Summary
The demand for modified peptides with improved stability profiles and pharmacokinetic properties is driving extensive research effort in this field. Many structural modifications of peptides guided by rational design and molecular modeling have been established to develop novel synthetic approaches. Recent advances in the synthesis of conformationally restricted building blocks and peptide bond isosteres are discussed.
Keywords: SAR, peptidomimetics, topographical constraints
Introduction
Peptidomimetics are compounds whose essential elements (pharmacophore) mimic a natural peptide or protein in 3D space and which retain the ability to interact with the biological target and produce the same biological effect. Peptidomimetics are designed to circumvent some of the problems associated with a natural peptide: e.g. stability against proteolysis (duration of activity) and poor bioavailability. Certain other properties, such as receptor selectivity or potency, often can be substantially improved. Hence mimics have great potential in drug discovery. The design process begins by developing structure-activity relationships (SAR) that can define a minimal active sequence or major pharmacophore elements, and identify the key residues that are responsible for the biological effect. Then structural constraints are applied to probe the 3-D arrangement(s) of these features[1–4]. In this process, the peptide complexity is reduced and the basic pharmacophore model is defined by its critical structural features in 3D space. This model then supports the re-assembly of the critical elements and non-peptide variants on a modified scaffold that presents the optimized pharmacophore to the receptor. The definition has been broadened to include any compound hit including from high throughput screens or one-bead-one-compound libraries[5], but a narrow definition requires rational modification of polyamide structure. Generally natural products, such as peptides, show much greater structural diversity than synthetic drugs which often are based on nitrogen-containing heteroaromatic scaffolds with a few or no stereogenic centers. Further, several key synthetic technologies, such as solid-phase synthesis of non-peptide libraries, extended the range of chemical space covered with peptidomimetics. If these tools exploit biologically relevant regions of chemical space (often they do not), they will present a partial-matrix-type of libraries of reasonable size (up to 109) that can be readily screened against biological targets. Despite of the fact that even this size is a very small fraction of total chemical space, those libraries will represent highly diverse and biologically relevant sets of molecules for drug discovery. Because of limited space, we will not discuss rational design and molecular modeling, but focus on novel synthetic tools enabling the peptide to mimetic transformation. We will also exclude de novo protein design, protein arrays and related chemistries (such as chemical protein ligation), because these methods utilize peptides assembled on various templates to form nonnatural architectures. Some of these methods have been thoroughly reviewed recently[6–9].
Peptides tend to be quite comformationally flexible at kT and conformations are highly dependent on environment. The relationship or lack thereof of conformations in solution and the receptor-bound conformation presents a major problem in this research. In most successful approaches to small molecule mimetics, the chemical modifications involve the restriction of conformations performed either by the cyclization of peptides or by the incorporation of conformationally restricted building blocks, mostly unnatural amino acids and dipeptide surrogates[10–12]. The other tactic involves replacing a particular peptide bond with its isostere.
Conformationally restricted β-turn dipeptide mimetics
The Hruby group has systematically studied the substitutes of side chain peptides, mainly conformationally restricted β-substituted amino acid analogues and their use as templates for preparation of peptidomimetics[13–19], especially external bicyclic β-turn dipeptide mimetics (Figure 1)[18]. This type of bicyclic template restricts conformations by a combination of structural constraints and steric interactions. Evidently, that is influenced by strereochemistry of the scaffold, the presence of substituents, and particularly the frame size. For example, a biologically active [3.3.0]-bicyclo-Leu-enkephalin analogue was shown by molecular modeling to adopt a type I β-turn conformation, which is consistent with the X-ray structure of Leu-enkephalin[20]. The relatively rigid bicyclic structure provides an excellent scaffold for the incorporation of various side chain groups to explore the importance of side chain topography that are often important for molecular recognition[11,13]. The β-substituted unnatural amino acids required for the synthesis of such mimetics are β-substituted ω-unsaturated amino acids 2 and β-substituted cysteine derivatives 3.
Figure 1.
External β-turn dipeptide mimetics
To place Ri+1 in as many as possible 3D regions relative to the rigid bicyclic scaffold, compounds 2 need to have different stereochemistry at the β-carbon and the value of n should be varied. The absolute stereochemical control of the β-substituent can be achieved by metal chelation with use of chiral ligands[21,22], chirality transfer from available chiral sources[23,24], or by chiral auxiliaries[25,26]. For example, optically active syn β-substituted γ,δ-unsaturated amino acids can be synthesized via chelate-Claisen rearrangement in the presence of chiral quinine[21,22]. On the other hand, Eschenmoser-Claisen rearrangement can be employed for the synthesis of anti β-substituted γ,δ-unsaturated amino acids[27] and incorporation of a C2-symmetric chiral auxiliary in the Eschenmoser-Claisen rearrangement can provide optically active amide analogues with various β-substituents[26]. Removal of the chiral auxiliary via iodolactonization/zinc reduction or reduction/oxidation provided the mimetics in high enantiomeric excess. In both cases, the Claisen rearrangement is highly diastereo- and enantio-selective due to the formation of a low energy chair-like transition state. For compounds in which the value of n is greater than 0, an efficient approach was developed via the direct alkylation of (R) or (S)-Ni(II)-complex {(2-(N-(N’-benzylprolyl)amino))benzophenone}with use of various alkyl halides[25]. The method can provide δ,ε-unsaturated, or ε,ζ-unsaturated, or other ω-unsaturated analogues in high yields and diastereoselectivity in 2 steps from the Ni(II)-complex.
Besides their application in bicyclic β-turn dipeptide mimetics, β-substituted ω-unsaturated amino acids can be used to make medium ring β-turn mimetics by incorporating them in the i and i+3 positions of a β-turn and linking their chains via metathesis. This kind of mimetic is less drug-like compared to bicyclic β-turn mimetics. However, they are resistant to enzymatic degradation and easy to synthesize. In addition, they may be closer mimetics of a natural β-turn with some flexibility which may be important for the effective interactions between a mimetic and a target protein, especially in the induced fit scenario. For example, analogues of H-Tyr-c[D-Cys-Gly-Phe-D-Cys]-OH in which the disulfide bridge is replaced with a dicarba bridge retained high biologically activities[28].
A method for the asymmetric synthesis of compound 3 (Figure 1) with either an anti or syn β-substituent was also developed[29]. The key intermediate of this method is a chiral aziridine that was synthesized from an α,β-unsaturated ester employing the Sharpless asymmetric dihydroxylation. Subsequent regio-selective ring opening with a sulfur nucleophile afforded β-substituted cysteine derivatives in high enantioselectivity.
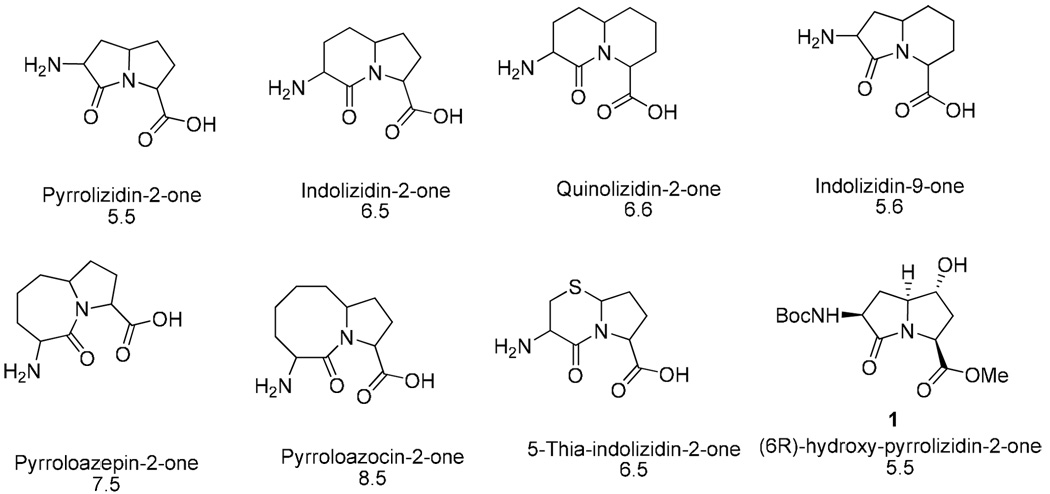
Other prominent groups have studied constrained peptidomimetics as reviewed recently[10,30]. Azabicycloalkanone amino acids scaffold as dipeptide surrogates[30] have proven to be effective to mimic type II’ β-turns. Many variations in size including 5.5-, 6.5-, 5.6-, 7.5-, 8.5 and 6.6-ring systems have been published and a variety of sites for substitution can give the desirable structural flexibility needed in drug discovery (see Figure 2).
Figure 2.
Azabicycloalkanone amino acid scaffolds.
The list of azabicycloalkanone amino acid scaffolds has been recently extended by synthesis of 3-amino-8-carboxylate 6-hydroxypyrrolizidin-2-one[31]. X-ray crystallographic analysis confirmed that the dihedral angles within the pyrrolizidine ring carboxylate were consistent with those of the central residues of a type II’ β-turn. Both enantiomers were synthesized by five-step synthesis in 23% and 14% overall yields from 4-acetoxy diaminosuberates (5R) and (5S). The introduction of functional groups onto the azabicycloalkane scaffold has typically proven challenging especially for pure enantiomers. Therefore, the hydroxyl functionality in position 6 opens the opportunity for substitutions to fine-tune scaffold geometry as well as to increase diversity. Indolizidine-2-one and -9-one amino acids, representing 6,5 and 5,6 scaffolds, were used as β-turn scans to explore the conformational requirements for activity of analogues of Calcitonin-gene related peptide (CGRP) antagonist, together with aza-aminoacid scans[32]. The importance of a type II’ β-turn centered at Gly33-Pro34 of antagonist [Asp31, Pro33, Phe35]CGRP27–37 has been illustrated by increased antagonistic potency of the aza- Gly33 and indolizidine-2-one amino acid33–34 analogues. In addition, the improved metabolic stability and longer duration of action qualifies those scans to be another peptidomimetic research tool. The older reviews[33,34] related to azabicycloalkane scaffolds provide a general overview of synthetic and conformational possibilities.
Peptide bond isosteres
Another appealing and broadly used technique lies in backbone amide replacement with amide bond look-a-like surrogates, or isosteres. Isostere replacement modifies a backbone, and therefore can be rarely used for the study of receptor interactions. Alternatively, peptide bond surrogates can have protease inhibitor activity at the substrate cleavage site, and therefore offer a fundamental strategy for understanding protease enzyme activity, especially if isosteres can imitate the transition state of bond degradation (hydrolysis). Moreover, strategically placed isosteres can also improve bioavailability and transport properties of native hormones. Generally, the isosteres do not restrict global conformations, but have influence on secondary structure through different hydrogen bonding and length of backbone. The most common isosteres used are depicted in Figure 3.
Figure 3.
Peptide bond isosteres.
In addition, small heterocycles also can serve as an isostere (comprehensively reviewed in Houben-Weyl[10]). Especially useful are those directly prepared from dipeptides such as oxazoles, oxazolines, oxazolidines, and their thio-derivatives[35]. Modern synthetic transformations, such as Cu-catalyzed Huisgen 1,3-dipolar additions (so called Click chemistry) have been thoroughly studied[36]. This highly chemo and regio-selective reaction has been used to insert 1,2,3-triazoles into peptide chains for macrocyclizations, and for quantitative conjugation of other subunits such as carbohydrates, polymers or labeling agents. The -NH- group in a peptide bond can be replaced by alkylated -NR- or -O- groups. N-Alkyl peptides are important modifications which commonly occur in natural peptides. The Nα-alkylation induces significant structural effects: the occurrence of the cis-isomer in the secondary amide bond, steric effects, no H-bonding possibility, increased basicity, and decreased polarity of the adjacent carbonyl group. The incorporation of Nα-alkyl (mostly methyl) has resulted in many peptidomimetics with improved properties[10] and systematic Nα-alkylation is a powerful approach to improve biological activity. Recently, convenient procedures to prepare protected Nα-methylamino acids or direct alkylation on solid supports have been described[37,38]. The optimized three-step procedure, compatible with the Fmoc-tBu protecting scheme, involves amine activation by a 2-nitrobenzenesulfonyl group followed by Nα-alkylation (directly by an electrophile or by the Mitsunobu reaction), and then removal of the sulfonamide group. Nα-Alkyl peptides are not limited to non-functional methylated or benzylated residues, but can contain functional pharmacophores. For example, a number of cyclic α-MSH analogues were designed on the basis of the MTII NMR structure, while the pharmacophore in arginine was mimicked via backbone Nα-alkylation with a guanidinylbutyryl group[39]. The binding affinity and adenylate cyclase activity assays of these peptidomimetics at human melanocortin receptors showed that three of the new α-MSH analogues act as antagonists and exhibited high selectivity toward the human melanocortin-4 receptor[39].
Conclusions
Gigantic amounts of genomic and proteomic data creates high demand for synthesis and screening of nature-like biopolymers and their more stable modified derivatives. The design and synthesis of peptidomimetics are most important because of the dominant position peptide and protein-protein interactions play in molecular recognition and signaling, especially in living systems. The design of peptide mimetics can be viewed from several different perspectives[3,12] and peptidomimetics can be categorized in a number of different ways[12,40]. Examination of the vast literature would suggest that medicinal and organic chemists, who deal with peptide mimics utilize these methods in many different ways. In any case, a variety of methodologies and strategies have been developed and continue to be developed to establish systematic tools for transformation of peptides into peptidomimetics or further into small drug-like molecules. Significant industrial as well as academic resources are invested in this effort and there is still much to learn to optimize these approaches. We envision that peptidomimetic research will continue to be an indispensable tool of structure-activity relationships in drug discovery for the foreseeable future.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Paper of particular interest has been highlighted as:
• of special interest
•• of outstanding interest
- 1.Hruby VJ, Qiu W, Okayama T, Soloshonok VA. Design of nonpeptides from peptide ligands for peptide receptors. Methods Enzymol. 2001;343:91–123. doi: 10.1016/s0076-6879(02)43129-1. [DOI] [PubMed] [Google Scholar]
- 2.Cowell SM, Lee YS, Cain JP, Hruby VJ. Exploring Ramachandran and Chi space: conformationally constrained amino acids and peptides in the design of bioactive polypeptide ligands. Curr Med Chem. 2004;11:2785–2798. doi: 10.2174/0929867043364270. [DOI] [PubMed] [Google Scholar]
- 3.Hruby VJ. Conformational restrictions of biologically active peptides via amino acid side chain groups. Life Sci. 1982;31:189–199. doi: 10.1016/0024-3205(82)90578-1. [DOI] [PubMed] [Google Scholar]
- 4.Kessler H. Peptide conformations. Part 19. Conformation and biological effects of cyclic peptides. Angewandte Chem Int Ed Engl. 1982;94:509–520. [Google Scholar]
- 5.Lam KS, Salmon SE, Hersh EM, Hruby VJ, Kazmierski WM, Knapp RJ. A new type of synthetic peptide library for identifying ligand-binding activity. Nature (London, United Kingdom) 1991;354:82–84. doi: 10.1038/354082a0. [DOI] [PubMed] [Google Scholar]
- 6.Singh Y, Dolphin GT, Razkin J, Dumy P. Synthetic peptide templates for molecular recognition: recent advances and applications. ChemBioChem. 2006;7:1298–1314. doi: 10.1002/cbic.200600078. [DOI] [PubMed] [Google Scholar]
- 7.Duffner JL, Clemons PA, Koehler AN. A pipeline for ligand discovery using small-molecule microarrays. Curr Opin Chem Biol. 2007;11:74–82. doi: 10.1016/j.cbpa.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Dorman G, Frank R. Small Molecule Microarrays - small and smart. QSAR & Comb Sci. 2006;25:1007–1008. [Google Scholar]
- 9.Frank R. High-density synthetic peptide microarrays: Emerging tools for functional genomics and proteomics. Combinatorial Chemistry and High Throughput Screening. 2002;5:429–440. doi: 10.2174/1386207023330165. [DOI] [PubMed] [Google Scholar]
- 10.Goodman M, editor. Houben-Weyl Methods in Organic Chemistry.Vol. E22C, Synthesis of Peptides and Peptidomimetics., edn Additional and Supplementary Volumes to the 4th Edition. Georg Thieme Verlag: 2003. [Google Scholar]
- 11.Hruby VJ, Al-Obeidi F, Kazmierski W. Emerging approaches in the molecular design of receptor-selective peptide ligands: conformational, topographical and dynamic considerations. Biochem J. 1990;268:249–262. doi: 10.1042/bj2680249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ripka AS, Rich DH. Peptidomimetic design. Curr Opin Chem Biol. 1998;2:441–452. doi: 10.1016/s1367-5931(98)80119-1. [DOI] [PubMed] [Google Scholar]
- 13.Hruby VJ, Li G, Haskell-Luevano C, Shenderovich M. Design of peptides, proteins, and peptidomimetics in chi space. Biopolymers. 1997;43:219–266. doi: 10.1002/(SICI)1097-0282(1997)43:3<219::AID-BIP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Yang J, Ying J, Xiong C, Zhang J, Cai C, Hruby VJ. Stereoselective Synthesis of Dipeptide β-Turn Mimetics: 7-Benzyl and 8-Phenyl Substituted Azabicyclo[4.3.0]nonane Amino Acid Esters. J Org Chem. 2002;67:6353–6360. doi: 10.1021/jo0203591. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Xiong C, Ying J, Wang W, Hruby VJ. Stereoselective Synthesis of Novel Dipeptide β-Turn Mimetics Targeting Melanocortin Peptide Receptors. Org Lett. 2003;5:3115–3118. doi: 10.1021/ol0351347. [DOI] [PubMed] [Google Scholar]
- 16.Ndungu JM, Gu X, Gross DE, Cain JP, Carducci MD, Hruby VJ. Synthesis of bicyclic dipeptide mimetics for the cholecystokinin and opioid receptors. Tetrahedron Lett. 2004;45:4139–4142. [Google Scholar]
- 17.Ndungu JM, Gu X, Gross DE, Ying J, Hruby VJ. A simple and efficient synthesis of an Asp-Gly dipeptide mimetic. Tetrahedron Lett. 2004;45:3245–3247. [Google Scholar]
- 18.Gu X, Ying J, Min B, Cain JP, Davis P, Willey P, Navratilova E, Yamamura HI, Porreca F, Hruby VJ. Parallel synthesis and biological evaluation of different sizes of bicyclo[2,3]-Leuenkephalin analogues. Biopolymers. 2005;80:151–163. doi: 10.1002/bip.20208. [DOI] [PubMed] [Google Scholar]
- 19.Ndungu JM, Cain JP, Davis P, Ma SW, Vanderah TW, Lai J, Porreca F, Hruby VJ. Synthesis of constrained analogs of cholecystokinin/opioid chimeric peptides. Tetrahedron Lett. 2006;47:2233–2236. doi: 10.1016/j.tetlet.2006.01.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu X, Ying J, Agnes RS, Navratilova E, Davis P, Stahl G, Porreca F, Yamamura HI, Hruby VJ. Novel Design of Bicyclic β-Turn Dipeptides on Solid-Phase Supports and Synthesis of [3.3.0]-Bicyclo[2,3]-Leu-enkephalin Analogues. Org Lett. 2004;6:3285–3288. doi: 10.1021/ol0488183. [DOI] [PubMed] [Google Scholar]
- 21.Kazmaier U, Mues H, Krebs A. Asymmetric chelated Claisen rearrangements in the presence of chiral ligands-scope and limitations. Chemistry--A European Journal. 2002;8:1850–1855. doi: 10.1002/1521-3765(20020415)8:8<1850::AID-CHEM1850>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 22.Kazmaier U, Krebs A. Synthesis of chiral γ,δ-unsaturated amino acids by asymmetric ester enolate Claisen rearrangement. Angewandte Chem Intl Ed Engl. 1995;34:2012–2014. [Google Scholar]
- 23.Sakaguchi K, Suzuki H, Ohfune Y. Chirality transferring [3,3] sigmatropic rearrangement of (1-Acyloxy-2-alkenyl)trianlkylsilane synthesis of optically active vinylsilane-containing alphaamino acid. Chirality. 2001;13:357–365. doi: 10.1002/chir.1045. [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi K, Yamamoto M, Kawamoto T, Yamada T, Shinada T, Shimamoto K, Ohfune Y. Synthesis of optically active β-alkyl aspartate via [3,3] sigmatropic rearrangement of a-acyloxytrialkylsilane. Tetrahedron Lett. 2004;45:5869–5872. [Google Scholar]
- 25.Gu X, Ndungu JM, Qiu W, Ying J, Carducci MD, Wooden H, Hruby VJ. Large scale enantiomeric synthesis, purification, and characterization of ω-unsaturated amino acids via a Gly-Ni(II)-BPB-complex. Tetrahedron. 2004;60:8233–8243. [Google Scholar]
- 26•.Qu H, Gu X, Liu Z, Min BJ, Hruby VJ. Asymmetric Eschenmoser-Claisen rearrangement for anti-β-substituted γ,δ-unsaturated amino acids. Org Lett. 2007;9:3997–4000. doi: 10.1021/ol701704h.Report of the first asymmetric synthesis of anti-β-substituted γ,δ-unsaturated amino acids via a novel design of the Eschenmoser-Claisen rearrangement of glycine amide derivatives. The rearrangement gives good isolated yields and excellent diastereoselectivity due to (Z)-N,O-ketene acetal formation and the pseudochair-like conformations of reaction intermediates.
- 27.Qu H, Gu X, Min BJ, Liu Z, Hruby VJ. Synthesis of Anti-β-Substituted γ,δ-Unsaturated Amino Acids via Eschenmoser-Claisen Rearrangement. Org Lett. 2006;8:4215–4218. doi: 10.1021/ol061414l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mollica A, Guardiani G, Davis P, Ma SW, Porreca F, Lai J, Mannina L, Sobolev AP, Hruby VJ. Synthesis of Stable and Potent δ/μ Opioid Peptides: Analogues of H-Tyr-c[D-Cys-Gly-Phe-DCys]-OH by Ring-Closing Metathesis. J Med Chem. 2007;50:3138–3142. doi: 10.1021/jm061048b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiong C, Wang W, Hruby VJ. A General Asymmetric Synthesis of syn- and anti-β-Substituted Cysteine and Serine Derivatives. J Org Chem. 2002;67:3514–3517. doi: 10.1021/jo011172x. [DOI] [PubMed] [Google Scholar]
- 30.Cluzeau J, Lubell WD. Design, synthesis, and application of azabicyclo[X.Y.O]alkanone amino acids as constrained dipeptide surrogates and peptide mimics. Biopolymers. 2005;80:98–150. doi: 10.1002/bip.20213. [DOI] [PubMed] [Google Scholar]
- 31•.Rao MHVR, Pinyol E, Lubell WD. Rigid dipeptide mimics: synthesis of enantiopure C6-functionalized pyrrolizidinone amino acids. J Org Chem. 2007;72:736–743. doi: 10.1021/jo0616761.Report on a spectacular synthetic route to hydroxypyrrolizidinones, constrained scaffold of dipeptide mimics such as aminoacid-hydroxyprolines. Enantiopure (3S,5S,6R,8S)- and (3S,5S,6S,8S)-6-hydroxy pyrrolizidinone-3-N-(Boc)amino 8-methyl carboxylates were synthesized in seven steps starting from (2S)-α-tert-Bu N-(9-phenylfluoren-9-yl) aspartate β-aldehyde. The X-ray study indicated dihedral angles constrained within the hydroxypyrrolizidinone that were consistent with the ideal values for the i + 1 and i + 2 residues of a type II' β-turn.
- 32.Boeglin D, Hamdan FF, Melendez RE, Cluzeau J, Laperriere A, Heroux M, Bouvier M, Lubell WD. Calcitonin gene-related peptide analogues with aza and indolizidinone amino acid residues reveal conformational requirements for antagonist activity at the human calcitonin generelated peptide 1 receptor. J Med Chem. 2007;50:1401–1408. doi: 10.1021/jm061343w. [DOI] [PubMed] [Google Scholar]
- 33.Hanessian S, Naughton-Smith G, Lombart HG, Lubell WD. Design and synthesis of conformationally constrained amino acids as versatile scaffolds and peptide mimetics. Tetrahedron. 1997;53:12789–12854. [Google Scholar]
- 34.Halab L, Gosselin F, Lubell WD. Design, synthesis, and conformational analysis of azacycloalkane amino acids as conformationally constrained probes for mimicry of peptide secondary structures. Biopolymers. 2000;55:101–122. doi: 10.1002/1097-0282(2000)55:2<101::AID-BIP20>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 35.Wipf P, Wang X. Parallel Synthesis of Oxazolines and Thiazolines by Tandem Condensation-Cyclodehydration of Carboxylic Acids with Amino Alcohols and Aminothiols. J Comb Chem. 2002;4:656–660. doi: 10.1021/cc020041m. [DOI] [PubMed] [Google Scholar]
- 36.Angell YL, Burgess K. Peptidomimetics via copper-catalyzed azide-alkyne cycloadditions. Chem Soc Rev. 2007;36:1674–1689. doi: 10.1039/b701444a. [DOI] [PubMed] [Google Scholar]
- 37.Biron E, Chatterjee J, Kessler H. Optimized selective N-methylation of peptides on solid support. J Peptide Sci. 2006;12:213–219. doi: 10.1002/psc.711. [DOI] [PubMed] [Google Scholar]
- 38•.Biron E, Kessler H. Convenient Synthesis of N-Methylamino Acids Compatible with Fmoc Solid-Phase Peptide Synthesis. J Org Chem. 2005;70:5183–5189. doi: 10.1021/jo050477z.Report of an efficient preparation of protected N-α-methyl-amino acids without extensive purification. The procedure is based on the improved N-alkylation of N-α-arylsulfonylamino esters. Compatibility of the synthesized N-α-methyl-amino acids with Fmoc/tBu solid-phase peptide synthesis is demonstrated.
- 39•.Ying J, Gu X, Cai M, Dedek M, Vagner J, Trivedi DB, Hruby VJ. Design, synthesis, and biological evaluation of new cyclic melanotropin peptide analogues selective for the human melanocortin-4 receptor. J Med Chem. 2006;49:6888–6896. doi: 10.1021/jm060768f.Report of a number of novel α-MSH analogues that lead to a discovery of very selective peptide-based antagonists for the hMC4R. In these analogues, a disulfide or lactam bridge was used as a conformational constraint of pharmacophore tetrapeptide His-DPhe-Arg-Trp to enhance the β-turn spanning His6 and D-Phe7, while the pharmacophore group in Arg8 was mimicked via N-α-alkylation of residues 8 or 9 with the guanidinylbutyl group. N-α-alkylation procedures other than N-α-methylation are very important but still rare.
- 40.Hruby VJ. Prospects for Peptidomimetic Drug Design. Drug Discovery Today. 1997;2:165–167. [Google Scholar]