Abstract
Microsomal triglyceride transfer protein (Mttp) is a key player in the assembly and secretion of hepatic very low density lipoproteins (VLDL). Here we determined the effects of Mttp overexpression on hepatic triglyceride (TG) and VLDL secretion in leptin-deficient (ob/ob) mice, specifically in relation to apolipoproteinB (apoB) isoforms. We crossed Apobec1−/− mice with congenic ob/ob mice to generate apoB100-only ob/ob mice (A-ob/ob). The obesity phenotype in both genotypes was similar, but A-ob/ob mice had greater hepatic TG content. Administration of recombinant adenovirus expressing murine Mttp cDNA (Ad-mMTP) increased hepatic Mttp content and activity and increased hepatic VLDL-TG secretion in A-ob/ob mice. However, despite equivalent overexpression of Mttp, there was no change in VLDL-TG secretion in ob/ob mice in a wild-type Apobec1 background. Metabolic labeling studies in primary hepatocytes from A-ob/ob mice demonstrated that Ad-mMTP increased triglyceride secretion without changing the synthesis and secretion of apoB100, suggesting greater incorporation of TG into existing VLDL particles rather than increased particle number. Ad-mMTP administration failed to increase hepatic VLDL secretion in lean Apobec1−/− mice or controls. By contrast, VLDL secretion increased and hepatic TG content decreased following Ad-mMTP administration to human APOB transgenic mice crossed into the Apobec1−/− line. These findings demonstrate that Ad-mMTP increases murine hepatic VLDL-TG secretion only in the apoB100 background, and even then only in situations with either increased hepatic TG accumulation or increased apoB100 expression.
Keywords: hepatic steatosis, VLDL secretion, apoB mRNA editing, apobec1
Biosynthesis of hepatic very low density lipoprotein (VLDL) is critically dependent on the coordinated interactions of two dominant proteins, namely apolipoprotein B (apoB) and the microsomal triglyceride transfer protein (Mttp) (as reviewed in Refs. 1–3). ApoB is an obligatory structural component of VLDL and requires progressive lipidation, mediated by the resident endoplasmic reticulum chaperone Mttp, in order to maintain optimal conformational integrity and folding during the process of lipoprotein assembly. In the setting of either limiting Mttp availability or inhibition of Mttp function, the nascent apoB polypeptide becomes misfolded and undergoes presecretory degradation (as reviewed in Refs. 2–4). The importance of these two genes and their interdependent function is illustrated by the phenotypes associated with deletion or mutations in either MTTP and/or APOB gene in humans, which is accompanied by impaired hepatic VLDL secretion and extremely low levels of apoB in plasma (as reviewed in Refs. 3 and 5). These phenotypes have been reproduced using experimentally induced mutations in murine models, suggesting that these integrated and codependent functions are highly conserved (5–7).
Studies in rodent hepatoma cells and in primary hepatocytes have revealed an apoB isoform dependence for Mttp-mediated lipidation, with apoB100 exhibiting dramatically more susceptibility to presecretory degradation following pharmacologic inhibition of Mttp, compared with the shorter isoform apoB48 (8–11). This apoB-isoform dependence upon Mttp was dramatically illustrated in studies using isolated hepatocytes from mice with liver-specific deletion of Mttp, where the secretion of apoB100 was virtually eliminated while the effects on apoB48 secretion were modest (12). Furthermore, similar studies in heterozygous Mttp knockout mice demonstrated that apoB100 secretion was also selectively reduced, suggesting that the secretion of apoB100 from mouse liver may be limited by as little as a 50% reduction in Mttp availability (7, 13).
The role of Mttp expression has been explored in leptin-deficient ob/ob mice, which exhibit severe hepatic steatosis due to dramatically induced hepatic lipogenesis (14–16) coupled with increased free fatty acid flux from adipose depots (14, 15). This combination would be expected a priori to induce hepatic VLDL-TG secretion, yet previous studies have shown that VLDL secretion is either unchanged (16) or decreased (17) in these mice. This discrepancy could conceivably be attributed to the finding that hepatic Mttp expression is reduced in ob/ob mice (18), raising the possibility that a relative deficiency of Mttp might potentially contribute to the dramatic accumulation of hepatic lipid in this genetic background (18). In support of this possibility, increased Mttp expression was observed in ob/ob mice following combined adenovirus-mediated hepatic expression of the transcriptional coactivators Pgc-1β and Foxa2, which in turn led to increased serum triglyceride levels and decreased hepatic steatosis (18). Weighed against this, however, is evidence from other studies that demonstrated either no change or even increased Mttp expression in livers from ob/ob mice (16, 19). While leaving open the question of whether an underlying defect in Mttp expression is responsible for hepatic steatosis in ob/ob mice, these studies collectively suggest the possibility that directly augmenting hepatic Mttp expression, for example through adenovirus-mediated delivery, might result in enhanced VLDL secretion in the ob/ob background.
In the current study, we addressed two related hypotheses emerging from these observations. We first addressed the possibility that hepatic steatosis in ob/ob mice would be exacerbated by imposing an apoB100-only genotype on this background. We reasoned that the selective requirement for Mttp-mediated lipidation of apoB100 would magnify the defect in VLDL secretion in the setting of the augmented triglyceride accumulation observed in an apoB100-only ob/ob model. In considering this possibility, however, we were also aware of the findings of Li, Grundy, and Patel (17) that the secretion of apoB100 in ob/ob mice was actually somewhat increased compared with lean controls, suggesting that there may be alternative possibilities in regard to the regulation of VLDL secretion in this model. Second, we examined the corollary hypothesis that liver-specific overexpression of Mttp might augment apoB100-VLDL secretion by “rescuing” this apparently selective impairment in either the ob/ob background or in an apoB100-only background with varying levels of apoB100.
To address these hypotheses, we generated Apobec1 null (apoB100-only) ob/ob mice and examined the effects of adenovirus-mediated hepatic overexpression of murine Mttp (Ad-mMTP) on plasma lipids and VLDL-TG secretion. We further examined the effects of adenovirus-mediated hepatic overexpression of Mttp in the Apobec1−/− background with crosses into a line of human APOB transgenic mice (HuBTg+/0). Our findings suggest that Ad-mMTP increases murine hepatic VLDL-TG secretion only in the apoB100 background, and even then only in situations of either increased hepatic TG accumulation or increased apoB100 expression.
METHODS
Materials
[35S]Promix (530 MBq/ml), an L-[35S]Met and L-[35S]Cys metabolic labeling solution, was from Amersham Bioscience Corp. (Piscataway, NJ). 2-[3H]glycerol (20 mCi/mmol) was from American Radiolabeled Chemicals, Inc. (St Louis, MO). Triton WR-1339 (Tyloxapol), rabbit antihuman albumin and antimouse actin antisera and oleic acid were from Sigma Chemical Co. (St. Louis, MO). Antimouse apoB antibodies have been described (20, 21).
Animals
Leptin deficient ob/ob mice in a C57BL/6 background were obtained from Jackson Laboratory (Bar Harbor, ME) and crossed with C57BL/6 congenic Apobec1−/− mice [generated previously as described (22)] to produce apoB100-only ob/ob mice (A-ob/ob). Transgenic human apoB expressing mice (HuBTg+/0) have been previously characterized in detail (13, 23) and were obtained from Stephen Young. HuBTg+/0 mice were also crossed into the Apobec1−/− background to generate a line referred to as A-HuBTg+/0. Mice were housed in a specific-pathogen-free barrier facility with a 12 h light/dark cycle and fed a regular mouse chow diet (Ralston Purina, St. Louis, MO) with free access to food and water. For body weight studies, mice were weighed weekly starting at 4 weeks. All animal experiments were approved by the Animal Studies Committee of Washington University School of Medicine, and conformed to criteria outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Phenotyping
Plasma TG and cholesterol measurements were determined using Infinity enzymatic assay kits (Fisher Scientific) and expressed as mg/dl. Glucose, phospholipid, and free fatty acid levels were determined using commercially available kits (Wako Chemicals, Richmond, VA). Serum alanine aminotransferase (ALT) levels were determined using a kit from Teco Diagnostics (Anaheim, CA). For analysis of the lipoprotein profile, serum was pooled (four animals/genotype, total volume ∼200μl) and fractionated on tandem Superose 6 columns using a Pharmacia fast-protein liquid chromatograph instrument. Fractions were stored overnight at 4°C before determination of cholesterol content using a commercially available kit (Wako Chemicals). Hepatic TG, phospholipid, and cholesterol levels were determined following extraction of lipid with chloroform:methanol and solubilization with Triton X-100 as described (20). Data were normalized to wet tissue weight (mg/g liver).
Construction of recombinant adenoviruses
A mouse Mttp full-length cDNA (∼2.7 kb) was generated from mouse liver RNA by RT-PCR using an upstream PCR primer 5′-gggagatctAGGGAGCCAGCATGATCCTCTTGGCAGTGC-3′ and a downstream primer 5′-gggaagctTGGAAGGGAACCCACGGGAATCAAAACCATCC-3, which contain Bgl II and Hind III restriction sites, respectively, to facilitate subcloning. The Mttp cDNA was subcloned into a pCR-XL-TOPO vector (Invitrogen) and the entire insert was sequenced to verify its identity and integrity. To construct recombinant adenoviral vector to overexpress mouse Mttp, the Mttp cDNA was excised from the pCR-XL-TOPO vector and subcloned into an adenoviral shuttle vector (Qbiogene, Madison, WI) that contains a green fluorescent protein (GFP) cDNA under the control of a separate CMV promoter. The shuttle vector expressing both Mttp and GFP was homologously recombined into an AdEasy-1 vector and the recombinant adenoviruses were purified and amplified using an AdEasy-1 system (Qbiogene) following the manufacturer's instruction. High-titer adenoviral particles were isolated from adenovirus-infected 293 cell lysates by double CsCl-gradient bandings. A recombinant adenovirus expressing GFP alone was also produced and used for control experiments. The adenoviral preparations were titered on 293 cells by monitoring cellular GFP signal.
In vivo studies
Mice used for in vivo studies were 10–20 weeks of age. Male and female mice were used, with no difference noted between the sexes. To produce hepatic Mttp overexpression, mice (12–14 weeks old) were intravenously infused with adenoviruses expressing mouse Mttp and GFP (Ad-mMTP) or GFP alone (Ad-GFP) at doses of 2–3 × 1011 viral particles (approximately 0.5 × 1010 pfu) for each lean mouse and 4–6 × 1011 viral particles (1.0 × 1010 pfu) for each mouse on the ob/ob genetic background. Pilot experiments demonstrated that hepatic Mttp protein expression peaked 4 days after adenoviral injection and was maintained at this level for at least 2 additional days (data not shown). Blood samples were taken at day 0 (before infusion) and at day 5 after adenoviral infusion. In vivo rates of VLDL-TG secretion were determined 5 days after adenoviral transduction using Triton WR-1339 (650 mg/kg body weight) as a blocker of VLDL-TG lipolysis/uptake as described (20, 21, 24, 25).
Metabolic labeling studies using primary hepatocytes
Primary mouse hepatocytes were isolated and cultured as described (20, 21). Adenoviral transductions were carried out by incubating hepatocytes with Ad-mMTP or Ad-GFP in 5% FBS/DMEM media overnight in 24-well plates (0.5 ml/well) or 6-well plates (2 ml/well) at a M.O.I. of 8. Experiments were carried out 24–30 h after adenoviral transduction. To measure rates of synthesis and secretion of TG, hepatocytes were incubated with 2-[3H]glycerol-containing DMEM (10 μCi/ml) for the specified time periods with 0.3 mM oleate complexed to BSA (21). [3H]labeled TG was isolated from cells and medium and [3H]radioactivity determined as described (20, 21). Data were expressed as DPM/mg protein/h. To determine apoB synthesis and secretion, hepatocytes were labeled with [35S]methionine for 3 h in the presence of 0.3 mM oleic acid (21). [35S]Labeled apoB and albumin in cells and media were immunoprecipitated and separated on a gradient SDS-gel (3–15%) and quantified as described (20, 21).
Analysis of Mttp mass and activity
The relative abundance of Mttp protein in the liver or in cultured hepatocytes was determined by Western blot using a monoclonal antimouse Mttp antibody (BD Transduction Laboratories, Lexington, KY). Mttp activity in the liver was measured with a fluorescent assay kit (Roar Biomedical, New York, NY) according to the manufacturer's protocol and as described (26), except that liver microsomes were used in our assay instead of whole liver homogenates to minimize interference from cellular GFP. Microsomes were prepared from the postnuclear fraction of liver tissue and resuspended with Mttp assay buffer as described (8, 27). Each assay was carried out in triplicate and activity expressed as pmol/min/mg protein. Protein content of liver and cell samples was determined using a BCA method (Pierce).
Statistics
All data are presented as mean ± SD, unless otherwise noted. Differences in the mean values between two groups were assessed by 2-tailed Student's t-test. Differences in mean values between more than two groups were determined by ANOVA. P < 0.05 was considered to be statistically significant.
RESULTS
ApoB isoform, obesity, and hepatic lipid phenotypes in ob/ob mice
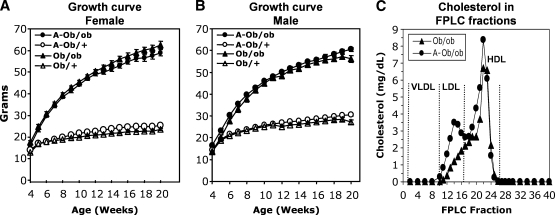
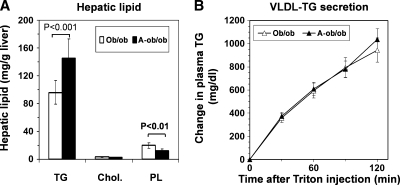
We crossed Apobec1−/− mice with ob/ob mice to generate a compound line (A-ob/ob). Growth curves of ob/ob and A-ob/ob mice are indistinguishable in both genders (Fig. 1A, B). These mice had similar plasma concentrations of triglycerides, free fatty acids, glucose, and plasma ALT levels, but plasma total cholesterol levels in A-ob/ob mice were significantly higher than those in Apobec1-sufficient ob/ob mice (Table 1). Fast-protein liquid chromatograph determination revealed that increased cholesterol was primarily associated with LDL-sized particles in A-ob/ob mice (Fig. 1C), as predicted based on the exclusive presence of apoB100. There was a similar distribution of plasma TG in both genotypes, but a more pronounced peak in the LDL range in A-ob/ob mice (data not shown). Hepatic TG content was significantly increased and phospholipid content decreased in A-ob/ob mice compared with Apobec1 sufficient ob/ob mice (Fig. 2A), but hepatic VLDL-TG secretion was comparable (Fig. 2B). Together, these data demonstrate that imposing an apoB100-only background onto ob/ob mice induces a subtle phenotype with regard to hepatic lipid metabolism, but does not change VLDL secretion.
Fig. 1.
Characterization of A-ob/ob, ob/ob, A-ob/+, and ob/+ mice. Body weight (g) of chow-fed female (A) and male (B) A-ob/ob, ob/ob, A-ob/+, and ob/+ mice from 4–20 weeks of age. Each data point represents mean and standard error of 9–18 mice per genotype. C: Cholesterol distribution among fast-protein liquid chromatograph-fractionated lipoproteins of serum obtained from 20-week-old ob/ob (black triangles) and A-ob/ob (black circles) mice. Serum from four animals per genotype was pooled. FPLC, fast-protein liquid chromatography.
TABLE 1.
Plasma concentrations of triglycerides, cholesterol, free fatty acids, ALT, and glucose of A-ob/ob and ob/ob mice
| Triglycerides | Cholesterol | Free fatty acids | ALT | Glucose | |
|---|---|---|---|---|---|
| (mg/dl) | (mg/dl) | (mM) | (IU/l) | (mg/dl) | |
| Ob/ob | 23.2 ± 10.08 | 109.3 ± 24 | 0.259 ± 0.108 | 98.9 ± 38 | 428 ± 100.4 |
| A-ob/ob | 30.4 ± 8.42 | 139.4 ± 29** | 0.204 ± 0.07 | 102.2 ± 36.5 | 359 ± 122.5 |
Fasted blood samples were obtained from 20-week-old ob/ob or A-ob/ob mice. Each value represents mean and SD of nine mice. ** A-ob/ob vs. ob/ob, P < 0.001.
Fig. 2.
Hepatic lipid metabolism in ob/ob and A-ob/ob mice. A: Hepatic lipid content of 20-week-old ob/ob (open bars) and A-ob/ob (black bars) mice. B: Hepatic VLDL-TG secretion in 12-week-old ob/ob (open triangles) and A-ob/ob (black triangles) mice following injection of Triton WR-1339. Each data point represents mean and SD (n = 9/genotype in A and n = 5/genotype in B).
Hepatic overexpression of Mttp increases VLDL-TG secretion in vivo in Apobec1-null but not Apobec1 sufficient ob/ob mice
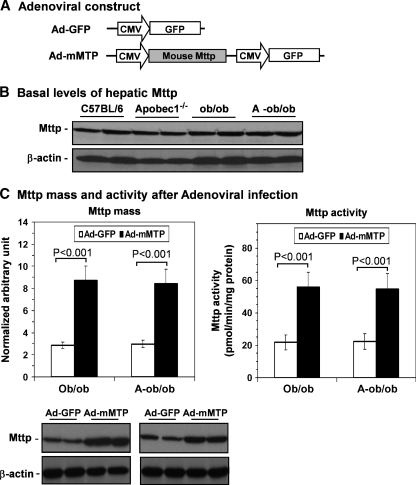
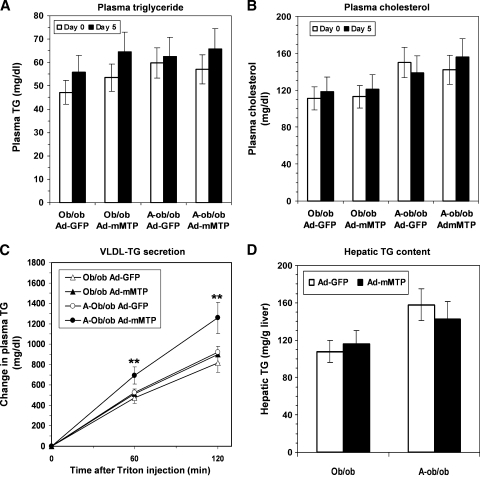
In order to pursue the hypothesis that augmenting Mttp expression might yield distinctive alterations in VLDL secretion in a background of apoB100 versus apoB48, we generated recombinant adenoviruses encoding either a murine Mttp cDNA (Ad-mMTP) or GFP (Ad-GFP) as a control (Fig. 3A). Relative protein levels of hepatic Mttp were quantified by Western blot analysis, which demonstrated that hepatic Mttp abundance was comparable in all genotypes (Fig. 3B). These results suggest that an intrinsic deficiency in Mttp expression was unlikely to account for the hepatic steatosis in our ob/ob lines. We next turned to the gain-of-function experiments and found that on day 5 after intravenous administration, both hepatic Mttp content and activity (Fig. 3C) were increased (in the range of 2- to 3-fold) in both ob/ob and A-ob/ob mice, suggesting that delivery and functional expression of the recombinant Mttp was successful. Nevertheless, expression of Ad-mMTP failed to impact plasma TG or cholesterol levels in either line of ob/ob mice (Fig. 4A, B). Moreover, hepatic Mttp overexpression failed to augment VLDL-TG secretion in ob/ob mice (Fig. 4C), contrary to our expectations. By contrast, Ad-mMTP administration significantly increased VLDL-TG secretion in A-ob/ob mice (132% at 2 h; P < 0.01, Fig. 4C). Hepatic TG content was unchanged in either genotype although there was a modest trend toward decreased TG in A-ob/ob mice following Ad-mMTP administration (Fig. 4D). These findings suggest that the gain-of-function phenotype associated with augmented abundance of Mttp may be differentially modified by the presence of apoB100.
Fig. 3.
Adenovirus-mediated overexpression of a mouse Mttp cDNA in livers of ob/ob and A-ob/ob mice. A: Schematic representation of Ad-green fluorescent protein (GFP) and Ad-mMTP constructs. B: Hepatic Mttp content in homogenates prepared from C57BL/6, Apobec1−/−, ob/ob, and A-ob/ob mice demonstrated by Western blot analysis (liver samples from four mice per genotype were pooled). Expression of β-actin was monitored as a loading control. C: Increased hepatic Mttp mass and activity following Ad-mMTP infection. Mice of the indicated genotype were treated with either Ad-mMTP (black bars) or control Ad-GFP (open bars) adenoviral construct as shown in the top panel. Infection of mice with Ad-mMTP resulted in a ∼3-fold increase in Mttp protein abundance and a 2.5-fold increase in Mttp activity 5 days following adenoviral infusion. A representative Western blot showing expression of Mttp protein in livers of mice infused with Ad-GFP or Ad-mMTP is shown, with β-actin being used as a loading control. Mttp activity was determined on liver microsomes using a fluoresence method as described in Materials and Methods. Each data point represents mean and SD of five mice.
Fig. 4.
Changes in serum and hepatic lipid levels in ob/ob and A-ob/ob mice following infusion of Ad-mMTP. Plasma triglyceride (A) and cholesterol (B) levels in ob/ob and A-ob/ob mice on day 0 (open bars) or day 5 (black bars) following infusion of either Ad-GFP or Ad-mMTP. C: Hepatic VLDL-triglyceride secretion following injection of Triton WR-1339 in ob/ob and A-ob/ob mice treated with Ad-GFP or Ad-mMTP. Asterisks indicate P < 0.01. D: Hepatic triglyceride content in ob/ob and A-ob/ob mice infused with Ad-GFP (open bars) or Ad-mMTP (black bars). Each data point represents mean and SD of five mice.
Ad-mMTP differentially modulates VLDL secretion in isolated hepatocytes from Apobec1-null ob/ob mice compared with the parental ob/ob strain
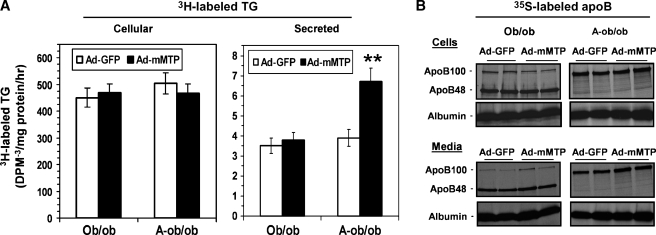
In order to determine more directly the impact of augmenting Mttp expression on hepatic VLDL secretion, we turned to studies in isolated hepatocytes from ob/ob or A-ob/ob mice infected in vitro with Ad-GFP or Ad-mMTP. We again achieved a 3- to 4-fold increase in Mttp abundance in all genotypes (data not shown). Metabolic labeling experiments revealed that Ad-mMTP expression specifically increased the secretion of [3H]glycerol-labeled TG by A-ob/ob hepatocytes (Fig. 5A, right panel), although intracellular accumulation of [3H]TG was unchanged by Mttp overexpression (Fig. 5A, left panel). These findings suggest that augmenting Mttp abundance and activity increases the fractional secretion of newly synthesized TG from A-ob/ob mice. By contrast, and in agreement with our results from the in vivo experiments noted above, neither the synthesis nor secretion of TG were modulated by Ad-mMTP in hepatocytes isolated from ob/ob mice (Fig. 5A). In order to examine whether the increased secretion of TG from isolated hepatocytes was associated with alterations in the secretion of apoB, we undertook radiolabeling and immunoprecipitation experiments in isolated hepatocytes following adenoviral infection. The findings demonstrate that the increased TG secretion by Ad-mMTP-infected A-ob/ob hepatocytes was not accompanied by enhanced secretion of [35S]labeled apoB100 (Fig. 5B and, in data not shown, confirmed by quantitative phosphorimaging of radioactivity incorporation), suggesting that augmenting Mttp abundance in isolated hepatocytes acts predominantly to increase TG incorporation into existing VLDL particles. In addition, there was no change (again confirmed by quantitative determination of radioactivity incorporation into apoB using phosphorimaging) in the secretion of either apoB100 or apoB48 in ob/ob hepatocytes following infection with Ad-mMTP (Fig. 5B and data not shown). Taken together, these findings raised the possibility that a gain-of-function phenotype associated with augmenting Mttp expression appears to be confined to apoB100-only expressing murine hepatocytes.
Fig. 5.
Synthesis and secretion of [3H]glycerol-labeled (3 h) triglyceride (A) and [35S]labeled (3 h) apoB (B) from primary hepatocytes prepared from ob/ob and A-ob/ob mice infected with Ad-GFP (open bars) or Ad-mMTP (black bars). Asterisks indicate P < 0.001. Radiolabeling of albumin was monitored as a control for equivalent incorporation of [35S]methionine/cysteine. Each data point represents mean and SD of four incubations. These data are representative findings from two independent experiments.
Ad-mMTP differentially modulates VLDL secretion in Apobec1−/− mice in response to increased apoB expression
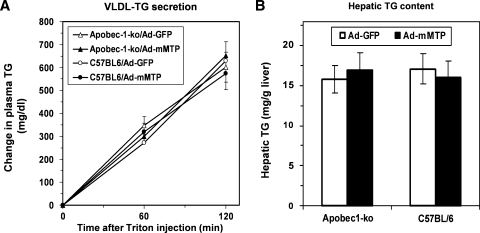
In order to pursue the hypothesis that apoB isoform dependence accounts for the gain-of-function phenotype with respect to augmented Mttp expression in murine hepatocytes, we administered Ad-mMTP to lean Apobec1−/− mice and lean wild-type controls. Hepatic Mttp abundance was increased ∼3-fold in both genotypes (data not shown), yet as noted above in neither instance was there a discernable effect on either VLDL-TG secretion (Fig. 6A) or hepatic TG content (Fig. 6B). These results suggest that merely augmenting hepatic Mttp activity alone is insufficient to increase hepatic VLDL secretion, even in an apoB100-only background. We extended these findings to studies of Ad-mMTP supplementation in isolated primary hepatocytes from wild-type and Apobec1−/− mice where again we found no effect on VLDL-TG or apoB100 secretion in either genotype (data not shown).
Fig. 6.
Hepatic lipid metabolism following Mttp overexpression in Apobec1−/− and C57BL/6 mice. A: Hepatic VLDL-TG secretion by lean Apobec1−/− (triangles) or lean C57BL/6 (circles) mice infused with Ad-GFP (open symbols) or Ad-mMTP (closed symbols) following injection of Triton WR-1339. B: Hepatic TG content in Apobec1−/− and C57BL/6 mice 5 days after infusion of Ad-GFP (open bars) or Ad-mMTP (closed bars). Each data point represents mean and SD of six mice.
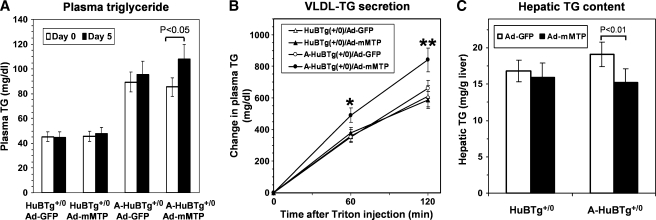
We next asked whether increasing the production of apoB100 molcules might allow us to elicit an effect of augmented Mttp abundance on VLDL secretion, in the absence of massive hepatic TG accumulation. In order to pursue this possibility, we compared VLDL-TG secretion in a line of human apoB-transgenic mice (HuBTg+/0)) in an Apobec1 sufficient background (ie, producing both apoB100 and apoB48) to this same line crossed into an Apobec1 null background (A-HuBTg+/0), in order to increase selectively the expression of apoB100. Animals of both genotypes were administered Ad-mMTP or Ad-GFP as detailed above and hepatic VLDL secretion examined 5 days later. Hepatic Mttp expression was again increased in Ad-mMTP infused animals by 2.5- to 3.0-fold in both genotypes (data not shown). In response to Ad-mMTP administration, plasma TG levels (Fig. 7A) and VLDL-TG secretion rates (Fig. 7B) increased significantly in the A-HuBTg+/0 mice, with a concomitant decrease in hepatic TG content (Fig. 7C). However, Ad-mMTP administration failed to alter either VLDL-TG secretion or hepatic TG content in the parental HuBTg+/0 line (Fig. 7B and 7C). These results suggest that augmenting Mttp abundance increases hepatic VLDL-TG secretion in association with a selective increase in intracellular apoB100.
Fig. 7.
Changes in serum and hepatic lipid levels in HuBTg+/0 and A-HuBTg+/0 mice following infusion of Ad-mMTP. A: Plasma triglyceride levels in HuBTg+/0 and A-HuBTg+/0 mice on day 0 (open bars) or day 5 (black bars) following infusion of either Ad-GFP or Ad-mMTP. B: Hepatic VLDL-triglyceride secretion in HuBTg+/0 (triangles) and A-HuBTg+/0 (circles) mice infused with Ad-GFP (open symbols) or Ad-mMTP (closed symbols). Single and double asterisks indicate P < 0.05 and P < 0.01, respectively. C: Hepatic triglyceride content in HuBTg+/0 and A-HuBTg+/0 mice 5 days after infusion with Ad-GFP (open bars) or Ad-mMTP (black bars). Each data point represents mean and SD of five mice.
DISCUSSION
There is a substantial body of evidence indicating that limiting expression of Mttp impairs the assembly and secretion of TG-rich lipoproteins, both from the liver (12, 13) and the small intestine (28, 29). In addition, numerous observations point to a disproportionate effect of limiting Mttp expression or pharmacologic Mttp inhibition on the secretion of apoB100-containing lipoproteins, in comparison to particles containing apoB48 (8–11). By contrast, little is known about the possible gain-of-function effects of augmented Mttp expression in relation to apoB genotype. The central conclusions of the present study suggest that augmenting Mttp expression in the liver of mice increases VLDL production only in the apoB100 background, and even then only in situations with either increased hepatic TG accumulation or increased apoB100 expression. Important features of these conclusions were unexpected and merit expanded discussion.
We were initially surprised by the observation that augmenting Mttp expression in the liver of Apobec1 sufficient ob/ob mice (expressing predominantly apoB48) failed to influence VLDL secretion. We had predicted a priori that augmenting Mttp expression in this background might restore VLDL secretion, based on two independent lines of evidence. First, prior studies demonstrated that VLDL production in the ob/ob background was impaired relative to lean controls (17) and second, codelivery of the transcriptional coactivators Pgc-1β and Foxa2 increased Mttp expression and in turn increased VLDL secretion in ob/ob mice (18). It is possible that the magnitude of the increase in Mttp expression (∼3-fold) achieved in our studies was insufficient to provide a clear gain-of-function effect, since the studies using adenovirus administration of Pgc-1β and Foxa2 increased Mttp expression ∼6-fold. Nevertheless, as discussed below, our strategy increased Mttp expression to levels in mouse liver that were comparable to those reported by others using adenovirus-mediated Mttp delivery (30), yet failed to influence VLDL-TG secretion. Our findings in Ad-mMTP infused mice further imply that Mttp abundance or activity is not itself limiting for hepatic VLDL secretion in ob/ob mice.
We next studied leptin-deficient ob/ob mice in an Apobec1 null background (A-ob/ob mice) in order to examine the direct effects of increased hepatic Mttp expression on VLDL secretion in an apoB100-only (Apobec1−/−) background. We found in this instance that adenovirus-mediated hepatic Mttp overexpression enhanced hepatic VLDL-TG secretion. This observation strongly suggests that there is a distinct requirement for apoB100 for additional Mttp molecules to augment VLDL secretion in this genetic background. Along these lines, we can propose at least two potential mechanisms that might account for this apoB isoform-specific effect. First, as outlined previously, secretion of apoB48 appears intrinsically less dependent upon Mttp function than is the secretion of apoB100 (9–12). Whether this property reflects the size differential alone or the role of unique domains present in the carboxyl terminus of apoB100 remains to be resolved. Second, apoB100 contains potential Mttp-binding domains that are not present in apoB48 (31) and which may play an important role in mediating the gain-of-function effect of Mttp on VLDL-TG secretion. Further study will be required to discriminate among these possibilities. We also entertained the possibility that differences in endoplasmic reticulum stress might contribute to the effects noted, particularly in the ob/ob background but our preliminary findings gave no such indication (Z. Chen and N.O. Davidson, unpublished observations).
As alluded to above, our current findings are broadly consistent with the results of Wolfrum and Stoffel (18) where an increase in hepatic Mttp expression was accompanied by a corresponding increase in hepatic TG secretion. However, there was an important distinction between our current findings and those earlier results. Specifically, those workers demonstrated a ∼5-fold increase in the secretion of apoB100 into the medium from isolated hepatocytes from ob/ob mice treated with adenoviruses expressing Pgc-1β and Foxa2 (18) and concluded that there was a corresponding increase in both apoB100 production and VLDL secretion following this intervention. In contrast, our findings revealed no increase in apoB100 secretion following Ad-mMTP administration in isolated A-ob/ob hepatocytes, despite increased VLDL-TG secretion (Fig. 5). While further study will be required, we can invoke at least two possibilities to account for these differences. The first is that the approach used by Wolfrum and Stoffel (18) led to a greater increase in Mttp expression with the concomitant overexpression of PGC1β and Foxa2 compared with our experience with Ad-mMTP, as noted above. A second possibility, however, is that the cocktail of adenoviruses used by Wolfrum and Stoffel (18) increased the expression of other target genes including DGAT2, and we speculate that this coordinated induction of Mttp and DGAT2 expression could be particularly potent in promoting hepatic VLDL secretion in the PGC-1β- and Foxa2-overexpressing ob/ob mice.
Extending our analyses, we were equally surprised by the finding that increasing hepatic Mttp activity and expression protein levels by 2- to 3-fold failed to modify VLDL secretion in either lean Apobec1−/− or wild-type mice. These results were particularly unexpected in view of earlier studies by Tietge et al. (30) who had demonstrated that in vivo administration of an adenovirus encoding human Mttp increased VLDL secretion in wild-type C57BL/6 mice. The reasons for this discrepancy are not immediately apparent, since we achieved comparable levels of augmented protein expression and transfer activity with murine Mttp to those reported in that study using human Mttp. It is conceivable that human and murine Mttp may differ in regard to a gain-of-function in murine hepatocytes but this possibility was not formally explored. Furthermore, we extended our analysis to mice in an Apobec1−/− background where we reasoned that a gain-of-function phenotype with apoB100-VLDL might become apparent. However, this again was not the case. Our results suggest that under physiologic conditions there is sufficient Mttp to support VLDL assembly in both wild-type and Apobec1−/− mice and that increasing Mttp expression alone is insufficient to augment VLDL secretion, even in hepatocytes where apoB100 is the dominant isoform.
To begin to address this apparent paradox, we recalled the findings of Leung et al. (13) who demonstrated that mice expressing a human apoB transgene manifested impaired secretion of apoB100 in the setting of half normal levels of Mttp. These findings suggested that augmented Mttp expression might elicit a gain-of-function phenotype in a setting where apoB100 expression itself was increased. Our results confirmed this prediction. We demonstrated that Ad-mMTP administration to A-HuBTg+/0 but not the parental HuBTg+/0 line resulted in increased VLDL secretion and decreased hepatic TG content. An important question emerging from this observation is whether apoB100 synthesis and secretion itself was modulated following Ad-mMTP administration. We speculate that augmenting Mttp availability might abrogate co and posttranslational degradation of both human and murine apoB and thereby increase the secretion of newly synthesized apoB100-containing particles, but this was not formally addressed in the context of the current study. Additional studies will be required to examine the response to Mttp supplementation in murine hepatocytes expressing the human apoB transgene.
In conclusion, our studies demonstrate a conditional gain-of-function phenotype associated with adenovirus-mediated increases in Mttp expression. In our hands, supplementation of Mttp increased murine hepatic VLDL-TG secretion only in the apoB100 background, and only when accompanied by either increased hepatic TG content or increased apoB100 expression. In a broader context, several studies have demonstrated that Mttp expression is upregulated in animal models of increased hepatic VLDL secretion, including high-fat diet feeding (32), type II diabetes, and insulin-resistance (33, 34). These findings have been widely interpreted to suggest that induction of hepatic Mttp gene expression may be a relevant consideration in the pathogenesis of the increased VLDL secretion found in human subjects with obesity and/or type II diabetes (35–38). In view of the fact that human liver expresses exclusively apoB100, these observations may have important implications.
Abbreviations
ALT, alanine aminotransferase
ApoB, apolipoprotein B
apobec1, apolipoprotein B mRNA editing catalytic polypeptide 1
GFP, green fluorescent protein
Mttp, microsomal triglyceride transfer protein
TG, triglyceride
VLDL, very low density lipoprotein
Published, JLR Papers in Press, June 2, 2008.
Footnotes
This work was supported by NIH grants DK-56260, HL-38180, and Digestive Diseases Research Core Center (DK-52574) (to N.O.D) and HL-73939 (to Z.C). This work was also supported, in part, by the Clinical Nutrition Research Unit (DK-56341) of Washington University.
References
- 1.Blasiole D. A., R. A. Davis, and A. D. Attie. 2007. The physiological and molecular regulation of lipoprotein assembly and secretion. Mol. Biosyst. 3 608–619. [DOI] [PubMed] [Google Scholar]
- 2.Davidson N. O., and G. S. Shelness. 2000. Apolipoprotein B: mRNA editing, lipoprotein assembly, and presecretory degradation. Annu. Rev. Nutr. 20 169–193. [DOI] [PubMed] [Google Scholar]
- 3.Chen, Z., and N. O. Davidson. 2006. Genetic Regulation of Intestinal Lipid Transport and Metabolism. In Physiology of the Gastrointestinal Tract. 4th Edition. LR Johnson, editor. Academic Press. Boston. 1711–1734.
- 4.Hussain M. M., J. Shi, and P. Dreizen. 2003. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J. Lipid Res. 44 22–32. [DOI] [PubMed] [Google Scholar]
- 5.Kim E., and S. G. Young. 1998. Genetically modified mice for the study of apolipoprotein B. J. Lipid Res. 39 703–723. [PubMed] [Google Scholar]
- 6.Farese R. V., Jr., S. L. Ruland, L. M. Flynn, R. P. Stokowski, and S. G. Young. 1995. Knockout of the mouse apolipoprotein B genes results in embryonic lethality in homozygotes and protection against diet-induced hypercholesterolemia in heterozygotes. Proc. Natl. Acad. Sci. USA. 92 1774–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raabe M., L. M. Flynn, C. H. Zlot, J. S. Wong, M. M. Véniant, R. L. Hamilton, and S. G. Young. 1998. Knockout of the abetalipoproteinemia gene in mice: reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc. Natl. Acad. Sci. USA. 95 8686–8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jamil H., D. A. Gordon, D. C. Eustice, C. M. Brooks, J. K. J. Dickson, Y. Chen, B. Ricci, C. H. Chu, T. W. Harrity, C. P. J. Ciosek, et al. 1996. An inhibitor of the microsomal triglyceride transfer protein inhibits apoB secretion from HepG2 cells. Proc. Natl. Acad. Sci. USA. 93 11991–11995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., R. S. McLeod, and Z. Yao. 1997. Normal activity of microsomal triglyceride transfer protein is required for the oleate-induced secretion of very low density lipoproteins containing apolipoprotein B from McA-RH7777 cells. J. Biol. Chem. 272 12272–12278. [DOI] [PubMed] [Google Scholar]
- 10.Kulinski A., S. Rustaeus, and J. E. Vance. 2002. Microsomal triglyceride transfer protein is required for lumenal accretion of triacylglycerol not associated with apoB, as well as for apoB lipidation. J. Biol. Chem. 277 31516–31525. [DOI] [PubMed] [Google Scholar]
- 11.Larsson S. L., J. Skogsberg, and J. Bjorkegren. 2004. The low density lipoprotein receptor prevents secretion of dense apoB100-containing lipoproteins from the liver. J. Biol. Chem. 279 831–836. [DOI] [PubMed] [Google Scholar]
- 12.Raabe M., M. M. Véniant, M. A. Sullivan, C. H. Zlot, J. Björkegren, L. B. Nielsen, J. S. Wong, R. L. Hamilton, and S. G. Young. 1999. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J. Clin. Invest. 103 1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung G. K., M. M. Veniant, S. K. Kim, C. H. Zlot, M. Raabe, K. Bjorkegren, R. A. Neese, M. K. Hellerstein, and S. G. Young. 2000. A deficiency of microsomal triglyceride transfer protein reduced apolipoprotein B secretion. J. Biol. Chem. 275 7515–7520. [DOI] [PubMed] [Google Scholar]
- 14.Yen T. T., J. A. Allan, P. L. Yu, M. A. Acton, and D. V. Pearson. 1976. Triacylglycerol contents and in vivo lipogenesis of ob/ob, db/db and Avy/a mice. Biochim. Biophys. Acta. 441 213–220. [DOI] [PubMed] [Google Scholar]
- 15.Memon R. A., J. Fuller, A. H. Moser, P. J. Smith, C. Grunfeld, and K. R. Feingold. 1999. Regulation of putative fatty acid transporters and Acyl-CoA synthetase in liver and adipose tissue in ob/ob mice. Diabetes. 48 121–127. [DOI] [PubMed] [Google Scholar]
- 16.Wiegman C. H., R. H. Bandsma, M. Ouwens, F. H. van der Sluijs, R. Havinga, T. Boer, D. J. Reijngoud, J. A. Romijn, and F. Kuipers. 2003. Hepatic VLDL production in ob/ob mice is not stimulated by massive de novo lipogenesis but is less sensitive to the suppressive effects of insulin. Diabetes. 52 1081–1089. [DOI] [PubMed] [Google Scholar]
- 17.Li X., S. M. Grundy, and S. B. Patel. 1997. Obesity in db and ob animals leads to impaired hepatic very low density lipoprotein secretion and differential secretion of apolipoprotein B-48 and B-100. J. Lipid Res. 38 1277–1288. [PubMed] [Google Scholar]
- 18.Wolfrum C., and M. Stoffel. 2006. Coactivation of Foxa2 through Pgc-1beta promotes liver fatty acid oxidation and triglyceride/VLDL secretion. Cell Metab. 3 99–110. [DOI] [PubMed] [Google Scholar]
- 19.Bartels E. D., M. Lauritsen, and L. B. Nielsen. 2002. Hepatic expression of microsomal triglyceride transfer protein and in vivo secretion of triglyceride-rich lipoproteins are increased in obese diabetic mice. Diabetes. 51 1233–1239. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z., R. L. Fitzgerald, M. R. Averna, and G. Schonfeld. 2000. A targeted apolipoprotein B-38.9-producing mutation causes fatty livers in mice due to the reduced ability of apolipoprotein B-38.9 to transport triglycerides. J. Biol. Chem. 275 32807–32815. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z., R. L. Fitzgerald, G. Li, N. O. Davidson, and G. Schonfeld. 2004. Hepatic secretion of apoB-100 is impaired in hypobetalipoproteinemic mice with an apoB-38.9-specifying allele. J. Lipid Res. 45 155–163. [DOI] [PubMed] [Google Scholar]
- 22.Hirano K-I., S. G. Young, R. V. Farese, Jr., J. Ng, E. Sande, C. Warburton, L-M. Powell-Braxton, and N. O. Davidson. 1996. Targeted disruption of the mouse apobec-1 gene abolishes apolipoprotein B mRNA editing and eliminates apolipoprotein B48. J. Biol. Chem. 271 9887–9890. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen L. B., S. P. McCormick, V. Pierotti, C. Tam, M. D. Gunn, H. Shizuya, and S. G. Young. 1997. Human apolipoprotein B transgenic mice generated with 207- and 145-kilobase pair bacterial artificial chromosomes. Evidence that a distant 5′-element confers appropriate transgene expression in the intestine. J. Biol. Chem. 272 29752–29758. [DOI] [PubMed] [Google Scholar]
- 24.Borensztajn J., M. S. Rone, and T. J. Kotlar. 1976. The inhibition in vivo of lipoprotein lipase (clearing-factor lipase) activity by Triton WR-1339. Biochem. J. 156 539–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishikawa T., and N. Fidge. 1979. Changes in the concentration of plasma lipoproteins and apoproteins following the administration of Triton WR 1339 to rats. J. Lipid Res. 20 254–264. [PubMed] [Google Scholar]
- 26.Améen C., U. Edvardsson, A. Ljungberg, L. Asp, P. Åkerblad, A. Tuneld, S-O. Olofsson, D. Lindén, and J. Oscarsson. 2005. Activation of peroxisome proliferator-activated receptor increases the expression and activity of microsomal triglyceride transfer protein in the liver. J. Biol. Chem. 280 1224–1229. [DOI] [PubMed] [Google Scholar]
- 27.Wetterau J. R., and D. B. Zilversmit. 1984. A triglyceride and cholesteryl ester transfer protein associated with liver microsomes. J. Biol. Chem. 259 10863–10866. [PubMed] [Google Scholar]
- 28.Xie Y., E. P. Newberry, S. G. Young, S. Robine, R. L. Hamilton, J. S. Wong, J. Luo, S. Kennedy, and N. O. Davidson. 2006. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J. Biol. Chem. 281 4075–4086. [DOI] [PubMed] [Google Scholar]
- 29.Xie Y., J. Luo, S. Kennedy, and N. O. Davidson. 2007. Conditional intestinal lipotoxicity in Apobec-1−/− Mttp-IKO mice: a survival advantage for mammalian intestinal apoblipoprotein B mRNA editing. J. Biol. Chem. 282 33043–33051. [DOI] [PubMed] [Google Scholar]
- 30.Tietge U. J., A. Bakillah, C. Maugeais, K. Tsukamoto, M. Hussain, and D. J. Rader. 1999. Hepatic overexpression of microsomal triglyceride transfer protein (MTP) results in increased in vivo secretion of VLDL triglycerides and apolipoprotein B. J. Lipid Res. 40 2134–2139. [PubMed] [Google Scholar]
- 31.Nicodeme E., F. Benoist, R. McLeod, Z. Yao, J. Scott, C. C. Shoulders, and T. Grand-Perret. 1999. Identification of domains in apolipoprotein B100 that confer a high requirement for the microsomal triglyceride transfer protein. J. Biol. Chem. 274 1986–1993. [DOI] [PubMed] [Google Scholar]
- 32.Petit V., L. Arnould, P. Martin, M-C. Monnot, T. Pineau, P. Besnard, and I. Niot. 2007. Chronic high-fat diet affects intestinal fat absorption and postprandial triglyceride levels in the mouse. J. Lipid Res. 48 278–287. [DOI] [PubMed] [Google Scholar]
- 33.Taghibiglou C., A. Carpentier, S. C. Van Iderstine, B. Chen, D. Rudy, A. Aiton, G. F. Lewis, and K. Adeli. 2000. Mechanisms of hepatic very low density lipoprotein overproduction in insulin resistance. Evidence for enhanced lipoprotein assembly, reduced intracellular apoB degradation, and increased microsomal triglyceride transfer protein in a fructose-fed hamster model. J. Biol. Chem. 275 8416–8425. [DOI] [PubMed] [Google Scholar]
- 34.Carpentier A., C. Taghibiglou, N. Leung, L. Szeto, S. C. Van Iderstine, K. D. Uffelman, R. Buckingham, K. Adeli, and G. F. Lewis. 2002. Ameliorated hepatic insulin resistance is associated with normalization of microsomal triglyceride transfer protein expression and reduction in very low density lipoprotein assembly and secretion in the fructose-fed hamster. J. Biol. Chem. 277 28795–28802. [DOI] [PubMed] [Google Scholar]
- 35.Grundy S. M., A. Chait, and J. D. Brunzell. 1987. Familial combined hyperlipidemia workshop. Arteriosclerosis. 7 203–207. [Google Scholar]
- 36.Gormsen L. C., M. D. Jensen, O. Schmitz, N. Moller, J. S. Christiansen, and S. Nielsen. 2006. Energy expenditure, insulin, and VLDL-triglyceride production in humans. J. Lipid Res. 47 2325–2332. [DOI] [PubMed] [Google Scholar]
- 37.Mittendorfer B., B. W. Patterson, S. Klein, and L. S. Sidossis. 2003. VLDL-triglyceride kinetics during hyperglycemia-hyperinsulinemia: effects of sex and obesity. Am. J. Physiol. Endocrinol. Metab. 284 E708–E715. [DOI] [PubMed] [Google Scholar]
- 38.Klein S., B. Mittendorfer, J. C. Eagon, B. Patterson, L. Grant, N. Feirt, E. Seki, D. Brenner, K. Korenblat, and J. McCrea. 2006. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology. 130 1564–1572. [DOI] [PubMed] [Google Scholar]