence of CVD (Wållberg-Jonsson et al 1997; Solomon et al 2003).
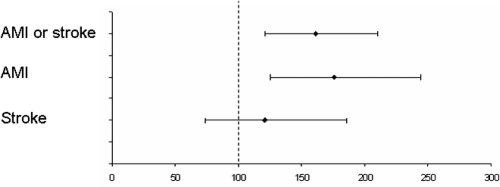
The magnitude of this increase varies in different studies due to differences in case selection and the comparator population. In a community based RA cohort from southern Sweden, the risk of first ever CVD events was estimated to be increased by 60%, mainly because of an excess of myocardial infarctions (MI) (Turesson et al 2004b) (Figure 1). The incidence of MI has been found to be increased to a similar extent in men and women with RA (Turesson et al 2004b). By contrast, the risk of stroke in this (Turesson et al 2004b) or other RA populations (Solomon 2003) was not significantly increased. This suggests that RA associated vascular abnormalities may specifically predispose to coronary artery disease, and not to cerebrovascular events, for which other risk factors, such as hypertension, may be more important.
Figure 1.
Cardiovascular morbidity in a community-based RA sample, compared with the general population. Standardized morbidity ratios; 95% confidence intervals. Based on data from Turesson et al 2004.
Abbreviations: AMI, acute myocardial infarction; RA, rheumatoid arthritis.
There is also some evidence for an increased overall mortality and CVD mortality in patients with other rheumatic diseases such as AS (Lehtinen 1993), although the available data are limited. The same holds true for psoriatic arthritis (Wong et al 1997), but selection bias may be a particular problem in studies of clinical cohorts with this heterogeneous disease (Bond et al 2005). Subclinical atherosclerosis may be more frequent in patients with psoriatic arthritis than in the general population (Gonzales-Juanatey et al 2007). In a population-based study from Finland, finger joint OA was associated with an increased mortality from CVD in men, but not in women (Haara et al 2003). By contrast, there was no significantly increased rate of vascular events or all-cause mortality in patients with OA overall in the UK General Practice Research Database (Watson et al 2003). Interestingly, in a long term population based survey of the Pima Indians in Arizona, USA, the presence of two or more swollen joints was a significant predictor of CVD mortality, independent of traditional risk factors (Jacobsson et al 2001). This effect was seen also in subjects without a diagnosis of RA, when studied separately, suggesting that joint swelling of various causes may be associated with CVD.
Inflammation and cardiovascular disease
Atherosclerosis is an inflammatory disorder, in which factors such as inflammation related dyslipidemia and chronic immune dysregulation contribute to the pathogenesis (Scherer and Shoenfeld 2006). The inflammatory biomarker high-sensitivity C-reactive protein (hs-CRP) predicts cardiovascular events in the general population (Ridker et al 2004), and higher levels of hs-CRP indicate a poor prognosis in acute coronary syndromes (Ridker et al 2004). Carotid artery intima-media thickness (IMT), a measure of early atherosclerosis, correlates with circulating markers of systemic inflammation in patients with RA as well as in subjects without the disease (Del Rincon et al 2003). In women with SLE, increased IMT has been associated with a higher total SLE organ damage score (Manzi et al 1999), indicating that atherosclerosis may be regarded as an effect of the inflammatory burden in patients with SLE and other rheumatic diseases. A recent study of surgical specimens from the aorta, taken from patients undergoing coronary artery bypass grafting, revealed a higher frequency of inflammatory infiltrates in the media and the adventitia of patients with inflammatory rheumatic disorders (including RA, SLE and various vasculitides) compared to other patients (Hollan 2007). Diffuse vascular inflammation in such patients may contribute to the development of atherosclerosis and possibly aneurysm formation. In addition, a number of more specific shared disease mechanisms in systemic rheumatic inflammation and atherosclerotic vascular disease have been suggested (Table 1).
Table 1.
Shared disease mechanisms in rheumatic diseases and CVD
| Mechanism | Pathway in vascular disease | References |
|---|---|---|
| Cytokine-induced macrophage migration and activation | Ox-LDL scavenging and formation of vulnerable atherosclerotic plaque | Ross 1999,Hansson 2005,Dixon and Symmons 2007 |
| Cytokine-induced upregulation of procoagulant factors | Increased systemic thrombodiatesis | McEntegart et al 2001; Sattar et al 2003 |
| Systemic endothelial activation with upregulation of MHC class II molecules | Endothelial dysfunction Increased T-cell migration and activation | Vallbracht et al 2002; Turesson 2004 |
| Clonal expansion and activation of abnormal T-cells | Cytotoxicity leading to plaque damage Local cytokine upregulation | Park et al 1997,Liuzzo et al 1999,Michel et al 2007 |
| Anti-phospholipid antibodies | Increased arterial and venous thrombodiatesis | Toloza et al 2004 |
Abbreviations: CVD, cardiovascular disease; LDL, low density lipoprotein; MHC, major hisocompatibility complex
Atherosclerotic plaque formation and hemostasis
There is extensive evidence for the importance of proinflammatory cytokines such as tumour necrosis factor (TNF) in the development and rupture of atherosclerotic plaques subsequently leading to cardiovascular events. This is of particular interest to this topic, since TNF is also a key factor in the pathogenesis of RA, and a target for specific therapy with biologic agents such as monoclonal anti-TNF antibodies and soluble TNF receptor fusion proteins (Taylor 2003). The formation of the atherosclerotic plaque is enhanced by endothelial activation, migration of monocytes into the vascular wall and scavenging of oxidized LDL (Ross 1999; Hansson 2005), all of which are augmented by high levels of TNF (Dixon and Symmons 2007). Activation of these processes may lead to a vicious cycle promoting plaque growth. Tissue studies have also shown that there is an abundance of inflammatory cells at the border of atherosclerotic plaques, which probably promotes the rupture of the plaque with resultant cardiovascular events (Ross 1999; Hansson 2005; Dixon and Symmons 2007).
Inflammation driven by proinflammatory cytokines seems to be crucial for this process. It has for instance been shown that knockout of the TNF gene in atherosclerotic prone apoE knock-out mice inhibits growth of atherosclerotic plaques (Brånen et al 2004). Moreover, treatment with a soluble receptor for TNF resulted in a dose dependent inhibitory effect on the development of atherosclerosis in the same strain (Brånen et al 2004).
Furthermore, TNF and other cytokines activate the endothelium, which is reflected by endothelial dysfunction and increased expression of adhesion molecules. In parallel, inflammation contributes to a systemic up-regulation of proteins involved in hemostasis. Patients with RA have increased serum levels of fibrinogen, von Willebrand factor and tissue plasminogen activator antigen compared with controls and these prothrombotic markers have been shown to predict cardiovascular events in patients with RA (McEntegart et al 2001; Sattar et al 2003).
Immunology
Immunological pathways associated with chronic inflammation are important in autoimmune diseases as well as in the development of CVD. The major histocompatibility complex (MHC) class II subtypes HLA-DR, -DQ and -DP are regulators of T-cell dependent immune responses, and aberrant expression of these tissue antigens in the endothelium has been demonstrated in autoimmune diseases such as RA and SLE (Turesson 2004a). Recent data suggest an association between such endothelial expression and diffuse endothelial dysfunction (Vallbracht et al 2002). Increased endothelial MHC class II expression may also be important for the activation and migration of T-cells and monocytes into the vascular wall.
In a chronic inflammatory environment, T-cells may feature particular phenotypes, such as lack of surface expression of the co-stimulatory molecule CD28 (Schmidt et al 1996). Increased levels of CD4+CD28null T-cells have been demonstrated in patients with chronic inflammatory disorders such as RA (Schmidt et al 1996), especially those with severe, systemic disease (Michel et al 2007), and unstable coronary artery disease (Liuzzo et al 1999). These T-cells are characterized by high production of IFN-γ (Park et al 1997), cytotoxic capabilities, and expression of natural killer cell markers including CD56 (Michel et al 2007) and killer immunoglobulin-like receptors. CD4+CD28null T-cells can also be isolated from culprit lesions in unstable coronary artery plaques (Liuzzo et al 2000) but not from stable plaques and have in addition been shown to correlate with intima-media thickness and impaired arterial flow mediated vasodilatation in RA (Gerli et al 2004).
Taken together, these data suggest a role for such cells in explaining the association between autoimmune vascular disease and CVD. The concept of modulation of such pathomechanisms by potent anti-inflammatory treatment, such as TNF inhibitors, is supported by the observed up-regulation of CD28 expression on T-cells in patients with RA treated with the monoclonal anti-TNF antibody infliximab by Bryl and colleagues (2005). In addition, in a recent study of CD4+CD28null T-cells isolated from patients with unstable angina, such cells were depleted in vitro when the infliximab was added to whole blood samples, suggesting that the persistence of this T-cell phenotype is TNF-dependent (Rizello et al 2006).
Risk factors for cardiovascular events in patients with rheumatic diseases
Traditional risk factors for vascular disease, such as smoking, hypertension, diabetes and hyperlipidemia, are important in, but do not fully account for, the increased risk of CVD in subjects with SLE (Esdaile et al 2001) or RA (Solomon et al 2004a). Although there is an association between smoking and RA (Silman et al 1996) and SLE (Bengtsson et al 2002), the excess CVD morbidity and mortality in these disorders has been shown to be independent of smoking (Manzi et al 1997; Maradit-Kremers et al 2005).
Disease severity has consistently been associated with an increased risk of CVD events in RA (Table 2). Disability, as measured by the Health Assessment Questionnaire (HAQ) (Fries et al 1982), has been shown to be a predictor of overall mortality (Wolfe et al 2003; Young et al 2007), CVD mortality (Young et al 2007), increased atherosclerosis (Kumeda et al 2002) and CVD events (Jacobsson et al 2005). Patients with severe extra-articular RA manifestations are at an increased risk of developing coronary artery disease (Turesson et al 2007a) as well as peripheral vascular disease (Liang et al 2006); and severe extra-articular RA is a predictor of both overall mortality (Gabriel et al 2003) and cardiovascular mortality (Maradit-Kremers et al 2005), indicating that systemic inflammation is a major determinant of vascular co-morbidity in RA. An elevated erythrocyte sedimentation rate (ESR) has been shown to predict CVD mortality in several RA populations (Wållberg-Jonsson et al 1999; Maradit-Kremers et al 2005), and baseline CRP was an independent predictor of cardiovascular death in an inception cohort of recently diagnosed inflammatory polyarthritis (Goodson et al 2005). In contrast with the general population, a low body mass index (BMI), rather than obesity, has been associated with increased CVD in patients with RA (Kremers et al 2004; Escalante et al 2005). This most likely reflects cachexia associated with severe inflammation. By contrast, in patients with active SLE, BMI, and total body fat tend to increase over time, although this may partly be due to treatment with high doses of corticosteroids (Kipen et al 1999).
Table 2.
Risk factors for cardiovascular disease in patients with rheumatoid arthritis
| Risk factor | Predicted outcome | References |
|---|---|---|
| Severe extraarticular manifestations | Total mortality and CVD mortality First-ever CVD events New onset PAD or venous thromboembolic events | Gabriel et al 2003,Maradit-Kremers et al 2005; Turesson et al 2007; Liang et al 2006 |
| Disability (HAQ score) | Total mortality and CVD mortality First-ever CVD events | Wolfe et al 2003,Young et al 2007,Jacobsson et al 2005 |
| ESR (≥60 mm/h at least 3 times) | Death from CVD | Maradit-Kremers et al 2005; |
| CVD events | Wållberg-Jonsson et al 1999; | |
| Last ESR | Death from CVD | Goodson et al 2005; |
| CRP | Death from CVD | Maradit-Kremers et al 2005; |
| Smoking | New onset PAD | Liang et al 2006 |
| Hypertension | Death from CVD | Wållberg-Jonsson et al 1999; Maradit-Kremers et al 2005 |
| Low BMI | Death from CVD | Kremers et al 2004,Escalante et al 2005 |
Abbreviations: CVD, cardiovascular disease; HAQ, health assessment questionnaire; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; BMI, body mass index; PAD, peripheral arterial disease
In SLE, smoking, elevated CRP, and antiphospholipid antibodies predicted CVD events in a multiethnic US cohort of patients (Toloza et al 2004). However, in a Swedish study of women with SLE, dyslipidemia was more frequently found in patients with a history of CVD compared with controls without CVD, whereas there was no difference for smoking, hypertension, diabetes or obesity (Svenungsson et al 2001). Such discrepancies may be due to differences between ethnic groups, or methodological issues, including selection bias.
There are conflicting evidence on the prevalence of hypertension (McEntegart et al 2001; Solomon et al 2004b) and hyperlipidemia (Park et al 1999; McEntegart et al 2001; Solomon et al 2004b) among patients with RA. This issue is further complicated by the impact of corticosteroid treatment on blood pressure and metabolism. Several studies of prevalent RA cases have shown an increased prevalence of former smokers, but not current smokers, compared with non-RA controls (McEntegart et al 2001; Solomon et al 2004b). Studies of prevalent RA and SLE cohorts may, however, be prone to bias from left censorship, because of premature death in patients with a high risk of CVD, or selection bias, because of accumulation of patients with treated vascular disease. In our opinion, long term follow-up of inception cohorts or community based samples with prospectively collected data give the most reliable information on co-morbidity and related risk factors. Future studies of CVD risk in patients with rheumatic diseases should include data on apolipoprotein levels as well as abdominal obesity, since the INTERHEART study and other surveys have suggested that the apoB/apoA1 ratio and the waist/hip ratio are actually better predictors of myocardial infarction than LDL cholesterol and BMI (Yusuf et al 2004).
In a recent survey of men with AS from Glasgow, Scotland, there was an increased number of smokers and a higher BMI compared with age-matched healthy controls (Divecha et al 2005). There were also significantly higher CRP, IL-6, fibrinogen, and von Willebrand factor levels in the AS group. This suggests an unfavorable cardiovascular risk profile in AS, including systemic inflammation that may predispose to CVD. A similar pattern probably exists in patients with psoriatic arthritis, where, in addition, dyslipidemia has been reported by several investigators (Peters et al 2004). In a study of patients with moderately severe to end-stage lower extremity OA, cases were found to have significantly greater mean BMI, waist-hip ratio, systolic blood pressure and fasting blood glucose, and a lower mean high density lipoprotein cholesterol than age and sex matched controls without OA (Philbin et al 1996). As expected, the patients with knee or hip OA reported less performed aerobic exercise than controls.
Preventive strategies
Traditional risk factors
Several different strategies for the prevention of CVD should be considered in patients with rheumatic disorders (Table 3). There is a strong rational for targeting traditional CVD risk factors in high risk groups, since the likelihood of preventing CVD on an individual level with such interventions is greater. Overall, there are three complementary approaches to reducing the risk of CVD co-morbidity in patients with rheumatic disorders by targeting traditional risk factors: 1) Screen patients for CVD risk factors regularly, and use treatment targets recommended for the general population; 2): Use modified targets closer to those recommended for other high risk groups (eg, patients with diabetes); 3): Use a modified risk score algorithm, taking into account the increased risk of CVD in patients with severe rheumatic disorders, and base treatment decisions on the individual risk score.
Table 3.
Strategies for prevention of CVD in patients with rheumatic diseases
| Preventive strategy | Suggested interventions | References |
| Achieve target levels for traditional risk factors including blood pressure, glucose, and lipids | Regular screening, counselling and pharmacotherapy according to guidelines | Wajed et al 2004,van Doornum et al 2006 |
| Reduce traditional risk factors in high risk individuals | Interventions based on decision algorithms, guided by risk scores | Wilson et al 1998 Yusuf et al 2003 |
| Increase physical exercise | Patient education, physiotherapy and structured rehabilitation | Wikström et al 2005; Turesson et al 2007b |
| Preventive statin treatment | Statins for patients with severe RA, despite moderate cholesterol levels | McCarey et al 2004; van Doornum et al 2004 |
| Reduce inflammatory burden in patients with RA | Use methotrexate as drug of choice in RA Add TNF inhibitor in refractory patients | Choi et al 2002,Jacobsson et al 2005 |
Abbreviations: CVD, cardiovascular disease; RA, rheumatoid arthritis;TNF, tumor necrosis factor
The first option may lead to a substantial improvement in the management of many patients. Traditionally, rheumatologists and other physicians treating patients with rheumatic diseases have focused on their musculoskeletal problems and paid limited attention to CVD risk factors. The cardiovascular health of these patients can be substantially improved if the managing physician takes and acts upon a smoking history, determines blood pressure and lipid levels regularly and screens for diabetes. This advice assumes that resources for such interventions are available and that guidelines for risk factor screening are implemented. It can also be argued that since the excess risk of CVD in RA and SLE could not be explained by increased frequency of traditional risk factors (Esdaile et al 2001; Solomon et al 2003), target values should be no different than those set for the general population.
The high risk of premature death from CVD in patients with SLE or severe RA is analogous to diabetes mellitus. Based on this, Wajed and colleagues (2004) suggested target levels for patients with SLE including a systolic blood pressure of <130/80 mm Hg and LDL cholesterol of <2.6 mmol/L (whereas the current recommendations for individuals without excess CVD risk are <140/90 mm Hg and 3.0 mmol/L, respectively). Similar guidelines have been proposed for patients with RA (van Doornum et al 2006). The value of adhering to these targets for managing traditional risk factors in patients with rheumatic diseases is only incompletely known, and forms an agenda for further investigation.
Risk assessment for CVD can be performed by applying decision algorithms such as the Framingham risk score (Wilson et al 1998) or the SCORE risk estimation system (Conroy et al 2003), which is recommended by the European Society for Cardiology. With theses algorithms, the increased risk of CVD in patients with, for example, SLE and co-existing antiphospholipid syndrome, or severe extra-articular RA, can be accounted for by classifying them as having a risk corresponding to existing CVD related organ damage, with a correspondingly lower threshold for intervention. This strategy directs interventions to patients with higher absolute risk of CVD. Ideally, such a modified strategy should be tested in controlled clinical trials, and its use in practice subjected to a structured evaluation.
Physical activity
Overall, patients with RA and other chronic rheumatic diseases have a reduced level of physical activity compared with the general population (Hootman et al 2003). A corresponding degree of inactivity is associated with a reduced life expectancy in the general population (Franco et al 2005). A number of studies confirm that regular, vigorous physical exercise is associated with a reduced mortality, in particular from CVD (Turesson et al 2007b). In the US Nurses’ Health Study, obesity (BMI ≥ 30 kg/m2) and a sedentary life style (exercise <1 hour/week) were independent predictors of coronary heart disease (CHD) (Li et al 2006). Of particular interest, even in women with a normal weight (BMI 18.5–24.9 kg/m2), sedentary living was associated with an increased risk of CHD (relative risk 1.48; 95% CI 1.24–1.77, adjusted for a number of covariates, including smoking) (Li et al 2006). Physical fitness thus likely has effects beyond those of weight reduction. Suggested mechanisms include reduced plasma fibrinogen levels (Lakka and Salonen 1993) and other positive rheologic effects of exercise (Eichner 1990) that may potentially even reverse the negative impact of chronic low-grade inflammation.
Although the benefits of regular physical exercise are well recognized, implementing this knowledge remains a challenge in the general population (Warburton et al 2006) and in patients with rheumatic diseases. Whereas structured day-care rehabilitation programs have been reported to increase the number of self reported performed physical activities (Wikström and Jacobsson 2005), another intervention study directed at adverse life style factors in patients with established RA led only to modest improvement (Gordon et al 2002). The current overall trend in most populations is rather towards decreasing physical activity and weight gain. The growing epidemic of obesity in the US and other countries has been projected to lead to a rapidly increasing incidence of OA (Hootman and Helmick 2006), creating a vicious circle of inactivity, obesity and arthritis, likely contributing to CVD morbidity. Efforts to change this pattern and reduce life style related risk factors for CVD are of major importance. In patients with rheumatic disorders, patient education, physiotherapy and structured day-care or in-patient rehabilitation are tools to increase physical exercise. As all other interventions, they should be thoroughly evaluated.
Statin treatment
Statins (3-hydroxy-3-methylglutarylcoenzyme-A reductase inhibitors) reduce CVD morbidity and mortality (Ridker et al 2005). Although they were originally used in this context because of their effects on lipid levels, it has become increasingly evident that they have other actions which may diminish CVD risk. The anti-inflammatory and immunomodulating effects of statins include suppression of leukocyte cytokine release, reduced MHC class II expression and reduced production of reactive oxygen species (Palinski and Napoli 2002). A low achieved hs-CRP level (<2 mg/L) has been shown to be a good marker of reduced risk of recurrent MI or coronary death in patients hospitalized because of acute coronary syndromes and treated with atorvastatin or pravastatin (Ridker et al 2005). This suggests that the anti-inflammatory properties of statins are clinically important.
Statin-related reduction in inflammatory biomarkers provided the rationale for a randomized controlled trial of atorvastatin for treatment of synovitis in RA (the Trial of Atorvastatin in Rheumatoid Arthritis [TARA]) (McCarey et al 2004). There was a significant reduction in CRP and ESR, as well as in the swollen joint count and the disease activity score, in patients treated with 40 mg atorvastatin daily for 6 months compared with placebo. A good or moderate clinical response (according to the European League Against Rheumatism criteria) was seen in 31% of patients treated with atorvastatin compared to 10% in the placebo group (p = 0.006). As expected, the anti-inflammatory effect was paralleled by a substantial reduction in total cholesterol and LDL cholesterol. Apart from reducing lipid levels, treatment with statins in patients with RA may also have a modest but clinically useful effect on arthritis. Atorvastatin has also been shown to reduce arterial stiffness in patients with RA and high disease activity (van Doornum et al 2004). Taken together, these findings may suggest a lower threshold for using statins in patients with RA.
The low cholesterol levels found in patients with active RA due to inflammation (Rantapää-Dahlqvist et al 1991) indicate that target levels from general guidelines may not be adequate for RA populations. In a recent placebo-controlled study of the effect of atorvastatin on markers of atherosclerosis in patients with SLE (the Lupus Atherosclerosis Prevention Study [LAPS]), there was no significant effect on the primary endpoints: the change in the coronary calcium score or the change in the carotid artery IMT (Petri et al 2006). It should be noted that this study excluded patients with hyperlipidemia for whom statin treatment would normally be indicated. Since surrogate markers are poor substitutes for hard CVD endpoints, additional studies in patients with SLE as well as RA are needed. Furthermore, although atorvastatin was well tolerated in the TARA study as well as in the LAPS, long term safety data in patients with rheumatic disorders are lacking. Based on the currently available data, we suggest that statins should be considered in patients with severe RA and an unfavorable CVD risk profile, and that individual lipid or apolipoprotein level targets may need to be modified.
Anti-rheumatic therapy
Corticosteroids are often used in the treatment of SLE, RA, and other inflammatory disorders. High dose treatment with corticosteroids has adverse effects on the cardiovascular system, including endothelial dysfunction, hypertension and dysregulated glucose metabolism (Boots et al 2004). To our knowledge, there is no evidence for similar clinical effects in patients treated with moderate or low doses (≤7.5 mg/day). On the other hand, a protective effect from CVD could be postulated based on control of inflammation. Indeed, in patients with SLE, it has been suggested that corticosteroid treatment may be associated with a reduced risk of atherosclerosis (Roman et al 2003). In RA, the net effect of low dose glucocorticoids is still not defined (Davis et al 2005). However, long term treatment with higher doses is likely to increase the risk of CVD, in addition to other side effects. In a recent report from a long term RA study, there was an interaction between the cumulative corticosteroid dose and RF status, with the highest risk for heart failure and death from CVD in RF positive patients receiving high doses over extended time periods (Davis et al 2007). The mechanisms underlying this relationship are unknown. Since the study included patients recruited between 1955 and 1995, it is not clear whether the results are applicable to patients receiving modern DMARD therapy from disease onset.
Recently, the risk of adverse vascular events associated with treatment with cyclooxygenase (COX) inhibitors, in particular selective COX-2 inhibitors, has been recognized. Based on a significant increase of CVD events in a randomized trial, rofecoxib has been withdrawn from the market, while other coxibs and traditional non-steroidal anti-inflammatory drugs (NSAIDs) may have less impact on the risk of CVD (Solomon et al 2006a).
In a survey of elderly individuals in the Pennsylvania Medicare database, where use of rofecoxib, but not of celecoxib, was associated with increased risk of CVD, a diagnosis of RA remained a significant predictor of CVD mortality in an analysis adjusted for traditional risk factors and NSAID treatment (Solomon et al 2004a). In an extension of this study, except for the confirmed association with rofecoxib, no significantly increased risk of MI in patients with RA using COX-inhibitors compared to non-users was detected (Solomon et al 2006b). It is unlikely therefore that the use of COX-inhibitors is the main reason for the observed increased risk of CVD in patients with RA.
A growing body of evidence indicates that traditional NSAIDs as well as coxibs have some impact on development of CVD in patients with RA and OA (Kearney et al 2006). In a large, recent trial, there was no significant difference in the occurrence of hard CVD endpoints between diclofenac (150 mg/day) and etoricoxib (60 or 90 mg/day), whereas discontinuation due to hypertension was more frequent among those treated with etoricoxib (Cannon et al 2006). Presently, a careful evaluation of the cardiovascular risk in the individual patients should precede the use of COX-inhibitors, and further studies attempting to estimate the relative impact of different agents should be performed. In addition, further observational studies of CVD in patients with rheumatic disorders should take the impact of COX-inhibitors into account.
Finally, consistent with the concept that disease activity as well as severity determines the risk of CVD in RA, treatment with methotrexate has been associated with a reduced cardiovascular mortality in a large observational study (Choi et al 2002). Recently, treatment with TNF inhibitors was associated with a lower risk of first-ever CVD events in a study of community based RA registers in Sweden (Jacobsson et al 2005). Additional follow-up of this cohort has revealed a reduced overall mortality in RA patients treated with TNF inhibitors, in particular among those with extensive disability indicated by high HAQ scores (Jacobsson et al 2007). A recent report from a survey of Spanish registers also reported a survival benefit from treatment with TNF inhibitors (Carmona et al 2007).
These findings contrast with the disappointing results from randomized controlled trials of treatment of established congestive heart failure (CHF) with the TNF inhibitors infliximab and etanercept. Especially noteworthy was an increased mortality rate associated with the administration of high dose of infliximab in patients with severe CHF (New York Heart Association class III and IV) (Anker et al 2002). Since the available data suggest that TNF inhibition in RA does not lead to an increased risk for developing CHF in RA patients (Wolfe et al 2004), it is likely that this negative effect is confined to patients already suffering from severe established CHF. The latter is therefore regarded as a contraindication for treatment with TNF inhibitors. Finally, although treatment with TNF blockers have a substantial effect on signs and symptoms in patients with severe AS and psoriatic arthritis, the long term effect on CVD co-morbidities in such patients has not been evaluated.
The reduced CVD morbidity and mortality associated with potent anti-inflammatory treatment in patients with RA could be partly due to a direct effect on the inflammatory burden, and partly due to reduced disability and increased physical activity. From a cardiovascular disease standpoint, management of rheumatic disorders should aim at disease modification and, ideally, remission. Such treatment would also reduce the need for treatment with NSAIDs and corticosteroids and their attendant CVD risks.
Conclusions
Patients with rheumatic disorders have an increased risk of CVD morbidity and premature mortality. This is to a major extent due to the chronic inflammatory burden and to reduced physical activity. Suppression of inflammation by potent anti-rheumatic drug treatment, such as TNF inhibitors for patients with RA, can reduce the risk of CVD events. Additional preventive strategies include regular screening for and intervention against traditional risk factors, preventive treatment with statins and efforts to increase regular physical exercise. Scientific evaluation of such interventions is of major importance. The shared disease mechanisms in chronic rheumatic disorders and atherosclerotic vascular disease are a field for further fruitful investigation.
References
- Anker SD, Coats AJ. How to RECOVER from RENAISSANCE? The significance of the results of RECOVER, RENAISSANCE, RENEWAL and ATTACH. Int J Cardiol. 2002;86:123–30. doi: 10.1016/s0167-5273(02)00470-9. [DOI] [PubMed] [Google Scholar]
- Bengtsson AA, Rylander L, Hagmar L, et al. Risk factors for developing systemic lupus erythematosus: a case-control study in southern Sweden. Rheumatology (Oxford) 2002;41:563–71. doi: 10.1093/rheumatology/41.5.563. [DOI] [PubMed] [Google Scholar]
- Bond S, Farewell VT, Schentag CT, et al. Reporting of mortality in a psoriatic arthritis clinic is primarily a function of the number of clinic contacts and not disease severity. J Rheumatol. 2005;32:2364–7. [PubMed] [Google Scholar]
- Boots JM, Christiaans MH, van Hoof JP. Effect of immunosuppressive agents on long-term survival of renal transplant recipients: focus on the cardiovascular risk. Drugs. 2004;64:2047–73. doi: 10.2165/00003495-200464180-00004. [DOI] [PubMed] [Google Scholar]
- Bryl E, Vallejo AN, Matteson EL, et al. Modulation of CD28 expression with anti-tumor necrosis factor alpha therapy in rheumatoid arthritis. Arthritis Rheum. 2005;52:2996–3003. doi: 10.1002/art.21353. [DOI] [PubMed] [Google Scholar]
- Brånen L, Hovgaard L, Nitulescu M, et al. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:2137–42. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- Carmona L, Descalzo MA, Perez-Pampin E, et al. All-cause and cause-specific mortality in rheumatoid arthritis are not greater than expected when treated with tumour necrosis factor antagonists. Ann Rheum Dis. 2007;66:880–85. doi: 10.1136/ard.2006.067660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon CP, Curtis SP, FitzGerald GA, et al. Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison. Lancet. 2006;368:1771–81. doi: 10.1016/S0140-6736(06)69666-9. [DOI] [PubMed] [Google Scholar]
- Chogle AR, Chakravarty A. Cardiovascular events in systemic lupus erythematosus and rheumatoid arthritis: Emerging concepts, early diagnosis and management. J Assoc Physicians India. 2007;55:32–40. [PubMed] [Google Scholar]
- Choi HK, Hernan MA, Seeger JD, et al. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359:1173–7. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- Conroy RM, Pyrölä K, Fitzgerald AP, et al. Estimation of 10-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- Davis JM, III, Maradit-Kremers H, Gabriel S. Use of low-dose glucocorticoids and the risk of cardiovascular morbidity and mortality in rheumatoid arthritis: what is the true direction of effect? J Rheumatol. 2005;32:1856–62. [PubMed] [Google Scholar]
- Davis JM, III, Maradit-Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis. Arthritis Rheum. 2007;56:820–30. doi: 10.1002/art.22418. [DOI] [PubMed] [Google Scholar]
- Del Rincon I, Williams K, Stern MP, et al. Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum. 2003;48:1833–40. doi: 10.1002/art.11078. [DOI] [PubMed] [Google Scholar]
- Divecha H, Sattar N, Rumley A, et al. Cardiovascular risk parameters in men with ankylosing spondylitis in comparison with non-inflammatory control subjects: relevance of systemic inflammation. Clin Sci (Lond) 2005;109:171–6. doi: 10.1042/CS20040326. [DOI] [PubMed] [Google Scholar]
- Dixon WG, Symmons DPM. What effects might anti-TNF[alpha] therapy be expected to have on cardiovascular morbidity and mortality in rheumatoid arthritis? A review of the role of TNF[alpha] in cardiovascular pathophysiology. Ann Rheum Dis. 2007;66:1132–36. doi: 10.1136/ard.2006.063867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria A, Iaccardino L, Ghirardello A, et al. Long-term prognosis and causes of death in systemic lupus erythematosus. Am J Med. 2006;119:700–6. doi: 10.1016/j.amjmed.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Eichner ER. Exercise and arthritis. The hematology of inactivity. Rheum Dis Clin North Am. 1990;16:815–25. [PubMed] [Google Scholar]
- Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624–9. doi: 10.1001/archinte.165.14.1624. [DOI] [PubMed] [Google Scholar]
- Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–7. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Franco OH, de Laet C, Peeters A, et al. Effects of physical activity on life expectancy with cardiovascular disease. Arch Intern Med. 2005;165:2355–60. doi: 10.1001/archinte.165.20.2355. [DOI] [PubMed] [Google Scholar]
- Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol. 1982;9:789–93. [PubMed] [Google Scholar]
- Gabriel SE, Crowson CS, Kremers HM, et al. Survival in rheumatoid arthritis: a population- based analysis of trends over 40 years. Arthritis Rheum. 2003;48:54–8. doi: 10.1002/art.10705. [DOI] [PubMed] [Google Scholar]
- Gerli R, Schillaci G, Giordano A, et al. CD4+CD28- T lymphocytes contribute to early atherosclerotic damage in rheumatoid arthritis patients. Circulation. 2004;109:2744–8. doi: 10.1161/01.CIR.0000131450.66017.B3. [DOI] [PubMed] [Google Scholar]
- Goodson NJ, Symmons DPM, Scott DG, et al. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: a ten-year followup study of a primary care-based inception cohort. Arthritis Rheum. 2005;52:2293–9. doi: 10.1002/art.21204. [DOI] [PubMed] [Google Scholar]
- Gonzales-Juanatey C, Llorca J, Amigo-Diaz E, et al. High prevalence of subclinical atherosclerosis in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Arthritis Rheum. 2007;57:1074–80. doi: 10.1002/art.22884. [DOI] [PubMed] [Google Scholar]
- Gordon M-M, Thomson EA, Madhok R, et al. Can intervention modify adverse lifestyle variables in a rheumatoid population? Results of a pilot study. Ann Rheum Dis. 2002;61:66–9. doi: 10.1136/ard.61.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haara MM, Manninen P, Kroger H, et al. Osteoarthritis of finger joints in Finns aged 30 or over: prevalence, determinants, and association with mortality. Ann Rheum Dis. 2003;62:151–8. doi: 10.1136/ard.62.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hollan I, Scott H, Satvedt K, et al. Inflammatory rheumatic disease and smoking are predictors of aortic inflammation: a controlled study of biopsy specimens obtained at coronary artery surgery. Arthritis Rheum. 2007;56:2072–9. doi: 10.1002/art.22690. [DOI] [PubMed] [Google Scholar]
- Hootman JM, Macera CA, Sam HA, et al. Physical activity levels among the general US adult population and in adults with and without arthritis. Arthritis Rheum. 2003;49:129–35. doi: 10.1002/art.10911. [DOI] [PubMed] [Google Scholar]
- Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226–9. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- Jacobsson LT, Knowler WC, Pillemer S, et al. Rheumatoid arthritis and mortality. A longitudinal study in Pima Indians. Arthritis Rheum. 1993;36:1045–53. doi: 10.1002/art.1780360804. [DOI] [PubMed] [Google Scholar]
- Jacobsson LT, Turesson C, Knowler WC, et al. Number of swollen joints predicts cardiovascular death; results from a population study of Pima Indians. Arthritis Rheum. 2001;44:1170–76. doi: 10.1002/1529-0131(200105)44:5<1170::AID-ANR200>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Jacobsson LT, Turesson C, Gülfe A, et al. Low incidence of first cardiovascular event in rheumatoid arthritis patients treated with TNF-blockers. J Rheumatol. 2005;32:1213–18. [PubMed] [Google Scholar]
- Jacobsson LTH, Turesson C, Nilsson J-Å, et al. Treatment with TNF-blockers and mortality risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:670–5. doi: 10.1136/ard.2006.062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332:1302–8. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipen Y, Briganti EM, Strauss BJ, et al. Three-year follow-up of body composition in pre-menopausal women with systemic lupus erythematosus. Rheumatology (Oxford) 1999;38:59–65. doi: 10.1093/rheumatology/38.1.59. [DOI] [PubMed] [Google Scholar]
- Kremers HM, Nicola PJ, Crowson CS, et al. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450–7. doi: 10.1002/art.20612. [DOI] [PubMed] [Google Scholar]
- Kumeda Y, Inaba M, Goto H, et al. Increased thickness of the arterial intima-media detected by ultrasonography in patients with rheumatoid arthritis. Arthritis Rheum. 2002;46:1489–97. doi: 10.1002/art.10269. [DOI] [PubMed] [Google Scholar]
- Lakka TA, Salonen JT. Moderate to high intensity conditioning leisure time physical activity and high cardiorespiratory fitness are associated with reduced plasma fibrinogen in eastern Finnish men. J Clin Epidemiol. 1993;46:1119–27. doi: 10.1016/0895-4356(93)90111-d. [DOI] [PubMed] [Google Scholar]
- Lehtinen K. Mortality and causes of death in 398 patients admitted to hospital with ankylosing spondylitis. Ann Rheum Dis. 1993;52:174–6. doi: 10.1136/ard.52.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TY, Rana JS, Manson JE, et al. Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation. 2006;113:499–506. doi: 10.1161/CIRCULATIONAHA.105.574087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KP, Liang KV, Matteson EL, et al. Incidence of noncardiac vascular disease in rheumatoid arthritis and relationship to extra-articular disease manifestations. Arthritis Rheum. 2006;54:642–8. doi: 10.1002/art.21628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzo G, Kopecky SL, Frye RL, et al. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation. 1999;100:2135–9. doi: 10.1161/01.cir.100.21.2135. [DOI] [PubMed] [Google Scholar]
- Liuzzo G, Goronzy JJ, Yang H, et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000;101:2883–8. doi: 10.1161/01.cir.101.25.2883. [DOI] [PubMed] [Google Scholar]
- Manzi S, Meliahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–15. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- Manzi S, Selzer F, Sutton-Tyrrell K, et al. Prevalence and risk factors of carotid plaque in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42:51–60. doi: 10.1002/1529-0131(199901)42:1<51::AID-ANR7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Maradit-Kremers HM, Nicola PJ, Crowson CS, et al. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52:722–32. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- McCarey DW, McInnes IB, Madhok R, et al. Trial of atorvastatin in rheumatoid arthritis (TARA): double blind, placebo-controlled trial. Lancet. 2004;363:2015–21. doi: 10.1016/S0140-6736(04)16449-0. [DOI] [PubMed] [Google Scholar]
- McEntegart A, Capell HA, Creran D, et al. Cardiovascular risk factors, including thrombotic variables, in a population with rheumatoid arthritis. Rheumatology (Oxford) 2001;40:640–4. doi: 10.1093/rheumatology/40.6.640. [DOI] [PubMed] [Google Scholar]
- Michel JJ, Turesson C, Lemster B, et al. CD56-expressing T cells that have senescent features are expanded in rheumatoid arthritis. Arthritis Rheum. 2007;56:43–57. doi: 10.1002/art.22310. [DOI] [PubMed] [Google Scholar]
- Minor MA, Lane NE. Recreational exercise in arthritis. Rheum Dis Clin North Am. 1996;22:563–77. doi: 10.1016/s0889-857x(05)70288-x. [DOI] [PubMed] [Google Scholar]
- Palinski W, Napoli C. Unraveling pleiotropic effects of statins on plaque rupture. Arterioscler Thromb Vasc Biol. 2002;22:1745–50. doi: 10.1161/01.atv.0000038754.39483.cd. [DOI] [PubMed] [Google Scholar]
- Park W, Weyand CM, Schmidt D, et al. Co-stimulatory pathways controlling activation and peripheral tolerance of human CD4+CD28- T cells. Eur J Immunol. 1997;27:1082–90. doi: 10.1002/eji.1830270507. [DOI] [PubMed] [Google Scholar]
- Park YB, Lee SK, Lee WK, et al. Lipid profiles in untreated patients with rheumatoid arthritis. J Rheumatol. 1999;26:1701–4. [PubMed] [Google Scholar]
- Peters MJ, van der Horst-Bruinsma IE, Dijkmans BA, et al. Cardiovascular risk profile of patients with spondylarthropathies, particularly ankylosing spondylitis and psoriatic arthritis. Semin Arthritis Rheum. 2004;34:585–92. doi: 10.1016/j.semarthrit.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Petri M, Kiani AN, Post W, et al. Lupus atherosclerosis prevention study (LAPS): Randomized double blind placebo controlled trial of atorvastatin versus placebo. Arthritis Rheum. 2006;54:S520. [Google Scholar]
- Philbin EF, Ries MD, Groff GD, et al. Osteoarthritis as a determinant of an adverse coronary heart disease risk profile. J Cardiovasc Risk. 1996;3:529–33. [PubMed] [Google Scholar]
- Rantapää-Dahlqvist S, Wållberg-Jonsson S, Dahlén G. Lipoprotein (a), lipids, and lipoproteins in patients with rheumatoid arthritis. Ann Rheum Dis. 1991;50:366–8. doi: 10.1136/ard.50.6.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Wilson PW, Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109:2818–25. doi: 10.1161/01.CIR.0000132467.45278.59. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Eng J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- Rizello V, Liuzzo G, Brugatella S, et al. Modulation of CD4(+)CD28null T lymphocytes by tumor necrosis factor-alpha blockade in patients with unstable angina. Circulation. 2006;113:2272–7. doi: 10.1161/CIRCULATIONAHA.105.588533. [DOI] [PubMed] [Google Scholar]
- Roman MJ, Schanker BA, Davis A, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2399–406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Sattar N, McInnes IB. Vascular comorbidity in rheumatoid arthritis: potential mechanisms and solutions. Curr Opin Rheumatol. 2005;17:286–92. doi: 10.1097/01.bor.0000158150.57154.f9. [DOI] [PubMed] [Google Scholar]
- Sattar N, McCarey DW, Capell H, et al. Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation. 2003;108:2957–63. doi: 10.1161/01.CIR.0000099844.31524.05. [DOI] [PubMed] [Google Scholar]
- Scherer Y, Shoenfeld Y. Mechanisms of disease: atherosclerosis in autoimmune diseases. Nat Clin Pract Rheumatol, 2006;2:99–106. doi: 10.1038/ncprheum0092. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Martens PB, Weyand CM, et al. The repertoire of CD4+ CD28− T cells in rheumatoid arthritis. Mol Med. 1996;2:608–18. [PMC free article] [PubMed] [Google Scholar]
- Silman A, Newman J, MacGregor AJ. Cigarette smoking increases the risk of rheumatoid arthritis. Results from a nationwide study of disease-discordant twins. Arthritis Rheum. 1996;39:732–5. doi: 10.1002/art.1780390504. [DOI] [PubMed] [Google Scholar]
- Silman AJ, Hochberg MC. Epidemiology of the rheumatic diseases. Oxford: Oxford University Press; 1993. [Google Scholar]
- Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–7. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- Solomon DH, Schneeweiss S, Glynn RJ, et al. Relationship between selective cyclooxygenase-2 inhibitors and acute myocardial infarction in older adults. Circulation. 2004;109:2068–73. doi: 10.1161/01.CIR.0000127578.21885.3E. [DOI] [PubMed] [Google Scholar]
- Solomon DH, Curhan GC, Rimm EB, et al. Cardiovascular risk factors in women with and without rheumatoid arthritis. Arthritis Rheum. 2004;50:3444–9. doi: 10.1002/art.20636. [DOI] [PubMed] [Google Scholar]
- Solomon DH, Avorn J, Stürmer T, et al. Cardiovascular outcomes in new users of coxibs and nonsteroidal antiinflammatory drugs. Arthritis Rheum. 2006;54:1378–89. doi: 10.1002/art.21887. [DOI] [PubMed] [Google Scholar]
- Solomon DH, Sturmer T, Glynn RJ, et al. NSAIDs, coxibs, and cardiovascular outcomes: Exploratory subgroup analyses. Arthritis Rheum. 2006;54:S335. doi: 10.1002/art.21887. [DOI] [PubMed] [Google Scholar]
- Svenungsson E, Jensen-Urstad K, Heimbürger M, et al. Risk factors for cardiovascular disease in systemic lupus erythematosus. Circulation. 2001;104:1887–93. doi: 10.1161/hc4101.097518. [DOI] [PubMed] [Google Scholar]
- Taylor PC. Anti-TNF alpha therapy for rheumatoid arthritis: an update. Intern Med. 2003;42:15–20. [PubMed] [Google Scholar]
- Toloza SM, Uribe AG, McGwin G, Jr, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA). XXIII. Baseline predictors of vascular events. Arthritis Rheum. 2004;50:3947–57. doi: 10.1002/art.20622. [DOI] [PubMed] [Google Scholar]
- Turesson C. Endothelial expression of MHC class II molecules in autoimmune disease. Curr Pharm Des. 2004;10:129–43. doi: 10.2174/1381612043453414. [DOI] [PubMed] [Google Scholar]
- Turesson C, Jarenros A, Jacobsson LT. Increased incidence of cardiovascular disease in patients with rheumatoid arthritis – results from a community based study. Ann Rheum Dis. 2004;63:952–5. doi: 10.1136/ard.2003.018101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turesson C, McClelland RL, Christianson TJ, et al. Severe extra-articular disease manifestations are associated with an increased risk of first ever cardiovascular events in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:59–64. doi: 10.1136/ard.2006.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turesson C, Matteson EL. Cardiovascular risk factors, fitness and physical activity in rheumatic diseases. Curr Opin Rheumatol. 2007;19:190–6. doi: 10.1097/BOR.0b013e3280147107. [DOI] [PubMed] [Google Scholar]
- Vallbracht KB, Schwimmbeck PL, Seeberg B, et al. Endothelial dysfunction of peripheral arteries in patients with immunohistologically confirmed myocardial inflammation correlates with endothelial expression of human leukocyte antigens and adhesion molecules in myocardial biopsies. J Am Coll Cardiol. 2002;40:515–20. doi: 10.1016/s0735-1097(02)01990-3. [DOI] [PubMed] [Google Scholar]
- van Doornum S, McColl G, Wicks IP. Atorvastatin reduces arterial stiffness in patients with rheumatoid arthritis. Ann Rheum Dis. 2004;63:1571–75. doi: 10.1136/ard.2003.018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doornum S, Jennings GLR, Wicks IP. Reducing the cardiovascular disease burden in rheumatoid arthritis. Med J Aust. 2006;184:287–90. doi: 10.5694/j.1326-5377.2006.tb00239.x. [DOI] [PubMed] [Google Scholar]
- Watson DJ, Rhodes T, Guess HA. All-cause mortality and vascular events among patients with rheumatoid arthritis, osteoarthritis, or no arthritis in the UK General Practice Research Database. J Rheumatol. 2003;30:1196–202. [PubMed] [Google Scholar]
- Wajed D, Ahmad Y, Durrington PN, et al. Prevention of cardiovascular disease in systemic lupus erythematosus. Proposed guidelines for risk factor management. Rheumatology (Oxford) 2004;43:7–12. doi: 10.1093/rheumatology/keg436. [DOI] [PubMed] [Google Scholar]
- Warburton DE, Nicol CW, Bredin SS. Prescribing exercise as preventive therapy. CMAJ. 2006;174:961–74. doi: 10.1503/cmaj.1040750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström I, Jacobsson LT. Change in and predictors of leisure activities among patients with rheumatoid arthritis: a prospective study. Scand J Rheumatol. 2005;34:367–71. [PubMed] [Google Scholar]
- Wilson PW, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Michaud K, Gefeller O, et al. Predicting mortality in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:1530–42. doi: 10.1002/art.11024. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Michaud K. Heart failure in rheumatoid arthritis: rates, predictors, and the effect of anti-tumor necrosis factor therapy. Am J Med. 2004;116:305–11. doi: 10.1016/j.amjmed.2003.09.039. [DOI] [PubMed] [Google Scholar]
- Wong K, Gladman DD, Husted J, et al. Mortality studies in psoriatic arthritis: results from a single outpatient clinic. I. Causes and risk of death. Arthritis Rheum. 1997;40:1868–72. doi: 10.1002/art.1780401021. [DOI] [PubMed] [Google Scholar]
- Wållberg-Jonsson S, Öhman M-L, Rantapää-Dahlqvist S. Cardiovascular morbidity and mortality in patients with seropositive rheumatoid arthritis in Northern Sweden. J Rheumatol. 1997;24:445–51. [PubMed] [Google Scholar]
- Wållberg-Jonsson S, Johansson H, Öhman M-L, et al. Extent of inflammation predicts cardiovascular disease and overall mortality in seropositive rheumatoid arthritis. A retrospective cohort study from disease onset. J Rheumatol. 1999;26:2562–71. [PubMed] [Google Scholar]
- Young A, Koduri G, Batley M, et al. Mortality in rheumatoid arthritis. Increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology (Oxford) 2007;46:350–59. doi: 10.1093/rheumatology/kel253. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]