Abstract
The pathogenesis that is primarily responsible for Alzheimer’s disease (AD) and cerebrovascular accidents (CVA) appears to involve chronic hypoperfusion. We studied the ultrastructural features of vascular lesions and mitochondria in brain vascular wall cells from human AD biopsy samples and two transgenic mouse models of AD, yeast artificial chromosome (YAC) and C57B6/SJL Tg (+), which overexpress human amyloid beta precursor protein (AβPP). In situ hybridization using probes for normal and 5 kb deleted human and mouse mitochondrial DNA (mtDNA) was performed along with immunocytochemistry using antibodies against the Aβ peptide processed from AβPP, 8-hydroxy-2’-guanosine (8OHG), and cytochrome c oxidase (COX). More amyloid deposition, oxidative stress markers as well as mitochondrial DNA deletions and structural abnormalities were present in the vascular walls of the human AD samples and the AβPP-YAC and C57B6/SJL Tg (+) transgenic mice compared to age-matched controls. Ultrastructural damage in perivascular cells highly correlated with endothelial lesions in all samples. Therefore, pharmacological interventions, directed at correcting the chronic hypoperfusion state, may change the natural course of the development of dementing neurodegeneration.
Keywords: atherosclerosis, Alzheimer’s disease, transgenic animals, brain hypoperfusion, vascular and mitochondrial lesions, electron microscopy
Introduction
Vascular endothelial cells, neurons and glia are all able to synthesize, store and release reactive oxygen species (ROS) and vasoactive substances in response to certain stimuli, especially those produced by chronic hypoxia/hypoperfusion (for review see Aliev et al 2003b). The contribution of these substances to the pathophysiology of stroke, cerebrovascular disease or CVAs and AD is extremely important. It has been suggested that hypoperfusion can be an initiator of AD (Aliev et al 2002b, 2003a, 2003b, 2007; de la Torre 2002a). This idea is based on a positive correlation between AD and cardiovascular diseases. ROS are generated at sites of injury and/or inflammation. We hypothesize that the cellular and molecular mechanisms, by which hypoperfusion-induced ROS-accumulation impairs endothelial barrier function and promotes leukocyte adhesion, induce alterations in normal vascular function and result in the development of AD (Aliev et al 2003b).
Sustained hypoperfusion promoting oxidative stress of brain tissues could also stimulate secondary damage via the overexpression of inducible and neuronal specific nitric oxide synthase (iNOS and nNOS, respectively) and endothelin-1 (ET-1) in brain cells (Aliev et al 2000; de la Torre 2002a; Aliyev et al 2004; de la Torre and Aliev 2005). The continuous accumulation of oxidative stress products, such as peroxynitrite accumulation (via the overexpression of the iNOS and/or nNOS), may be secondary as well as an accelerating factor for the damage and compromise of the blood brain barrier (BBB) in hypoxia/hypoperfusion or AD (Aliyev et al 2004).
One of the main effects of chronic hypoperfusion induced vascular abnormality in AD appears to be tissue oxygen deficiency. Chronic cerebral hypoperfusion induces reduction of tissue oxygen delivery that causes the development of cognitive impairments such as AD (Kumar et al 1990; Friston and Frackowiak 1991; De Jong et al 1997; de la Torre 1997, 2002a; Aliev et al 2007). De la Torre (2000) proposes that advanced aging with a comorbid condition, such as a vascular risk factor that further decreases cerebral perfusion, promotes a critically attained threshold of cerebral hypoperfusion (CATCH). With time, CATCH induces brain capillary degeneration and suboptimal delivery of energy substrates to neuronal tissue (de la Torre 2000). Because glucose is the main fuel of brain cells, its impaired delivery together with a deficient delivery of oxygen, compromises neuronal stability because the supplies for aerobic glycolysis fail to meet the brain tissue demand. The outcome of CATCH is a metabolic cascade that involves, among other things, mitochondrial dysfunction, oxidative stress, decreased adenosine triphosphate (ATP) production and increased calcium entry, abnormal protein synthesis, cell ionic pump deficiency, signal transduction defects, and neurotransmission failure. These events contribute to the progressive cognitive decline characteristic of patients with AD, as well as regional anatomic pathology consisting of synaptic loss, senile plaques (SP), neurofibrillary tangles (NFT), tissue atrophy, and neurodegeneration. CATCH encompasses the clinical heterogenic pattern that characterizes AD and provides compelling evidence that any of a multitude of different etiopathophysiologic vascular risk factors, in the presence of advanced aging, can lead to AD (de la Torre 2000, 2002b; de la Torre and Aliev 2005; Aliev et al 2007).
The aim of the present study was to investigate the features of the vascular lesions in human AD and two transgenic mouse models of AD, lines overexpressing a mutated form of the amyloid beta precursor protein (AβPP). We compared the ultrastructural features of each and identified common changes relative to age-matched controls that may give clues to underlying mechanisms affecting brain neuronal function.
Materials and methods
All experimental procedures were performed in accordance with University of Texas San Antonio and Case Western Reserve University guidelines for the use of human biopsy and post-mortem tissues and the care and use of laboratory animals for research. Human AD brain biopsy and postmortem tissues for ultrastructural studies were taken as described earlier and processed for future electron microscopic (EM) and in situ hybridization studies (Hirai et al 2001; Aliev et al 2002a, 2002b, 2003a, 2007). Mouse brain samples were obtained from a normal, nontransgenic line (Py8.9-Hemi [wild type]) and 2 transgenic lines, AβPP-YAC and C57B6/SJL Tg (+), were obtained from Bruce Lamb and colleagues (1999). Animals were 22–26 months old (n = 6 for each group). All animals received a standard laboratory diet ad libitum. Mice, under terminal anesthesia were perfusion fixed via the heart as described previously (Aliev et al 2002a, 2003a; Aliyev et al 2005). Brain samples were processed for future analysis by electron microscope and in situ hybridization for cytological detection of mtDNA on the electron microscopic level. In situ hybridization was performed using wild type and Δ5kb deleted human and mouse specific probes as described recently (Aliev et al 2003a; Aliev 2007). Finally, all the sections were exposed to OsO4 for 1 h at RT, rinsed, dehydrated and flat embedded in Spurr’s embedding media. Ultrathin sections were stained with uranylacetate and lead citrate and viewed in a JEOL 100CX or Jeol 1230 EX electron microscope at 80 kV.
Results and discussion
Endothelial cells (EC) and perivascular cells of human brain microvessels from age-matched control cases showed no visible changes in their ultrastructure. Mitochondria in the EC were intact (data not shown).
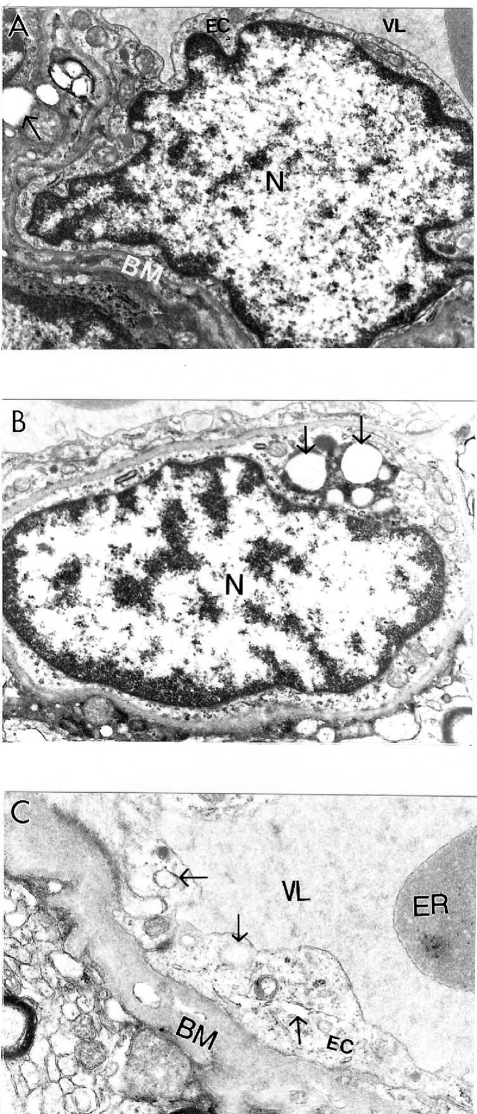
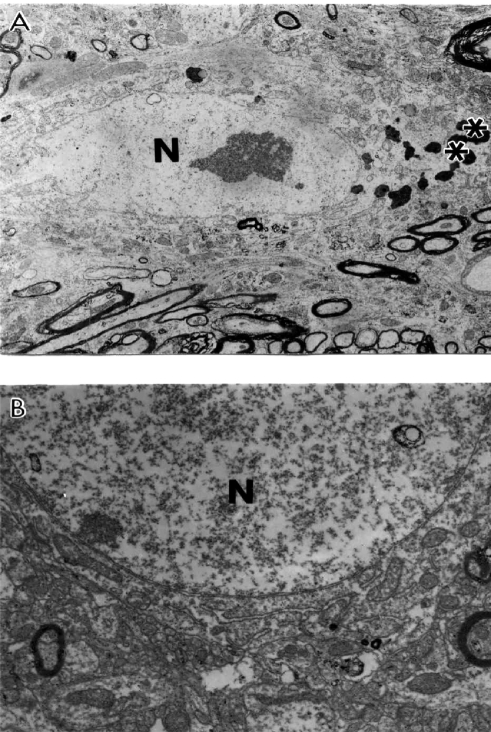
Conversely, cortical microvessels from AD brain biopsies were characterized by different degrees of damage, and heterogeneous lesions (Figures 1A–C). In some areas microvessels showed multiple lesions such as the presence of a cluster of mitochondria-derived lysosomes and necrotic changes in the ultrastructure of the vascular EC and perivascular cells (Figures 1A–B). Very often capillary endothelium cells showed the presence of “giant” sized lipid vacuoles in their matrices. Transformation of the mitochondria to mitochondria-derived lysosomes was generalized to all brain cellular compartments (Figures 1B–C). In addition, EC occupied only a small part of the vessel wall. Perivascular cells showed the presence of a large number of the mitochondria-derived vacuoles in their matrices (Figure 1C). Sometimes microvascular endothelium, at the early stages of AD, did not show any damage in their ultrastructure. However, the luminal plasma membrane of this EC sharply protruded into the vessel lumen, indicating the effect of hypoperfusion before any visible ultrastructural damage (Figure 1A).
Figure 1.
Ultrastructural characteristics of brain microvessels from human Alzheimer disease brain biopsies. A: Microvessels with minimal changes did not show any particular pathology in the ultrastructure of the vascular endothelium; however, perivascular cells show the presence of vacuolar degenerative structures in the matrix (indicated by single arrow). B: Vascular endothelium and perivascular cells of damaged microvessels display ultrastructural lesions in their cytoplasmic organelles especially mitochondria. Completely damaged matrices appeared to be permanent features of all of these cells (indicated by single arrow). Original magnification ×20,000 (A and B). C: Vascular endothelium with non-reversible damage have completely damaged mitochondria and destructive changes in the membranous structures. The basal membrane (BM) is very thick and occupies a large area of the vascular wall. Original magnification ×12,000.
The ultrastructural features of vascular lesions and mitochondria changes in neuronal cell bodies in transgenic and non-transgenic age-matched control mice were analyzed following perfusion fixation (Aliev et al 2000, 2003a, 2005). Ultrastructural changes in the brain samples of AβPP-YAC, but not age-matched control mice, coexisted with different degrees of amyloid deposition (Aliev 2002; Aliev et al 2002a).
Very often, clusters of Aβ positive immunoreactivity were observed in the neuronal cell bodies of perivascular paired cortical neurons (Aliev et al 2000; Aliev 2002). EM immunocytochemistry revealed different sizes of fibrils and extracellular types of amyloid deposits in the brain tissues (Figure 2A–F). The amyloid depositions were associated with the formation of parietal helical filamental (PHF) structures (Figure 2D–F), which is a permanent feature of neuronal lesions in AD brains (Aliev et al 2000, 2003a).
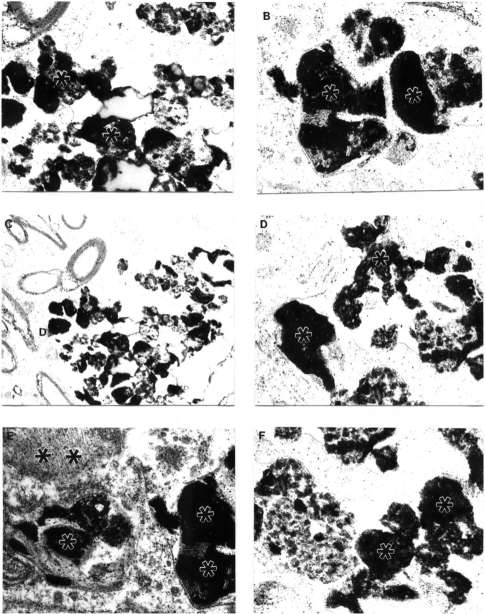
Figure 2.
Features of amyloid deposition in a neuronal cell body and the extracellular matrix in AβPP-YAC aged transgenic mouse brain (PAP pre-embedding immunocytochemistry). Amyloid depositions are associated with the formation of PHF-like structures (double asterisk in figure E). Original magnification A–D ×20,000. E–F ×25,000.
Brain microvessels showed immunopositive staining for Aβ and were characterized by the presence of large-sized lipid-laden vacuoles in the matrices of EC and perivascular cells (Figure 3A–D). These changes were generalized to cortical microvessels as in AD samples (Aliev 2002). The ultrastructural abnormalities of vascular wall cells were dependent on the presence of Aβ deposits around the microvessels (Figures 3-4). In contrast with these observations, age-matched control mouse brain vessels did not show any particular changes in the ultrastructure of vascular EC at different levels of microcirculation. Only sometimes did a very small number of lipid droplets appear in the matrices of perivascular cells (Aliev 2002; Aliev et al 2003a). This data clearly indicates that disruption of BBB function in vascular EC may be a major factor in lipid accumulation and amyloid deposition during the development of AD-like pathology in AβPP-YAC mice without cholesterol feeding (Aliev 2002; Shi et al 2002b; Aliev et al 2003a).
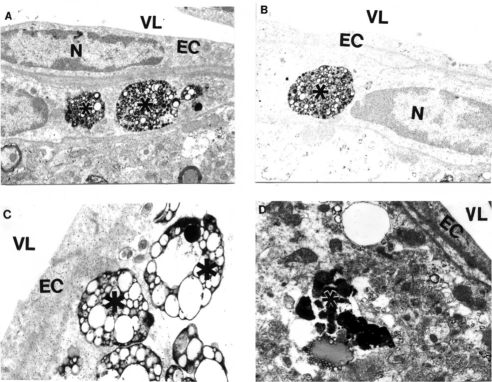
Figure 3.
Features of APP immunostaining in frontal cortical microvessels in aged AβPP-YAC mice determined by PAP pre-embedding immunocytochemistry. A, B and C are without counter staining. D is with counter staining. Asterisks indicate amyloid deposition. Original magnification ×12,000.
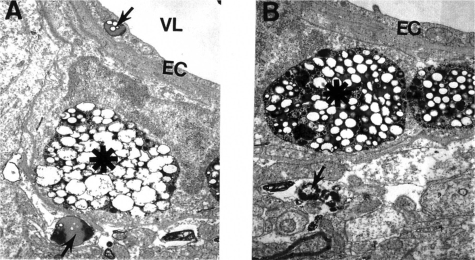
Figure 4.
Electron microscopic subcellular features of brain cortical microvessels from 24-month old AβPP-YAC mice. A: Vascular endothelial cells display lipid vacuoles in their cytoplasmic matrices (single arrow). Perivascular cells have “giant”- sized lipid/amyloid granules in their cytoplasmic matrices (indicated by asterisk). Original magnification: ×12,000. B: Microvessels with amyloid “angiopathy” are characterized by the accumulation of large-sized amyloid/lipid granules in the matrices of perivascular cells (indicated by asterisk). Vacuolar structures with lipid filled amyloid deposition (single arrow) are also present in the matrices of perivascular cells. Original magnification: ×12,000.
Ultrastructural immunocytochemical analyses using peroxidase-anti-peroxidase (PAP) and/or colloidal gold probes indicated that the damaged vascular walls in AβPP-YAC transgenic mice possess atherosclerotic lesions (Figure 4A–B), while control and non-damaged vessels from AβPP-YAC mice do not show Aβ immunopositive staining (data not shown).
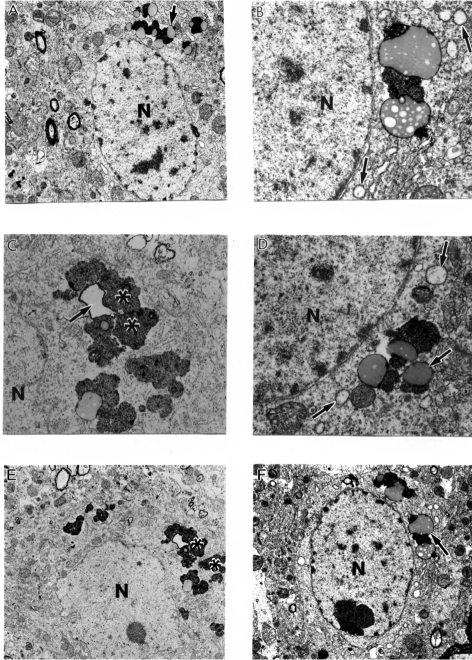
Age-matched control mice showed no particular changes in their neuronal ultrastructure (Figure 5A–B). Cortical neuronal cell bodies in AβPP-YAC mice were characterized by different degrees of ultrastructural alterations in their mitochondrial structures (Figure 6A–F) similar to human AD samples (Aliev et al 1999, 2000). Giant and electron- dense (ED) mitochondria appeared to be permanent features of the neuronal abnormality and was associated with the AβPP overexpression (Aliev et al 2000, 2003a). We also found that mitochondrial lesions in neuronal cell bodies appeared to be associated with the absence of microtubules and with lipofuscin formation. The same observations were seen in the populations of vulnerable neurons in AD brain biopsies indicating neuronal cytoskeletal abnormalities (Cash et al 2003).
Figure 5.
Age-matched control, non-transgenic mice did not show any particular changes in their neuronal ultrastructure. Lipofuscin was present in some neurons (indicate by asterisk in figure A). Original magnification of A and B: ×5,000, and ×20,000, respectively.
Figure 6.
Ultrastructural characteristics of the neuronal damage in AβPP-YAC transgenic mouse hippocampus. Mitochondrial lesions were associated with lipofuscin formation in the neuronal cell body (asterisk) and mitochondria appeared to be a major substrate for lipofuscin formation (single arrows). Original magnification: A, E, and F ×5,000. B, C, and D ×20,000 respectively.
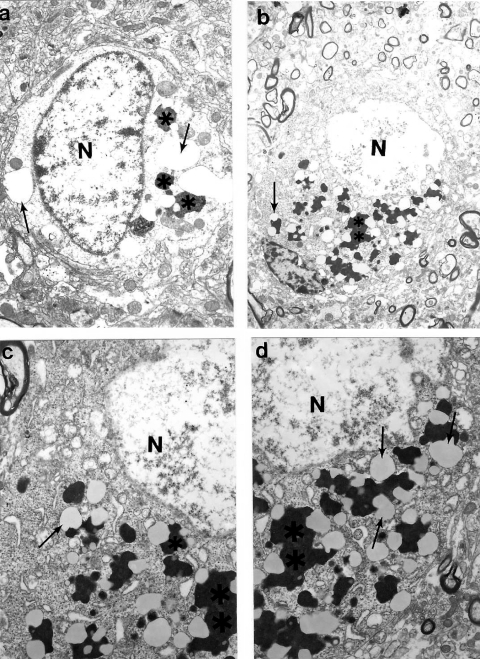
We looked at ultrastructural features of brain tissue from C57B6/SJL Tg (+) transgenic mice overexpressing AβPP. We demonstrated that the binding of basic fibroblast growth factor (bFGF) and serum amyloid P (SAP) to Aβ is a marker of a lack of BBB integrity in this mouse model. Adjacent sections of brain were stained with 4G8, a monoclonal antibody to the Aβ peptide (aa17–24), followed by 48.1, a monoclonal antibody against bFGF. The binding of bFGF in this mouse line is similar to that of AD cases in which bFGF binds specifically to Aβ neuritic plaques and the basement membrane of cerebral microvessels (Shi et al 1999, 2002a). In addition, the cores of amyloid plaques were intensely stained with 4G8, and bFGF binding is co-localized with amyloid immunoreactivity as visualized by polyclonal antiserum to amyloid. Our ultrastructural study indicated that bFGF immunostaining in aged C57B6/SJL Tg (+) mice was associated with damaged, but not normal neurons (Aliev et al 2000, 2002a, 2003a, 2007). Moreover, this was associated with different degrees of Aβ immunostaining in the neuronal cell body and vascular wall cells (Aliev 2002; Aliev et al 2003a; Aliyev et al 2005). The degree of mitochondrial abnormality, such as ED mitochondria-derived lysosomes and lipofuscin formation appeared to be features of damaged neurons in 24-month old aged C57B6/SJL Tg (+) but not age-matched control mice (Figure 7A–D). Vascular abnormality was associated with the selective damage to cortical neurons suggesting a direct relationship between vascular abnormality, BBB breakdown, neuronal loss and amyloid depositions during the maturation of AD-like pathology (Aliev 2002; Aliev et al 2003a).
Figure 7.
Ultrastructural characteristics of cortical neurons in C57B6/SJL transgenic mouse (Tg +) brain. The main characteristic of neuronal damage appeared to be the transformation of completely damaged mitochondria (mitochondria without any residues of mitochondrial cristae; indicated by single arrows) into the lipofuscin granules, characterised by their cluster type localization in the neuronal cell body. Original magnification: A and B ×5,000; C and D ×20,000, respectively.
Previously we demonstrated that the ultrastructural features of cortical neurons from AD brain biopsies are characterized by the selective localization of mitochondria abnormalities to the cell bodies (Aliev et al 1999, 2000; Hirai et al 2001; Aliev 2002; Shi et al 2002b). The majority of the neurons, which were closely associated with lesioned vessels, possessed different degrees of ultrastructural abnormality. Partially and completely damaged mitochondria were associated with lipofuscin formation and mitochondria appeared to be a major substrate for this process (Figures 6-7). In many cases, neuronal cell bodies showed an absence of cellular organelles. Different stages of mitochondrial abnormality, such as formation of mitochondria-derived lysosomes and lipofuscin, were seen in damaged but not in normal neurons (Figures 6-7). The mitochondria-derived lysosomes and lipofuscin deposits of varied density and sizes were permanent features of the neuronal abnormality (Aliev et al 2003a). Mitochondrial lesions and lipofuscinogenesis were also present in glial cells of the brain parenchyma (Aliev et al 2003a).
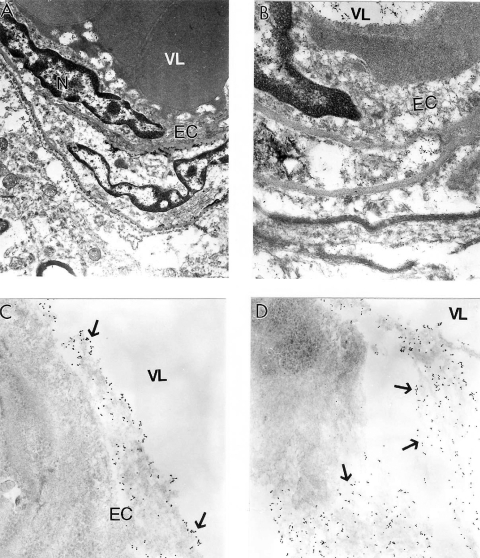
Quantitative morphometric measurements of the percentage of the different types of mitochondria (normal, partially damaged, and completely damaged) indicate that age-matched control groups have a significantly higher percentage of normal mitochondria compared with AD cases (Hirai et al 2001). Cytological in situ hybridization studies using probes for human normal and 5 kB deleted mtDNA found that mtDNA signals were associated with severely damaged or mitochondria-derived lysosomal structures (Figure 8). However, the areas containing lipofuscin did not show any mtDNA containing positive signals. On the ultrastructural level, clusters of 5 kb deleted mtDNA containing gold particles (17 nm) were localized in mitochondria-derived lysosomal structures, indicating that mtDNA deletions and turnover occur at the late stages of the mitochondrial lesion formation. Similarly, areas of neuronal cell bodies containing normal mtDNA and lipofuscin were free from any 5 kb deleted mtDNA positive signals (Hirai et al 2001; Aliev et al 2002a). In contrast to this observation, hippocampal neuronal mitochondria of age-matched control subjects did not show mtDNA positive signals containing gold particles in their matrices.
Figure 8.
Electron microscopic determination of mitochondrial DNA signals visualized by using wild type and chimeric 5 kb deleted mtDNA probes in a human postmortem Alzheimer’s disease (AD) brain (A–B) and 24-month old AβPP-YAC transgenic mouse brain (C–D). A and B: AD brain microvessels endothelium and perivascular cells show clusters of wild type mtDNA containing positive signals visualized by indirect colloidal gold techniques (indicated by the dark dots). Original magnification: A and B ×10,000 and ×25,000, respectively. C–D: AβPP-YAC transgenic mice show the presence of clusters of chimeric 5 kb deleted mitochondria DNA positive signals throughout the matrices of vascular and perivascular cells (single arrows). Original magnification: C and D ×30,000 and ×20,000, respectively.
Abbreviations: BM, basal membrane; EC, endothelial cell; ER, erythrocytes; N, cell nucleus; VL, vessel lumen; PAP, peroxidase-anti-peroxidase; PHF, paired helical filaments.
Our detailed immunocytochemical analysis also demonstrated that the mitochondrial abnormalities in neurons are associated with increased markers of lipid peroxidation (Moreira et al 2007). Moreover, lipid peroxidation markers were associated with RNA oxidation (staining by 8OHG). Clusters of 8OHG containing immunopositive gold particles (17 nm) were localized to the matrices of completely and/or partially damaged mitochondria (Nunomura et al 2001; Aliev et al 2002, 2002a). Large quantities of immunopositive gold particles were also associated with the cytoplasmic matrix. In addition, the cytoplasmic matrices, especially of the regions where damaged mitochondria or mitochondria-lipofuscin were present, showed clusters of 8OHG containing immunopositive gold particles (Aliev et al 2002, 2002a). Our quantitative study indicated that the extent of oxidative damage (eg, 8OHG staining) is highly dependent on the degree of mitochondrial abnormalities (Nunomura et al 2001; Aliev et al 2003a). Based on the evidence presented in this study we theorize the hypothetical timeline of the damage in the brain during maturation of AD is much more complex than the previously proposed hypothetical schematic (Aliev et al 2003b). With the onset of AD, normal neurons develop numerous forms of oxidative damage including nitration (nitrotyrosine), lipid peroxide adducts (lipid peroxidation), and nucleic acid oxidation prior to the formation of pre-neurofibrillary tangles (pre-NFT) that induce nonreversible damage to neurons and therefore finally failure of neurotransmission that occurs in AD brain.
The detailed analysis of 8OHG immunostaining demonstrated that only vulnerable neurons show immunopositive staining for 8OHG in AD, but not in age-matched controls. By using ultrastructural analysis we have found that 8OHG immunostaining was selectively associated in vulnerable neurons and microvessels of AD brain (Aliev et al 2002b; Aliyev et al 2005). The 8OHG immunogold labeling (17 nm) was seen throughout the cytoplasm, including the damaged mitochondria or electron dense abnormal mitochondria (Hirai et al 2001; Nunomura et al 2001; Aliev et al 2002b; Moreira et al 2007). However, we did not find 8OHG in normal mitochondria or in lipofuscin. The capillary EC and perivascular pericytes showed a high intensity of 8OHG immunostaining (Aliev et al 2002b).
In this study, the main location of the mtDNA was in damaged mitochondria and mitochondria derived lysosomes, but not in lipofuscin in all brain cellular compartments including the vascular wall cells (Figure 8A–B). Endothelial cells (EC) of vessels with atherosclerotic lesions and nearby perivascular cells contain clusters of normal and deleted mtDNA positive signals (Figure 8A–B). These observations emphasize the key role of hypoperfusion, mitochondrial abnormality and oxidative stress in the pathogenesis of vascular and nonvascular cells lesions during the development of AD-like pathology in AβPP-YAC mice (Aliev et al 2002a; Aliyev et al 2005). Many of these characteristics overlap the neuropathology of human AD (Aliev et al 2002a).
In situ hybridization analysis with mouse mtDNA probes found abundant deleted mtDNA in AβPP-YAC mice compared to age-matched controls (Aliev et al 2000, 2002a, 2003a). Moreover, the majority of deleted mtDNA was found in mitochondria-derived lysosomes (Figure 8C–D) in regions closely associated with lipofuscin, suggesting that proliferation, deletion and duplication of mtDNA occur in mitochondria, many of which have been fused with lysosomes (Aliev et al 2000). These finding suggest that abnormalities in mitochondrial structures and mtDNA are features of damaged neurons in AβPP-YAC mice, and may also play a key role in the pathogenesis of AD (Aliev et al 2000). We found that neurons in AD samples were dominated by abnormal mitochondria as compared with the control group (Aliyev et al 2005). By in situ hybridization analyses with a chimeric cDNA probe to the 5 kb common deletion (Hirai et al 2001), we found that deleted mtDNA is increased at least 3 fold for the AD cases compared to the controls (Hirai et al 2001). In situ hybridization detected mtDNA proliferation, deletion and duplication in abnormal mitochondria, many of which were fused with lysosomes, indicating they were being turned over (see Figure 8A–B).
A growing body of evidence suggests that there are direct links between heart disease, hypertension, stroke and neurodegeneration (Roher et al 2003; de la Torre 2006; Skoog and Gustafson 2006). The relationship between atherosclerosis, vascular amyloidosis and brain hypoperfusion in the pathogenesis of sporadic Alzheimer’s disease has been well documented (Roher et al 2003; Kalback et al 2004). Hypertension has been suggested to be an initiator of the ischemic pathway leading to AD (Aliev et al 2003, 2007; de la Torre 2006; Skoog et al 2006). Recently, studies by Choi and colleagues (2007) showed that preconditioning with chronic cerebral hypoperfusion reduces a focal cerebral ischemic injury and increases apurinic/apyrimidinic endonuclease/redox factor-1 and matrix metalloproteinase-2 expression. Another report by Annaházi and colleagues (2007) demonstrated that the pre- and post-treatment with alpha-tocopherol attenuates hippocampal neuronal damage in experimental cerebral hypoperfusion. Moreover, circulating CD34 positive T-cells are able to provide an index of cerebrovascular function (Taguchi et al 2004). The beneficial effect of carotid endarterectomy on the cerebral blood flow and cerebral blood volume improvement was studied by SPECT (Lushmanov et al 1997).
Based on this literature evidence and our present study we believed that managing AD as a vascular disease will open a new window of insight not only for better understanding of the etiopathogenesis but also new and more effective treatment strategies.
We theorize that the oxidative stress markers seen in the human AD and AD mouse models selectively affect the population of vulnerable neurons, vascular EC and perivascular cells. We believe that hypoperfusion-induced oxidative stress plays a key role in the pathogenesis of vascular and nonvascular cell lesions during the maturation of AD.
Conclusion
Our data for the first time demonstrates that a chronic injury stimulus induces the hypoperfusion seen in the microcirculation of vulnerable brain regions. Determining the mechanisms behind these imbalances may provide crucial information in the development of new, more effective therapies for the treatment of cerebrovascular diseases, including AD. Future studies must seek to answer the following questions: (1) What are the major factors altering and/or controlling cerebral blood flow during the progression of chronic hypoperfusion and/or the development of atherosclerotic changes in brain microvessels? (2) What are the roles of endothelial content vasoactive substances (especially nitric oxide [NO] and endothelin-1 [ET-1]) during the development of this pathophysiology? (3) Does acute or chronic hypoperfusion with concomitant oxidative stress accelerate the vascular and neuronal lesions during normal aging and/or when brain is exposed to chronic hypoperfusion induced factors? Resolving these issues will allow for novel therapeutic approaches that will modify the natural history of these chronic age onset disorders in the 21st century.
Acknowledgments
Supported by the Philip Morris USA Research Management Group and Alzheimer’s Association. The authors report no conflict of interest.
References
- Aliev G. Is non-genetic Alzheimer’s disease a vascular disorder with neurodegenerative consequences? J Alzheimer’s Dis. 2002;4:513–16. doi: 10.3233/jad-2002-4607. [DOI] [PubMed] [Google Scholar]
- Aliev G, Liu J, Xu K, et al. ApoE4 in mice induces age-dependent brain hypoperfusion, neuronal, glial and microvascular damage, and cognitive impairment, which can be prevented by feeding Acetyl-L-Carnitine and R-Lipoic Acid. 8th International Conference on Alzheimer’s and Parkinson’s Diseases AD/PD (Salzburg, Austria); Proceeding AD/PD Conference; Monduzzi Editore, Bologna, Italy. 2007. in press. [Google Scholar]
- Aliev G, Obrenovich ME, Seyidova D, et al. Exploring ischemia-induced vascular lesions and potential pharmacological intervention strategies. Histol Histopathol. 2005;20:261–73. doi: 10.14670/HH-20.261. [DOI] [PubMed] [Google Scholar]
- Aliev G, Seyidova D, Lamb BT, et al. Mitochondria and vascular lesions as a central target for the development of Alzheimer’s disease and Alzheimer disease-like pathology in transgenic mice. Neurol Res. 2003a;25:665–74. doi: 10.1179/016164103101201977. [DOI] [PubMed] [Google Scholar]
- Aliev G, Seyidova D, Neal ML, et al. Atherosclerotic lesions and mitochondria DNA deletions in brain microvessels as a central target for the development of human AD and AD-like pathology in aged transgenic mice. Ann N Y Acad Sci. 2002a;977:45–64. doi: 10.1111/j.1749-6632.2002.tb04798.x. [DOI] [PubMed] [Google Scholar]
- Aliev G, Shi J, Perry G, et al. Neuronal mitochondrial abnormalities in yeast artificial chromosome (YAC) transgenic mice overexpressing amyloid precursor protein (APP) Society for Neuroscience Abstracts Book. 2000:30. [Google Scholar]
- Aliev G, Smith MA, Obrenovich ME, et al. Role of vascular hypoperfusion-induced oxidative stress and mitochondria failure in the pathogenesis of Alzheimer disease. Neurotox Res. 2003b;5:491–504. doi: 10.1007/BF03033159. [DOI] [PubMed] [Google Scholar]
- Aliev G, Smith MA, Seyidov D, et al. The role of oxidative stress in the pathophysiology of cerebrovascular lesions in Alzheimer’s disease. Brain Pathol. 2002b;12:21–35. doi: 10.1111/j.1750-3639.2002.tb00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliev G, Smith MA, Vinters H, et al. Mitochondria abnormalities mark vulnerable neurons in Alzheimer’s disease. J Neuropathol Exp Neurol. 1999;58:511. [Google Scholar]
- Aliyev A, Chen SG, Seyidova D, et al. Mitochondria DNA deletions in atherosclerotic hypoperfused brain microvessels as a primary target for the development of Alzheimer’s disease. J Neurol Sci. 2005;229–230:285–92. doi: 10.1016/j.jns.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Aliyev A, Seyidova D, Rzayev N, et al. Is nitric oxide a key target in the pathogenesis of brain lesions during the development of Alzheimer’s disease? Neurol Res. 2004;26:547–53. doi: 10.1179/01610425017613. [DOI] [PubMed] [Google Scholar]
- Annaházi A, Mracskó E, Süle Z, et al. Pre-treatment and post-treatment with alpha-tocopherol attenuates hippocampal neuronal damage in experimental cerebral hypoperfusion. Eur J Pharmacol. 2007;571(2–3):120–8. doi: 10.1016/j.ejphar.2007.05.048. [DOI] [PubMed] [Google Scholar]
- Cash AD, Aliev G, Siedlak SL, et al. Microtubule reduction in Alzheimer’s disease and aging is independent of tau filament formation. Am J Pathol. 2003;162:1623–7. doi: 10.1016/s0002-9440(10)64296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SA, Kim EH, Lee JY, et al. Preconditioning with chronic cerebral hypoperfusion reduces a focal cerebral ischemic injury and increases apurinic/apyrimidinic endonuc-lease/redox factor-1 and matrix metalloproteinase-2 expression. Curr Neurovasc Res. 2007;4:89–97. doi: 10.2174/156720207780637252. [DOI] [PubMed] [Google Scholar]
- De Jong GI, De Vos RA, Steur EN, et al. Cerebrovascular hypoperfusion: a risk factor for Alzheimer’s disease? Animal model and postmortem human studies. Ann N Y Acad Sci. 1997;826:56–74. doi: 10.1111/j.1749-6632.1997.tb48461.x. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Hemodynamic consequences of deformed microvessels in the brain in Alzheimer’s disease. Ann N Y Acad Sci. 1997;826:75–91. doi: 10.1111/j.1749-6632.1997.tb48462.x. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Critically attained threshold of cerebral hypoperfusion: the CATCH hypothesis of Alzheimer’s pathogenesis. Neurobiol Aging. 2000;21:331–42. doi: 10.1016/s0197-4580(00)00111-1. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Alzheimer’s disease: how does it start? J Alzheimer’s Dis. 2002a;4:497–512. doi: 10.3233/jad-2002-4606. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Alzheimer disease as a vascular disorder: nosological evidence. Stroke. 2002b;33:1152–62. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. How do heart disease and stroke become risk factors for Alzheimer’s disease? Neurol Res. 2006;6:637–44. doi: 10.1179/016164106X130362. [DOI] [PubMed] [Google Scholar]
- de la Torre JC, Aliev G. Inhibition of vascular nitric oxide after rat chronic brain hypoperfusion: spatial memory and immunocytochemical changes. J Cereb Blood Flow Metab. 2005;25:663–72. doi: 10.1038/sj.jcbfm.9600057. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frackowiak RS. Cerebral function in aging and Alzheimer’s disease: the role of PET. Electroencephalogr Clin Neurophysiol Suppl. 1991;42:355–65. [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, et al. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–23. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb BA, Bardel KA, Kulnane LS, et al. Amyloid production and deposition in mutant amyloid precursor protein and presenilin-1 yeast artificial chromosome transgenic mice. Nat Neurosci. 1999;2:695–7. doi: 10.1038/11154. [DOI] [PubMed] [Google Scholar]
- Kumar A, Schapiro MB, Haxby JV, et al. Cerebral metabolic and cognitive studies in dementia with frontal lobe behavioral features. J Psychiatr Res. 1990;24:97–109. doi: 10.1016/0022-3956(90)90050-z. [DOI] [PubMed] [Google Scholar]
- Kalback W, Esh C, Castaño EM, et al. Atherosclerosis, vascular amyloidosis and brain hypoperfusion in the pathogenesis of sporadic Alzheimer’s disease. Neurol Res. 2004;26:525–39. doi: 10.1179/016164104225017668. [DOI] [PubMed] [Google Scholar]
- Lishmanov Y, Shvera I, Ussov W, et al. The effect of carotid endarterectomy on cerebral blood flow and cerebral blood volume studied by SPECT. J Neuroradiol. 1997;24:155–62. [PubMed] [Google Scholar]
- Moreira PI, Siedlak SL, Wang X, et al. Autophagocytosis of mitochondria is prominent in Alzheimer disease. J Neuropathol Exp Neurol. 2007;66:525–32. doi: 10.1097/01.jnen.0000240476.73532.b0. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–67. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- Roher AE, Esh C, Kokjohn TA, et al. Circle of willis atherosclerosis is a risk factor for sporadic Alzheimer’s disease. Arterioscler Thromb Vasc Biol. 2003;23:2055–62. doi: 10.1161/01.ATV.0000095973.42032.44. [DOI] [PubMed] [Google Scholar]
- Shi J, Perry G, Aliev G, et al. Serum amyloid P is not present in amyloid beta deposits of a transgenic animal model. Neuro Report. 1999;10:3229–32. doi: 10.1097/00001756-199910190-00019. [DOI] [PubMed] [Google Scholar]
- Shi J, Perry G, Berridge MS, et al. Labeling of cerebral amyloid beta deposits in vivo using intranasal basic fibroblast growth factor and serum amyloid P component in mice. J Nucl Med. 2002a;43:1044–51. [PubMed] [Google Scholar]
- Shi J, Seyidova D, Perry G, et al. Neuronal mitochondria abnormalities in a transgenic mouse model overexpressing amyloid beta. Society for Neuroscience. 2002b;295:20. [Google Scholar]
- Skoog I, Gustafson D. Update on hypertension and Alzheimer’s disease. Neurol Res. 2006;28:605–11. doi: 10.1179/016164106X130506. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Matsuyama T, Moriwaki H, et al. Circulating CD34-positive cells provide an index of cerebrovascular function. Circulation. 2004;109:2972–5. doi: 10.1161/01.CIR.0000133311.25587.DE. [DOI] [PubMed] [Google Scholar]