Abstract
Endocytic pathways have been implicated in polyamine transport in mammalian cells, but specific mechanisms have not been described. We have shown that expression of a dominant negative (DN) form of the GTPase Dynamin, but not Eps15, diminished polyamine uptake in colon cancer cells indicating a caveolar and nonclathrin uptake mode. Polyamines co-sediment with lipid raft/caveolin-1 rich fractions, of the plasma membrane in a sucrose density gradient. Knock down of caveolin-1 significantly increased polyamine uptake. Conversely, ectopic expression of this protein resulted in diminished polyamine uptake. We also found that presence of an activated K-RAS oncogene significantly increased polyamine uptake by colon cancer cells. This effect is through an increase in caveolin-1 phosphorylation at tyrosine residue 14. Caveolin-1 is a negative regulator of caveolar endocytosis and phosphorylation in a K-RAS dependent manner leads to an increase in caveolar endocytosis. In cells expressing wild type K-RAS, addition of exogenous uPA was sufficient to stimulate caveolar endocytosis of polyamines. This effect was abrogated by the addition of a SRC kinase inhibitor. These data indicate that polyamine transport follows a dynamin-dependent and clathrin-independent endocytic uptake route, and this route is positively regulated by the oncogenic expression of K-RAS in a caveolin-1 dependent manner.
Keywords: polyamine uptake, endocytosis, caveolin-1, K-RAS, colon cancer
Introduction
The polyamines putrescine, spermidine, and spermine are ubiquitous, low molecular weight aliphatic amines [1,2]. They are essential for normal growth and development, and their tissue levels are often increased in cancer [3]. The polyamines can influence gene expression, interact with membrane phospholipids, and influence ion channels to name a few of their functions. Their intracellular pools are tightly regulated. A loss in pool homeostasis leads to apoptosis [4]. Intracellular polyamine contents are regulated by four processes: biosynthesis, catabolism, uptake, and efflux [1,2]. The key enzymes in polyamine biosynthesis and catabolism have been cloned and characterized. Genes involved in polyamine transport in prokaryotes (the PotD, PotE, and PotF set of genes in E. coli) and unicellular eukaryotes (LmPOT1 in L. major), have been identified [5,6]. Despite sustained efforts in the last two decades, mechanisms of polyamine uptake in mammals are still largely unknown. Several groups have alluded to an endocytic route of uptake in mammalian cells. Fransson et al. were the first to show involvement of cell surface heparin sulfate proteoglycans (HSPG) in polyamine uptake. They used a genetic approach and demonstrated that a mutant CHO cell line, deficient in HSPG biosynthesis, had reduced uptake, and an inhibitor of HSPG biosynthesis abrogated uptake in normal cells [7]. Following this observation, two independent groups reported that fluorescent derivatives of polyamines localized in mammalian cells in discrete vesicles [8,9]. The Fransson group then showed that treatment of cells in vitro with difluoromethyl ornithine (DFMO), a polyamine biosyhthesis inhibitor, led to an increase in uptake, because of an increased affinity of HSPG to exogenous polyamines [10]. Their studies also showed that polyamine uptake in CHO cells was mediated by the HSPG, Glypican-1 and that polyamine bound HSPG colocalised with caveolin-1 positive endocytic vesicles [11]. Recently, Soulet et al. [12] have shown that a fluorescent analog of polyamines accumulates in the cytoplasm of CHO cells, via a two-step mechanism into small vesicles, reminiscent of an endocytic mechanism. These prior studies allude to endocyotsis as a possible mechanism of polyamine uptake in mammalian cells. However, the nature of the endocytic pathway, involved in polyamine uptake by mammalian cells is not known.
Endocytosis is a highly dynamic process, involved in various cellular processes, including uptake of nutrients and growth factor signaling [13]. It is classified into various sub-types based on the requirement of certain proteins. Endocytosis is broadly classified into dynamin-dependent and -independent processes [14]. The GTPase dynamin is ubiquitously expressed and is required for a large subset of endocytic processes. Dynamin dependent processes are further sub-classified as clathrin-dependent and independent [15]. Lipid raft/caveolar endocytosis is a subset of dynamin-dependent, clathrin-independent endocytosis. Lipid rafts are highly specialized microdomains in the plasma membrane [15]. Caveolin-1 is a lipid raft protein required for the formation of flask shaped invaginations in the plasma membrane, called caveolae. These invaginations are highly dynamic and are involved in constitutive endocytic trafficking of ligands like albumin and cholera toxin [14,16]. Caveolae formation is impaired in cells without caveolin-1 [17]. Thus, caveolin-1 stabilizes caveolae at the plasma membrane.
Polyamine levels are found to be elevated in cancerous, as compared to normal tissue. This is because of an upregulation of biosynthesis and a downregualtion of polyamine catabolism [18]. Polyamine biosynthesis has been a target for drug development for cancer therapy [3]. Though the biosynthesis inhibitor DFMO shows promise as a chemopreventive and chemotherapeutic agent in vitro, its effect on inhibition of polyamine biosynthesis is abrogated in vivo, because there is an compensatory increase in uptake of extracellular polyamines [19]. In a recent study by Nilson et al. [20] polyamine uptake seems to play a significant role in the progression of lymphomagenesis. Two independent researchers have shown that removal of the two main sources of exogenous polyamines in the gut can circumvent compensatory uptake and increase the efficacy of biosynthesis inhibitors like DFMO. Hessels et al. [21] have shown that decontamination of the gut with antibiotics gets rid of polyamines from microbial flora activity and increases the efficacy of DFMO, in an in vivo mouse model. Similarly, Quemener et al. [22] have reported that a polyamine-free diet enhances the efficacy of DFMO as a polyamine biosynthesis inhibitor. Thus, an understanding of polyamine uptake is critical for the development of efficacious anticancer agents. A hallmark of cancer is the accumulation of mutations in oncogenes. Bachrach and Seiler [23] have shown that various oncogenes can increase polyamine uptake. However, a mechanism for this oncogene-dependent uptake process has not been proposed.
Previous work from others has suggested that polyamine uptake may occur via an endocytic mechanism. However, no specific endocytic pathway has been attributed to this process. In the present study, we have reevaluated the role of different endocytic pathways in polyamine uptake by human colon-tumor derived cells, using a combination of genetic, biochemical, and pharmacological approaches. Our work reveals that polyamine uptake follows a dynamin dependent, clathrin independent endocytic route. Polyamines tend to associate with lipid rafts in these cells, and their uptake is negatively regulated by caveolin-1. We also show that oncogenes like K-RAS regulate polyamine uptake by modulating caveolin-1 phosphorylation in a SRC dependent manner.
Materials and Methods
Cell Culture
The human colon cancer cell line, HCT116 was purchased from the American Type Culture Collection and maintained in McCoy's 5A medium, supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S), in a humidified incubator at 37°C with 5% CO2. The HCT116—Mock and Caveolin-1 antisense transfected cells were a kind gift from Dr. B. Sloane and Dr. D. Cadavello-Medved [24]. They were maintained in Dulbecco's Modified Essential Medium (DMEM) supplemented with 10% FBS and P/S and 0.5 μg/mL puromycin. The HT29 cells transfected with an inducible caveolin-1 expression vector were obtained from Dr. E. Felley-Bosco [25]. They were maintained in DMEM supplemented with 10% heat-inactivated FBS and P/S. Caveolin-1 expression was induced by the addition of 1 mM isopropyl-beta-d-thiogalactopyranoside (IPTG) for 24 h. All experiments were carried out after 24 h of IPTG addition. The HCT116 cells have an activating K-RAS mutation (G13V) in one of the K-RAS alleles. The Hkh2 cell line, which is a clone of the HCT116 cells where the activated K-RAS allele has been disrupted by homologous recombination, was a kind gift from Dr. Shirasawa and was maintained in DMEM supplemented with 10% FBS and P/S and 600 μg/mL G418 [26]. Cells were routinely tested for mycoplasma contamination.
Reagents and Antibodies
All chemicals and reagents were of the highest grade. Chelerythrine chloride (Protein Kinase C inhibitor) and PP2 (Src inhibitor) were obtained from Calbiochem (San Diego, CA). Methyl-β-cyclo-dextrin (membrane cholesterol extracting agent), putrescine and spermidine were purchased from Sigma (St. Louis, MO). UPA was purchased for Chemicon (Temecula, CA). Tritiated spermidine (specific activity 18 Ci/mmol) was purchased from Perkin Elmer, and tritiated putrescine (specific activity 26 Ci/mmol) was purchased from Amersham Biosciences, Piscataway, NJ and alexa fluor 546/633 labeled transferrin were purchased from Molecular Probes (Eugene, OR). Lipofectamine 2000 and all media were from Invitrogen, Carlsbad, CA. The caveolin-1 rabbit polyclonal antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). The flotillin-1 antibody was from BD Transduction Laboratories (Franklin Lakes, NJ). All secondary antibodies were from Santa Cruz Biotechnology. The phospho-caveolin-1 (tyrosine 14), phospho-Src (tyrosine 416), and total Src antibodies were purchased from Cell Signaling Tehcnology, Inc. (Danvers, MA).
Plasmids
The GFP-tagged dynamin II wild type (WT) and dynamin K44A (DN) plasmids were from Dr. M. McNiven [27,28]. The GFP-tagged EpsD3Δ2 (WT) and Eps15 (95/295 (DN) were from Dr. A. Dautry-Varsat [28].
Endocytosis of Fluorescent Ligands
For uptake of fluorescent transferrin, 5 × 105 cells were plated on Lab-Tek Chamber slides for 18 h. The next day, they were transfected with GFP-tagged plasmids (1 μg/well) with Lipofectamine 2000, according to the manufacturer's protocol. Twenty-four hours after transfection, the cells were serum-starved for 30 min for transferrin uptake. Labeled transferrin (10 μg/mL) was added and the cells were incubated for 15 and 30 min. Cells were washed three times with serum-free medium and once with ice-cold PBS. For flowcytometric analysis of transferrin uptake, HCT116 cells were transfected as described above. Following labeling with transferrin, cells were trypsinized and collected for flow cytometry. Cells were analyzed using a BD FACSAria scanning machine and mean fluorescence intensity (MFI) was obtained. A control reaction at 4°C was carried out to control for surface-bound transferrin and the MFI was subtracted from the MFI obtained at 37°C.
RAS Activity Assay
Cells (2 × 106 cells/100 mm plate, or 3 × 106 cells/100 mm plate for serum-starved cells) were plated in complete growth medium for 24 h. The next day, serum starvation was carried out as described in the TPA treatment. After overnight incubation, cells were lysed in magnesium lysis buffer (MLB) and activated RAS levels were measured using the RAS activation assay kit (Catalog No: 17-218) from Upstate, Millipore (Billerica, MA). The RAS activity assay is based on the principle that GTP-bound RAS will bind to RAF. The RAS-binding domain (RBD) of RAF is conjugated to Agarose beads. These beads are then used to pull down GTP-bound (active) RAS from a cell lysate and the beads probed with an anti-RAS antibody after precipitation and immunoblotting.
Polyamine Uptake Assay
For uptake of radio-labeled polyamines, 3 × 105 cells were plated in 6-well plates. After 48 h, cells were washed three times with serum-free medium. Uptake was initiated by the addition of 5–10 μM radio-labeled polyamine. Cells were incubated at 37°C for the indicated time and then washed three times with PBS containing 100× cold polyamines, followed by one wash with cold PBS alone. They were then lysed in 0.5% SDS in PBS at 37°C for 30 min. A control reaction was carried out at 4°C for the same time, to control for cell-surface bound polyamines. Lysates were counted using a Beckman LS 5000 TD scintillation counter, and uptake was normalized to total cellular protein content. Temperature-dependent uptake of polyamines at 37°C was calculated by subtracting the uptake at 4°C from the total uptake at 37°C. For inhibitor experiments, HCT116 were washed two times with serum free medium and incubated with inhibitors (5 mM methyl-β-cyclo-dextrin, 5 μM chelerythrine chloride, 10 μM PP2) for 1 h at 37°C. Inhibitors were present in all subsequent steps of the experiment. Polyamine uptake was carried out as described above.
Uptake of Polyamines by GFP-Transfected Cells
Ten million HCT116 cells were plated in 150 mm dishes for 18 h. They were then transfected with GFP-tagged plasmids with Lipofectamine 2000, as per the manufacturer's protocol. Twenty-four hours after transfection, cells were washed three times with serum-free medium and 5 μM unlabeled polyamine in serum-free medium was added to initiate uptake. Cells were incubated at 37°C for the indicated time and then washed three times with PBS. A control reaction was carried out at 4°C for the same time, to account for cell-surface bound polyamines. Cells were then trypsinized and GFP-positive cells were sorted using a BD FACSAria scanning machine. GFP-positive cells were then lysed and polyamine content was measured using the method of Seiler and Knodgen [29].
Lipid Raft Fractionation
Lipid raft fractionation was carried out by using a detergent-free, alkaline lysis method as described previously [30]. Briefly, HCT116 cells (7 × 106 cells/150 mm plate) were plated for 48 h. Each plate was then lysed with 2 mL of 500 mM sodium carbonate (pH-11.0). The lysate was sonicated for three 20-s bursts using a SONIC Vibra Cell sonicator. The lysate was then adjusted to 45% sucrose by mixing with equal volumes of 90% sucrose prepared in Mes-buffered saline (MBS—25 mM Mes, pH 6.5, 0.15 M NaCl), and placed at the bottom of an ultracentrifuge tube. A 5–35% discontinuous sucrose gradient was formed above (4 mL of 5% sucrose/4 mL of 35% sucrose; both in MBS containing 250 mM sodium carbonate) and centrifuged at 39 000 rpm for 16 h in an SW40-Ti rotor (Beckman Instruments, Palo Alto, CA). A light-scattering band at the 5–35% sucrose interface was observed. This fraction contains caveolin-1/lipid raft proteins. Twelve 1 mL fractions were collected from top to bottom of the tube. For detection of caveolin-1 in the fractions, equal volume from each fraction were loaded on a 12.5% SDS–PAGE gel and visualized as described in Western Blotting. For labeling cells with radioactive spermidine, cells were plated for 48 h and washed twice with serum-free medium. Tritiated spermidine was added to the cells in serum-free medium and incubated at 4°C for 15 min. The cells were then lysed as previously described and 250 μL from each fraction was counted in a Beckman LS 5000 TD scintillation counter.
Uptake of Cholera Toxin and Labeling of Lipid Rafts
For uptake of Alexa Fluor labeled-cholera toxin sub-unit B, 3 × 105 cells were plated in 6-well plates. After 48 h, cells were washed three times with PBS and medium containing 1 μg/mL of toxin was added for 30 min at 4 and 37°C. Following uptake, cells were washed three times with ice-cold PBS and lysed in 1× Reporter Lysis Buffer, Roche Applied Science (Catalog # 11897675001). Fluorescence intensity of lysates was measured using a Spectra Max Gemini fluorescence plate reader from Molecular Devices, and intensity was normalized to protein content.
Western Blotting
Cells (2 × 106 cells/100 mm plate) were plated for 48 h. They were lysed in radio-immunoprecipitation assay (RIPA) buffer with protease inhibitors. Samples were kept on ice for 30 min, followed by the centrifugation at 14 000 rpm for 10 min. Supernatants were collected and protein concentration was determined with the Bio-Rad DC protein assay. Twenty micrograms of protein were resolved on a 12.5% SDS–PAGE gel and transferred overnight to a Hybond-C nitrocellulose membrane, at 4°. The next day, the membrane was blocked in 5% nonfat dry milk in Tris-buffered saline with 0.05% Tween-20 (TBST) for 1 h at room temperature. Membranes were probed with caveolin-1 and flotillin-1 antibody in blocking buffer (1:1000 dilution) for 2 h at room temperature. Phospho-caveolin-1, phospho-Src, and total Src antibody were applied overnight at 4°C, diluted in 5% bovine serum albumin (BSA) in TBST. After washing with TBST, the membrane was probed with horse-radish peroxidase (HRP)-conjugated secondary antibody, washed and protein was detected with an enhanced chemiluminescence detection reagent (Amersham).
Statistical Analysis
Statistical analysis was carried out using the paired t-test with Microsoft Excel Software.
Results
Dynamin-Dependent Endocytosis Regulates Polyamine Uptake in HCT116 Cells
To investigate the role of endocytosis in polyamine uptake, a human colon-derived tumor cell line, HCT116, was treated with an inhibitor of endocytosis, brefeldin A, or incubated at 4°C [31]. As a control, we used uptake of transferrin and cholera toxin for clathrin-dependent and caveolar endocytosis respectively. Both low temperature (Figure 1A and B) and Brefeldin A (Figure 2A and B) impaired the uptake of these two ligands indicating that both these pathways were perturbed. We then investigated the effect of low temperature and Brefeldin A on polyamine uptake. As shown in Figures 1C and 2C, both low temperature and brefeldin A inhibited spermidine uptake in HCT116 cells, indicating an endocytic mechanism. Also, we found that treatment with a known inhibitor of dynamin-independent macropinocytosis, amiloride had no effect of polyamine uptake in HCT116 cells (Figure 3C) [31]. Uptake of transferrin and cholera toxin were unaffected by amiloride (Figure 3A and B). In order to further dissect the exact nature of the endocytic pathways involved in polyamine uptake, the HCT116 cells were transfected with a GFP-tagged form of the GTPase dynamin II (referred to as WT) and its DN form (K44A). To assess whether the construct was functional, uptake of Alexa-Fluor 633-conjugated transferrin in transfected HCT116 cells was assessed. Transferrin is an iron-binding protein and is internalized via a dynamin-dependent, clathrin-dependent endocytic route by mammalian cells [28,32]. When transferrin-positive, GFP-positive cells were sorted, the cells expressing wild type dynamin had significantly more MFI as compared to the cells expressing the DN isoform (Figure 4A). Next, we evaluated whether dynamin played a role in putrescine and spermidine uptake. HCT116 cells were transfected with the GFP-tagged constructs. Twenty-four hours after transfection, the cells were treated with cold polyamines for an hour and GFP-positive cells were collected by FACS. This method ensures that a pure population of cells, expressing the construct of interest, is analyzed. Intracellular polyamine content of WT and K44A-expressing HCT116 cells revealed that putrescine uptake was decreased by about 75% (P<0.01) in the K44A-expressing cells and compared to the WT (Figure 4B). Spermidine uptake was also significantly decreased in cells expressing the DN form of dynamin II (Figure 4C). The adaptor protein, Eps15 plays a pivotal role in the internalization of clathrin-coated pits. To investigate the role of this protein in polyamine uptake, the HCT116 cells were transiently transfected with GFP-tagged forms of the wild type form (D3Δ2) and its DN counterpart (Δ95/295). Uptake of transferrin was assessed in the transfected cells to ensure that the construct was functioning. As shown in Figure 5A, there was a significant reduction (50%) in MFI on fluorescent transferrin between the WT and DN Eps15 expressing cells. The HCT116 cells were then transfected with the constructs and polyamine uptake was measured. As seen in Figure 5B, expression of a DN form of the adaptor protein, Eps15 had no effect on putrescine internalization by the HCT116 cells. Similarly, internalization of spermidine was unperturbed in cells expressing Eps15Δ95/295 (Figure 5C).
Figure 1.
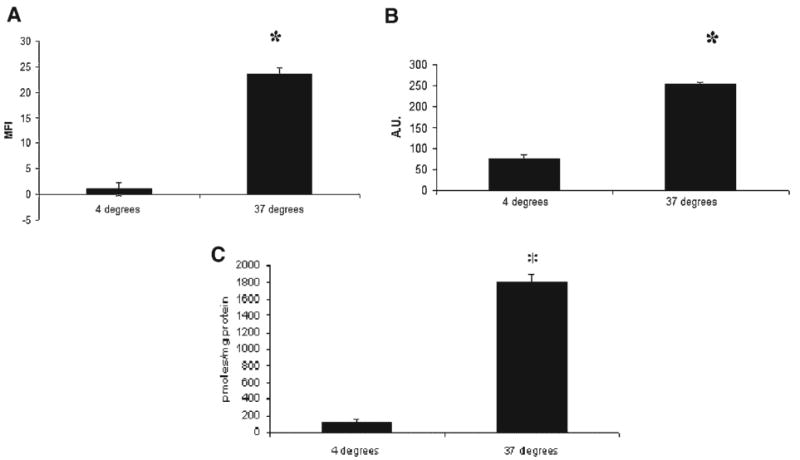
Incubation at a low temperature (4°C) inhibits uptake of transferrin, cholera toxin and spermidine in HCT116 cells. (A) HCT116 were plated for 48 h and then incubated at 4° or 37° for 30 min. Transferrin uptake with AlexaFluor 488-conjugated transferrin was assessed, as described in Materials and Methods Section. (B) HCT116 were plated for 48 h and then incubated at 4° or 37° for 30 min. Cholera toxin uptake with AlexaFluor 488-conjugated cholera toxin subunit-B was assessed, as described in Materials and Methods Section. (C) HCT116 were plated for 48 h and then incubated at 4° or 37° for 30 min. Polyamine uptake with radiolabeled spermidine was assessed, as described in Materials and Methods Section.
Figure 2.

Treatment with Brefeldin A, an inhibitor of endocytosis, abrogates transferrin, cholera toxin and spermidine uptake in HCT116 cells. (A) HCT116 were plated for 48 h and then incubated with 10 μg/mL Brefeldin A at 37° for 30 min. Transferrin uptake with AlexaFluor 488-conjugated transferrin was assessed, as described in Materials and Methods Section. (B) HCT116 were plated for 48 h and then incubated with 10 μg/mL Brefeldin A at 37° for 30 min. Cholera toxin uptake with AlexaFluor 488-conjugated cholera toxin subunit-B was assessed, as described in Materials and Methods Section. (C) HCT116 were plated for 48 h and then incubated with 10 μg/mL Brefeldin A at 37° for 30 min. Polyamine uptake with radiolabeled spermidine was assessed, as described in Materials and Methods Section.
Figure 3.

The macropinocytosis inhibitor, Amiloride, has no effect on transferrin, cholera toxin and spermidine uptake in HCT116 cells. (A) HCT116 were plated for 48 h and then incubated with 10 μM Amiloride at 37° for 30 min. Transferrin uptake with AlexaFluor 488-conjugated transferrin was assessed, as described in Materials and Methods Section. (B) HCT116 were plated for 48 h and then incubated with 10 μM Amiloride at 37° for 30 min. Cholera toxin uptake with AlexaFluor 488-conjugated cholera toxin subunit-B was assessed, as described in Materials and Methods Section. (C) HCT116 were plated for 48 h and then incubated with 10 μM Amiloride A at 37° for 30 min. Polyamine uptake with radiolabeled spermidine was assessed, as described in Materials and Methods Section.
Figure 4.
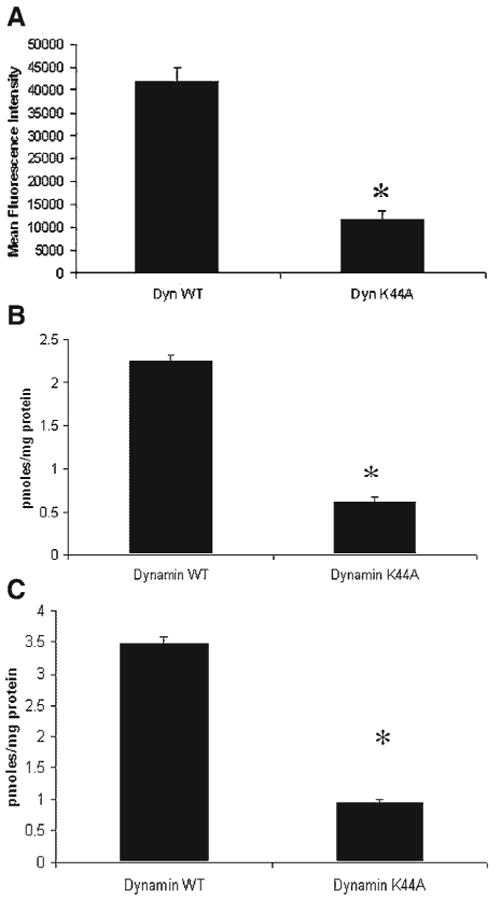
Perturbation of dynamin-dependent endocytosis blocks polyamine uptake in HCT116 cells. (A) HCT116 cells were transfected with DynWT and DynK44A, as described in Materials and Methods Section. Eighteen hours after transfection, cells were serum-starved for 1 h and treated with 10 μL of Alexa-Fluor 633 transferrin for 30 min. Cells were trypsinized and collected for FACS analysis. MFI was calculated after cells were sorted in a BDFacs Aria machine. In all figures, *P-value of <0.01. (B) HCT116 cells were transfected with DynWT and DynK44A. Twenty-four hours after transfection, cells were washed twice with serum free medium. Putrescine uptake was assayed as described in Materials and Methods Section. (C) HCT116 cells were transfected with DynWT and DynK44A. Twenty-four hours after transfection, cells were washed twice with serum free medium. Spermidine uptake was assayed as described in Materials and Methods Section. In all figures, *P-value of 0.01.
Figure 5.

Clathrin-dependent endocytosis does not affect polyamine uptake in HCT116 cells. (A) HCT116 cells were transfected with Eps15 WT and Eps15 DN, as described in Materials and Methods Section. Eighteen hours after transfection, cells were serum-starved for 1 h and treated with 10 μL of Alexa-Fluor 633 transferrin for 30 min. Cells were trypsinized and collected for FACS analysis. MFI was calculated after cells were sorted in a BDFacs Aria machine. In all figures, *P-value of <0.01. (B) HCT116 cells were transfected with Eps15 WT and Eps15 DN. Twenty-four hours after transfection, cells were washed twice with serum free medium. Putrescine uptake was assayed as described in Materials and Methods Section. (C) HCT116 cells were transfected with Eps15 WT and Eps15 DN. Twenty-four hours after transfection, cells were washed twice with serum free medium. Spermidine uptake was assayed as described in Materials and Methods Section. In all figures, *P-value of <0.01.
Polyamines Associate With Lipid Raft/Caveolae Fractions in Sucrose Density Gradient Centrifugation and Their Uptake Is Inhibited by Drugs Disrupting Caveolar Endocytosis
A distinct endocytic pathway, different from the traditional clathrin-coated pathway has been characterized, and involves the internalization of ligands and small molecules via lipid rafts. Lipid raft rich areas in the plasma membrane can be readily separated from the rest of the plasma membrane components based on their density. The technique usually involves lysing cells in a hypertonic alkaline buffer and fractionating the lysate on a discontinuous sucrose density gradient. By this method, lipid raft/caveolin-1 rich membrane micro-domains typically migrate to the interface of the 5–35% layer in the gradient. The heavier organelles are found in the lower fractions. In order to assess the purity of the fractionation, we assessed the migration of a common lipid raft marker flotillin-1 in the fractions by Western blotting. Flotillin-1 was primarily found in fractions 4, 5, and 6 of the sucrose density gradient, indicating that these fractions correspond to lipid raft/caveolae rich domains (Figure 6A). Immunoblotting for caveolin-1 revealed that its migration was similar to that of flotillin-1. In order to assess whether polyamines associate with lipid raft/caveolar fractions, HCT116 cells were labeled at 4°C with tritiated spermidine for 15 min and then lysed as described in Materials and Methods Section. Lysates were fractionated on a discontinuous sucrose density gradient. The radiolabel primarily localized to flotillin-1/caveolin-1 rich fractions 4–6 with small amounts seen in the heavier fractions (Figure 6A). We also found that the association of spermidine with lipid raft fractions is independent of the expression of caveolin-1 (Figure 6B).
Figure 6.


Polyamines associate with lipid raft/caveolin-1 rich fractions in a sucrose density gradient, and their uptake is inhibited with drugs that perturb caveolar endocytosis. (A) HCT116 cells were grown to subconfluency in 150 mm plates. Plates were washed twice with serum free medium and labeled with tritiated spermidine for 15 min at 4°C. Cells were then lyzed, fractions collected and counted as described in Materials and Methods Section. Same experiments were run without the radiolabel. Fractions were collected and resolved on an SDS–PAGE gel and immunoblotted for caveolin-1 and flotillin-1. (B) HCT116 caveolin-1 antisense transfected cells were analyzed as in Figure 6A. (C) HCT116 cells were plated for 48 h. They were then serum starved and treated with various inhibitors of endocytosis for 30–60 min at 37°C. Transferrin uptake with AlexaFluor 488-conjugated transferrin was assessed, as described in Materials and Methods Section. (D) HCT116 cells were plated for 48 h. They were then serum starved and treated with various inhibitors of endocytosis for 30–60 min at 37°C. Cholera toxin uptake with AlexaFluor 488-conjugated cholera toxin subunit-B was assessed, as described in Materials and Methods Section. (E) HCT116 cells were plated for 48 h. They were then serum starved and treated with various inhibitors of endocytosis for 30–60 min at 37°C. Polyamine uptake with radiolabeled putrescine was assessed, as described in Materials and Methods Section. (F) HCT116 cells were plated for 48 h. They were then serum starved and treated with various inhibitors of endocytosis for 30–60 min at 37°C. Polyamine uptake with radiolabeled spermidine was assessed, as described in Materials and Methods Section. In all figures, *, **, and ***P-value of <0.01. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Internalization of lipid rafts can be blocked by various agents including methyl-β-cyclodextrin, PP2, and chelerythrine chloride [33]. As shown in Figure 6C (transferrin uptake) and 6D (cholera toxin uptake), these agents perturbed caveolar endocytosis without having an effect on the uptake of transferrin. Methyl-β-cyclodextrin decreased putrescine uptake to about 30% of the control (Figure 6E). The same effect was seen on spermidine uptake (Figure 6F). The Src kinase inhibitor, PP2, decreased putrescine and spermidine uptake by about 40% and 70%, respectively (Figure 6E and F). Chelerythrine chloride, a pan-protein kinase C inhibitor also significantly reduced polyamine uptake in HCT116 cells (Figure 6E and F).
Loss of Caveolin-1 Increases Polyamine Uptake in Colon Cancer Cells
Caveolin-1 is a lipid raft-associated protein and is involved in the formation of caveolae [16]. To assess whether the lipid-raft dependent uptake was affected by caveolin-1 expression, we used a HCT116-derived cell line where caveolin-1 expression had been ablated using an antisense construct. As shown in Figure 7A, the antisense transfected cells show almost no expression of caveolin-1, as compared to the mock-transfected counterpart. As a control to assess whether loss of caveolin-1 increased caveolar endocytosis, we assessed the uptake of cholera toxin subunit B. There was an increase in uptake of fluorescent labeled cholera toxin subunit B in the HCT116 cells expressing the caveolin-1 antisense construct, as compared to the mock transfected controls (Figure 7B). We sought to determine whether caveolin-1 expression affected polyamine uptake in these cells. Caveolin-1 loss dramatically increased putrescine uptake in the HCT116 cells, with a twofold increase in putrescine uptake seen in the caveolin-1 antisense transfected cells (Figure 7C). Similar results were seen with spermidine uptake with a twofold increase seen with the antisense transfected cells (Figure 7D). This effect was not relegated to the HCT116 cells. We found that ectopic expression of caveolin-1 in HT29 cells (which express low levels of caveolin-1) was sufficient to reduce polyamine uptake (Figure 8A–C).
Figure 7.
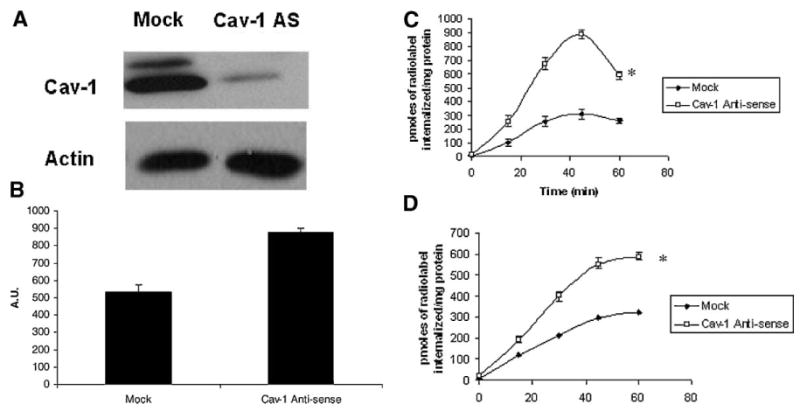
Loss of caveolin-1 increases polyamine uptake in HCT116 cells. (A) HCT116-Mock and Caveolin-1 Antisense transfected cells were grown to sub-confluency and lysed in RIPA buffer. Equal amounts of protein were resolved on SDS–PAGE gels and probed for caveolin-1. (B) HCT116-Mock and Caveolin-1 Antisense transfected cells were plated for 48 h. They were then serum starved for 60 min at 37°C. Cholera toxin uptake with AlexaFluor 488-conjugated cholera toxin subunit-B was assessed, as described in Materials and Methods Section. (C) HCT116-Mock and Caveolin-1 Antisense transfected cells were plated for 48 h. Polyamine uptake with radiolabeled putrescine was assessed, as described in Materials and Methods Section. (D) HCT116-Mock and Caveolin-1 Antisense transfected cells were plated for 48 h. Polyamine uptake with radiolabeled spermidine was assessed, as described in Materials and Methods Section.
Figure 8.
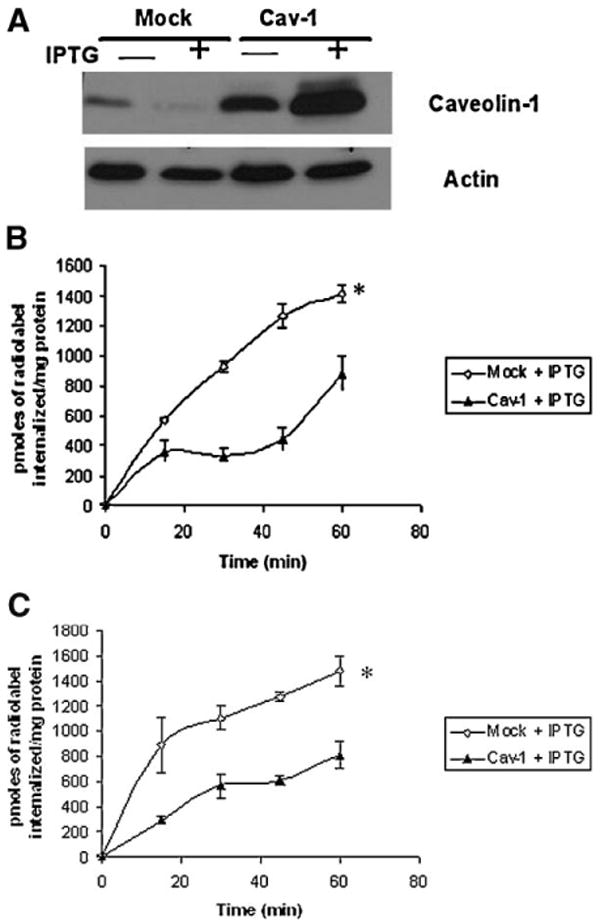
Ectopic expression of caveolin-1 decreases polyamine uptake in HT29 cells. (A) HT29-Mock and Caveolin-1 transfected cells were plated for 24 h. Following incubation, IPTG (1 mM) was added for an additional 24 h. Cells were then lysed, and equal amounts of protein were resolved on SDS–PAGE gels and probed for caveolin-1. (B) HT29-Mock and Caveolin-1 transfected cells were plated for 24 h. Following incubation, IPTG (1 mM) was added for an additional 24 h. Polyamine uptake with radiolabeled putrescine was assessed, as described in Materials and Methods Section. (C) HT29-Mock and Caveolin-1 transfected cells were plated for 24 h. Following incubation, IPTG (1 mM) was added for an additional 24 h. Polyamine uptake with radiolabeled spermidine was assessed, as described in Materials and Methods Section.
Activated K-RAS Increases Polyamine Uptake in Colon Cancer Cells Through SRC
Oncogenes like K-RAS can increase polyamine uptake in transformed cells. To test the effect of an activated K-RAS on polyamine uptake, we employed the HCT116 and a derived clone Hkh2 where the activated K-RAS has been disrupted by homologous recombination. As seen in Figure 9A and B, an activated K-RAS oncogene dramatically increases both putrescine and spermidine uptake in human colon cancer cells. This effect of an activated K-RAS was not restricted to the HCT116 cell system. In another isogenic cell line system, ectopic expression of an activated K-RAS was sufficient to increase putrescine uptake (Figure 9C). These cells have been developed and previously described by our laboratory [34] We wanted to understand whether an activated K-RAS influences polyamine uptake by modulating caveolar endocytosis. The HCT116 cells have higher levels of active (GTP-bound) K-RAS (Figure 10A), higher uptake of cholera toxin subunit B (Figure 10B) and higher levels of phopho-caveolin-1 as compared to HKh2 cells (Figure 10C). They also have a higher level of activated Src as seen by increased phosphorylated tyrosine 416 levels in Figure 10D. Treatment of the HCT116 cells with the Src kinase inhibitor, PP2 decreased both activated Src and phospho-caveolin-1 levels (Figure 10C and D). There was also a significant decrease in uptake of cholera toxin subunit B after PP2 treatment (Figure 10B).
Figure 9.

Activated K-RAS increases polyamine uptake in HCT116 cells, by increasing caveolin-1 tyrosine residue 14 phosphorylation. (A) HCT116 and Hkh2 cells were plated for 48 h. Polyamine uptake with radiolabeled putrescine was assessed, as described in Materials and Methods Section. (B) HCT116 and Hkh2 cells were plated for 48 h. Polyamine uptake with radiolabeled spermidine was assessed, as described in Materials and Methods Section. (C) Caco-Neo and Caco-K-Ras#26 cells were plated for 48 h. Polyamine uptake with radiolabeled putrescine was assessed, as described in Materials and Methods Section.
Figure 10.
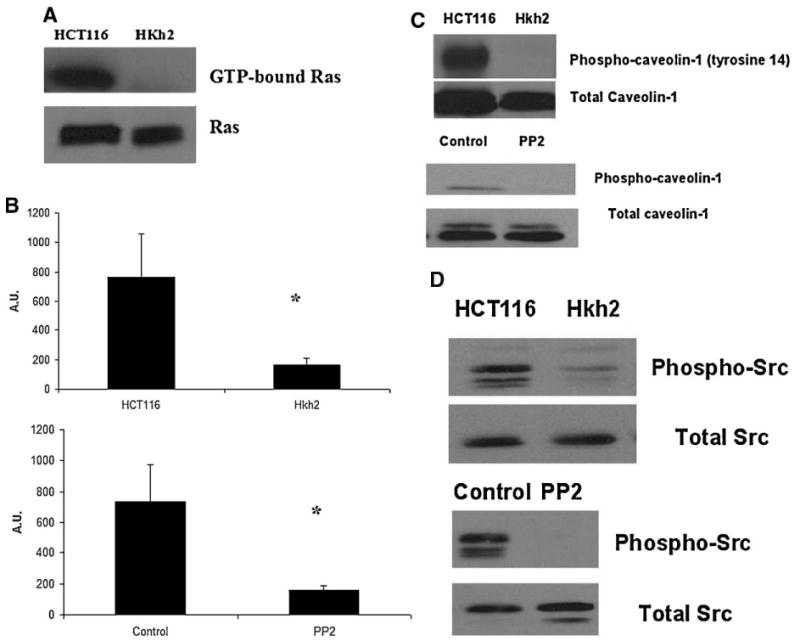
Activated K-RAS increases caveolin-1 phosphorylation in HCT116 cells in a SRC-dependent manner. (A) HCT116 and Hkh2 cells were plated for 48 h RAS activity status using the Ras Activation Assay Kit (Catalog # 17-218) from Upstate, Millipore Corporation using the manufacturer's protocol, as described in Materials and Methods Section. (B) HCT116 and Hkh2 cells were plated for 48 h. They were then serum starved for 60 min at 37°C. Cholera toxin uptake with AlexaFluor 488-conjugated cholera toxin subunit-B was assessed, as described in Materials and Methods Section. (C,D) HCT116 and Hkh2 cells were grown to sub-confluency and lysed in RIPA buffer. Equal amounts of protein were resolved on SDS–PAGE gels and probed for phospho-caveolin-1, caveolin-1, phospho-Src, and total Src. For PP2 treatment, cells were serum-starved for 1 h. PP2 (5 μM) was added for an additional 30 min before lysis.
Activated K-RAS Increases Polyamine Uptake in Colon Cancer Cells Through SRC
The previous results prompted us to understand how an activated K-RAS leads to SRC activation in the HCT116 cells. SRC localizes to caveolae, whereas K-RAS is found in nonlipid raft areas of the membrane [35,36]. So a direct interaction was ruled out. In a cDNA microarray study carried out in our laboratory, the urokinase plasminogen activated receptor (uPAR) was found to be downregulated in the Hkh2 cells (Table 1). Our microarray data corroborated the findings of two independent groups who have shown that uPAR is downregulated in the Hkh2 cells [37,38]. Cavallo-Medved et al. [37] also showed that an activated K-RAS altered the sub-cellular distribution of the uPAR ligand, uPA. In order to test whether the uPA–uPAR pathway was involved in SRC activation, we treated the Hkh2 cells with exogenous uPA.
Table 1.
Fold Decrease in Urokinase Plasminogen Activated Receptor (uPAR) Expression in Hkh2 Cells as Compared to HCT116 Cells
| Systemic | Normalized | Standard deviation | P-value from t-test | Gene name |
|---|---|---|---|---|
| AA455222 | 0.483342 | 0.144974 | 0.012472 | uPAR |
Exogenous uPA was sufficient to increase SRC activation, caveolin-1 phosphorylation and an increase in cholera toxin uptake (Figure 11A and B). Under the same conditions, an increase in spermidine uptake was observed (Figure 11C). The effects of exogenous uPA were abolished with the use of a SRC kinase inhibitor, PP2 (Figure 11A–C).
Figure 11.
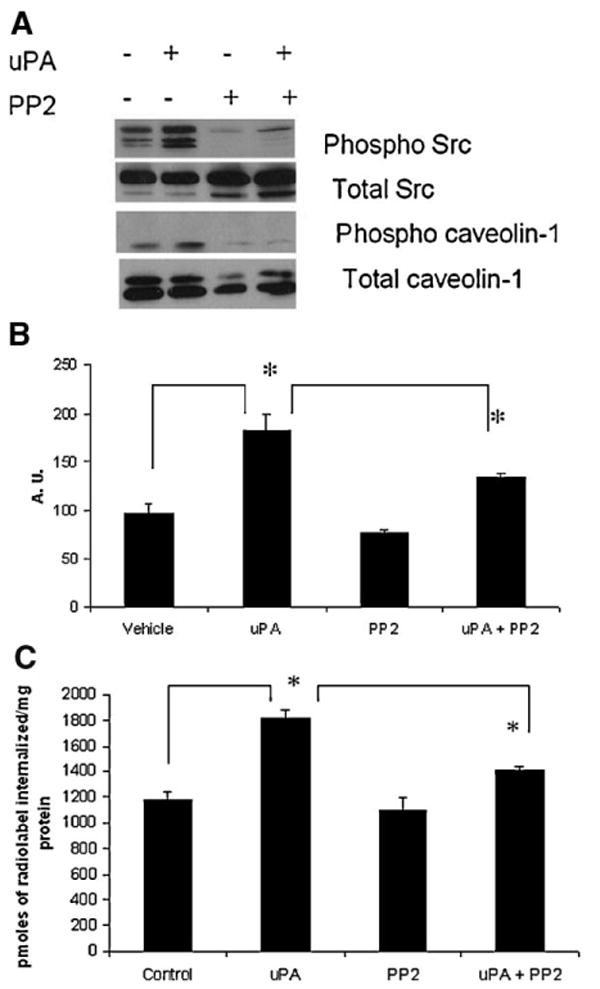
Exogenous uPA increases caveolin-1 phosphorylation and polyamine uptake in Hkh2 cells, in a SRC dependent manner. (A) Hkh2 cells were grown to sub-confluency and treated with 50 ng/mL uPA for 30 min. They were lysed in RIPA buffer. Equal amounts of protein were resolved on SDS–PAGE gels and probed for phospho-caveolin-1, caveolin-1, phospho-Src, and total Src. For PP2 treatment, cells were serum-starved for 1 h. PP2 (5 μM) was added for an additional 30 min before lysis. This was followed by uPA treatment for 30 min after which cells were lysed in RIPA buffer. (B) Hkh2 cells were grown to sub-confluency and treated with 50 ng/mL uPA for 30 min. Cholera toxin uptake was carried out as described in Materials and Methods Section. For PP2 treatment, cells were serum-starved for 1 h. PP2 (5 μM) was added for an additional 30 min uptake. (C) Hkh2 cells were plated for 48 h. They were then serum-starved for 1 h after which PP2 (5 μM) was added for 30 min. This was followed by the addition of 50 ng/mL uPA for an additional 30 min. Polyamine uptake with radiolabeled spermidine was assessed, as described in Materials and Methods Section.
Discussion
We have reevaluated the role of various proteins involved in endocytosis in relationship to polyamine uptake. Previous work done by others has suggested that polyamine uptake occurs by an endocytic pathway involving caveolin-1 associated endosomes [7,11]. An endocytic mechanism was subsequently disputed, based on transport studies in mutant cell lines that were defective in clathrin-coated pit-dependent endocytosis [12]. Our studies provide genetic evidence for the involvement of a specific endocytic pathway. We show, for the first time, that polyamines are internalised via a dynamin-dependent, clathrin-independent endocytic route. Our results support the conclusion of Cheng et al. that these ubiquitous cations are internalized via a caveolar endocytic pathway, but provide novel data indicating that the polyamines localize to lipid rafts and that caveolin-1 negatively regulates this process. Finally, we provide a mechanism by which oncogenes like K-RAS can increase polyamine uptake in human colon cancer cells by increasing caveolin-1 phosphorylation in a SRC-dependent manner.
Our study shows that polyamines are taken up by a dynamin-dependent mechanism in human colon tumor derived cells. The GTPase dynamin regulates a major subset of endocytosis-dependent uptake and trafficking. It is involved in the blebbing of the plasma membrane and subsequent internalization. Expression of a DN form of this protein dramatically reduced the uptake of spermidine and putrescine. Dynamin dependent endocytosis is further sub-classified as clathrin-depenent, caveolin-1/lipid raft dependent, and clathrin-independent/caveolin-1 independent [39]. In order to further understand the involvement of these pathways, we used a DN form of the adaptor protein, Eps15 to perturb clathrin-dependent endocytosis. Eps15 is involved in the assembly of clathrin triskelions at the site of endocytosis. The DN form impedes the formation of these pits [28]. Expression of Eps15 Δ95/295 completely inhibited the uptake of transferrin by HCT116 cells, indicating cessation of clathrin-dependent endocytosis. However, this construct did not have any effect on the internalization of putrescine and spermidine. This pointed toward a dynamin-dependent/clathrin-independent pathway of polyamine internalization.
Lipid raft/caveolar endocytosis is a subset of dynamin-dependent endocytic processes. We first fractionated the HCT116 cells on a sucrose density gradient to check whether caveolin-1 localized in raft rich fractions. Immunoblotting analysis of the fractions revealed the presence of caveolin-1 in the same fractions as the lipid-raft marker protein, flotillin-1. Flotlillin-1 is known to localize to lipid rafts and can form a hetero-oligomeric complex with caveolin-1 in the membrane microdomains [40,41]. To assess whether polyamines associate with these caveolae fractions, we labeled HCT116 cells with tritiated spermidine. Following fractionation of the labeled cells on a sucrose density gradient, we found that spermidine was primarily localized to the caveolae rich plasma membrane fractions. This association with lipid rafts was also found in the HCT116-caveolin-1 antisense cells, indicating that polyamines associate with lipid rafts independent of caveolin-1. The cholesterol extracting agent methyl-β-cyclodextrin has been shown to inhibit lipid raft endocytosis. Treatment with methyl-β-cyclodextrin decreased putrescine and spermidine uptake to about 30% of the vehicle controls. Also, lipid raft endocytosis requires the participation of tyrosine kinases like Src, and protein kinase C. Use of the Src kinase inhibitor PP2 decreased putrescine and spermidine uptake by HCT116 cells, by 60% and 30%, respectively. Protein Kinase C has been implicated in caveolar endocytosis [42]. Treatment with an inhibitor, chelerythrine choride, decreased the uptake of both putrescine and spermidine indicating that Protein Kinase C is involved in the internalization of polyamines. At this stage, it needs to be mentioned that some of the inhibitors are not selective and can perturb clathrin-dependent endocytosis [43].
We used a genetic approach to determine if caveolar endocytosis plays a role in polyamine uptake. Caveolin-1 is a negative regulator of caveolar endocytosis and acts to stabilize caveolae at the plasma membrane [44]. This effect was also seen in the internalization of the polyamines, putrescine, and spermidine. The uptake of these two polyamines significantly increased with loss of caveolin-1 in the HCT116 cells, as reported for other ligands of caveolar endocytosis [45,46].
Polyamine metabolism is de-regulated during carcinogenesis. Activated oncogenes have been shown to increase polyamine uptake in transformed cells [23]. However, no mechanism for this observation has been proposed. We saw that an activated K-RAS increases polyamine uptake in colon cancer cells. This is through the phosphorylation of caveolin-1 at tyrosine residue 14. This phosphorylation event leads to increased budding of caveolae at the plasma membrane with a concurrent increase in caveolar endocytosis rate [47,48]. This effect was blocked with the use of the Src kinase inhibitor, PP2 as indicated by a decrease in polyamine uptake and a decrease in amount of phospho-caveolin-1 following PP2 treatment [49]. Thus, our data show that an activated K-RAS increases polyamine uptake by increasing SRC-dependent caveolin-1 phosphorylation at tyrosine residue 14. Our finding is consistent with the observation that autocrine motility factor receptor (AMF-R) endocytosis, which is endocytosed via caveolae, is increased in RAS transformed—NIH3T3 fibroblasts [44]. An activated K-RAS increase polyamine uptake in these cells via the modulation of the uPA-UPAR pathway. uPAR localizes to lipid rafts and could serve as a potential link between K-RAS and SRC signaling in these cells [15]. Our model for polyamine uptake is indicated in Figure 12. Though we have restricted ourselves to uPAR, other receptors with tyrosine kinase activity like the EGFR receptor could also modulate endocytosis of polyamine through tyrosine phosphorylation of caveolin-1. Indeed, exogenous EGF has been show to increase polyamine uptake in a colon cancer cell line, Caco2 [50].
Figure 12.

Model for an activated K-RAS dependent polyamine uptake in colon cancer cells. 1,2: An activated K-RAS oncogene increases uPAR gene expression. 3: Increased uPAR expression leads to more uPA/uPAR interaction and subsequent SRC activation. 4,5: This in turn increases phosphorylation of caveolin-1 at tyrosine residue 14 and more polyamine internalization via caveolar endocytosis.
Antizyme, a polyamine-regulated gene, has been shown to negatively regulate polyamine uptake and biosynthesis [51]. Our study does not address the role of antizyme in regulation of polyamine uptake. Uptake of polyamines at 4°C was minimal, indicating that passive diffusion was not a major route of uptake in human colon tumor-derived cells. Several groups have alluded to an endocytic dependent mechanism for polyamine uptake in mammlian cells. Belting et al. [7] have shown that the polyamine spermine colocalises with caveolin-1 positive endosomes in CHO cells. Caveolin-1 acts to stabilize caveolae at the plasma membrane and is a negative regulator of caveolar endocytosis. Our work provides a mechanism for internalization of polyamines via a dynamin-dependent and clathrin-independent mechanism. Belting et al. have also shown the involvement of HSPG as a possible vehicle for polyamine uptake. HSPG are localized to lipid rafts in various cell types [52]. Binding of polyamines to HSPG and their subsequent internalization via caveolae is an interesting mechanism for polyamine uptake. HSPG like glypicans can activate Src and polyamine binding to such molecules might possibly lead to clustering of HSPG and activation of their caveolar endocytic process [53]. Soulet et al. have used a fluorescent probe for studying polyamine uptake in CHO cells. Their studies reveal that receptor-mediated endocytosis is not involved in polyamine uptake by these cells [12]. However, it is necessary to point out that they employed the End1, LEX1, and LEX2 mutant cells. The End1 mutant cells show impaired endosomal acidification and transferrin uptake, indicating a defect in clathrin-dependent endocytosis [54]. The LEX1 cells are defective in fusion between late endosome and lysosomes [55]. The LEX2 mutants show impaired maturation of multivesicular bodies [56]. Thus, the studies by this group have mainly focused on polyamine trafficking following internalization. Our work is different from other studies, in that it focuses primarily on internalization of polyamines rather than trafficking. We have not addressed the fate of internalized polyamines using fluorescent labeled probes, because we are concerned that bulky fluorescent adducts may influence the intracellular trafficking of these small cations.
Our work sheds light on how colon cells can take up polyamines. The intestinal tract is a rich source of polyamines originating from enteric bacteria and diet [57]. Caveolar endocytosis might be a potential mechanism by which polyamines from the gut can be internalized. The colonic epithelium is highly polarized and there might be different mechanisms of uptake in the apical and basolateral sides of the colonic epithelium. Several groups have shown that caveolin-1 is primarily expressed on the apical side of the lumen [58]. Other groups have shown that the expression of caveolin-1 is not restricted to the apical side [59,60]. Thus, there is still an apparent dichotomy regarding caveolin-1 expression in the colon. Nonetheless, caveolin-1 is a potential regulator of polyamine uptake by human colon-tumor derived cells. Caveolin-1 is often down-regulated in human colon cancers [25]. Thus, one could speculate that loss of caveolin-1 would lead to an increase in polyamine uptake during colon carcinogenesis. Also, mutations like K-RAS, which occur at later stages of colon carcinogenesis could possibly confer resistance to DFMO, since the cells can rapidly develop compensatory endocytic uptake mechanisms. Thus, a caveolin-1 dependent uptake route could prove to be a potential therapeutic target in cancer chemotherapy.
Acknowledgments
This work was supported by NIH/NCI grants CA95060 and CA72008. The authors wish to thank Dr. M. McNiven and Dr. A. Dautry-Varsat for the plasmids, Dr. B. Sloane for the HCT116-Caveolin-1 antisense cells, Dr. E. Felley-Bosco for the HT29-caveolin-1 cells and Dr. Shirasawa for the Hkh2 cells. In addition, they would like to thank David E. Stringer, Dr. H. Yerushalmi and Dr. N. Ignatenko for assistance with experimental set-up, and Barbara Carolus of the Biotechnology Imaging Facility, University of Arizona for help with flow cytometry and confocal microscopy.
Abbreviations
- HSPG
heparin sulfate proteoglycans
- DFMO
difluoromethyl ornithine
- FBS
fetal bovine serum
- P/S
penicillin/streptomycin
- IPTG
isopropyl-beta-d-thiogalactopyranoside
- WT
wild type
- DN
dominant negative
- MFI
mean fluorescence intensity
- uPAR
urokinase plasminogen activated receptor
References
- 1.Wallace HM, Fraser AV, Hughes A. A perspective of polyamine metabolism. Biochem J. 2003;376:1–14. doi: 10.1042/BJ20031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basuroy UK, Gerner EW. Emerging concepts in targeting the polyamine metabolic pathway in epithelial cancer chemo-prevention and chemotherapy. J Biochem (Tokyo) 2006;139:27–33. doi: 10.1093/jb/mvj022. [DOI] [PubMed] [Google Scholar]
- 3.Gerner EW, Meyskens FL., Jr Polyamines and cancer: Old molecules, new understanding. Nat Rev Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 4.Xie X, Tome ME, Gerner EW. Loss of intracellular putrescine pool-size regulation induces apoptosis. Exp Cell Res. 1997;230:386–392. doi: 10.1006/excr.1996.3442. [DOI] [PubMed] [Google Scholar]
- 5.Igarashi K, Kashiwagi K. Polyamine transport in bacteria and yeast. Biochem J. 1999;344:633–642. [PMC free article] [PubMed] [Google Scholar]
- 6.Hasne MP, Ullman B. Identification and characterization of a polyamine permease from the protozoan parasite Leishmania major. J Biol Chem. 2005;280:15188–15194. doi: 10.1074/jbc.M411331200. [DOI] [PubMed] [Google Scholar]
- 7.Belting M, Persson S, Fransson LA. Proteoglycan involvement in polyamine uptake. Biochem J. 1999;338:317–323. [PMC free article] [PubMed] [Google Scholar]
- 8.Soulet D, Covassin L, Kaouass M, Charest-Gaudreault R, Audette M, Poulin R. Role of endocytosis in the internalization of spermidine-C(2)-BODIPY, a highly fluorescent probe of polyamine transport. Biochem J. 2002;367:347–357. doi: 10.1042/BJ20020764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cullis PM, Green RE, Merson-Davies L, Travis N. Probing the mechanism of transport and compartmentalisation of polyamines in mammalian cells. Chem Biol. 1999;6:717–729. doi: 10.1016/s1074-5521(00)80019-8. [DOI] [PubMed] [Google Scholar]
- 10.Ding K, Sandgren S, Mani K, Belting M, Fransson LA. Modulations of glypican-1 heparan sulfate structure by inhibition of endogenous polyamine synthesis. Mapping of spermine-binding sites and heparanase, heparin lyase, and nitric oxide/nitrite cleavage sites. J Biol Chem. 2001;276:46779–46791. doi: 10.1074/jbc.M105419200. [DOI] [PubMed] [Google Scholar]
- 11.Cheng F, Mani K, van den Born J, Ding K, Belting M, Fransson LA. Nitric oxide-dependent processing of heparan sulfate in recycling S-nitrosylated glypican-1 takes place in caveolin-1-containing endosomes. J Biol Chem. 2002;277:44431–44439. doi: 10.1074/jbc.M205241200. [DOI] [PubMed] [Google Scholar]
- 12.Soulet D, Gagnon B, Rivest S, Audette M, Poulin R. A fluorescent probe of polyamine transport accumulates into intracellular acidic vesicles via a two-step mechanism. J Biol Chem. 2004;279:49355–49366. doi: 10.1074/jbc.M401287200. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 14.Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol. 2003;161:673–677. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 16.Parton RG. Caveolae and caveolins. Curr Opin Cell Biol. 1996;8:542–548. doi: 10.1016/s0955-0674(96)80033-0. [DOI] [PubMed] [Google Scholar]
- 17.Fra AM, Williamson E, Simons K, Parton RG. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc Natl Acad Sci USA. 1995;92:8655–8659. doi: 10.1073/pnas.92.19.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milovic V, Turchanowa L. Polyamines and colon cancer. Biochem Soc Trans. 2003;31:381–383. doi: 10.1042/bst0310381. [DOI] [PubMed] [Google Scholar]
- 19.Thomas T, Thomas TJ. Polyamines in cell growth and cell death: Molecular mechanisms and therapeutic applications. Cell Mol Life Sci. 2001;58:244–258. doi: 10.1007/PL00000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nilsson JA, Keller UB, Baudino TA, et al. Targeting ornithine decarboxylase in Myc-induced lymphomagenesis prevents tumor formation. Cancer Cell. 2005;7:433–444. doi: 10.1016/j.ccr.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 21.Hessels J, Kingma AW, Ferwerda H, Keij J, van den Berg GA, Muskiet FA. Microbial flora in the gastrointestinal tract abolishes cytostatic effects of alpha-difluoromethylornithine in vivo. Int J Cancer. 1989;43:1155–1164. doi: 10.1002/ijc.2910430632. [DOI] [PubMed] [Google Scholar]
- 22.Quemener V, Moulinoux JP, Havouis R, Seiler N. Polyamine deprivation enhances antitumoral efficacy of chemotherapy. Anticancer Res. 1992;12:1447–1453. [PubMed] [Google Scholar]
- 23.Bachrach U, Seiler N. Formation of acetylpolyamines and putrescine from spermidine by normal and transformed chick embryo fibroblasts. Cancer Res. 1981;41:1205–1208. [PubMed] [Google Scholar]
- 24.Cavallo-Medved D, Mai J, Dosescu J, Sameni M, Sloane BF. Caveolin-1 mediates the expression and localization of cathepsin B, pro-urokinase plasminogen activator and their cell-surface receptors in human colorectal carcinoma cells. J Cell Sci. 2005;118:1493–1503. doi: 10.1242/jcs.02278. [DOI] [PubMed] [Google Scholar]
- 25.Bender FC, Reymond MA, Bron C, Quest AF. Caveolin-1 levels are down-regulated in human colon tumors, and ectopic expression of caveolin-1 in colon carcinoma cell lines reduces cell tumorigenicity. Cancer Res. 2000;60:5870–5878. [PubMed] [Google Scholar]
- 26.Shirasawa S, Furuse M, Yokoyama N, Sasazuki T. Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science. 1993;260:85–88. doi: 10.1126/science.8465203. [DOI] [PubMed] [Google Scholar]
- 27.Cao H, Garcia F, McNiven MA. Differential distribution of dynamin isoforms in mammalian cells. Mol Biol Cell. 1998;9:2595–2609. doi: 10.1091/mbc.9.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benmerah A, Bayrou M, Cerf-Bensussan N, Dautry-Varsat A. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J Cell Sci. 1999;112:1303–1311. doi: 10.1242/jcs.112.9.1303. [DOI] [PubMed] [Google Scholar]
- 29.Seiler N, Knodgen B. High-performance liquid chromatographic procedure for the simultaneous determination of the natural polyamines and their monoacetyl derivatives. J Chromatogr. 1980;221:227–235. doi: 10.1016/s0378-4347(00)84307-8. [DOI] [PubMed] [Google Scholar]
- 30.Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae micro-domains. Detergent-free purification of caveolae micro-domains. J Biol Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- 31.Damm EM, Pelkmans L, Kartenbeck J, Mezzacasa A, Kurzchalia T, Helenius A. Clathrin- and caveolin-1-independent endocytosis: Entry of simian virus 40 into cells devoid of caveolae. J Cell Biol. 2005;168:477–488. doi: 10.1083/jcb.200407113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Schmid SL. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marks DL, Singh RD, Choudhury A, Wheatley CL, Pagano RE. Use of fluorescent sphingolipid analogs to study lipid transport along the endocytic pathway. Methods. 2005;36:186–195. doi: 10.1016/j.ymeth.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Lawson KR, Ignatenko NA, Piazza GA, Cui H, Gerner EW. Influence of K-ras activation on the survival responses of Caco-2 cells to the chemopreventive agents sulindac and difluoromethylornithine. Cancer Epidemiol Biomarkers Prev. 2000;9:1155–1162. [PubMed] [Google Scholar]
- 35.Roy S, Luetterforst R, Harding A, et al. Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat Cell Biol. 1999;1:98–105. doi: 10.1038/10067. [DOI] [PubMed] [Google Scholar]
- 36.Smart EJ, Graf GA, McNiven MA, et al. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cavallo-Medved D, Dosescu J, Linebaugh BE, Sameni M, Rudy D, Sloane BF. Mutant K-ras regulates cathepsin B localization on the surface of human colorectal carcinoma cells. Neoplasia. 2003;5:507–519. doi: 10.1016/s1476-5586(03)80035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allgayer H, Wang H, Shirasawa S, Sasazuki T, Boyd D. Targeted disruption of the K-ras oncogene in an invasive colon cancer cell line down-regulates urokinase receptor expression and plasminogen-dependent proteolysis. Br J Cancer. 1999;80:1884–1891. doi: 10.1038/sj.bjc.6690616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nichols B. Caveosomes and endocytosis of lipid rafts. J Cell Sci. 2003;116:4707–4714. doi: 10.1242/jcs.00840. [DOI] [PubMed] [Google Scholar]
- 40.Dermine JF, Duclos S, Garin J, et al. Flotillin-1-enriched lipid raft domains accumulate on maturing phagosomes. J Biol Chem. 2001;276:18507–18512. doi: 10.1074/jbc.M101113200. [DOI] [PubMed] [Google Scholar]
- 41.Volonte D, Galbiati F, Li S, Nishiyama K, Okamoto T, Lisanti MP. Flotillins/cavatellins are differentially expressed in cells and tissues and form a hetero-oligomeric complex with caveolins in vivo. Characterization and epitope-mapping of a novel flotillin-1 monoclonal antibody probe. J Biol Chem. 1999;274:12702–12709. doi: 10.1074/jbc.274.18.12702. [DOI] [PubMed] [Google Scholar]
- 42.Sharma DK, Brown JC, Choudhury A, et al. Selective stimulation of caveolar endocytosis by glycosphingolipids and cholesterol. Mol Biol Cell. 2004;15:3114–3122. doi: 10.1091/mbc.E04-03-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodal SK, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell. 1999;10:961–974. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le PU, Guay G, Altschuler Y, Nabi IR. Caveolin-1 is a negative regulator of caveolae-mediated endocytosis to the endoplasmic reticulum. J Biol Chem. 2002;277:3371–3379. doi: 10.1074/jbc.M111240200. [DOI] [PubMed] [Google Scholar]
- 45.Minshall RD, Tiruppathi C, Vogel SM, et al. Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J Cell Biol. 2000;150:1057–1070. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le PU, Nabi IR. Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endoplasmic reticulum. J Cell Sci. 2003;116:1059–1071. doi: 10.1242/jcs.00327. [DOI] [PubMed] [Google Scholar]
- 47.Aoki T, Nomura R, Fujimoto T. Tyrosine phosphorylation of caveolin-1 in the endothelium. Exp Cell Res. 1999;253:629–636. doi: 10.1006/excr.1999.4652. [DOI] [PubMed] [Google Scholar]
- 48.del Pozo MA, Balasubramanian N, Alderson NB, et al. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–908. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim YN, Dam P, Bertics PJ. Caveolin-1 phosphorylation in human squamous and epidermoid carcinoma cells: Dependence on ErbB1 expression and Src activation. Exp Cell Res. 2002;280:134–147. doi: 10.1006/excr.2002.5623. [DOI] [PubMed] [Google Scholar]
- 50.Milovic V, Deubner C, Zeuzem S, Piiper A, Caspary WF, Stein J. EGF stimulates polyamine uptake in Caco-2 cells. Biochem Biophys Res Commun. 1995;206:962–968. doi: 10.1006/bbrc.1995.1136. [DOI] [PubMed] [Google Scholar]
- 51.Pegg AE. Regulation of ornithine decarboxylase. J Biol Chem. 2006;281:14529–14532. doi: 10.1074/jbc.R500031200. [DOI] [PubMed] [Google Scholar]
- 52.Chu CL, Buczek-Thomas JA, Nugent MA. Heparan sulphate proteoglycans modulate fibroblast growth factor-2 binding through a lipid raft-mediated mechanism. Biochem J. 2004;379:331–341. doi: 10.1042/BJ20031082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bianco C, Strizzi L, Rehman A, et al. A Nodal- and ALK4-independent signaling pathway activated by Cripto-1 through Glypican-1 and c-Src. Cancer Res. 2003;63:1192–1197. [PubMed] [Google Scholar]
- 54.Roff CF, Fuchs R, Mellman I, Robbins AR. Chinese hamster ovary cell mutants with temperature-sensitive defects in endocytosis. I. Loss of function on shifting to the non-permissive temperature. J Cell Biol. 1986;103:2283–2297. doi: 10.1083/jcb.103.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohashi M, Miwako I, Nakamura K, et al. An arrested late endosome-lysosome intermediate aggregate observed in a Chinese hamster ovary cell mutant isolated by novel three-step screening. J Cell Sci. 1999;112:1125–1138. doi: 10.1242/jcs.112.8.1125. [DOI] [PubMed] [Google Scholar]
- 56.Ohashi M, Miwako I, Yamamoto A, Nagayama K. Arrested maturing multivesicular endosomes observed in a Chinese hamster ovary cell mutant, LEX2, isolated by repeated flowcytometric cell sorting. J Cell Sci. 2000;113:2187–2205. doi: 10.1242/jcs.113.12.2187. [DOI] [PubMed] [Google Scholar]
- 57.Milovic V. Polyamines in the gut lumen: Bioavailability and biodistribution. Eur J Gastroenterol Hepatol. 2001;13:1021–1025. doi: 10.1097/00042737-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 58.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell. 2004;15:176–188. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vogel U, Sandvig K, van Deurs B. Expression of caveolin-1 and polarized formation of invaginated caveolae in Caco-2 and MDCK II cells. J Cell Sci. 1998;111:825–832. doi: 10.1242/jcs.111.6.825. [DOI] [PubMed] [Google Scholar]
- 60.von Ruhland CJ, Campbell L, Gumbleton M, Jasani B, Newman GR. Immunolocalization of caveolin-1 in rat and human mesothelium. J Histochem Cytochem. 2004;52:1415–1425. doi: 10.1369/jhc.4A6334.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


