Abstract
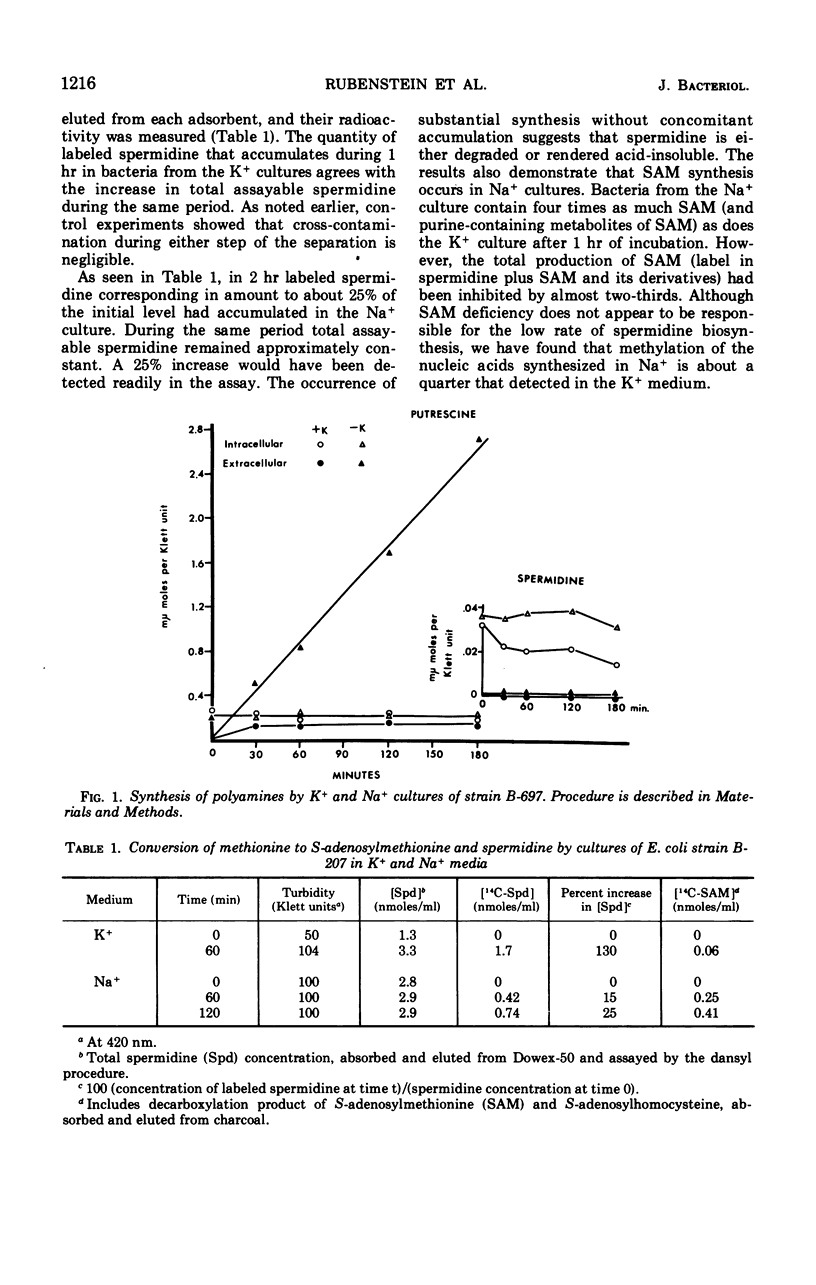
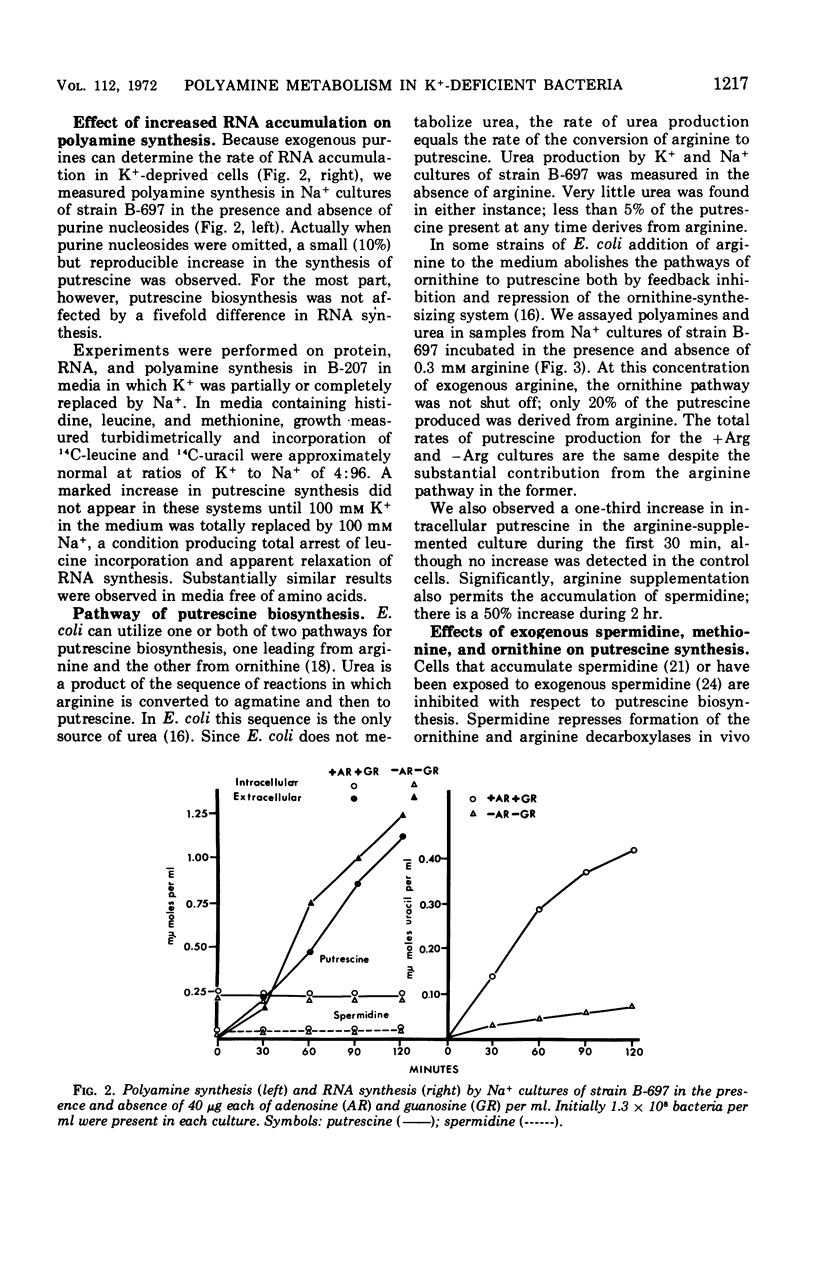
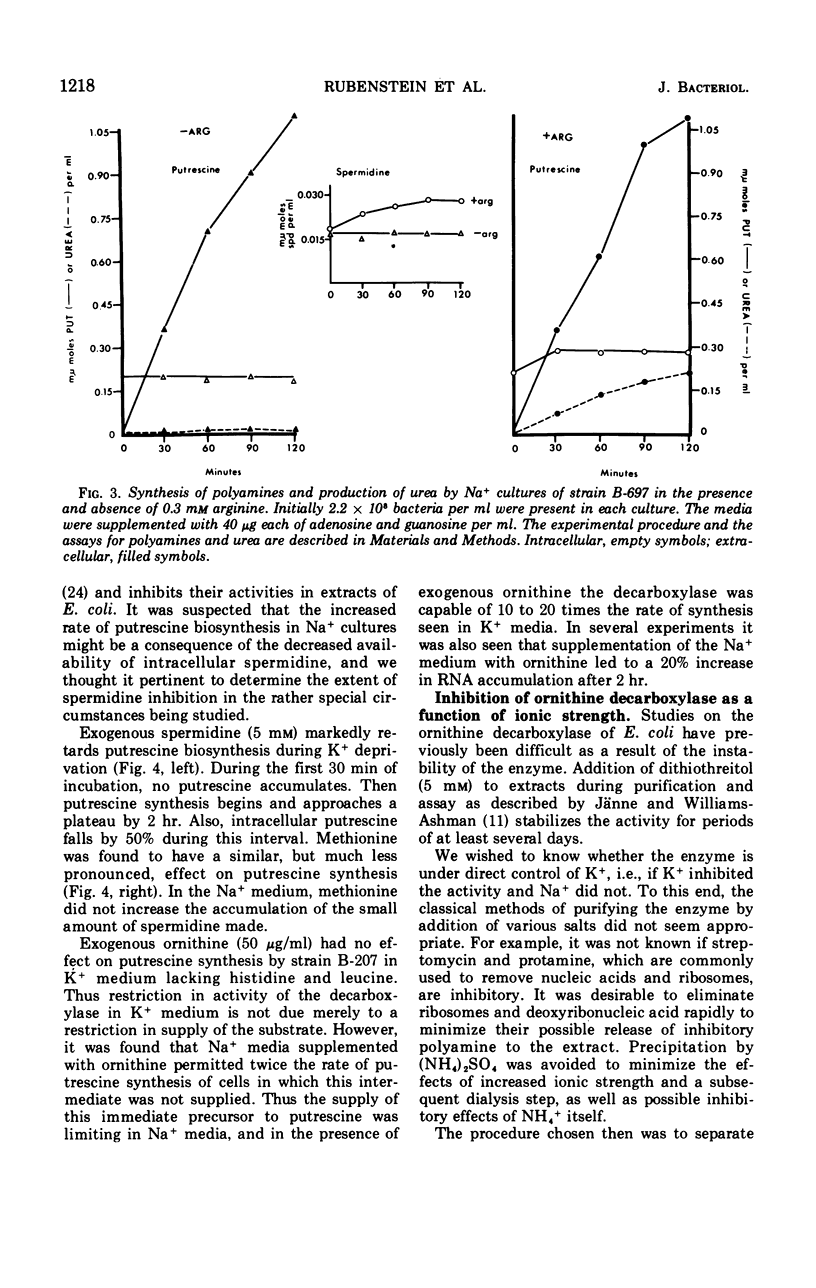
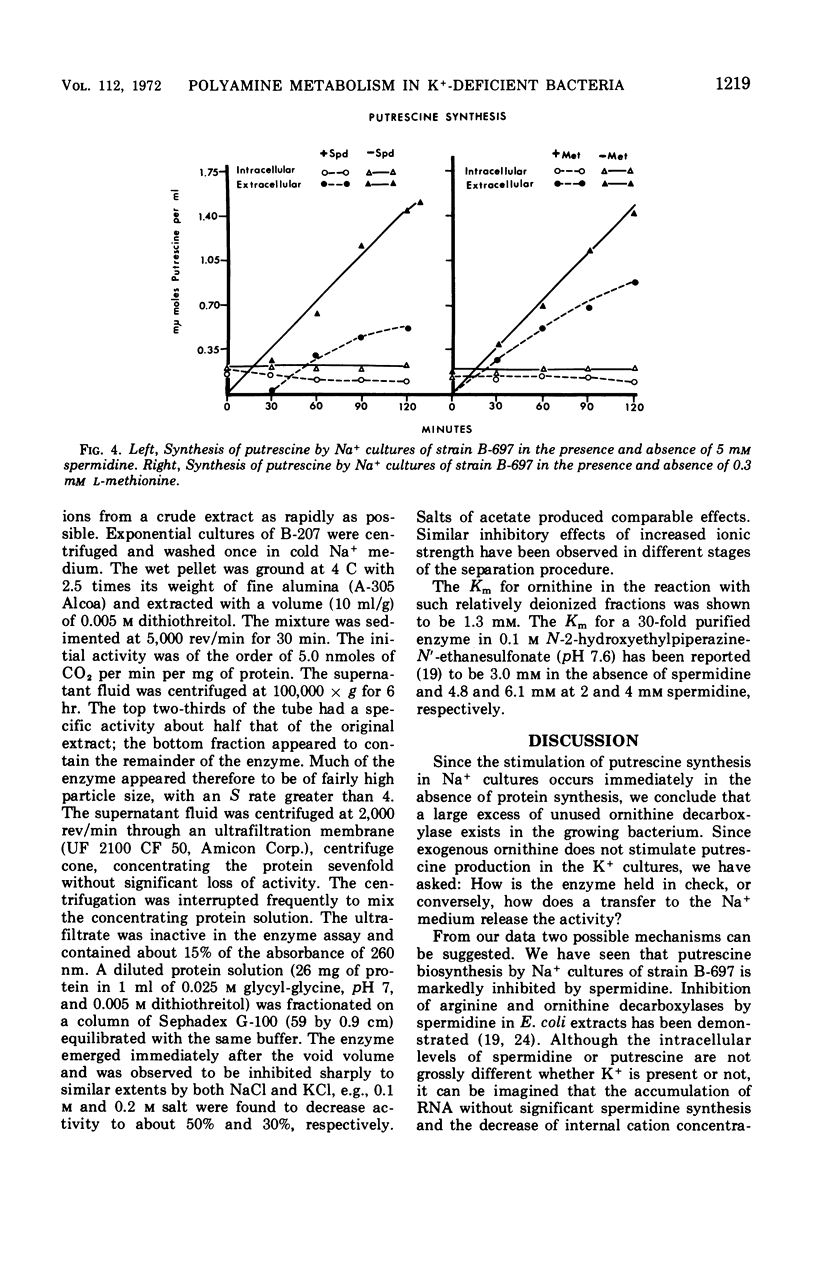
The metabolism of polyamines was studied in K+-dependent strains of Escherichia coli. When these stringent organisms were in a medium containing Na+ instead of K+, protein synthesis was arrested, but synthesis of ribonucleic acid continued as it would in a relaxed organism. The Na+ medium inhibited synthesis of spermidine and S-adenosylmethionine. However, the synthesis of putrescine was accelerated at least five- to eightfold. Exogenous ornithine doubled even this rate of putrescine synthesis but did not increase the low level of putrescine synthesis in the K+ medium. In K+ or Na+ media, with or without 0.3 mm arginine, putrescine was derived almost entirely from ornithine via ornithine decarboxylase. Addition of spermidine (5 mm) to a Na+ culture markedly inhibited putrescine synthesis. The ornithine decarboxylase of an extract of a K−-dependent strain prepared at low ionic strength was separated from ribosomes, deoxyribonucleic acid, and associated polyamines by centrifugation, and from many ions by ultrafiltration and fractionation on Sephadex G-100. Addition of Na+ and K+ salts to 200 mm was markedly inhibitory. The combined reductions both in synthesis of the inhibitor spermidine and in intracellular ionic strength may explain the in vivo activation of this enzyme.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen S. S., Hoffner N., Jansen M., Moore M., Raina A. POLYAMINES, RNA SYNTHESIS, AND STREPTOMYCIN LETHALITY IN A RELAXED MUTANT OF E. coli STRAIN 15 TAU. Proc Natl Acad Sci U S A. 1967 Mar;57(3):721–728. doi: 10.1073/pnas.57.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S., Morgan S., Streibel E. The polyamine content of the tRNA of E. coli. Proc Natl Acad Sci U S A. 1969 Oct;64(2):669–676. doi: 10.1073/pnas.64.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion A. S., Cohen S. S. Polyamine stimulation of nucleic acid synthesis in an uninfected and phage-infected polyamine auxotroph of Escherichia coli K12 (arginine-agmatine ureohydrolase-putrescine-spermidine-lysine-cadaverine). Proc Natl Acad Sci U S A. 1972 Jan;69(1):213–217. doi: 10.1073/pnas.69.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENNIS H. L., LUBIN M. Dissociation of ribonucleic acid and protein synthesis in bacteria deprived of potassium. Biochim Biophys Acta. 1961 Jun 24;50:399–402. doi: 10.1016/0006-3002(61)90355-9. [DOI] [PubMed] [Google Scholar]
- ENNIS H. L., LUBIN M. PRE-RIBOSOMAL PARTICLES FORMED IN POTASSIUM-DEPLETED CELLS. STUDIES ON DEGRADATION AND STABILIZATION. Biochim Biophys Acta. 1965 Apr 19;95:605–623. doi: 10.1016/0005-2787(65)90515-0. [DOI] [PubMed] [Google Scholar]
- Ennis H. L. Role of potassium in the regulation of polysome content and protein synthesis in Escherichia coli. Arch Biochem Biophys. 1971 Jan;142(1):190–200. doi: 10.1016/0003-9861(71)90275-x. [DOI] [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. Mammalian ornithine decarboxylase: activation and alteration of physical behaviour by thiol compounds. Biochem J. 1970 Sep;119(3):595–597. doi: 10.1042/bj1190595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUBIN M., ENNIS H. L. ON THE ROLE OF INTRACELLULAR POTASSIUM IN PROTEIN SYNTHESIS. Biochim Biophys Acta. 1964 Apr 27;80:614–631. doi: 10.1016/0926-6550(64)90306-8. [DOI] [PubMed] [Google Scholar]
- LUBIN M. INTRACELLULAR POTASSIUM AND CONTROL OF PROTEIN SYNTHESIS. Fed Proc. 1964 Sep-Oct;23:994–1001. [PubMed] [Google Scholar]
- LUBOCHINSKY B., MEURY J., STOLKOWSKI J. CIN'ETIQUE DES 'ECHANGES DE POTASSIUM CHEZ L'ESCHERICHIA COLI, SOUCHE B 207, QUI NE PEUT CRO ITRE NORMALEMENT QU'EN PR'ESENCE DE CONCENTRATIONS 'ELEV'EES EN POTASSIUM. C R Hebd Seances Acad Sci. 1964 May 20;258:5106–5109. [PubMed] [Google Scholar]
- MUDD S. H., CANTONI G. L. Activation of methionine for transmethylation. III. The methionine-activating enzyme of Bakers' yeast. J Biol Chem. 1958 Mar;231(1):481–492. [PubMed] [Google Scholar]
- Morris D. R., Koffron K. L. Urea production and putrescine biosynthesis by Escherichia coli. J Bacteriol. 1967 Nov;94(5):1516–1519. doi: 10.1128/jb.94.5.1516-1519.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Pardee A. B. A biosynthetic ornithine decarboxylase in Escherichia coli. Biochem Biophys Res Commun. 1965 Sep 22;20(6):697–702. doi: 10.1016/0006-291x(65)90072-0. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Pardee A. B. Multiple pathways of putrescine biosynthesis in Escherichia coli. J Biol Chem. 1966 Jul 10;241(13):3129–3135. [PubMed] [Google Scholar]
- Raina A., Jansen M., Cohen S. S. Polyamines and the accumulation of ribonucleic acid in some polyauxotrophic strains of Escherichia coli. J Bacteriol. 1967 Nov;94(5):1684–1696. doi: 10.1128/jb.94.5.1684-1696.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W. The effects of temperature on the acetylation of spermidine. Biochem Biophys Res Commun. 1968 Feb 26;30(4):339–342. doi: 10.1016/0006-291x(68)90747-x. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Formation of 1,4-diaminobutane and of spermidine by an ornithine auxotroph of Escherichia coli grown on limiting ornithine or arginine. J Biol Chem. 1969 May 10;244(9):2286–2292. [PubMed] [Google Scholar]
- VYAS S., MAAS W. K. Feedback inhibition of acetylglutamate synthetase by arginine in Escherichia coli. Arch Biochem Biophys. 1963 Mar;100:542–546. doi: 10.1016/0003-9861(63)90124-3. [DOI] [PubMed] [Google Scholar]
- Van Dijk-Salkinoja M. S., Planta R. J. Effects of tris and oligoamines on the ribosomal size distribution in lysates of B. licheniformis. FEBS Lett. 1970 Dec 23;12(1):9–13. doi: 10.1016/0014-5793(70)80581-6. [DOI] [PubMed] [Google Scholar]


