Abstract
A common pathophysiological course in vascular diseases is an overwhelming activation and aggregation of blood platelets, which results in atherothrombosis. By causing the last decisive step of cerebral, coronary, or peripheral arterial ischemia thrombotic complications of atherosclerotic disease represent a major player in death cause statistics of most western countries. The development of novel therapies against platelet-dependent thrombosis and the concurrent improvement of existing therapeutic strategies thus is a paramount focus of pharmaceutical research. Currently, efficiency, dosing and indications of established antiplatelet substances are being re-evaluated, whilst new, so far unrecognized molecular targets for inhibition of platelet activity come up front. This not only allows for interesting new therapeutical options, but also widens our insight into the role platelets play in atherosclerosis in general. This article summarizes the relevant pathophysiology of platelet activation, presents current concepts in antiplatelet drug therapy, and highlights the role of platelets in vascular diseases apart from atherothrombosis.
Keywords: atherothrombosis, antiplatelet drug therapy, pathophysiology, platelet activation
Introduction
Pathophysiology of platelet activation in atherothrombosis
Platelet activation within a blood vessel that is altered by atherosclerosis consists of numerous single steps originating with an initial transient interaction of the platelet with the still intact endothelium. On atherosclerotically activated endothelium, this may be followed by firm interaction with intact endothelium, but ultimately leads to a cascade of events resulting in thrombotic occlusion of a the blood vessel when platelets firmly adhere and become activated at sites of ruptured endothelium. This last event then causes myocardial infarction when a coronary artery is affected (Fuster et al 1992) and other catastrophes associated with atherothrombosis, depending on the location involved. In all these processes, endothelial and platelet adhesion receptors, platelet born activatory receptors, endothelium-released anti- and pro-platelet factors, platelet-released auto-activatory factors, and components of the subendothelial matrix each have their specific roles. Based on the detailed characterization of these single steps and factors involved in platelet activation, the development of novel antiplatelet drugs aims at specifically targeting some of these entities.
According to our current understanding of pathophysiology within blood vessels, it is assumed that under conditions of arterial blood flow, an initial contact of a circulating platelet with the intact endothelium is mediated either through von-Willebrandt Factor (vWF) or the endothelial surface molecule P-selectin, which both are involved in rolling of the platelet at the subendothelium (Jackson et al 2003). Following platelet rolling, the next escalation of platelet activation is also mediated by vWF, which, through firmly binding to the platelet GPIb receptor also mediates the first firm adhesion (Jackson et al 2003; Ruggeri 2003). This initial reversible interaction may already lead to preactivation of platelets in terms of platelet release of factors that either mediate platelet auto-activation, such as adenosine diphosphate (ADP) or thromboxane A2 (TxA2), or these factors that have vasoactive properties such as epinephrine, serotonin, CD40 ligand (CD40L), and others. Finally, at sites of ruptured endothelium, collagen fibrils contained in the subendothelial matrix gain contact with flowing blood, an important step in longer lasting platelet activation, which goes along with platelet shape change, intracellular calcium elevation, and release of contents of platelet granules, which contain the already mentioned auto-activatory and vasoactive substances (Siess 1989; Jackson et al 2003; Nieswandt and Watson 2003; Ruggeri 2003).
Platelets possess various receptors for collagen, such as the GPIa/IIa integrin, which mediates firm adhesion or the GPVI receptor, which, according to discoveries made during the last ten years, mediates strong platelet activation (Nieswandt and Watson 2003). The latter seems to represent the decisive step leading to profound platelet activation with its sequele of the aggregation cascade and the ultimate thrombotic occlusion of a vessel following injury to its wall.
Especially the release of auto-activating ADP and TxA2 sustain the cascade of aggregation once it is initiated eg, by collagen. These factors lead to activation of further, not yet activated platelets and to their recruitment to the site of aggregation. Platelet receptors for ADP are the purinergic P2Y1 and P2Y12 receptors, each of which activates specific signaling pathways. Whilst the P2Y1 receptor is involved in shape change and a transient type of aggregation, the P2Y12 receptor is the target molecule of thienopyridine drugs and physiologically mediates sustained aggregation (Daniel et al 1998; Jin et al 1998; Dorsam and Kunapuli 2004). On the other hand, TxA2 activates platelets through the TP prostanoid receptor, which initiates a cAMP dependent signaling cascade (Siess 1989).
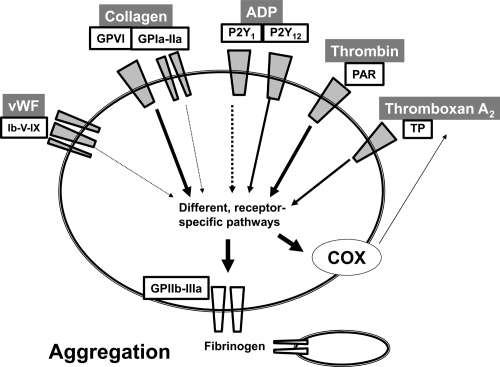
A necessary precondition for the binding of two platelets to each others and thus for platelet aggregation following the initial receptor ligand interaction dependent activation, is the presence of numerous mechanical platelet receptors, the GPIIb/IIIa integrin receptors (Shattil 1995). They function as fibrinogen receptors and mediate binding of two platelets through one molecule of fibrinogen. By breaking down fibrinogen to fibrin, thrombin then manifests the irreversibility of binding of two platelets two each others, as until then aggregation is a reversible phenomenon. By this action, thrombin also has a central role in thrombus formation and itself is the most potent amongst the pathophysiologically relevant platelet activators (collagen, ADP, TxA2, thrombin, to a lesser degree also epinephrine and others) (Jackson et al 2003; Shankar et al 2006) (Figure 1).
Figure 1.
Signaling pathways that activate platelets intraarterially. Platelets possess different receptors that are specific for physiological stimuli such as collagen, ADP, TxA2, thrombin, or vWF. Receptors for ADP are the purinergic P2Y1 and P2Y12 receptors, Collagen binds a GPIa/IIa integrin-receptor and activates platelets through the GPVI receptor. Thrombin activates via protease-activated receptors (PAR), of which 4 isotypes have been described. It also binds GPIb/V/IX, which is equally a receptor for vWF. TxA2 activates through the TP-receptor, a prostanoid-receptor (not shown are epinephrine and serotonin, which may also activate platelets in vivo). Following receptor mediated platelet activation the platelet GPIIb/IIIa integrin receptor is activated and secreted from platelet granules, which ultimately mediates platelet-platelet binding through fibrinogen and thus is indispensable for aggregation. Other sequele of platelet activation include activation of platelet cyclooxygenase-1 (COX) which induces formation and release of auto-activating TxA2.
Abbreviations: ADP, adenosine diphosphate; TxA2, thromboxane A2; vWF, von-Willebrandt Factor.
A role for platelets in atherosclerosis beyond thrombosis
Apart from dissecting the single steps of platelet activation and thus contributing to innovative therapeutical strategies targeting atherothrombosis, platelet research nowadays has come up with a plethora of evidence for an involvement of platelets in inflammatory and proatherogenic pathophysiological vascular processes ( Huo et al 2003; Gawaz et al 2005). Other data point to the fact that platelets might contribute to angiogenesis through secretion of granule contents (Pinedo et al 1998). These insights allow the implication that antiplatelet therapies in the future might not only be useful for preventing atherothrombosis, but may also positively influence the progression of atherosclerosis in general. In this case, data pointing at a use of certain antiplatelet substances in primary prevention, such as present at least for aspirin, could be brought to a new light and extend the scope of indications of antiplatelet therapies. Of note, there repeatedly have been observations about a use of low-dose aspirin in primary prevention of certain solid tumors, which on a speculative base could be in connection with a potential proangiogenic effect of platelet secreted mediators such as platelet-derived growth factor (Dube et al 2007). However these assumptions so far remain speculative and need further backup by clinical or experimental evidence.
Clinically established antiplatelet strategies
Low-dose aspirin
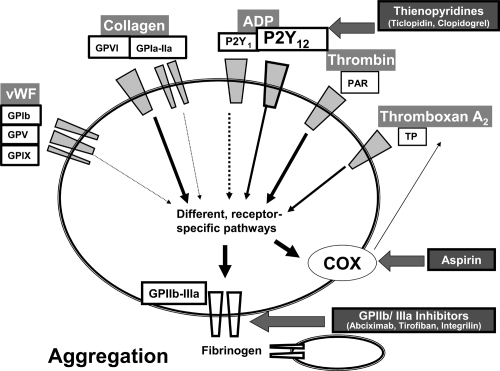
Aspirin inhibits activation of the enzyme cyclooxygenase and thus cyclooxygenase-dependent formation of autoactivating TxA2 in platelets (Figure 2). It does so by irreversibly acetylating a serine residue within the active centre of the enzyme (Loll et al 1995). In anucleate cells, which do not contain mRNA and the cellular organelles for protein synthesis and thus cannot resynthesize for cyclooxygenase, such as in platelets, this leads to an inhibition of for as long as the specific platelet circulates (Evangelista et al 2006). As a single dose of low-dose aspirin only inhibits cyclooxygenase incompletely, but as the accumulation caused by irreversible binding after repeated delivery leads to an effective inhibition, this inhibiting effect is quite specific for platelets, because nucleate cells overcome the accumulation of low-dose aspirin by novel synthesis of cyclooxygenase. Thus, there is a preferential inhibition for platelet cyclooxygenase in comparison with cyclooxygenases of other vascular cells such as endothelial or smooth muscle cells, when low-dose aspirin is repeatedly delivered.
Figure 2.
Established antiplatelet substances. Thienopyridines such as ticlopidine or clopidogrel need to be hepatically converted into an unknown active metabolite in order to exert antagonistic action at the P2Y12 ADP receptor. Aspirin acts as an irreversible inhibitor of cyclooxygenase (COX), which leads to a decreased production of platelet TxA2. GPIIb/IIIa Inhibitors in contrast prevent a mechanic event in platelet activation, which is the binding of fibrinogen, the most important prerequisite for platelet-platelet binding and thus aggregation. GPIIb/IIIa inhibitors are either monoclonal antibodies (Abciximab) or peptides of low molecular weight (Eptifibatide, Tirofiban). (Not shown are the antiplatelet drugs dipyridamol, which is contained in Aggrenox™, or cilostazol, both inhibitors of phosphodiesterases).
Abbreviation: ADP, adenosine diphosphate.
A beneficial effect of aspirin that is related to inhibition of platelet activity has first been suspected in the 50s of the past century (Craven 1953) and was first described in the following decade by Weiss and colleagues (1968).
Rapid absorption following its intake and the mentioned irreversible binding to platelet cyclooxygenase lead to an inhibition of platelet function as soon as about one hour after intake of aspirin, an effect which lasts for several days (Patrono et al 2004). Repeated delivery increases the antiplatelet effect and a maximum effect is reached at about 5 days following application of low-dose aspirin (Patrignani et al 1982; Cipollone et al 1997).
Clinical indications of low-dose aspirin span from primary to secondary prevention and also to acute treatment of atherothrombotic events (ISIS-2 Collaborative Group 1988; Wallentin 1991; Nymanet al 1992). However, whereas the use in secondary prevention is uncontradicted, this is not clearly the case in primary prevention and especially with regard to gender or site of manifestation of the atherothrombotic event (cerebrovascular or coronaries) that is to be prevented, there are contradictory reports (Peto et al 1988; Physician’s Health Study Group 1989; Manson et al 1991; Thrombosis Prevention Trial Study Group 1998; Awtry and Loscalzo 2000; Antithrombotic Trialists’ Collaboration 2002; Ridker et al 2005). In terms of gender, the larger ones of the studies addressing primary prevention with aspirin have enrolled mainly males, such as the Physicians’ health study (Physician’s Health Study Group 1989), which showed a strong decrease in the risk for myocardial infarction, or the British Physicians’ Study, which failed to do so (Peto et al 1988). Interestingly, aspirin for primary prevention of cardiovascular thrombosis did not decrease mortality, which could have been to a fairly low intrinsic risk within the study populations (Awtry and Loscalzo 2000). In contrast to this, the Thrombosis Prevention Trial investigated a group of patients at higher risk, and was able to show that primary prevention by aspirin reduced the risk of ischemic heart disease in more than 5000 men (Thrombosis Prevention Trial Study Group 1998). This effect was not observed in a recently published prospective study in women, which failed to show that aspirin affects the risk of myocardial infarction or death from cardiovascular causes. However, in this study, there was a beneficial effect on the risk of stroke (Ridker et al 2005). In contrast to this, a similar randomized study that had previously been performed in nurses taking aspirin infrequently, did show a positive effect in primary prevention of first myocardial infarction (Manson et al 1991).
There have repeatedly been reports about a “resistance” of certain patients to aspirin with respect to its antiplatelet efficacy (Wang et al 2006). Indeed, there can be situations in which low-dose aspirin therapy is ineffective. There are for example numerous indications that aspirin may be less effective in certain patient groups at high risk for vascular events, such as in diabetics (Angiolillo et al 2005; Coccheri 2007). Specific interactions of other drugs with the active centre of cyclooxygenase that aspirin also binds to, can reduce aspirin efficiency. This can be the case when some nonsteroidal anti-inflammatory drugs, which do not necessarily exhibit the same antiplatelet activity as aspirin, are taken before aspirin is ingested. Other reasons for insufficient efficiency of aspirin are reduced absorption after oral ingestion, extra-platelet TxA2 formation (eg, in vascular smooth muscle cells) or genetic variabilities in cyclooxygenase sensitivity to aspirin (Patrono and Rocca 2007). In no case, however, there is an underlying resistance in a pharmacological sense, which would mean a loss of sensitivity after initial exposure to the drug. Decreased aspirin efficiency is always caused by either individual factors or by specific pathophysiological situations. Thus, the term ‘resistance’ is incorrect.
Nevertheless, altogether, daily intake of low-dose aspirin represents the best established, safest and cheapest strategy for prevention of atherothrombotic events such as myocardial infarction or stroke, with lesser data at hand dealing with the prevention of peripheral thrombotic events.
Dipyridamole, an inhibitor of nucleoside transport that inhibits platelet activation by increasing adenosine levels, is being used in a fixed combination with aspirin as an antiplatelet agent (Schaper 2005). Although this combination has been shown to be superior to aspirin alone in secondary prevention of vascular thrombotic events following stroke in the ESPRIT trial (Halkes et al 2006), this effect could not been extended to other atherothrombotic diseases so far (De Schryver et al 2007).
ADP receptor blockade: Thienopyridines
As mentioned, the release of autoactivating substances such as TxA2 or ADP is an important step in the amplification of platelet activation leading to complete aggregation. ADP activates platelets by binding its purinergic P2Y1 and P2Y12 receptors. Whereas stimulation of the P2Y1 receptor mediates calcium mobilisation, shape change and a transient, reversible form of aggregation, stimulation of the P2Y12 receptor induces lasting aggregation and a decrease in cAMP (Cattaneo 2007).
Thienopyridines, which were known to inhibit platelet activity before the underlying mechanism was discovered, are antagonists at the P2Y12 receptor (Figure 2). Currently two substances are available: Clopidogrel and the older drug ticlopidine, which, due to its side effects, has been largely driven off the market. Both substances are prodrugs that have an active metabolite, which is only liberated after hepatic metabolism by the cytochrome P450 3A4 (CYP3A4) system. This so far unknown metabolite then irreversibly binds the P2Y12 receptor.
The efficiency of thienopyridines in the treatment of atherothrombotic disorders was first described for ticlopidine, which proved to have similar outcomes as aspirin in unstable angina (Balsano et al 1990). Moreover, it showed to have additional benefit in combination with aspirin in preventing stent thrombosis (Eshaghian et al 2007). Later, when the CLASSICS study showed comparable efficiency for the second thienopyridine, clopidogrel, with markedly reduced rates of side effects, namely a lack of dangerous thrombocytopenia in comparison to ticlopidine, the clinical use of ticlopidine was thrust into the background and clopidogrel prevailed (Bertrand et al 2000).
The first large randomized study assessing the efficiency of clopidogrel, the so called CAPRIE study, compared a monotherapy of clopidogrel versus aspirin in secondary prevention of atherothrombotic diseases. The CAPRIE study proved the effectiveness of clopidogrel, which, when compared with aspirin even afforded a statistically significant reduction of cumulative atherothrombotic events at 36 month follow-up. (CAPRIE Steering Committee 1996). Only because of markedly more expensive daily costs, these data did not lead to a displacement of aspirin as the drug of first choice in secondary prevention of cardiovascular events, so that in clinical practice clopidogrel as a monotherapy is merely used as an alternative of second choice to aspirin. Thus, clopidogrel so far is mainly used in the settings of acute coronary syndromes, myocardial infarction, and as a partner of aspirin in the so called dual antithrombotic therapy regimen following coronary angioplasty. Numerous studies, such as CURE, PCI-CURE, CREDO, COMMIT or CLARITY-TIMI 28 have underscored the usefulness of a dual antiplatelet therapy with aspirin and clopidogrel in the post-procedural treatment of coronary angioplasty (Eshaghian et al 2007). Interestingly, the CHARISMA study also tested the dual antiplatelet regimen in the setting of primary prevention and was not able to show additional benefit in this clinical setting in comparison to aspirin alone (Bhatt et al 2006). It needs to be mentioned that a dual antiplatelet therapy with aspirin and clopidogrel failed to prove superior to clopidogrel alone in the secondary prevention of ischemic cerebrovascular events in the MATCH study (Diener et al 2004).
More recently, there have been intensive attempts to maximize the efficiency of clopidogrel treatment by establishing an optimized dosing. This has led to an increase of the initial loading dose from 300 mg to 600 mg and has also prompted the setup of trials such as the CURRENT study, which investigate the increase of a maintenance daily dose from 75 mg to 150 mg. Smaller studies in healthy volunteers suggest that this increase in maintenance dose might also enhance the antiplatelet properties of clopidogrel (Angiolillo et al 2007; von Beckerath et al 2007).
Another recent focus of periinterventional drug treatment with clopidogrel has been the optimal duration of postinterventional dual antiplatelet therapy. This has been prompted by the enormous clinical success of drug-eluting stents in the first years after introduction of these devices into therapy. Although it currently cannot be taken as a fact that drug-eluting stents increase the rates of late stent thrombosis, as has been suggested by many observations (Mauri et al 2007), it can be stated that an extension of the duration of antiplatelet therapy following percutaneous coronary intervention leads to a significant reduction of the risk for death or myocardial infarction (Eisenstein et al 2007), an effect, which could already be observed in some studies with bare-metal stents such as CREDO or PCI-CURE. More recent data show clearly that a profound success of coronary stenting not only in the context of drug-eluting stents, will critically depend upon the establishment of a feasible, safe and cheap postinterventional antiplatelet therapy (Stone et al 2007).
Similarly described for aspirin, there also have been discussions about a potential resistance of certain patients to clopidogrel (Wang et al 2006). Also in case of clopidogrel, the term ‘resistence’, however, is inappropriate in this context, because the effects that have been observed are more likely because of eg, a decreased sensitivity of some patients to the clopidogrel effects. Other potential reasons for clopidogrel inefficiency can be diminished bioavailability of the active metabolite due to pharmacological interaction with other CYP3A4 metabolized drugs or inappropriate dosing when 75 mg/day are taken. Situational factors, such as increased platelet activity in smokers, diabetics or hypercholesterolemic patients aggravate the situation (Eshaghian et al 2007; Maree and Fitzgerald 2007).
GPIIb/IIIa receptor blockade
Nowadays, blockade of the most important and highly expressed platelet receptor, the fibrinogen-binding integrin receptor GPIIb/IIIa (or ανβ3-Integrin) is an indispensable part of interventional treatment of acute coronary syndromes (Figure 2). In additional to acute coronary syndromes, the three clinically available intravenous GPIIb/IIIa inhibitors abciximab (chimeric monoclonal antibody), eptifibatide and tirofiban (both RGD-inhibiting peptides of low molecular weight) are also used as adjunct therapy in high risk coronary interventions (Kong et al 1998).
The mechanism of antiplatelet action of GPIIb/IIIa inhibitors differs from other antiplatelet drugs because of their mechanism of action; they do not necessarily inhibit the activation of single platelets. However, as blockade of fibrinogen-binding completely prevents the most crucial mechanical step in aggregation, these drugs have the strongest antithrombotic potential. Still, GPIIb/IIIa inhibitors may not necessarily prevent platelet granule secretion or initial activation as eg, induced by collagen. This circumstance and underdosing or pharmacokinetic matters in respective studies may be have formed the basis for ineffectiveness of orally available GPIIb/IIIa inhibitors, which also caused increased bleeding and thus have no role in clinical practice today (Chew et al 2001).
It is interesting to note that – in contrast to aspirin and clopidogrel – resistances against or ineffectivenesses of GPIIb/ IIIa inhibitors have not been described so far.
New developments
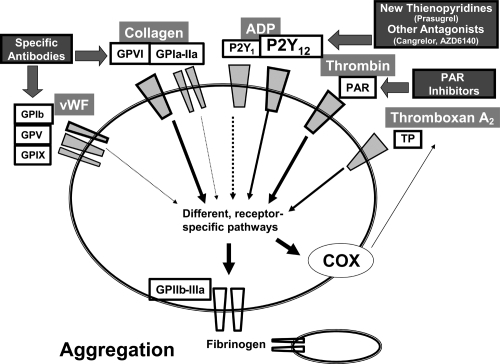
Among the many new antiplatelet substances that are currently being developed, different new P2Y12 antagonists are most likely to soon be introduced into clinical routine. The third orally available Thienopyridine drug, prasugrel, and the two non-thienopyridine antagonists of P2Y12 cangrelor (intravenous administration) and AZD6140 (orally applicable), are currently being tested in phase III studies or are already being launched by the manufacturers, after having shown promising results in phase II studies in terms of efficiency and safety (Figure 3).
Figure 3.
Potential new targets of antiplatelet substances. Aside from the development of further P2Y12 ADP receptor antagonists, which are either thienopyridines or non-thienopyridine structured and may have improved efficacy and advantageous pharmacokinetics, there are receptors for important physiological platelet agonists that clinically have not been targeted yet, but represent structures with potential for pharmacological development, due to insights from experimental studies. Amongst these are the platelet collagen receptor GPVI, the GPIb receptor and thrombin binding protease activated receptors (PAR). Substances against the latter are already in preclinical testing.
Similarly to clopidogrel, prasugrel is a prodrug, which only after hepatic metabolism releases its clinically active compound. In preclinical evaluation, it is 10 times more potent than other thienopyridines. Its safety and a comparable efficiency of prasugrel in comparison with clopidogrel (300 mg loading dose, 75 mg maintenance dose) was shown in the (JUMBO)-TIMI 26 study (Wiviott et al 2005). In the recently published TRITON-TIMI 38 study, prasugrel treatment was associated with a significantly reduced rate of the primary end point, which was death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke in patients with acute coronary syndromes, when compared with clopidogrel 300 mg loading and 75 mg maintenance dose. However, this advantage did not result in a difference in overall mortality, and, more importantly there was an increase in rates of major bleeding, including fatal bleeding (Wiviott et al 2007). As the currently running phase III study (PRINCIPLE)-TIMI 44 also incorporates more up to date, increased doses of clopidogrel it will not only deliver important information about prasugrel, but will also provide valuable information about the clinical consequences of an increased dose of clopidogrel, which so far is not sufficiently backed by data from large patient cohorts.
Cangrelor or AZD6140, on the other hand, are directly acting reversible inhibitors at the P2Y12 receptor. Potential advantages of cangrelor and AZD6140 in comparison to clopidogrel likely lie in their different pharmacokinetics, which include more rapid onset and loss of action (Greenbaum et al 2006). Due to this, cangrelor is also being investigated as an alternative to GPIIb/IIIa receptor antagonists in the CHAMPION-PCI and CHAMPION-PLATFORM studies, whereas AZD6140 is being tested against clopidogrel in the setting of STEMI and NSTEMI in the PLATO study. These drugs, due to the quicker loss of action, bear the potential to have improved safety profile in specific clinical situations such as in the setup of perioperative coronary angioplasty.
Besides the newly developed P2Y12 receptor antagonists, there are antiplatelet strategies currently under development, which target structures of platelets that so far have not been subject to pharmacological treatment and thus truly incorporate innovative mechanisms of action. Examples are antibodies or aptamers that target the collagen receptor GPVI or the vWF receptor GPIb (Figure 3). Both of these targets have gained attention rather late in comparison to the above mentioned platelet receptors as therapeutic targets, yet due to the important role of these structures in platelet pathophysiology may pose interesting alternatives for treatment and even extent the scope of indications of antiplatelet drug. Moreover, in animal experiments, these antibodies not only have antiplatelet, but also antiatherosclerotic properties and also may be associated with lower bleeding risks (Kleinschnitz et al 2007).
Thromboxane receptors represent interesting targets for newly developed antiplatelet agents. Interestingly, in animal models, drugs like S18886, a selective thromboxane receptor antagonist, exert properties beyond inhibition of platelet aggregation, by also improving endothelial function (Belhassen et al 2003), or inhibiting atherosclerosis development (Worth et al 2005). Although no clinical studies for S18886 are at hand yet, it seems to have excellent antithrombotic activity in an animal model of stent thrombosis (Vilahur et al 2007). Another substance that targets the thromboxane receptor also inhibits thromboxane synthase, and thus exerts dual inhibitory action. This drug, picotanide, has proved to be more effective than aspirin in the DAVID study that addresses its use in the prevention of mortality and major cardiovascular events in diabetics with peripheral arterial disease (Neri Serneri et al 2004).
Other interesting targets are the protease-activated receptors (PAR), which are physiological targets of thrombin that are not only expressed on platelets, but also on endothelial and vascular smooth muscle cells and on macrophages (Figure 3). As experimental findings have shown that PAR-dependent thrombin actions not only include platelet activation, but also the development of intimal hyperplasia, regulation of vessel tone, inflammation and endothelial barrier function, the various drugs that are currently being developed to target these structures, could potentially not only afford antithrombotic, but different, more profound antiatherosclerotic actions in patients (Leger et al 2006).
Conclusion
Antiplatelet therapy has come to be a main pillar in treatment of atherosclerosis. Well-established antiplatelet strategies include aspirin, one of the oldest and best-described drugs at hand, as well as clopidogrel or GPIIb/IIIa inhibitors, which both have become essential for periinterventional cardiology. New developments that are being tested either in clinical studies or are still in experimental preclinical phases include either alternative P2Y12 inhibitors or drugs that attack completely new targets. In any case, the concurrent progress in pathophysiological insights into platelet pathophysiology will likely bring about interesting even more new targets for antiplatelet substances. Similarly, it will continue to be of paramount importance to optimise existing antiplatelet strategies. In the near future, it will be exciting to witness the clinical impact of newly developed antiplatelet substances that currently are either in clinical testing or in still preclinical phases. Due to their innovative molecular targets or new pharmacokinetics, clinical situations in which they may make sense can easily be imagined.
References
- Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005;54:2430–5. doi: 10.2337/diabetes.54.8.2430. [DOI] [PubMed] [Google Scholar]
- Angiolillo DJ, Shoemaker SB, Desai B, et al. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation. 2007;115:708–16. doi: 10.1161/CIRCULATIONAHA.106.667741. [DOI] [PubMed] [Google Scholar]
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awtry EH, Loscalzo J. Aspirin. Circulation. 2000;101:1206–18. doi: 10.1161/01.cir.101.10.1206. [DOI] [PubMed] [Google Scholar]
- Balsano F, Violi F, Cimminiello C. Ticlopidine in unstable angina. Circulation. 1990;82:2282–3. doi: 10.1161/01.cir.82.6.2282. [DOI] [PubMed] [Google Scholar]
- Belhassen L, Pelle G, Dubois-Rande JL, et al. Improved endothelial function by the thromboxane A2 receptor antagonist S 18886 in patients with coronary artery disease treated with aspirin. J Am Coll Cardiol. 2003;41:1198–204. doi: 10.1016/s0735-1097(03)00048-2. [DOI] [PubMed] [Google Scholar]
- Bertrand ME, Rupprecht HJ, Urban P, et al. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: the clopidogrel aspirin stent international cooperative study (CLASSICS) Circulation. 2000;102:624–9. doi: 10.1161/01.cir.102.6.624. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–17. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- CAPRIE Steering Committee. A randomised blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348∧9038:1329–39. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- Cattaneo M. Platelet P2 receptors: old and new targets for antithrombotic drugs. Expert Rev Cardiovasc Ther. 2007;5:45–55. doi: 10.1586/14779072.5.1.45. [DOI] [PubMed] [Google Scholar]
- Chew DP, Bhatt DL, Sapp S, et al. Increased mortality with oral platelet glycoprotein IIb/IIIa antagonists: a meta-analysis of phase III multicenter randomized trials. Circulation. 2001;103:201–6. doi: 10.1161/01.cir.103.2.201. [DOI] [PubMed] [Google Scholar]
- Cipollone F, Patrignani P, Greco A, et al. Differential suppression of thromboxane biosynthesis by indobufen and aspirin in patients with unstable angina. Circulation. 1997;96:1109–16. doi: 10.1161/01.cir.96.4.1109. [DOI] [PubMed] [Google Scholar]
- Coccheri S. Approaches to prevention of cardiovascular complications and events in diabetes mellitus. Drugs. 2007;67:997–1026. doi: 10.2165/00003495-200767070-00005. [DOI] [PubMed] [Google Scholar]
- Craven LL. Experiences with aspirin (Acetylsalicylic acid) in the nonspecific prophylaxis of coronary thrombosis. Miss Valley Med J. 1953;75:38–44. [PubMed] [Google Scholar]
- Daniel JL, Dangelmaier C, Jin J, et al. Molecular basis for ADP-induced platelet activation. I. Evidence for three distinct ADP receptors on human platelets. J Biol Chem. 1998;273:2024–9. doi: 10.1074/jbc.273.4.2024. [DOI] [PubMed] [Google Scholar]
- De Schryver EL, Algra A, van Gijn J. Dipyridamole for preventing stroke and other vascular events in patients with vascular disease. Cochrane Database Syst Rev. 2007;3:CD001820. doi: 10.1002/14651858.CD001820.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364(9431):331–7. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- Dorsam RT, Kunapuli SP. Central role of the P2Y12 receptor in platelet activation. J Clin Invest. 2004;113:340–5. doi: 10.1172/JCI20986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube C, Rostom A, Lewin G, et al. The use of aspirin for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146:365–75. doi: 10.7326/0003-4819-146-5-200703060-00009. [DOI] [PubMed] [Google Scholar]
- Eisenstein EL, Anstrom KJ, Kong DF, et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA. 2007;297:159–68. doi: 10.1001/jama.297.2.joc60179. [DOI] [PubMed] [Google Scholar]
- Eshaghian S, Kaul S, Amin S, et al. Role of clopidogrel in managing atherothrombotic cardiovascular disease. Ann Intern Med. 2007;146:434–41. doi: 10.7326/0003-4819-146-6-200703200-00008. [DOI] [PubMed] [Google Scholar]
- Evangelista V, Manarini S, Di Santo A, et al. De novo synthesis of cyclooxygenase-1 counteracts the suppression of platelet thromboxane biosynthesis by aspirin. Circ Res. 2006;98:593–5. doi: 10.1161/01.RES.0000214553.37930.3e. [DOI] [PubMed] [Google Scholar]
- Fuster V, Badimon L, Badimon JJ, et al. The pathogenesis of coronary artery disease and the acute coronary syndromes (2) N Engl J Med. 1992;326:310–18. doi: 10.1056/NEJM199201303260506. [DOI] [PubMed] [Google Scholar]
- Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–84. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum AB, Grines CL, Bittl JA, et al. Initial experience with an intravenous P2Y12 platelet receptor antagonist in patients undergoing percutaneous coronary intervention: results from a 2-part, phase II, multicenter, randomized, placebo- and active-controlled trial. Am Heart J. 2006;151:689. doi: 10.1016/j.ahj.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Halkes PH, van Gijn J, Kappelle LJ, et al. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367(9523):1665–73. doi: 10.1016/S0140-6736(06)68734-5. [DOI] [PubMed] [Google Scholar]
- Huo Y, Schober A, Forlow SB, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–7. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- ISIS-2 Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet. 1988;2(8607):349–60. [PubMed] [Google Scholar]
- Jackson SP, Nesbitt WS, Kulkarni S. Signaling events underlying thrombus formation. J Thromb Haemost. 2003;1:1602–12. doi: 10.1046/j.1538-7836.2003.00267.x. [DOI] [PubMed] [Google Scholar]
- Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem. 1998;273:2030–4. doi: 10.1074/jbc.273.4.2030. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Pozgajova M, Pham M, et al. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. 2007;115:2323–30. doi: 10.1161/CIRCULATIONAHA.107.691279. [DOI] [PubMed] [Google Scholar]
- Kong DF, Califf RM, Miller DP, et al. Clinical outcomes of therapeutic agents that block the platelet glycoprotein IIb/IIIa integrin in ischemic heart disease. Circulation. 1998;98:2829–35. doi: 10.1161/01.cir.98.25.2829. [DOI] [PubMed] [Google Scholar]
- Leger AJ, Covic L, Kuliopulos A. Protease-activated receptors in cardiovascular diseases. Circulation. 2006;114:1070–7. doi: 10.1161/CIRCULATIONAHA.105.574830. [DOI] [PubMed] [Google Scholar]
- Loll PJ, Picot D, Garavito RM. The structural basis of aspirin activity inferred from the crystal structure of inactivated prostaglandin H2 synthase. Nat Struct Biol. 1995;2:637–43. doi: 10.1038/nsb0895-637. [DOI] [PubMed] [Google Scholar]
- Manson JE, Stampfer MJ, Colditz GA, et al. A prospective study of aspirin use and primary prevention of cardiovascular disease in women. JAMA. 1991;266:521–7. [PubMed] [Google Scholar]
- Maree AO, Fitzgerald DJ. Variable platelet response to aspirin and clopidogrel in atherothrombotic disease. Circulation. 2007;115:2196–207. doi: 10.1161/CIRCULATIONAHA.106.675991. [DOI] [PubMed] [Google Scholar]
- Mauri L, Hsieh WH, Massaro JM, et al. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020–9. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- Neri Serneri GG, Coccheri S, Marubini E, et al. Picotamide, a combined inhibitor of thromboxane A2 synthase and receptor, reduces 2-year mortality in diabetics with peripheral arterial disease: the DAVID study. Eur Heart J. 2004;25:1845–52. doi: 10.1016/j.ehj.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–61. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- Nyman I, Larsson H, Wallentin L. Prevention of serious cardiac events by low-dose aspirin in patients with silent myocardial ischaemia. The Research Group on Instability in Coronary Artery Disease in Southeast Sweden. Lancet. 1992;340(8818):497–501. doi: 10.1016/0140-6736(92)91706-e. [DOI] [PubMed] [Google Scholar]
- Patrignani P, Filabozzi P, Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J Clin Invest. 1982;69:1366–72. doi: 10.1172/JCI110576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrono C, Coller B, FitzGerald GA, et al. Platelet-active drugs: the relationships among dose, effectiveness, and side effects: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):234S–264S. doi: 10.1378/chest.126.3_suppl.234S. [DOI] [PubMed] [Google Scholar]
- Patrono C, Rocca B. Drug insight: aspirin resistance – fact or fashion? Nat Clin Pract Cardiovasc Med. 2007;4:42–50. doi: 10.1038/ncpcardio0728. [DOI] [PubMed] [Google Scholar]
- Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. BMJ (Clin Res Ed) 1988;296(6618):313–16. doi: 10.1136/bmj.296.6618.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Physician’s Health Study Group. Physician’s health study: aspirin and primary prevention of coronary heart disease. N Engl J Med. 1989;321:1825–8. doi: 10.1056/NEJM198912283212610. [DOI] [PubMed] [Google Scholar]
- Pinedo HM, Verheul HM, D’Amato RJ, et al. Involvement of platelets in tumour angiogenesis? Lancet. 1998;352(9142):1775–7. doi: 10.1016/s0140-6736(98)05095-8. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- Ruggeri ZM. Von Willebrand factor, platelets and endothelial cell interactions. J Thromb Haemost. 2003;1:1335–42. doi: 10.1046/j.1538-7836.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- Schaper W. Dipyridamole, an underestimated vascular protective drug. Cardiovasc Drugs Ther. 2005;19:357–63. doi: 10.1007/s10557-005-4659-6. [DOI] [PubMed] [Google Scholar]
- Shankar H, Kahner B, Kunapuli SP. G-protein dependent platelet signaling--perspectives for therapy. Curr Drug Targets. 2006;7:1253–63. doi: 10.2174/138945006778559166. [DOI] [PubMed] [Google Scholar]
- Shattil SJ. Function and regulation of the beta 3 integrins in hemostasis and vascular biology. Thromb Haemost. 1995;74:149–55. [PubMed] [Google Scholar]
- Siess W. Molecular mechanisms of platelet activation. Physiol Rev. 1989;69:58–178. doi: 10.1152/physrev.1989.69.1.58. [DOI] [PubMed] [Google Scholar]
- Stone GW, Ellis SG, Colombo A, et al. Offsetting impact of thrombosis and restenosis on the occurrence of death and myocardial infarction after paclitaxel-eluting and bare metal stent implantation. Circulation. 2007;115:2842–7. doi: 10.1161/CIRCULATIONAHA.106.687186. [DOI] [PubMed] [Google Scholar]
- Thrombosis Prevention Trial Study Group. Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council’s General Practice Research Framework. Lancet. 1998;351(9098):233–41. [PubMed] [Google Scholar]
- Vilahur G, Casani L, Badimon L. A thromboxane A2/prostaglandin H2 receptor antagonist (S18886) shows high antithrombotic efficacy in an experimental model of stent-induced thrombosis. Thromb Haemost. 2007;98:662–9. [PubMed] [Google Scholar]
- von Beckerath N, Kastrati A, Wieczorek A, et al. A double-blind, randomized study on platelet aggregation in patients treated with a daily dose of 150 or 75 mg of clopidogrel for 30 days. Eur Heart J. 2007;28:1814–19. doi: 10.1093/eurheartj/ehl489. [DOI] [PubMed] [Google Scholar]
- Wallentin LC. Aspirin (75 mg/day) after an episode of unstable coronary artery disease: long-term effects on the risk for myocardial infarction, occurrence of severe angina and the need for revascularization. Research Group on Instability in Coronary Artery Disease in Southeast Sweden. J Am Coll Cardiol. 1991;18:1587–93. doi: 10.1016/0735-1097(91)90489-v. [DOI] [PubMed] [Google Scholar]
- Wang TH, Bhatt DL, Topol EJ. Aspirin and clopidogrel resistance: an emerging clinical entity. Eur Heart J. 2006;27:647–54. doi: 10.1093/eurheartj/ehi684. [DOI] [PubMed] [Google Scholar]
- Weiss HJ, Aledort LM, Kochwa S. The effect of salicylates on the hemostatic properties of platelets in man. J Clin Invest. 1968;47:2169–80. doi: 10.1172/JCI105903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiviott SD, Antman EM, Winters KJ, et al. Randomized comparison of prasugrel (CS-747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention: results of the Joint Utilization of Medications to Block Platelets Optimally (JUMBO)-TIMI 26 trial. Circulation. 2005;111:3366–73. doi: 10.1161/CIRCULATIONAHA.104.502815. [DOI] [PubMed] [Google Scholar]
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- Worth NF, Berry CL, Thomas AC, et al. S18886, a selective TP receptor antagonist, inhibits development of atherosclerosis in rabbits. Atherosclerosis. 2005;183:65–73. doi: 10.1016/j.atherosclerosis.2005.02.034. [DOI] [PubMed] [Google Scholar]