Abstract
Inflammatory lipid mediators derived from arachidonic acid (AA) and docosahexaenoic acid (DHA) modify the pathophysiology of brain ischemia. The goal of this work was to investigate the formation of eicosanoids and docosanoids generated from AA and DHA, respectively, during no-flow cerebral ischemia. Rats were subjected to head-focused microwave irradiation 5 min following decapitation (complete ischemia) or prior to decapitation (controls). Brain lipids were extracted and analyzed by reverse-phase liquid chromatography-tandem mass spectrometry. After complete ischemia, brain AA, DHA, and docosapentaenoic acid concentrations increased 18-, 5- and 4-fold compared with controls, respectively. Prostaglandin E2 (PGE2) and PGD2 could not be detected in control microwaved rat brain, suggesting little endogenous PGE2/D2 production in the brain in the absence of experimental manipulation. Concentrations of thromboxane B2, E2/D2-isoprostanes, 5-hydroxyeicosatetraenoic acid (5-HETE), 5-oxo-eicosatetraenoic acid, and 12-HETE were significantly elevated in ischemic brains. In addition, DHA products such as mono-, di- and trihydroxy-DHA were detected in control and ischemic brains. Monohydroxy-DHA, identified as 17-hydroxy-DHA and thought to be the immediate precursor of neuroprotectin D1, was 6.5-fold higher in ischemic than in control brain. The present study demonstrated increased formation of eicosanoids, E2/D2-IsoPs, and docosanoids following cerebral ischemia. A balance of these lipid mediators may mediate immediate events of ischemic injury and recovery.
Keywords: arachidonic, docosahexaenoic, eicosanoids, docosanoids, isoprostanes, brain ischemia
Cerebral ischemia is characterized by a critical reduction in cerebral blood flow and is accompanied by edema, blood-brain barrier opening, and formation of free radicals and neuroinflammatory mediators (1). The accumulation of bioactive lipids, such as prostaglandins, leukotrienes (LTs), and docosanoids, during brain ischemia is thought to play an important role in brain injury (2). The production of such lipid mediators is complex and involves enzymatic as well as nonenzymatic pathways.
During brain ischemia, phospholipases A2 (EC 3.1.1.4; PLA2) are activated, resulting in hydrolysis of membrane phospholipids and release of unesterified fatty acids, including arachidonic acid (AA, 20:4n-6) and docosahexaenoic acid (DHA, 22:6n-3), which are highly enriched in brain phospholipids (3, 4). The unesterified AA is rapidly esterified to available lysophospholipids (5, 6), or is converted into eicosanoids via cyclooxygenase (COX), lipoxygenase (LOX), or cytochrome P450 epoxygenase enzymes. Increased brain levels of prostaglandin E2 (PGE2), PGD2, and thromboxane B2 (TXB2), formed via COX pathways, and of hydroxyeicosatetraenoic acid (HETE), LTB4, and LTC4, formed via LOX pathways, have been reported following cerebral ischemia (7–16).
Brain ischemia leads also to the formation of free radicals or reactive oxygen species (ROS) (17). These unstable molecules may convert AA through peroxidation to bioactive prostaglandin-like compounds known as isoprostanes (IsoPs) (18). Although studies of IsoP formation have centered on F2-IsoPs, other IsoPs, such as E2/D2-IsoPs also can be formed (19). Levels of IsoPs, specifically of F2-IsoPs, have been used as markers of oxidative stress (20) and were found to be increased during brain ischemia-reperfusion (21); however, the formation of E2/D2-IsoPs has not been reported during ischemia. One of the E2-IsoP isomers, 8-iso-PGE2, has a very potent biological activity and thus may contribute to brain damage associated with oxidative stress (18).
DHA metabolites known as docosanoids have been identified in trout and mouse brain cells challenged with a calcium ionophore, in mouse brain during ischemia-reperfusion (22–24) and in the retina (25). The major products were 17S-hydroperoxy-DHA, 10R,17S-dihydroxy-DHA [also called 10,17S-docosatriene or neuroprotectin D1 (NPD1)], and trihydroxy-DHA (such as resolvins D1 and D2). DHA is initially converted to 17S-hydroperoxy-DHA by 15-LOX, and then further enzymatically transformed to NPD1 and resolvins D via epoxide intermediates. Some DHA metabolites were found to stop leukocyte infiltration and cytokine production as well as to protect against ischemia in mouse brain (22, 24, 26, 27).
We hypothesized that decapitation-induced ischemia would increase unesterified brain concentrations of the polyunsaturated long-chain fatty acids, of which a fraction would be transformed into a complex mixture of eicosanoid and docosanoid metabolites. In this paper, we thought it would be of interest to simultaneously characterize the formation of unesterified AA, DHA, and docosapentaenoic acid (DPA, 22:5n-3) and their products in high-energy-microwaved rat brain, under normal and no-flow 5 min ischemic conditions, using reverse-phase liquid chromatography-tandem mass spectrometry (LC/MS/MS). We report that PGE2 and PGD2 are absent in control rat brain tissue but can be formed as artifacts of tissue manipulation if PGH synthase is not inactivated by microwave irradiation. For the first time, E2/D2-IsoPs were found markedly increased (48-fold) during brain ischemia.
MATERIALS AND METHODS
Materials
Reagents and solvents were purchased from Fisher Scientific (Pittsburgh, PA). Standards [d8]5-HETE (98 atom % D), [d8]AA (99 atom % D), [d5]DHA (98 atom % D), [d4]TXB2 (98 atom % D), [d4]PGD2 (98 atom % D), [d4]PGE2 (99 atom % D), [d4]LTB4 (97 atom % D), and 17-hydroxy-DHA were purchased from Cayman Chemical Co. (Ann Arbor, MI). Solid-phase extraction cartridges were purchased from Phenomenex (Strata C18-E, 100 mg/ml) (Torrance, CA) and Waters (Oasis HLB, 30 mg; Milford, MA). Resolvin D1 (7S,8,17S-trihydroxy-docosa-4Z,9E,11E,13Z,15E,19Z-hexaenoic acid) and NPD1 (10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid) were generously provided by Dr. Charles Serhan (Harvard University, Boston, MA).
Animals
Experiments were conducted following the “Guide for the Care and Use of Laboratory Animals” (National Institutes of Health Publication No. 86-23), under a protocol approved by the Animal Care and Use Committee of the National Institute of Child Health and Human Development. Three-month-old male Fischer CDF (F-344)/CrlBR rats (Charles River Laboratories, Wilmington, MA) were housed for 1 week before study in an animal facility with regulated temperature, humidity, and light cycle, and with free access to water and food (NIH-31 diet; Zeigler, Gardners, PA). The diet contained (as percent of total fatty acids) 20.1% saturated, 22.5% monounsaturated, 47.9% linoleic, 5.1% α-linolenic, 0.02% AA, 2.0% eicosapentaenoic, and 2.3% DHA. Rats were randomly allocated to either a control (n = 10) or ischemic (n = 10) group. Control rats were anesthetized with pentobarbital (40 mg/kg, ip) and then subjected to head-focused microwave irradiation to stop brain metabolism (5.5 kW, 3.6 s; Cober Electronics, Stamford, CT) prior to decapitation (9, 16, 28, 29). In order to produce complete ischemia, a rat (n = 10) was anesthetized with pentobarbital (40 mg/kg, ip) and decapitated, after which the head was put in a plastic bag at 37°C for up to 5 min as previously described (30, 31). The head then was subjected to head-focused microwave irradiation. After cooling the control and ischemic microwaved heads on dry ice, the brains were excised, frozen in 2-methylbutane, maintained at −40°C with dry ice, and stored at −80°C until use. The right and left cerebral hemispheres were homogenized separately in 4 ml of 80% methanol at 25°C using a dounce homogenizer (Pirex No. 7727).
Injection of deuterated PGE2 and PGD2 into the left brain hemisphere
Rats (n = 6) were anesthetized with Nembutal (40 mg/kg) and positioned in a stereotaxic apparatus (Stoelting, Wood Dale, IL). A mixture of 50 ng d4-PGE2 and 50 ng d4-PGD2 (in sterile saline, 5 μl) was administered into the left side of the caudate putamen using a 10 μl Hamilton syringe (26 ga Bevel tip) operated manually for 13 ± 3 min. The coordinates were 6.0 mm dorsal/ventral, 3.0 mm lateral, and 0.2 mm posterior from the bregma. The scalp was immediately closed with sutures, and rats were killed by microwave irradiation (5.5 kW, 3.6 s). After cooling the microwaved heads on dry ice, the brains were excised, frozen in 2-methylbutane, and stored at −80°C until use. Brain hemispheres were homogenized in 4 ml of cold 80% methanol, and a mixture of 50 ng d4-PGE2/50 ng d4-PGD2 (in sterile saline, 5 μl) was added to the homogenate corresponding to the right brain hemisphere.
Extraction and analysis of lipids
Deuterated internal standards (d8-5-HETE, d8-AA, d5-DHA, d4-TXB2, d4-PGE2) were added to the homogenate. Samples were centrifuged at 1,050 g for 10 min to precipitate the insoluble material, and the supernatant was diluted with water to a concentration of methanol lower than 15% and then extracted using a solid-phase extraction cartridge (Strata C18-E; 100 mg/1 ml) for analysis of all lipids except IsoPs. Oasis HLB (30 mg) was used for analysis of IsoPs. The eluate (1 ml of MeOH) was taken to dryness and reconstituted in 70 μl of HPLC solvent A (8.3 mM acetic acid buffered to pH 5.7 with NH4OH) + 20 μl of solvent B (acetonitrile-MeOH, 65:35, v/v).
An aliquot of each sample (35 μl) was injected into an HPLC system (LC-10AD, Shimadzu, Japan), subjected to reverse-phase chromatography using a C18 column (Columbus 150 × 1 mm, 5 mm; Phenomenex, Macclesfield, UK), and eluted at a flow rate of 50 μl/min, with a linear gradient from 25% to 100% of mobile phase B. Solvent B was increased from 25% to 85% in 24 min, to 100% in 26 min, and held at 100% for a further 12 min. The HPLC effluent was directly connected to the electrospray source of a triple quadrupole mass spectrometer (Sciex API 2000; PE-Sciex, Thornhill, ON, Canada). Mass spectrometric analyses were performed in negative-ion mode using multiple reaction monitoring (MRM) of the specific transitions, m/z 303 → 205 for AA; m/z 327 → 283 for DHA; m/z 329 → 285 for DPA; m/z 351 → 271, m/z 351 → 233, m/z 351 → 333, m/z 351 → 315 for E2/D2-IsoPs; m/z 353 → 193 for PGF2α and 8-iso-PGF2α; 351 m/z 369 → 169 for TXB2; m/z 319 → 115 for 5-HETE; m/z 317 → 113 for 5-oxo-eicosatetraenoic acid (5-oxo-ETE); m/z 319 → 179 for 12-HETE; m/z 624 → 272 for LTC4; m/z 335 → 195 for LTB4; m/z 375 → 141, m/z 375 → 277 for trihydroxy-DHA; m/z 359 → 206, mz 359 → 153 for dihydroxy-DHA; m/z 343 → 245, m/z 343 → 273 for monohydroxy-DHA; m/z 311 → 267 for d8-AA; m/z 332 → 288 for d5-DHA; m/z 355 → 275 for d4-PGE2; m/z 355 → 237 for d4-PGD2; m/z 373 → 173 for d4-TXB2; m/z 327 → 116 for d8-5-HETE; and m/z 339 → 197 for d4-LTB4.
Quantitation of DHA, AA, DPA, TXB2, 12-HETE, 5-HETE, 5-oxo-ETE, and LTC4 was performed using a standard isotope dilution curve as previously described (32). The lower limits of quantitation were 0.16 pmol for TXB2, 1.15 pmol for 5-HETE, 0.13 pmol for AA, 0.60 pmol for DHA, 0.36 pmol for DPA, 0.43 pmol for 12-HETE, 0.25 pmol for 5-oxo-ETE, 0.25 pmol for LTC4, and 0.23 pmol for LTB4. The detection limit of the method used was adequate to measure levels of these metabolites in ischemic brain. The concentration of E2/D2-IsoPs was calculated as the ratio between the area under the peaks for the transition m/z 351 → 271 between 12–19 min and the internal standard d4-PGE2 (m/z 355 → 275).
For the identification of the monohydroxy-DHA in the ischemic brain, another set of samples was subjected to the same extraction procedure and HPLC gradient conditions. The HPLC effluent was directly connected to the electrospray source of a Q-trap hybrid triple quadrupole/linear ion trap mass spectrometer (Sciex API 2000, PE-Sciex). Mass spectrometric analyses were performed using the enhanced product ion for m/z 343 in the negative-ion mode.
Statistical analysis
Data are expressed in pmol/g or nmol/g wet weight of brain, and are reported as means ± SD, with statistical significance taken as P ≤ 0.05. An unpaired two-tailed t-test was used to compare means between ischemic and control concentrations using GraphPad Prism, version 4.0b (GraphPad Software, San Diego, CA, www.graphpad.com).
RESULTS
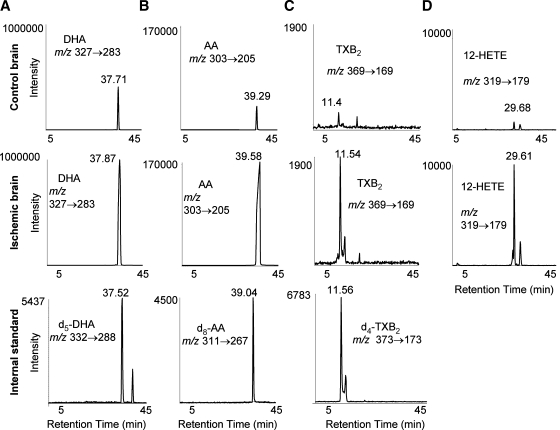
Lipids from both cerebral hemispheres in ischemic and control brains were extracted by solid-phase extraction and subjected to reverse-phase LC/MS/MS analysis. DHA (Fig. 1A), AA (Fig. 1B), TXB2 (Fig. 1C), and 12-HETE (Fig. 1D) could be detected in both control and ischemic brains. However, much higher levels of these metabolites were found in the ischemic brains. Identification of each of these metabolites was based on the specific single-reaction monitoring ion transition in the tandem quadrupole mass spectrometer and HPLC retention time (RT). The HPLC RT for each of these compounds was established by measuring the RT of the corresponding deuterated standard that was added to each sample (Fig. 1). The exogenous deuterated standard for 12-HETE was not added to the sample, but its RT was verified by comparing it with the elution time of an authentic 12-HETE standard run immediately after the sample analysis (data not shown).
Fig. 1.
Increase of lipid levels after global brain ischemia. Levels of docosahexaenoic acid (DHA) (A), arachidonic acid (AA) (B), thromboxane B2 (TXB2) (C), and 12-hydroxyeicosatetraenoic acid (12-HETE) (D) in ischemic and control brains analyzed by liquid chromatography-tandem mass spectrometry (LC/MS/MS). The specific HPLC elution times for each of these metabolites are consistent with their corresponding deuterated standard.
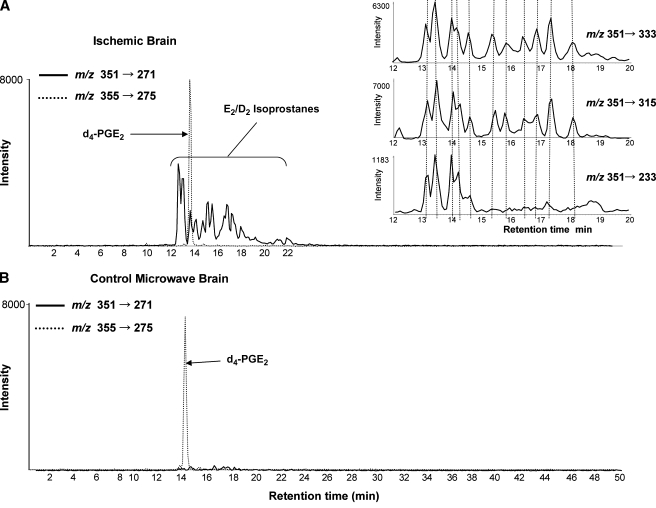
The HPLC profile corresponding to MRM transition m/z 351 → 271 revealed the formation of a series of new eicosanoids in ischemic brain (Fig. 2A) that were absent in control brain (Fig. 2B). The exogenously added d4-PGE2 (m/z 355 → 275) coeluted with one these components in ischemic brain, suggesting that PGE2 might have been formed during brain ischemia (Fig. 2A). The compound eluting right after PGE2 might be PGD2, based on the expected chromatographic RT, because PGD2 shares the MRM transition m/z 351 → 271 with PGE2. The large number of separable components that shared this ion transition (m/z 351 → 271) suggested that isomeric eicosanoids were present in ischemic brain, probably corresponding to a complex mixture of nonenzymatic E2/D2-IsoPs. Because the enzymatic products PGE2 and PGD2 were not the most abundant in the complex mixture of E2/D2-IsoPs in ischemic brain, it was not possible to unambiguously assign their formation. To confirm identification of E2/D2-IsoPs, additional MRM transitions m/z 351 → 233, 351 → 315, and 353 → 333, which are specific for PGE2/D2 and its isomers, were used in the product analysis and found to overlay the peaks observed for transition m/z 351 → 271 (Fig. 2A, inset). These eicosanoids eluted from the HPLC column in a specific time range (12–22 min), consistent with the lipophilicity of such compounds. The typical marker to assess oxidative stress, F2-IsoPs, measured by the MRM transition m/z 353 → 193, revealed many components eluting between 12 and 20 min from the HPLC column and were also substantially increased (3- to 6-fold) in ischemic brain (data not shown).
Fig. 2.
Formation of E2/D2 isoprostanes (E2/D2-IsoPs) in brain ischemia. The LC/MS/MS analysis corresponding transition m/z 351 → 271 shows the formation of E2/D2-IsoPs in ischemic brain (A). PGE2/PGD2 were not found in control brain. The exogenously added d4-PGE2 can be seen as well by the transition m/z 355 → 275 (B). Multiple reaction monitoring (MRM) transitions m/z 351 → 333, m/z 351 → 315, and m/z 351 → 233 revealed a series of peaks that coincided with transition m/z 351 → 271 (inset in A).
To determine the effect of microwave irradiation on the stability of PGE2 and PGD2, the recovery of exogenous d4-PGE2 and d4-PGD2 added to left and right hemispheres corresponding to pre and post microwave irradiation addition were compared (Fig. 3, left column). The amount of deuterated PGE2 and PGD2 recovered from the left hemisphere (pre microwaved standard addition) was between 60% and 70% of that recovered from the right hemisphere (post microwave standard addition) (67 ± 2% d4-PGE2, 61 ± 6% d4-PGD2). Endogenous PGE2 and PGD2 were detected in the left hemisphere at significantly higher concentrations compared with the right (Fig. 3, right column). The selective production of prostaglandins in the left hemisphere could be explained by the intracranial needle penetration during the deuterium-labeled prostanoid injection, which has been shown to induce an inflammation reaction (33). These results suggest that microwave irradiation does not substantially degrade prostaglandins.
Fig. 3.
Effect of microwave irradiation on prostaglandin stability and endogenous formation of PGE2 and PGD2 in the left brain hemisphere. Recovery of deuterated PGE2 and PGD2 (50 ng each, m/z 355 → 275) injected into the left brain hemisphere prior to microwave irradiation (pre microwave addition) (A) or added to the right hemisphere tissue homogenate after microwave exposure (post microwave irradiation) (B). C: Intracranial needle penetrationinduced PGE2 and PGD2 formation in the left hemisphere detected by ion transition m/z 351 → 271. D: Analysis of the right hemisphere showed little if any endogenous PGE2/D2.
Unesterified fatty acids
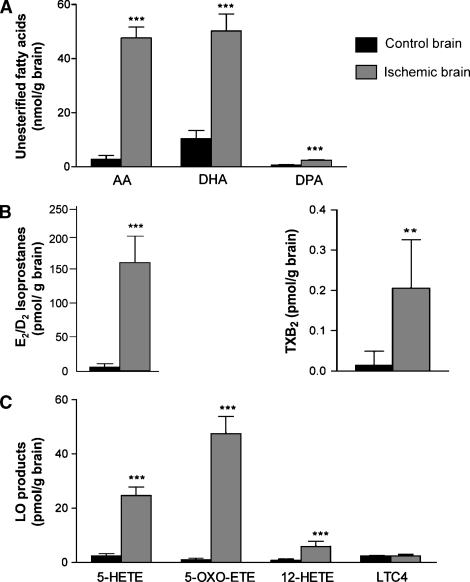
Quantitation of brain unesterified fatty acids AA, DHA, and DPA is shown in Fig. 4A. The mean unesterified AA concentration was 18-fold higher in ischemic than in control brain (47.61 ± 4.08 nmol/g vs. 2.71 ± 1.47 nmol/g, respectively). The concentration of AA in decapitated brains (without microwave) was 35.9 ± 4.1 nmol/g of brain (data not shown). The DHA concentration in ischemic brain was 5-fold greater than in control brain (50.24 ± 6.26 nmol/g vs. 10.40 ± 3.03 nmol/g, respectively). Although the final concentrations of unesterified AA and DHA were similar in ischemic brain, the unesterified DHA concentration was higher than the unesterified AA concentration in control brain. The unesterified DPA concentration in ischemic brain was 4-fold greater than in control brain (2.37 ± 0.26 nmol/g vs. 0.63 ± 0.17 nmol/g, respectively), but this PUFA was at least 20-fold less abundant than DHA and AA in ischemic brain.
Fig. 4.
Lipid concentrations in control and ischemic brains. Unesterified fatty acids AA, DHA, and DPA (A), E2/D2 isoprostanes and TXB2 (B), and LOX products 5-HETE, 5-oxo-ETE, 12-HETE, and leukotriene C4 (LTC4) (C) in control and ischemic brains. Data are expressed as means ± SD. The concentration of E2/D2-IsoPs was increased 48-fold after brain ischemia (B). ** P ≤ 0.01; *** P ≤ 0.001.
Eicosanoids
Using the d4-PGE2 as internal standard, the quantity of the E2/D2-IsoPs was estimated as 164 ± 41 pmol/g in ischemic brain and 3.7 ± 0.9 pmol/g in control microwaved brain. The TXB2 concentration was 8-fold higher in ischemic brain than in control brain, which was at the limit of detection (0.21 ± 0.09 pmol/g vs. 0.01 ± 0.03 pmol/g, respectively) (Fig. 4B). There was no PGF2α detected more abundant than any single isomer of the F2-IsoPs (see above).
Products of LOX activity and HETE-derived metabolites of AA were more abundant in ischemic than in control brain (Fig. 4C). The concentration of 5-HETE, derived via 5-LOX, was 10-fold higher in ischemic brain than in control brain (24.70 ± 3.04 pmol/g vs. 2.42 ± 0.79 pmol/g, respectively). The concentration of 12-HETE, derived via 12-LOX, was 7-fold higher in ischemic brain than in control brain (5.86 ± 1.91 pmol/g vs. 0.86 ± 0.43 pmol/g, respectively). The concentration of 5-oxo-ETE, the oxidation product of 5-HETE, was increased 48-fold in ischemic compared with control brain (47.52 ± 6.42 pmol/g vs. 0.99 ± 0.48 pmol/g, respectively). On the other hand, the concentration of LTC4, which is formed through the sequential action of 5-LOX and LTC4 synthase, was not increased in ischemic compared with control brain (2.49 ± 0.42 pmol/g vs. 2.33 ± 0.17 pmol/g, respectively, P = 0.3933). LTB4 was not detected in either control or ischemic brain.
Docosanoids
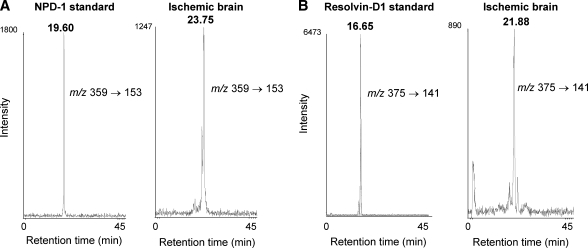
To investigate whether DHA-derived hydroxylated metabolites were formed during the 5 min ischemia, different MRM transitions based on the mass spectrum of hydroxylated DHA standards resolvin-D1 (7S,8,17S-trihydroxy-docosa-4Z,9E,11E,13Z,15E,19Z-hexaenoic acid), NPD1 (10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid), and 17-hydroxy-DHA were monitored. Mono-, di- and trihydroxy-DHA were detected in ischemic brain at levels higher than in control brain (Fig. 5). However, the HPLC RTs of di- (23.75 min) and tri- (21.88 min) hydroxy-DHA metabolites detected in ischemic brain differed from the respective reference standards, NPD1 (RT: 19.60 min) and resolvin-D1 (RT: 16.65 min), by 4–5 min (Fig. 6). The RT differences between di- and trihydroxy-DHA metabolites formed in ischemic brain, and their respective NPD1 and resolvin-D1 standards, could not be explained by variations of RT from sample to sample. This was evident when the elution time of each compound was compared with that of its deuterated standard (d4-PGE2 and d5-DHA), which had at most a difference of less than 1 min between runs. The reverse-phase HPLC RT for each of these metabolites was consistent with the expected decrease in lipophilicity: tri- < di- < monohydroxy-DHA. These data showed that di- and trihydroxy-DHA metabolites were not NPD1 and resolvin-D1, respectively.
Fig. 5.
Docosanoid formation in ischemic brain. LC/MS/MS trace showing MRM transition corresponding to tri- (A), di- (B), and mono- (C) hydroxy-DHA metabolites for ischemic and control brains.
Fig. 6.
Di- and trihydroxy-DHA HPLC retention times (RTs). Comparison of HPLC elution times between neuroprotectin D1 (NPD-1) standard (RT: 19.60 min) to ischemic brain di-hydroxy-DHA (RT: 23.75 min) (A) and resolvin-D1 standard (RT: 16.65 min) to ischemic brain tri-hydroxy-DHA (RT: 21.88 min) (B).
The most-abundant DHA metabolite found in ischemic brain was a monohydroxylated product that eluted between 27and 28 min. To identify the chemical structure of this monohydroxy-DHA, a hybrid triple quadrupole/linear ion trap mass spectrometer was employed, and collisional activation of the [M-H]− ion at m/z 343 was performed in the negative mode. The mass spectra of the HPLC effluent between 27 and 28 min from both the sample and 17-hydroxy-DHA standard were virtually identical (Fig. 7). The product ions at m/z 245, 201, 229, and 273 were consistent with the cleavage adjacent to the 17-hydroxy group (α cleavage) (Fig. 7, structural inset). Additionally, nonspecific ions corresponding to loss of H2O, CO2, and CO2 + H2O were of similar relative abundance between the docosanoid isolated from the ischemic brain and the authentic 17-OH-DHA standard.
Fig. 7.
Structural identification of monohydroxy-DHA from ischemic brain as 17-hydroxy-DHA. Enhanced product ion spectrum for the specific ion m/z 343 for both 17-OH-DHA standard (A) and the monohydroxy-DHA (B) from ischemic brain. Chemical structure for 17-OH-DHA shows diagnostic cleavages for this molecule. Inset to B: Quantitative determination of 17-OH-DHA concentration in control and ischemic brains. Data are expressed as means ± SD. *** P < 0.001.
The concentration of 17-OH-DHA was 6- to 7-fold higher in ischemic than control brain (13.40 ± 4.83 pmol/g vs. 2.06 ± 1.58 pmol/g, respectively) (Fig. 7 inset).
DISCUSSION
In this study, a 5 min decapitation-induced complete cerebral ischemia of adult rat brain led to marked increases in brain concentrations of unesterified AA, DHA, and DPA. Products of these PUFAs, including, E2/D2-IsoPs, TXB2, 5-HETE, 5-oxo-ETE, and 12-HETE, as well as mono-, di- and trihydroxy-DHA, were increased significantly as well.
The brain was subjected to high-energy microwave irradiation to irreversibly inactivate enzymes in the entire brain within a few seconds (8, 9, 12, 16, 28–30, 34–37), which permitted comparison of fatty acid and metabolite levels in ischemic and control brain. A 5 min ischemia was chosen because it was used previously in our laboratory (30, 31); this period includes a rapid phase followed by a much slower phase of fatty acid release from phospholipids (37, 38).
Although PGE2 and PGD2 have long been considered to be major prostanoids generated as a result of brain ischemia (8, 9, 13), our results suggest that this might not be the case. When decapitation after a 5 min ischemic period was followed by enzymatic inactivation by microwave prior to tissue manipulation, these prostanoids were minor products at best and only constituted a fraction of the E2/D2-IsoPs that were formed by free radical arachidonate peroxidation. PGE2 and PGD2 were absent in control brains subjected to head-focus microwave irradiation, in contrast to previous studies that used microwave euthanasia to detect these eicosanoids and employed immunoassay techniques without chromatographic identity of the immunoreactive agents present in the extracts (9, 13). There is a likelihood of molecules with some cross-reactivity occurring in the brain giving a false positive, but even in these studies, microwave inactivation of enzymes significantly reduced PGD2 and PGE2 to levels found when COX was inhibited in mice with indomethacin (8). To rule out the possibility that prostaglandins within brain were degraded by the microwave irradiation, we compared the recovery of pre and post microwave addition of deuterated PGE2 and PGD2 (Fig. 3, left column). Prostaglandins were not degraded substantially by microwaving, validating the finding that prostaglandins were not measurable in control microwaved brains.
Levels of IsoPs, specifically of F2-IsoPs, are widely used as markers of oxidative stress and have been observed to increase during brain ischemia-reperfusion (20, 21). Although an increase in F2-IsoPs was observed (3- to 6-fold) in our study, the total quantity found and the extent of the increase after ischemia was minor compared with the increment in E2/D2-IsoP concentration (48-fold). This suggested that E2/D2-IsoPs could be used as markers of oxidative stress in brain ischemia. The differential formation of F2-IsoPs or of E2/D2-IsoPs depends on whether the IsoP endoperoxide intermediate undergoes reduction or isomerization, respectively. Biological factors such as oxygen tension or glutathione levels dictate preferential formation of either E2/D2-IsoPs or F2-IsoPs (19). In this study, E2/D2-IsoP formation was greatly favored during brain ischemia, which may mean that reducing agents like glutathione were depleted during the ischemic period (39). F2-IsoPs and E2/D2-IsoPs also have been found to be increased in the brain of patients with Alzheimer's disease, and formation of E2/D2-IsoPs was favored over F2-IsoPs as well (40).
The concentrations of unesterified AA and DPA in control microwaved brain were consistent with previously reported values using microwave irradiation and gas-liquid chromatography (29, 41, 42), whereas the unesterified DHA concentration was slightly higher in the present study. The discrepancy may be due to differences in diet. Our concentration of unesterified AA in decapitated (nonmicrowaved) brain (35.9 ± 4.1 nmol/g) was much higher than that in control microwaved brain (2.71 ± 1.47 nmol/g), consistent with reports on nonmicrowaved brain for basal and ischemia-induced conditions (9, 38, 43–45). Additionally, when liquid nitrogen was used to rapidly freeze the brain, Ikeda et al. (46) and Rao et al. (14) reported values similar to ours, although they did not use an anesthetic or administer halothane before decapitation and liquid nitrogen immersion. The increased concentrations of AA, DHA, and DPA following ischemia agree with reports of PLA2 activation and of increased concentrations using either microwave irradiation or liquid nitrogen (3, 14, 29, 46). The percent increase was highest for AA, in agreement with previous reports (29, 47). However, it is important to point out that the reservoirs of esterified AA and DHA are quite large and have been previously found in control brains to be 13 μmol/g for AA and 15 μmol/g for DHA (48), which are several orders of magnitude above that found for the free AA and DHA released during ischemia (47.61 ± 4.08 nmol/g and 50.24 ± 6.26 nmol/g, respectively). Therefore, it is most probable that the free radical products formed during ischemia are derived from the esterified PUFAs rather than from the smaller pool of free AA and DHA.
The high concentration of 5-oxo-ETE in ischemic brain, which has been previously reported to be produced by hematopoietic cells, suggests that this compound was produced in the brain, inasmuch as microvessels account only for 0.15% of total brain wet weight (49). At least two pathways are known that can lead to the formation of this bioactive eicosanoid; one is an enzymatic pathway from 5(S)-HETE (50, 51), the other a nonenzymatic pathway from racemic 5-HpETE (52), which would be a free radical-generated eicosanoid.
The concentrations of TXB2 and 12-HETE (0.2 and 6 pmol/g brain, respectively) in ischemic brain could have resulted from an increased concentration of precursor unesterified AA and the activity of COX-2 and 12-LOX in neurons and COX-1 in microglia and astrocytes (53–58). Their formation only in blood platelets would be insufficient because of the small fraction of microvessels in brain (49). Nonmeasurable levels of PGE2, PGD2, and LTB4 and the nonsignificant increase in LTC4 suggest that most AA released following ischemia was not available to the tightly coupled enzyme complexes COX/PGD2 synthase or 5-LOX/LTC4 synthase within neurons and glia and eventually was reesterified.
Docosanoids were found in both control and ischemic brain. Following the 5 min no-flow ischemia, we identified 17-hydroxy-DHA and the structurally uncharacterized di- and trihydroxy-DHA. The accumulation of these docosanoids was consistent with an increased unesterified precursor DHA concentration (29, 47) and increased 15-LOX activity following ischemia (57). Interestingly, 17-hydroxy-DHA in control brain was in the same concentration range as were the 12-LOX products (2 pmol/g), but higher than COX products. The 10,17S-docosatriene (NPD1) and resolvin-D1 could not be detected, suggesting that 5 min of no-flow ischemia was too short to observe brain NPD1 accumulation. In mouse brain undergoing ischemia/reperfusion, NPD1 increased during the initial 8 h of reperfusion after 1 h of ischemia (24). However the proposed precursor of NPD1 (17-OH-DHA) was found even after 5 min of ischemia.
Although resolvin-1 and NPD1 following 5 min of global ischemia could not be detected, the MRM transitions set to measure these compounds (m/z 375 → 141 and m/z 375 → 277 for trihydroxy-DHA, and m/z 359 → 206 and mz 359 → 153 for dihydroxy-DHA) did reveal additional isomers of DHA metabolism during initial brain ischemia in the rat (59, 60). The identification of di- and trihydroxy-DHA isomers will require more investigation, but free radical oxidation of DHA to form polyhydroxylated DHA may be taking place.
In summary, concentrations of unesterified AA and its eicosanoid derivatives in this study in high-energy-microwaved brains were comparable to concentrations reported in brains fixed by liquid nitrogen immersion but lower than reported concentrations following decapitation without either microwaving or freezing. This confirms that microwave irradiation is an effective method to stop ischemic metabolic processes. Measurable concentrations of PGE2 and PGD2 were not found in control or even ischemic microwaved rat brain. Thus, reported measurements of these eicosanoids may have been due to artifactual formation when care was not taken to inactivate COX prior to tissue isolation and sample work up, or when nonspecific immunological methods were used for analysis. E2/D2-IsoPs were dramatically increased during rat brain ischemia, and their fractional increments were much higher than increments in the oxidative marker, F2-IsoPs. That 5-oxo-ETE was formed during ischemia further points to free radical-based synthetic pathways of biologically active eicosanoid production. Metabolites of DHA (including mono-, di- and trihydroxy-DHA products) also were elevated in rat ischemic brain. Monohydroxy-DHA was characterized as 17-OH-DHA, which is the precursor of NPD1. Because eicosanoids and docosanoids are considered to have pro- and anti-inflammatory properties, respectively (22, 24, 26, 27, 61), initiation of both pro- and anti-inflammatory pathways occurs in the ischemic rat brain. The interactive balance of eicosanoids and docosanoids probably dictates the severity of brain injury and the extent of recovery if reflow occurs.
Acknowledgments
The authors thank Dr. Charles Serhan (Harvard Medical School) for the resolvin-D1 and NPD1 standards.
Abbreviations
AA, arachidonic acid
COX, cyclooxygenase
DHA, docosahexaenoic acid
DPA, docosapentaenoic acid
5-oxo-ETE, 5-oxo-eicosatetraenoic acid
HETE, hydroxyeicosatetraenoic acid
IsoP, isoprostane
LC/MS/MS, liquid chromatography-tandem mass spectrometry
LOX, lipoxygenase
LT, leukotriene
MRM, multiple reaction monitoring
NPD1, neuroprotectin D1
PGD2, prostaglandin D2
RT, retention time
TXB2, thromboxane B2
Published, JLR Papers in Press, May 23, 2008.
Footnotes
This work was supported in part by the Heart, Lung and Blood Institute, National Institutes of Health, Grant HL-025785, and a grant from General Medical Sciences (GM-069338) (Lipid Maps), as well as by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. No author has a conflict of interest or financial interest with regard to this work.
References
- 1.Raichle M. E. 1983. The pathophysiology of brain ischemia. Ann. Neurol. 13 2–10. [DOI] [PubMed] [Google Scholar]
- 2.Farooqui A. A., and L. A. Horrocks. 2006. Phospholipase A2-generated lipid mediators in the brain: the good, the bad, and the ugly. Neuroscientist. 12 245–260. [DOI] [PubMed] [Google Scholar]
- 3.Adibhatla R. M., and J. F. Hatcher. 2006. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic. Biol. Med. 40 376–387. [DOI] [PubMed] [Google Scholar]
- 4.Phillis J. W., and M. H. O'Regan. 2003. The role of phospholipases, cyclooxygenases, and lipoxygenases in cerebral ischemic/traumatic injuries. Crit. Rev. Neurobiol. 15 61–90. [DOI] [PubMed] [Google Scholar]
- 5.Rapoport S. I. 2003. In vivo approaches to quantifying and imaging brain arachidonic and docosahexaenoic acid metabolism. J. Pediatr. 143 (Suppl.): 26–34. [DOI] [PubMed] [Google Scholar]
- 6.Sun G. Y., and R. A. MacQuarrie. 1989. Deacylation-reacylation of arachidonoyl groups in cerebral phospholipids. Ann. N. Y. Acad. Sci. 559 37–55. [DOI] [PubMed] [Google Scholar]
- 7.Aktan S., C. Aykut, S. Oktay, B. Yegen, E. Keles, I. Aykac, and S. Ercan. 1991. The alterations of leukotriene C4 and prostaglandin E2 levels following different ischemic periods in rat brain tissue. Prostaglandins Leukot. Essent. Fatty Acids. 42 67–71. [DOI] [PubMed] [Google Scholar]
- 8.Anton R. F., C. Wallis, and C. L. Randall. 1983. In vivo regional levels of PGE and thromboxane in mouse brain: effect of decapitation, focused microwave fixation, and indomethacin. Prostaglandins. 26 421–429. [DOI] [PubMed] [Google Scholar]
- 9.Bosisio E., C. Galli, G. Galli, S. Nicosia, C. Spagnuolo, and L. Tosi. 1976. Correlation between release of free arachidonic acid and prostaglandin formation in brain cortex and cerebellum. Prostaglandins. 11 773–781. [DOI] [PubMed] [Google Scholar]
- 10.Moskowitz M. A., K. J. Kiwak, K. Hekimian, and L. Levine. 1984. Synthesis of compounds with properties of leukotrienes C4 and D4 in gerbil brains after ischemia and reperfusion. Science. 224 886–889. [DOI] [PubMed] [Google Scholar]
- 11.Petroni A., A. Bertazzo, S. Sarti, and C. Galli. 1989. Accumulation of arachidonic acid cyclo- and lipoxygenase products in rat brain during ischemia and reperfusion: effects of treatment with GM1-lactone. J. Neurochem. 53 747–752. [DOI] [PubMed] [Google Scholar]
- 12.Petroni A., A. Socini, M. Blasevich, A. Borghi, and C. Galli. 1985. Differential effects of various vasoactive drugs on basal and stimulated levels of TXB2 and 6-keto-PGF1 alpha in rat brain. Prostaglandins. 29 579–587. [DOI] [PubMed] [Google Scholar]
- 13.Poddubiuk Z. M., J. B. Blumberg, and I. J. Kopin. 1982. Brain prostaglandin content in rats sacrificed by decapitation vs focused microwave irradiation. Experientia. 38 987–988. [DOI] [PubMed] [Google Scholar]
- 14.Rao A. M., J. F. Hatcher, M. S. Kindy, and R. J. Dempsey. 1999. Arachidonic acid and leukotriene C4: role in transient cerebral ischemia of gerbils. Neurochem. Res. 24 1225–1232. [DOI] [PubMed] [Google Scholar]
- 15.Usui M., T. Asano, and K. Takakura. 1987. Identification and quantitative analysis of hydroxy-eicosatetraenoic acids in rat brains exposed to regional ischemia. Stroke. 18 490–494. [DOI] [PubMed] [Google Scholar]
- 16.Golovko M. Y., and E. J. Murphy. 2008. An improved LC-MS/MS procedure for brain prostanoid analysis using brain fixation with head-focused microwave irradiation and liquid-liquid extraction. J. Lipid Res. 49 893–902. [DOI] [PubMed] [Google Scholar]
- 17.Peters O., T. Back, U. Lindauer, C. Busch, D. Megow, J. Dreier, and U. Dirnagl. 1998. Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J. Cereb. Blood Flow Metab. 18 196–205. [DOI] [PubMed] [Google Scholar]
- 18.Morrow J. D., and L. J. Roberts II. 1994. Mass spectrometry of prostanoids: F2-isoprostanes produced by non-cyclooxygenase free radical-catalyzed mechanism. Methods Enzymol. 233 163–174. [DOI] [PubMed] [Google Scholar]
- 19.Morrow J. D., L. J. Roberts, V. C. Daniel, J. A. Awad, O. Mirochnitchenko, L. L. Swift, and R. F. Burk. 1998. Comparison of formation of D2/E2-isoprostanes and F2-isoprostanes in vitro and in vivo—effects of oxygen tension and glutathione. Arch. Biochem. Biophys. 353 160–171. [DOI] [PubMed] [Google Scholar]
- 20.Milne G. L., S. C. Sanchez, E. S. Musiek, and J. D. Morrow. 2007. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat. Protocols. 2 221–226. [DOI] [PubMed] [Google Scholar]
- 21.Marin J. G., S. Cornet, B. Spinnewyn, C. Demerle-Pallardy, M. Auguet, and P. E. Chabrier. 2000. BN 80933 inhibits F2-isoprostane elevation in focal cerebral ischaemia and hypoxic neuronal cultures. Neuroreport. 11 1357–1360. [DOI] [PubMed] [Google Scholar]
- 22.Hong S., K. Gronert, P. R. Devchand, R. L. Moussignac, and C. N. Serhan. 2003. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J. Biol. Chem. 278 14677–14687. [DOI] [PubMed] [Google Scholar]
- 23.Hong S., E. Tjonahen, E. L. Morgan, Y. Lu, C. N. Serhan, and A. F. Rowley. 2005. Rainbow trout (Oncorhynchus mykiss) brain cells biosynthesize novel docosahexaenoic acid-derived resolvins and protectins-Mediator lipidomic analysis. Prostaglandins Other Lipid Mediat. 78 107–116. [DOI] [PubMed] [Google Scholar]
- 24.Marcheselli V. L., S. Hong, W. J. Lukiw, X. H. Tian, K. Gronert, A. Musto, M. Hardy, J. M. Gimenez, N. Chiang, C. N. Serhan, et al. 2003. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J. Biol. Chem. 278 43807–43817. [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee P. K., V. L. Marcheselli, C. N. Serhan, and N. G. Bazan. 2004. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA. 101 8491–8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwab J. M., N. Chiang, M. Arita, and C. N. Serhan. 2007. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 447 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serhan C. N. 2007. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 25 101–137. [DOI] [PubMed] [Google Scholar]
- 28.Cenedella R. J., C. Galli, and R. Paoletti. 1975. Brain free fatty levels in rats sacrificed by decapitation versus focused microwave irradiation. Lipids. 10 290–293. [DOI] [PubMed] [Google Scholar]
- 29.Deutsch J., S. I. Rapoport, and A. D. Purdon. 1997. Relation between free fatty acid and acyl-CoA concentrations in rat brain following decapitation. Neurochem. Res. 22 759–765. [DOI] [PubMed] [Google Scholar]
- 30.Bazinet R. P., H. J. Lee, C. C. Felder, A. C. Porter, S. I. Rapoport, and T. A. Rosenberger. 2005. Rapid high-energy microwave fixation is required to determine the anandamide (N-arachidonoylethanolamine) concentration of rat brain. Neurochem. Res. 30 597–601. [DOI] [PubMed] [Google Scholar]
- 31.Deutsch J., S. I. Rapoport, and T. A. Rosenberger. 2002. Coenzyme A and short-chain acyl-CoA species in control and ischemic rat brain. Neurochem. Res. 27 1577–1582. [DOI] [PubMed] [Google Scholar]
- 32.Hall L. M., and R. C. Murphy. 1998. Electrospray mass spectrometric analysis of 5-hydroperoxy and 5-hydroxyeicosatetraenoic acids generated by lipid peroxidation of red blood cell ghost phospholipids. J. Am. Soc. Mass Spectrom. 9 527–532. [DOI] [PubMed] [Google Scholar]
- 33.Williams L. R., H. L. Vahlsing, T. Lindamood, S. Varon, F. H. Gage, and M. Manthorpe. 1987. A small-gauge cannula device for continuous infusion of exogenous agents into the brain. Exp. Neurol. 95 743–754. [DOI] [PubMed] [Google Scholar]
- 34.Guidotti A., D. L. Cheney, M. Trabucchi, M. Doteuchi, and C. Wang. 1974. Focussed microwave radiation: a technique to minimize post mortem changes of cyclic nucleotides, dopa and choline and to preserve brain morphology. Neuropharmacology. 13 1115–1122. [DOI] [PubMed] [Google Scholar]
- 35.Merritt J. H., and J. W. Frazer. 1977. Microwave fixation of brain tissue as a neurochemical technique—a review. J. Microw. Power. 12 133–139. [DOI] [PubMed] [Google Scholar]
- 36.Narumiya S., T. Ogorochi, K. Nakao, and O. Hayaishi. 1982. Prostaglandin D2 in rat brain, spinal cord and pituitary: basal level and regional distribution. Life Sci. 31 2093–2103. [DOI] [PubMed] [Google Scholar]
- 37.Pediconi M. F., and E. B. Rodriguez de Turco. 1984. Free fatty acid content and release kinetics as manifestations of cerebral lateralization in mouse brain. J. Neurochem. 43 1–7. [DOI] [PubMed] [Google Scholar]
- 38.Yasuda H., K. Kishiro, N. Izumi, and M. Nakanishi. 1985. Biphasic liberation of arachidonic and stearic acids during cerebral ischemia. J. Neurochem. 45 168–172. [DOI] [PubMed] [Google Scholar]
- 39.Rehncrona S., J. Folbergrova, D. S. Smith, and B. K. Siesjo. 1980. Influence of complete and pronounced incomplete cerebral ischemia and subsequent recirculation on cortical concentrations of oxidized and reduced glutathione in the rat. J. Neurochem. 34 477–486. [DOI] [PubMed] [Google Scholar]
- 40.Reich E. E., W. R. Markesbery, L. J. Roberts 2nd, L. L. Swift, J. D. Morrow, and T. J. Montine. 2001. Brain regional quantification of F-ring and D-/E-ring isoprostanes and neuroprostanes in Alzheimer's disease. Am. J. Pathol. 158 293–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demar J. C., Jr., K. Ma, L. Chang, J. M. Bell, and S. I. Rapoport. 2005. Alpha-linolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. J. Neurochem. 94 1063–1076. [DOI] [PubMed] [Google Scholar]
- 42.Igarashi M., J. C. Demar, Jr., K. Ma, L. Chang, J. M. Bell, and S. I. Rapoport. 2007. Docosahexaenoic acid synthesis from alpha-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation. J. Lipid Res. 48 1150–1158. [DOI] [PubMed] [Google Scholar]
- 43.Rehncrona S., E. Westerberg, B. Akesson, and B. K. Siesjo. 1982. Brain cortical fatty acids and phospholipids during and following complete and severe incomplete ischemia. J. Neurochem. 38 84–93. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida S., M. Ikeda, R. Busto, M. Santiso, E. Martinez, and M. D. Ginsberg. 1986. Cerebral phosphoinositide, triacylglycerol, and energy metabolism in reversible ischemia: origin and fate of free fatty acids. J. Neurochem. 47 744–757. [DOI] [PubMed] [Google Scholar]
- 45.Yue H., S. A. Jansen, K. I. Strauss, M. R. Borenstein, M. F. Barbe, L. J. Rossi, and E. Murphy. 2007. A liquid chromatography/mass spectrometric method for simultaneous analysis of arachidonic acid and its endogenous eicosanoid metabolites prostaglandins, dihydroxyeicosatrienoic acids, hydroxyeicosatetraenoic acids, and epoxyeicosatrienoic acids in rat brain tissue. J. Pharm. Biomed. Anal. 43 1122–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikeda M., S. Yoshida, R. Busto, M. Santiso, and M. D. Ginsberg. 1986. Polyphosphoinositides as a probable source of brain free fatty acids accumulated at the onset of ischemia. J. Neurochem. 47 123–132. [DOI] [PubMed] [Google Scholar]
- 47.Sun G. Y., F. L. Lu, S. E. Lin, and M. R. Ko. 1992. Decapitation ischemia-induced release of free fatty acids in mouse brain. Relationship with diacylglycerols and lysophospholipids. Mol. Chem. Neuropathol. 1 39–50. [DOI] [PubMed] [Google Scholar]
- 48.Chang M. C., J. M. Bell, A. D. Purdon, E. G. Chikhale, and E. Grange. 1999. Dynamics of docosahexaenoic acid metabolism in the central nervous system: lack of effect of chronic lithium treatment. Neurochem. Res. 24 399–406. [DOI] [PubMed] [Google Scholar]
- 49.Williams W. M., T. Hayakawa, E. Grange, and S. I. Rapoport. 1998. Arecoline stimulation of radiolabeled arachidonate incorporation from plasma into brain microvessels of awake rat. Neurochem. Res. 23 551–555. [DOI] [PubMed] [Google Scholar]
- 50.Erlemann K. R., C. Cossette, G. E. Grant, G. J. Lee, P. Patel, J. Rokach, and W. S. Powell. 2007. Regulation of 5-hydroxyeicosanoid dehydrogenase activity in monocytic cells. Biochem. J. 403 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Powell W. S., F. Gravelle, and S. Gravel. 1992. Metabolism of 5(S)-hydroxy-6,8,11,14-eicosatetraenoic acid and other 5(S)-hydroxyeicosanoids by a specific dehydrogenase in human polymorphonuclear leukocytes. J. Biol. Chem. 267 19233–19241. [PubMed] [Google Scholar]
- 52.Zarini S., and R. C. Murphy. 2003. Biosynthesis of 5-oxo-6,8,11,14-eicosatetraenoic acid from 5-hydroperoxyeicosatetraenoic acid in the murine macrophage. J. Biol. Chem. 278 11190–11196. [DOI] [PubMed] [Google Scholar]
- 53.Choi J. S., H. Y. Kim, M. H. Chun, J. W. Chung, and M. Y. Lee. 2006. Differential regulation of cyclooxygenase-2 in the rat hippocampus after cerebral ischemia and ischemic tolerance. Neurosci. Lett. 393 231–236. [DOI] [PubMed] [Google Scholar]
- 54.Kaufmann W. E., P. F. Worley, J. Pegg, M. Bremer, and P. Isakson. 1996. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc. Natl. Acad. Sci. USA. 93 2317–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nogawa S., F. Zhang, M. E. Ross, and C. Iadecola. 1997. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J. Neurosci. 17 2746–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palluy O., M. Bendani, J. M. Vallat, and M. Rigaud. 1994. 12-Lipoxygenase mRNA expression by cultured neurons. C. R Acad. Sci. III. 317 813–818. [PubMed] [Google Scholar]
- 57.van Leyen K., H. Y. Kim, S. R. Lee, G. Jin, K. Arai, and E. H. Lo. 2006. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke. 37 3014–3018. [DOI] [PubMed] [Google Scholar]
- 58.Taniura S., H. Kamitani, T. Watanabe, and T. E. Eling. 2002. Transcriptional regulation of cyclooxygenase-1 by histone deacetylase inhibitors in normal human astrocyte cells. J. Biol. Chem. 277 16823–16830. [DOI] [PubMed] [Google Scholar]
- 59.Hong S., Y. Lu, R. Yang, K. H. Gotlinger, N. A. Petasis, and C. N. Serhan. 2007. Resolvin D1, protectin D1, and related docosahexaenoic acid-derived products: analysis via electrospray/low energy tandem mass spectrometry based on spectra and fragmentation mechanisms. J. Am. Soc. Mass Spectrom. 18 128–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serhan C. N., K. Gotlinger, S. Hong, Y. Lu, J. Siegelman, T. Baer, R. Yang, S. P. Colgan, and N. A. Petasis. 2006. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J. Immunol. 176 1848–1859. [DOI] [PubMed] [Google Scholar]
- 61.Phillis J. W., L. A. Horrocks, and A. A. Farooqui. 2006. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res. Brain Res. Rev. 52 201–243. [DOI] [PubMed] [Google Scholar]