Abstract
Background
The cardioprotective role of hormonal replacement therapy remains in doubt, but interest is increasing in the vascular effects of estrogens especially in coronary circulation.
Methods
Coronary blood flow (CBF) was measured in 24 postmenopausal women (age 55 ± 3 years), whose coronary arteries appeared angiographically normal, during incremental atrial pacing (AP) before and 20 minutes after intracoronary administration of either 75 ng/mL 17-β estradiol (treated group, n = 18) or 0.9% saline (controls, n = 6).
Results
Before estrogen, no differences in the coronary vasomotor responses at AP between the two groups (p = NS) could be detected. After estrogen, in the treated group, at the peak of the second AP, the coronary artery diameter decreased by 0.17 mm (p < 0.005) while the CBF increased by 61 mL/min (p < 0.05). These changes differed significantly from those observed at the peak of first AP (p < 0.001 for both cases). In contrast, in the control group no such changes were observed. The endothelin-1 (ET-1) levels in the coronary sinus were significantly reduced after estrogen infusion, which was negatively correlated with the degree of coronary artery constriction (r = −0.40, p = 0.03) and positively correlated with the increase in CBF (r = 0.54, p = 0.01).
Conclusions
In postmenopausal women without coronary artery disease, the intracoronary estrogen infusion mediates a greater increase in CBF and is positively correlated with the reduction of the coronary sinus ET-1 levels at the peak of AP.
Keywords: estrogens, coronary blood flow, endothelin-1, coronary interventions
Introduction
Nowadays, the beneficial effects of estrogen replacement therapy (ERT) on cardiovascular events among postmenopausal women are in doubt (Hsia et al 2000; Grady et al 2001). Many observational studies have reported a reduced incidence of cardiovascular disorders in pre-menopausal women and in post-menopausal women receiving replacement therapy (Stampfer et al 1991; Grodstein et al 2000). However the Heart and Estrogen/Progestin Replacement Study showed no benefit after 4 years of ERT in women with prior coronary artery disease (Hulley et al 1998). To re-examine the controversy, a meticulous study of the large variety of favorable biological mechanisms of estrogen action is needed, including not only its beneficial effect on the plasma lipids profile but also the direct effects of estrogens on vasculature (Gilligan et al 1994; Reis et al 1994; Austin et al 2000; Herrington et al 2000; Kallikazaros et al 2002). Acute exposure to estrogens may alter vascular tone via different mechanisms, both genomic and nongenomic. Short-term administration of 17β-estradiol has been shown to improve exercise-induced myocardial ischemia in postmenopausal female patients with coronary heart disease (Rosano et al 1993, 1997). Estrogen reverses acetylcholine-induced vasoconstriction of the coronary arteries in animals and in humans, maybe via a possible enhancement of endothelium-derived nitrous oxide (NO) production (Gueta et al 1997; Sughir et al 1996). Furthermore, plasma endothelin-1 (ET-1) concentrations seem to participate in the atherogenic process and to play a pivotal role in the coronary syndrome. Researchers have shown that estrogen decreases the production or the release of endothelium-derived constrictor factors such as ET-1 (Jiang et al 1992; Polderman et al 1993; Best et al 1998).
This study aimed to assess the effects of the intracoronary infusion of estrogens on coronary vasomotor responses during atrial pacing (AP), and to investigate any possible relationship with the levels of the coronary sinus ET-1 in postmenopausal women without coronary artery disease.
Methods
Study population
The enrolled subjects were all postmenopausal women (not receiving replacement therapy) aged 48 to 60 years old who had been referred for cardiac catheterization because of symptoms suggesting ischemic heart disease within the previous 6 months. Menopause was defined as the absence of a menstrual cycle for at least 12 months and was confirmed by serum levels of 17β-estradiol <50 pg/mL. Women were excluded from the study if they had acute coronary syndrome, cardiac failure, valvular disease, uncontrolled hypertension, diabetes mellitus, or any other major systemic illness before catheterization. Women who were included in the study were not current smokers and had angiographically smooth, normal-appearing coronary arteries. From this larger group, 26 women fulfilling the above criteria were selected, randomly assigned to treated or nontreated groups, and constituted our study population.
The Ethics Committee of our hospital approved the study and all patients provided written consent after being provided with a detailed description of the whole procedure.
Study protocol
All medications were stopped at least five drug half-lives before the study. Sublingual nitrates were allowed, to control any symptomatic episodes of myocardial ischemia, up to 8 hours before the study. Cardiac catheterizations were performed after overnight fasting with 5–10 mg diazepam PO given as premedication.
After diagnostic left-side cardiac catheterization and administration of 10,000 U heparin IV for anticoagulation, a 6F Judkins guiding catheter was advanced to the ostium of the left coronary artery. In 5 cases with angiographically rather irregular coronary arteries, an intravascular ultrasound study was performed, to confirm the absence of coronary stenoses. A 0.014-inch Doppler wire (FloWire®; Cardiometrics Inc.©, Mountain View, CA, USA) was advanced through the guiding catheter into the proximal part of the left anterior descending artery in 18 women, and the left circumflex artery in 8 women. The wire tip was positioned in an arterial segment without branch point so that a characteristic and stable flow velocity waveform could be recorded. After that, a 7F multipurpose catheter was introduced into the coronary sinus via the left femoral vein for blood sampling, and its position checked by fluoroscopy. A 5F pacing wire was inserted via the right femoral vein and placed into the right atrium. Aortic pressure, heart rate, and electrocardiogram (ECG) were displayed continuously. Once the cardiac catheterization and instrumentation was complete, a period of 15 minutes allowed women to relax, and hemodynamic parameters to stabilize. Firstly, measurements of both the dimension of the studied coronary artery and the average peak coronary blood flow (CBF) velocity were performed at pre-infusion of study medications, at a first AP protocol; at baseline (baseline-1), during the peak of AP (peak-1), and at 7 minutes in the recovery period (recovery-1) (Figure 1). Once these measurements were complete, a 20-minute intracoronary infusion of 17β-estradiol (75 ng/ml) (Sigma, Pomezia, Italy) at a rate of 1 mL/min was administered in 20 women, called the “treated group”. In 6 women, used as controls, an equivalent dose of 0.9% saline was administered by intracoronary infusion for 20 minutes. When infusion was complete, a second similar AP protocol was performed and the above measurements were obtained again at baseline-2, at peak-2, and at recovery-2. Coronary sinus levels of ET-1 and estradiol were evaluated before and after the end of estrogen or saline administration.
Figure 1.
Schematic presentation of the study protocol indicating time-points when coronary sinus samples and recordings of coronary blood flow (CBF) and diameter were taken.
Quantitative coronary angiography and calculation of flow
Coronary angiograms were obtained in the right oblique projections, to measure epicardial coronary diameters using hand injections of 5 mg iopromide. Measurements of coronary artery dimensions were taken in the proximal artery 0.5 cm distal to the wire tip using a computer-based edge detection enhancement technique, analyzed with a commercially available automated coronary analysis system (DCI-S Automated Coronary Analysis System, Philips Medical System, Eindhoven, Netherlands).
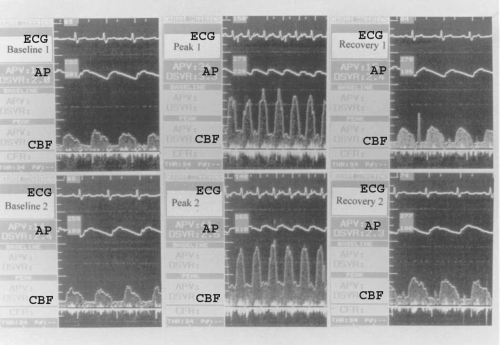
Average peak flow velocity was measured on line and each value was taken as the average of three cardiac cycles (Figure 2). A quantitative estimate of CBF was calculated from the Doppler flow velocity and quantitative angiographic data using the following equation: Q = 0.125 × π × D2 × APV × 0.6 where Q is flow (mL/min), D is the vessel diameter (mm), and APV is average peak velocity (cm/sec) (Gueta et al 1997).
Figure 2.
Representative coronary blood flow (CBF) of left anterior descending artery of a postmenopausal woman in the treated group at the two atrial pacing (AP) protocols before (upper panel) and after estrogen administration (low panel).
Abbreviations: AP, aortic pressure; ECG, electrocardiogram.
AP protocol
Incremental AP was started at 100 bpm, and the frequency of stimulation was gradually increased by 20 beats every 1 minute for a heart rate of up to 160 bpm, which stayed constant for 2 minutes.
Blood sampling
Coronary sinus blood was sampled at baseline and at the end of the intra-coronary infusions, to measure 17 β-estradiol and ET-1 levels with radio-immunoassay techniques.
Statistical analysis
Values are expressed as mean ± SD. Subject characteristics were compared between the treated and control groups using the independent Student’s t-test for continuous data and x2 analysis for categorical data. Comparisons of the evaluated parameters before and after estrogen or saline administration were performed using the paired Student’s t-test. Within each group, the influence of AP on coronary artery diameter and CBF was assessed using the paired Student’s t-test. The degree of coronary constriction was defined as the percentage decrease in coronary diameter [(Dbefore − Dafter)/Dbefore * 100]. The Pearson correlation coefficient was used to detect possible significant correlation between coronary vasomotor responses and demographic and laboratory parameters. A value of p < 0.05 was considered statistically significant.
Results
Of the 26 postmenopausal women without coronary artery disease studied, 2 of the treated group developed a second-degree atrioventricular block during AP and were excluded from the study. The clinical characteristics (Table1) of treated (n = 18) and control subjects (n = 6) did not differ significantly.
Table 1.
Clinical characteristics of the study population
| Parameters | Treated group (n = 18) | Controls (n = 6) | p |
|---|---|---|---|
| Age (years) | 55.6 ± 3.7 | 56.2 ± 3.2 | NS |
| Time since menopause (years) | 5.7 ± 2.8 | 5.4 ± 3.1 | NS |
| Body mass index (kgr/m2) | 27.2 ± 1.1 | 27.1 ± 1.1 | NS |
| Office systolic blood pressure (mmHg) | 142 ± 10 | 141 ± 10 | NS |
| Office diastolic blood pressure (mmHg) | 88 ± 4 | 89 ± 4 | NS |
| Heart rate (bpm) | 72 ± 8 | 72 ± 8 | NS |
| Total cholesterol (mg/dl) | 228 ± 25 | 230 ± 20 | NS |
| LDL cholesterol (mg/dl) | 150 ± 40 | 145 ± 36 | NS |
| HDL cholesterol (mg/dl) | 43 ± 14 | 45 ± 14 | NS |
| Serum estradiol levels (pg/ml) | 40 ± 14 | 38 ± 14 | NS |
| Coronary sinus estradiol levels (pg/ml) | 39 ± 12 | 39 ± 13 | NS |
| Coronary sinus ET-1 levels (mol/L) | 1.60 ± 0.48 | 1.64 ± 0.29 | NS |
Abbreviations: ET-1, endothelin-1; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Coronary sinus 17β-estradiol and ET-1 concentrations
At baseline, there were no significant differences between the serum levels of 17β-estradiol presented in Table 1 and those found in the coronary sinus. As expected, after the intracoronary infusions, there was a significant increase in the 17β-estradiol coronary sinus levels in the treated group, equivalent to pre-menopausal levels (39 ± 12 versus 350 ± 34 pg/ml, p < 0.001) but not in the control group (39 ± 13 versus 46 ± 12 pg/ml, p = NS). Coronary sinus ET-1 levels were significantly decreased after estrogen infusion by 0.66 pmol/L (41%) in the treated group (1.60 ± 0.48 versus 0.94 ± 0.53 pmol/L, p < 0.0001), but remained unchanged in the control group after saline infusion (1.64 ± 0.29 versus 1.58 ± 0.33 pmol/L, p = NS).
Coronary artery responses at the first AP protocol (before the study medications infusion)
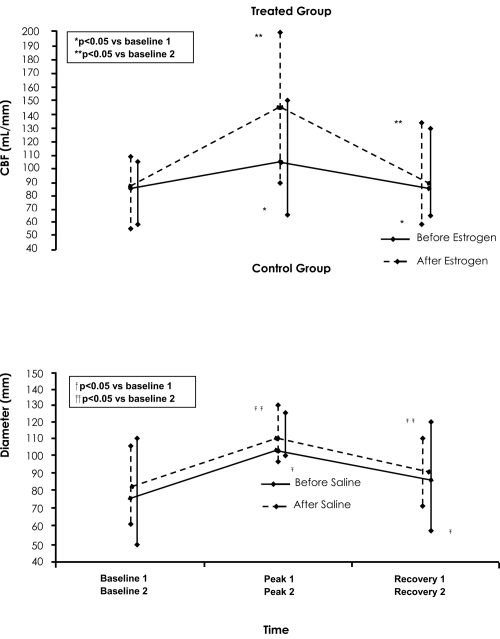
None of the subjects experienced typical chest pain or showed significant (>1 mm) ST-segment depression during the AP protocols. In the treated group, at peak-1 of AP, the coronary diameter was significantly reduced by 0.45 ± 0.22 mm (p < 0.005) while the CBF was significantly increased by 24.2 ± 21 mL/min (p < 0.05) compared with baseline-1 values (Table 2, Figure 3 and 4, upper level). At recovery-1, the coronary diameter remained significantly decreased and the CBF remained significantly increased compared to baseline-1 values.
Table 2.
Coronary artery responses at first atrial pacing protocol in the treated group (n = 18)
| Baseline-1 | Peak-1 | Recovery-1 | |
|---|---|---|---|
| Aortic pressure (mmHg) | 145/90 | 155/88 | 142/88 |
| Heart rate (bpm) | 75 ± 5 | 160 ± 3* | 78 ± 6 |
| Coronary artery diameter (mm) | 3.47 ± 0.42 | 3.02 ± 0.41* | 3.28 ± 0.49* |
| Average peak velocity (cm/sec) | 27.94 ± 3.31 | 47.61 ± 5.00* | 35.44 ± 4.98* |
| Coronary blood flow (mL/min) | 82.28 ± 30.14 | 106.44 ± 43.51* | 89.51 ± 38.86* |
Notes: p < 0.05 vs (baseline-1).
Figure 3.
Plot of coronary artery diameter at baseline-1, peak-1, recovery-1, and baseline-2, peak-2, recovery-2 of the first and second atrial pacing (AP) protocol, respectively. Upper panel: For the treated group before (solid line) and after estrogen administration (dotted line). Low panel: For the control group before (solid line) and after saline administration (dotted line).
Figure 4.
Plot of coronary blood flow (CBF) at baseline-1, peak-1, recovery-1 and baseline-2, peak-2, recovery-2 of the first and second atrial pacing (AP) protocol, respectively. Upper panel: For the treated group before (solid line) and after estrogen administration (dotted line). Low panel: For the control group before (solid line) and after saline administration (dotted line).
Similarly, in the control group, at peak-1 of AP the coronary artery diameter was significantly reduced by 0.42 ± 0.19 mm (p < 0.005) while the CBF was significantly increased by 23.8 ± 16 mL/min (p < 0.05) compared with baseline-1 values (Figure 3 and 4, low level). Furthermore, at 7 minutes into the recovery period the diameter remained significantly decreased, and the CBF remained significantly increased compared to baseline-1 values. There were no significant differences between the treated and control group in the above coronary vasomotor responses, at the three phases of the first AP protocol (p = NS for all cases).
Coronary artery responses at the second AP protocol (after the study medications infusion)
At baseline-2 in either the treated or control group, infusion of estradiol or saline had no significant effect on epicardial coronary artery diameter and CBF at baseline-2 compared with the preceding baseline-1 measurements.
In the treated group, at peak-2 of AP, the coronary artery diameter was significantly reduced by 0.17 ± 0.20 mm (p < 0.005) while CBF increased by 61 ± 32 ml/min (p < 0.05) compared to baseline-2 values (Table 3, Figure 3 and 4, upper level). These changes were significantly different compared with those observed at the first AP protocol (0.17 ± 0.20 vs 0.45 ± 0.22 mm and 61 ± 32 vs 24 ± 20 mL/min, respectively, p < 0.0001 for both cases). At recovery-2, the diameter remained significantly decreased and CBF remained significantly increased compared with baseline-2 values. Again, these changes were significantly different to those observed at the first AP protocol (0.05 ± 0.03 vs 0.19 ± 0.14 mm and 11 ± 9.6 vs 7 ± 6 mL/min, respectively, p < 0.0005 for both cases).
Table 3.
Coronary artery responses at second atrial pacing protocol in the treated group (n = 18)
| Baseline-2 | Pacing-2 | Recovery-2 | |
|---|---|---|---|
| Aortic pressure (mmHg) | 141/87 | 153/85 | 142/88 |
| Heart rate (bpm) | 77 ± 4 | 160 ± 4* | 80 ± 5 |
| Coronary artery diameter (mm) | 3.49 ± 0.41 | 3.32 ± 0.41* | 3.44 ± 0.40* |
| Average peak velocity (cm/sec) | 28.61 ± 2.70 | 53.77 ± 6.55* | 34.37 ± 4.02* |
| Coronary blood flow (mL/min) | 84.82 ± 28.95 | 145.01 ± 54.42* | 95.58 ± 35.01* |
Notes: p < 0.05 vs (baseline-2).
In the control group at peak-2, the diameter was significantly reduced by 0.41 ± 0.18 mm (p < 0.005) while CBF significantly increased by 24.9 ± 16 mL/min (p < 0.05) compared with baseline-2 values. Also at recovery-2 (Figure 3 and 4, low level) the diameter remained significantly decreased and CBF remained significantly increased compared to baseline-2 values. The above observed changes at the second AP protocol in both coronary diameter and CBF did not differ from those observed at the first AP protocol (p = NS for all cases).
Correlations
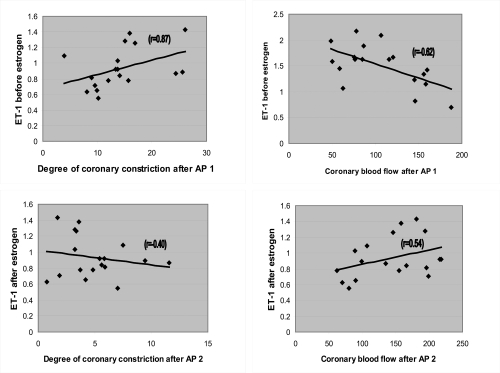
Before estrogen administration, the baseline-1 coronary sinus ET-1 levels had a positive correlation with the degree [(Diameterbefore − Diameterafter)/Diameterbefore * 100] of coronary artery constriction (r = 0.87) (p < 0.0001) and a negative correlation with CBF at peak-1 of AP (r = −0.62) (p < 0.005) (Figure 5). The estrogen-induced changes in coronary sinus ET-1 levels after peak-2 of AP were negatively correlated with the degree of coronary artery constriction (r = −0.40, p = 0.03) and positively correlated with CBF (r = 0.54, p = 0.01). Thus patients receiving estrogen had a greater reduction in ET-1 levels, less constriction of the coronary artery, and a greater increase in CBF at AP (Figure 5) than patients who did not receive estrogen treatment.
Figure 5.
Graphs of correlations of coronary blood flow (CBF) or degree of constriction, and levels of endothelin before and after estrogen infusion when atrial pacing (AP) is incorporated.
Discussion
The present study demonstrates that in postmenopausal women without coronary artery disease, intracoronary estrogen administration significantly attenuates the coronary artery responses at AP. In addition, the 17β-estradiol-induced improvement in these responses at AP is closely related to changes in coronary sinus ET-1 levels. The greater reduction in ET-1 levels was accompanied by both less constriction of the artery and greater increase in CBF at the peak of AP in this setting.
Effects of estrogen on flow mediated dilation and endothelium
The mechanisms of the possible putative antiatherosclerotic effect of estrogen remain unclear, and are the subject of intense investigation. Apart from the beneficial effect of estrogen on serum lipid profile and oxidative stress, a favorable modulation of coronary vasoactivity has also been proposed (Gilligan et al 1994; Reis et al 1994; Collins et al 1995; Austin et al 2000; Alexander et al 2001; Kallikazaros et al 2002). Our study found that estrogen significantly reduced the degree of coronary artery constriction and significantly increased the CBF, not only at the peak of AP but also in the recovery period compared to baseline. These findings are in agreement with previous studies and support the hypothesis that estrogen may possess anti-ischemic effects (Rosano et al 1993, 1997). The observed reduction of coronary constriction and improvement of flow during the second period of AP may be due to warm up effect, since the two AP protocols could potentially have caused cardiac preconditioning. However, our control subjects did not show any change in the degree of AP-induced changes in the coronary cross-sectional area or CBF; therefore, no effect resulting in cardiac preconditioning was observed in this protocol. In addition, the two AP protocols were separated by at least 30 minutes, so that the recovery period was long enough to avoid any preconditioning effect.
The effects of AP on coronary artery diameter in previous studies are controversial (Quyyumi et al 1995; Vojacek et al 1995; Nishikawa et al 1997). In our study, AP at baseline-1 resulted in a decrease in coronary artery diameter. This finding, although it differs from the results of previous studies (Quyyumi et al 1995; Nishikawa et al 1997) could be explained by the differences in AP protocol, the extent of coronary atherosclerosis, the study population, and the presence of different risk factors.
Effects of estrogen on endothelin-1 levels
The above vasoactive coronary properties of estrogen may be mediated via promotion of the release or action of potentially beneficial substances, such as nitric oxide (Gueta et al 1997; Rosano et al 1997; Best et al 1998) or via reduction of the release or action of potentially adverse mediators such as ET-1 (Jiang et al 1992; Lerman et al 1995; Lamping et al 1996; Sudhir et al 1996, 1997; Webb et al 2000). Researchers have reported that short-term 17β-estradiol administration into the coronary circulation decreases coronary blood ET-1 level (Webb et al 2000). Our study confirmed that acute administration of estrogen results in a significant decrease in intracoronary ET-1 levels. Furthermore, our study is unique because we showed for the first time that a relationship exists between the estrogen-induced reduction in coronary sinus ET-1 levels and the degree of both the constriction of the coronary artery and the increase in CBF at the peak of AP in postmenopausal women without atherosclerotic coronary arteries. Demonstrating a possible interrelationship between coronary vasomotor responses at exercise, by means of AP, and ET-1 levels may provide important information on the effects of estrogen on coronary circulation in this setting. Carolyn Webb and colleagues (2000) reported no change in coronary vasomotor or flow responses associated with a decrease in ET-1 levels in estradiol alone. However, there are some significant differences between these two studies, which could account for this controversy. First, our study demonstrated a nearly twofold decrease in ET-1 level (41%) compared with those observed (23%) in Webb’s study. Thus we can suggest that the degree of vasomotor response may be determined, at least partially, by the degree of decrease in ET-1 level. Secondly, the test used for endothelial function was different because the estrogen induced coronary vasomotor responses to substance P may not be so similar to those observed with AP. Third, the effects of estrogen on coronary vasomotor responses as well as the changes in the vasoconstrictor mediators may depend on the severity of the underlying coronary artery disease.
The above mentioned estrogen-induced decrease in plasma ET-1 levels may possibly explain some of the acute anti-ischemic effects of estrogen. In addition, in postmenopausal women, long-term ERT decreased plasma ET-1 levels in the systematic circulation, associated with an increased NO-to-ET-1 ratio (Polderman et al 1993; Lieberman et al 1994). Furthermore, one study reported that one month of treatment with 2 mg oral estradiol substantially reduced the forearm vasoconstrictor responses to ET-1, an effect which was lost three months later. Researchers have suggested that one potentially beneficial vascular action of ERT is subject to tachyphylaxis (Jhund et al 2001). If that is true, the combination of ERT with ET-1 antagonists may be a more suitable project in this setting.
In conclusion, intra-coronary 17β-estradiol administration affects the coronary vasomotor response favorably during AP in postmenopausal women without coronary artery disease. The improvement in both coronary artery constriction and CBF is closely related to estrogen-induced changes in the coronary sinus ET-1 levels. These findings provide new insights on the favorable effects of estrogen on vascular endothelium and may strengthen the growing interest in its potential benefit in postmenopausal women without coronary artery disease.
References
- Alexander KP, Newby LK, Hellkamp AS, et al. Initiation of hormone replacement therapy after acute myocardial infarction is associated with more cardiac events during follow-up. JACC. 2001;38:1–7. doi: 10.1016/s0735-1097(01)01329-8. [DOI] [PubMed] [Google Scholar]
- Austin C. Chronic and acute effects of estrogens on vascular contractility. J Hypertens. 2000;18:1365–78. doi: 10.1097/00004872-200018100-00003. [DOI] [PubMed] [Google Scholar]
- Best PJM, Berger PB, Miller VM, et al. The effect of estrogen replacement on plasma nitric oxide and endothelin-1 levels in post-menopausal women. Ann Intern Med. 1998;128:285–8. doi: 10.7326/0003-4819-128-4-199802150-00006. [DOI] [PubMed] [Google Scholar]
- Collins P, Rosano GMC, Sarrel PM, et al. Estradiol-17β attenuates acetylcholine-induced coronary arterial constriction in women but not men with coronary heart disease. Circulation. 1995;92:24–30. doi: 10.1161/01.cir.92.1.24. [DOI] [PubMed] [Google Scholar]
- Gilligan DM, Quyyumi AA, Cannon RO. Effects of physiological levels of estrogen on coronary vasomotor function in postmenopausal women. Circulation. 1994;89:2545–51. doi: 10.1161/01.cir.89.6.2545. [DOI] [PubMed] [Google Scholar]
- Grady D, Hulley SB. Postmenopausal hormones and heart disease. JACC. 2001;38:8–10. doi: 10.1016/s0735-1097(01)01331-6. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Manson JE, Colditz GA, et al. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933–41. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- Gueta V, Quyyumi A, Prasan D, et al. The role of nitric oxide in coronary vascular effects of estrogen in postmenopausal women. Circulation. 1997;96:2795–801. doi: 10.1161/01.cir.96.9.2795. [DOI] [PubMed] [Google Scholar]
- Herrington DM, Reboussin DM, Brosnihan KB, et al. Effects of estrogen on the progression of coronary artery atherosclerosis. N Engl J Med. 2000;343:522–9. doi: 10.1056/NEJM200008243430801. [DOI] [PubMed] [Google Scholar]
- Hsia J, Simon J, Lin F, et al. Peripheral arterial disease in randomized trial of estrogen with progestin in women with coronary artery disease. The Heart and Progestin Replacement Study. Circulation. 2000;102:2228–32. doi: 10.1161/01.cir.102.18.2228. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women: Heart and Estrogen/progestin Replacement Study (HERS) research group. JAMA. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Jhund P, Dawson N, Davie A, et al. Attenuation of endotheline-1 induced vasoconstriction by 17β -estradiol is not sustained during long term therapy in postmenopausal women with coronary artery disease. JACC. 2001;37:1367–73. doi: 10.1016/s0735-1097(01)01168-8. [DOI] [PubMed] [Google Scholar]
- Jiang C, Sarrel PM, Poole-Wilson PA, et al. Acute effect of 17 beta-estradiol on rabbit coronary artery contractile responses to endothelin-1. Am J Physiol. 1992;263:H271–5. doi: 10.1152/ajpheart.1992.263.1.H271. [DOI] [PubMed] [Google Scholar]
- Kallikazaros I, Tsioufis C, Zambaras P, et al. Conjugated estrogen administration improves common carotid artery elastic properties in normotensive postmenopausal women. Clin Cardiol. 2002;25:167–73. doi: 10.1002/clc.4960250407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamping KG, Nuno DW. Effects of 17 beta-estradiol on coronary microvascular responses to endothelin-1. Am J Physiol. 1996;271:H1117–24. doi: 10.1152/ajpheart.1996.271.3.H1117. [DOI] [PubMed] [Google Scholar]
- Lerman A, Holmes DRJ, Bell MR, et al. Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation. 1995;92:2426–31. doi: 10.1161/01.cir.92.9.2426. [DOI] [PubMed] [Google Scholar]
- Lieberman EH, Gerhard MD, Uehata A, et al. Estrogen improves endothelium-dependent flow-mediated vasodilation in postmenopausal women. Ann Intern Med. 1994;121:936–41. doi: 10.7326/0003-4819-121-12-199412150-00005. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Ogawa S. Importance of nitric oxide in the coronary artery at rest and during pacing in humans. JACC. 1997;29:85–92. doi: 10.1016/s0735-1097(96)00429-9. [DOI] [PubMed] [Google Scholar]
- Polderman K, Stehouwer C, Van Kamp G, et al. Influence of sex hormones on plasma endotheline levels. Ann Inter Med. 1993;118:429–32. doi: 10.7326/0003-4819-118-6-199303150-00006. [DOI] [PubMed] [Google Scholar]
- Quyyumi A, Dakak N, Andrews N, et al. Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation. 1995;92:320–6. doi: 10.1161/01.cir.92.3.320. [DOI] [PubMed] [Google Scholar]
- Reis SE, Gloth ST, Blumenthal RS, et al. Ethinyl estradiol acutely attenuates abnormal coronary vasomotor responses to acetylcholine in postmenopausal women. Circulation. 1994;89:52–60. doi: 10.1161/01.cir.89.1.52. [DOI] [PubMed] [Google Scholar]
- Rosano G, Caixeta AM, Chierchia S, et al. Short-tem anti-ischemic effect of 17β-estradiol in postmenopausal women with coronary artery disease. Circulation. 1997;96:2837–41. doi: 10.1161/01.cir.96.9.2837. [DOI] [PubMed] [Google Scholar]
- Rosano G, Sarrel PM, Poole-Wilson PA, et al. Beneficial effect of estrogen on exercise-induced myocardial ischaemia in women with coronary artery disease. Lancet. 1993;342:133–6. doi: 10.1016/0140-6736(93)91343-k. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Colditz GA, Willett WC, et al. Postmenopausal estrogen therapy and cardiovascular disease: ten-year follow-up from the nurses health study. N Engl J Med. 1991;325:756–62. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Jennings GL, Funder JW, et al. Estrogen enhances basal nitric oxide release in the forearm vasculature in perimenopausal women. Hypertension. 1996;28:3330–4. doi: 10.1161/01.hyp.28.3.330. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Ko E, Zellner C, et al. Physiological concentrations of estradiol attenuate endothelin 1-induced coronary vasoconstriction in vivo. Circulation. 1997;96:3626–32. doi: 10.1161/01.cir.96.10.3626. [DOI] [PubMed] [Google Scholar]
- Vojacek J, Rohac J, Kirkeelde R, et al. Changes in proximal and distal coronary artery diameter during atrial pacing-induced myocardial ischemia. Coron Artery Dis. 1995;6:685–91. [PubMed] [Google Scholar]
- Webb C, Ghatel M, McNeill J, et al. 17β-estradiol decreases endothelin-1 levels in the coronary circulation of postmenopausal women with coronary artery disease. Circulation. 2000;102:1617–22. doi: 10.1161/01.cir.102.14.1617. [DOI] [PubMed] [Google Scholar]