Abstract
Delineating neurons that underlie complex behaviors is of fundamental interest. Using adeno-associated virus 2, we expressed the Drosophila allatostatin receptor in somatostatin (Sst)-expressing neurons in the preBötzinger Complex (preBötC). Rapid silencing of these neurons in awake rats induced a persistent apnea without any respiratory movements to rescue their breathing. We hypothesize that breathing requires preBötC Sst neurons and that their sudden depression can lead to serious, even fatal, respiratory failure.
The preBötC in the ventrolateral medulla is hypothesized to be a kernel for the generation of respiratory rhythm in vitro and in vivo1–4. Adult rats with slow (~days), toxin-induced neurodegeneration of > 80% of neurokinin 1 receptor (NK1R)-expressing preBötC neurons survive with an ataxic rhythm during wakefulness and apnea during sleep1,5. Whether this pathological breathing pattern is driven by neurons that normally control respiratory-related muscles, including those underlying volitional or emotional behaviors, or by a compensatory reorganization in response to the slow neurodegeneration is unknown. To eliminate adaptation resulting from slow lesions, we rapidly (~minutes) decreased excitability in a glutamatergic subpopulation of preBötC neurons that express Sst6. This population overlaps with neurons expressing NK1R; 28 ± 2% of preBötC Sst neurons expressed NK1R and 41 ± 1% of preBötC NK1R neurons expressed Sst (5–6-week-old rats, n = 3; Supplementary Fig. 1 online), consistent with their overlap in neonatal rat preBötC7. We hypothesized that preBötC Sst neurons are essential for normal breathing and predicted that silencing these neurons would cause acute apnea in awake adult rats; as hypoxia and hypercapnea worsened with apnea, we presumed that other mechanisms, particularly in relation to volitional or emotional drives, would restore breathing, as appears to be the case in central congenital hypoventilation syndrome8. Rapid, reversible silencing of genotypic neuronal subpopulations can illuminate their role in behavior. This can be done by targeted expression of allatostatin receptor (AlstR), a G protein–coupled receptor that is neither expressed nor activated by any endogenous ligand in mammals9–11. Mammalian cortical and spinal cord neurons that are made to express AlstR can be rapidly and reversibly inactivated in vitro12,13 and in vivo under anesthesia10 by administration of allatostatin, which opens K+ channels9,13 to hyperpolarize them. We expressed AlstR and enhanced green fluorescent protein (EGFP) in targeted preBötC neurons and studied the effect of allatostatin application on breathing in adult rats.
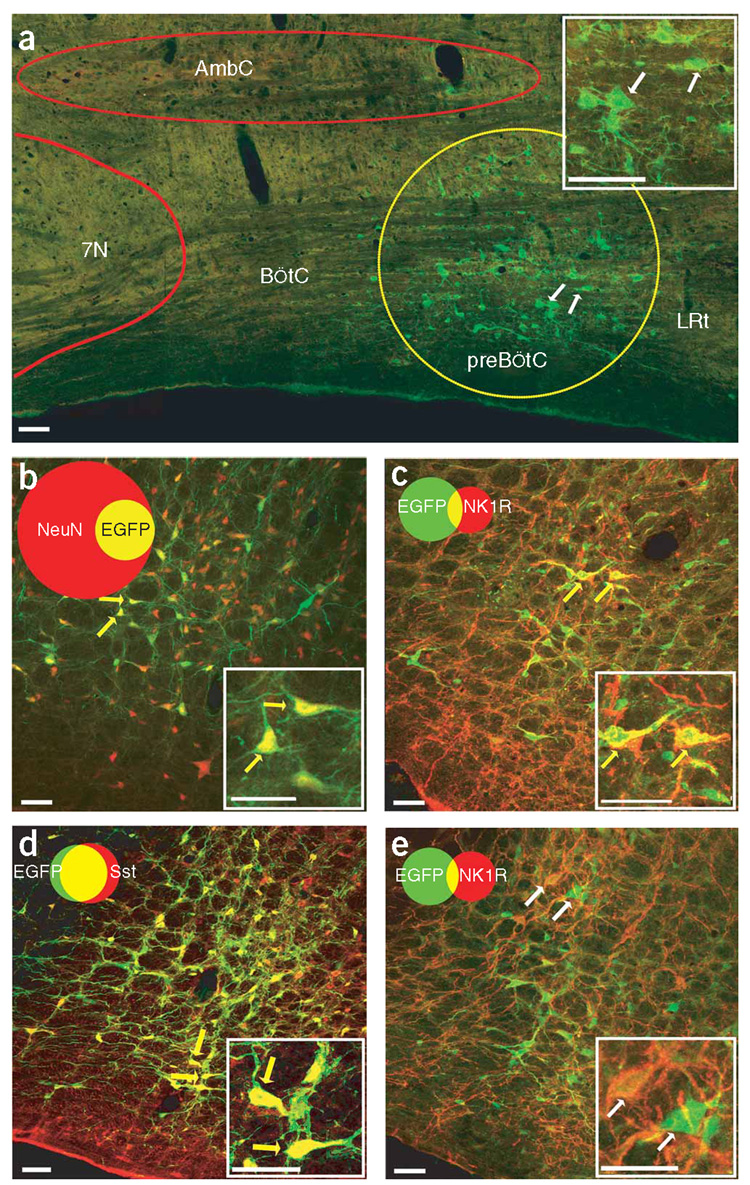
To obtain stable and reliable expression of exogenous genes in adult rat preBötC neurons, we used adeno-associated virus 2 (AAV2) to ensure high infection efficiency and long-term expression with low cytotoxicity or inflammation10,14. First, we describe the infection profile using AAV2 with the synapsin promoter to drive EGFP expression (Syn-EGFP; Supplementary Methods online). In 3 weeks after preBötC injection of Syn-EGFP10, EGFP could be detected in neuronal somas up to ~250 µm from the injection site. There were very few EGFP-positive cells in adjacent regions (for example, the Bötzinger Complex; Fig. 1a). In contrast with the wide expression that is inherent in transgenic mouse models (for example, see ref. 12), these viral microinjections produced site-specific expression of target genes that was highly localized in three dimensions around the injection site, as we did not find any EGFP expression elsewhere in the brainstem, spinal cord or cerebellum (Supplementary Table 1 online).
Figure 1.
preBötC neurons infected by AAV2 containing a Syn or Sst promoter. (a) EGFP expression (green) in adult rat preBötC (yellow circle) 3 weeks after microinjection of Syn-EGFP AAV2. 7N, facial nucleus; AmbC, ambiguous nucleus compact; BötC, Bötzinger Complex; LRt, Lateral reticular nucleus. (b) Syn-AlstR-EGFP AAV2–infected preBötC cells (green) were NeuN immunoreactive (red); that is, they were neurons. (c) Some Syn-AlstR-EGFP AAV2–infected preBötC neurons (green) were NK1R immunoreactive (red). (d) Sst-EGFP AAV2–infected preBötC neurons (green) were Sst immunoreactive (red). (e) Small portion of Sst-AlstR-EGFP AAV2–infected preBötC neurons (green) were NK1R immunoreactive (red). Images were obtained using a confocal microscope. Insets are high magnification of indicated neurons. Venn diagrams represent the relative number of neurons and the overlap of the relevant markers (b–e) normalized to NeuN immunoreactivity (n ≈ 3,000 neurons on each side of the preBötC). Yellow arrows represent neurons coexpressing and white arrows represent neurons not coexpressing the relevant markers. Scale bars represent 50 µm.
To modulate preBötC neuronal excitability in vivo, we made an AAV2 construct using the synapsin promoter to drive AlstR cDNA with an internal ribosome entry site linked to the EGFP gene (Syn-AlstR-EGFP)10. EGFP was detectable 4 weeks after viral injection in the preBötC. Almost all of the EGFP-positive cells (99 ± 1%, n = 7 rats) were immunoreactive for NeuN, a neuronal specific marker (Fig. 1b), and represented 64 ± 8% of all the neurons in 100 µm and 20 ± 2% of all the neurons in 250 µm of the injection site (n = 7). This pattern of viral infection probably depends on genotypic differences in the infectious preference of AAV2 in various subpopulations of preBötC neurons and decreased probability of infection with distance from the injection site. We found that 21 ± 4% of the preBötC neurons that expressed NK1R also expressed EGFP, and 10 ± 2% of the neurons that expressed EGFP were also immunoreactive for NK1R (n = 9; Fig. 1c). Of the preBötC neurons that expressed Sst, 20 ± 4% also expressed EGFP, and 15 ± 4% of the neurons that expressed EGF were also Sst positive (n = 3; Supplementary Fig. 2 and Supplementary Table 2 online). Neurons that were immunoreactive for tyrosine hydroxylase, adjacent to the preBötC2,6, were poorly infected; only 2 ± 1% of EGFP neurons were immunoreactive for tyrosine hydroxylase and <5% of the neurons that were immunoreactive for tyrosine hydroxylase (4 ± 3%; n = 7) expressed EGFP (Supplementary Fig. 2).
The synapsin promoter is not selective for Sst neurons. To specifically target these neurons, we replaced the synapsin promoter with the mouse Sst promoter in the AAV2 vectors (Supplementary Fig. 3 online). EGFP was exclusively expressed in 465 ± 26 preBötC neurons on each side (n = 6) 3 weeks postinjection of Sst-EGFP (Fig. 1d) or 6 weeks post-injection of Sst-AlstR-EGFP (Supplementary Fig. 3). We found that 66 ± 7% of neurons in 100 µm and 16 ± 1% of neurons in 250 µm of the injection site expressed EGFP (n = 6; Supplementary Fig. 3), and that 81 ± 8% of preBötC Sst neurons expressed EGFP and 80 ± 6% of preBötC EGFP neurons were Sst positive (n = 4; Supplementary Fig. 3 and Supplementary Table 2). Some preBötC NK1R neurons (16 ± 5%, n = 5) were EGFP positive and 11 ± 3% (n = 5) of EGFP neurons were immunoreactive for NK1R (Fig. 1e); no neurons were immunoreactive for tyrosine hydroxylase (n = 6; Supplementary Fig. 3).
Could activation of AlstR in infected preBötC neurons change the breathing pattern in anesthetized rats where volitional and emotional drives to breathe are suppressed? We examined the effects on breathing of inactivating larger numbers of neurons (590 ± 62 per side) using Syn-AlstR-EGFP AAV2. Allatostatin was applied in anesthetized rats 4–6 weeks postinjection of Syn-AlstR-EGFP into the preBötC. Tidal volume (that is, peak inspiratory amplitude) tended to decrease in 30 s, dropping to 50% at ~90 s, whereas frequency tended to decrease in ~60 s, dropping to 50% at 2 min with apnea onset at ~2.5 min (n = 5; Supplementary Fig. 4 online). After 1.5 min of apnea, rats were mechanically ventilated. The ventilator was stopped for 1 min (or less if cardiac arrhythmia was noticed) every 10 min to check for spontaneous breathing, but no breathing was observed before 40 min, at which point the rats started to breathe (n = 5; Supplementary Fig. 4). We observed that 3 out of 5 rats showed a significant transient hyperventilation in 20 s of allatostatin application (P < 0.05; Supplementary Fig. 5 online). Administration of allatostatin into Syn-EGFP–injected anesthetized rats did not change their breathing patterns (Supplementary Fig. 4).
Could viral expression of AlstR in preBötC neurons change the breathing pattern in behaving adult rats? When we administered allatostatin to awake rats without viral injection (n = 4; Supplementary Fig. 6 online) or in rats 4–6 weeks postinjection of Syn-EGFP into the preBötC (n = 4; Supplementary Fig. 7 online), we did not observe a substantial change in breathing. In contrast, similar administration of allatostatin to awake rats 4–6 weeks postinjection of Syn-AlstR-EGFP into the preBötC resulted in a transient (~1 min) increase in 30 s of both amplitude (214 ± 89%) and frequency (157 ± 43%; Supplementary Fig. 7), followed by a gradual decrease of both. At 90 s, breathing was back to baseline (amplitude, 117 ± 53%; frequency, 106 ± 29%). Between 2 and 2.5 min, amplitude and frequency decreased to 50% of baseline. At 4.5 min, these awake rats became apneic (Supplementary Fig. 7). Despite being awake, the rats did not breathe, even as their uninterrupted apnea progressed to severe hypoxia (indicated by bluish skin); after 1.5 min of apnea they were mechanically ventilated to prevent asphyxiation. A lower dose of allatostatin (0.34 µmol per kg of body weight) affected amplitude (66 ± 17% versus 93 ± 6%, P < 0.05, n = 6, Supplementary Fig. 8 online), but had little effect on frequency (P = 0.084) and did not lead to apnea; lowering the dose further had no effect (Supplementary Fig. 8). Heart rate was elevated during the early transient hyperventilation period, but was minimally affected just before the onset of apnea (Supplementary Fig. 9 online), indicating that induction of apnea was not secondary to cardiovascular changes. Thus, rapid silencing of a heterogeneous subset of preBötC neurons can produce persistent apnea.
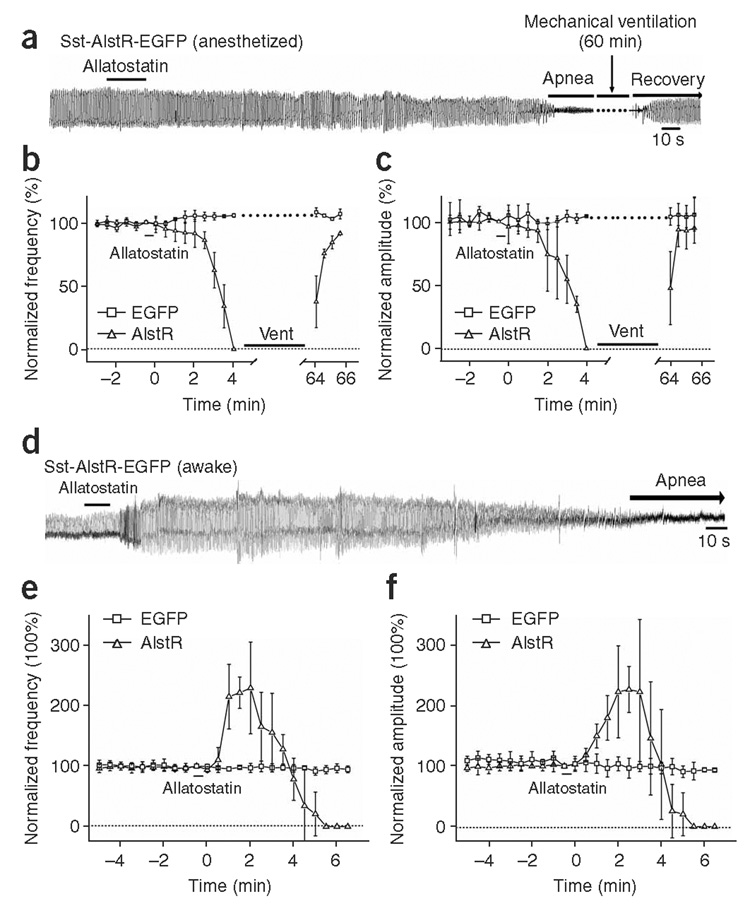
Next we tested whether silencing Sst neurons could cause apnea. We microinjected Sst-AlstR-EGFP (Supplementary Fig. 3) into adult rat preBötC. Subsequent (~6–8 weeks) administration of allatostatin to these rats during anesthesia produced similar respiratory depression compared to Syn-AlstR-EGFP–injected anesthetized rats, but took slightly longer (~4 min) to progress to prolonged (~60 min) apnea (n = 4; Fig. 2a–c). Two anesthetized rats showed a significant transient hyperventilation in 20 s of allatostatin application (P < 0.0001, Supplementary Fig. 5). Similar administration of allatostatin to awake rats 6–8 weeks postinjection produced a transient hyperventilation (amplitude, 150 ± 20%; frequency, 215 ± 54%) that lasted ~3 min before ventilation decreased markedly, progressing at ~5 min to apnea (n = 6; Fig. 2d–f). Despite being awake, the rats did not breathe and they required mechanical ventilation to prevent asphyxiation. Sst-EGFP–injected anesthetized (n = 4) or awake (n = 4) rats did not show any substantial respiratory depression after allatostatin application (Fig. 2b,c,e,f). Thus, selective and rapid silencing of preBötC Sst neurons induces a persistent apnea in awake adult rats.
Figure 2.
Rapid silencing of AlstR-expressing preBötC Sst neurons induced apnea in anesthetized and awake rats. Allatostatin was administered (0.42 µmol per kg, intracerebellomedullary cisternally) after prior (6–8 week) bilateral preBötC microinjection of Sst-AlstR-EGFP. (a) Changes in the breathing pattern (airflow) of anesthetized rat on allatostatin application; mechanical ventilation was used for the duration of apnea (~60 min). (b,c) Allatostatin administration induced a gradual decline of both the frequency (b) and tidal volume (c) until apnea developed at ~4 min post-allatostatin application. After mechanical ventilation, the rats resumed spontaneous breathing (n = 4). It should be noted that, in n = 2 out of 4 anesthetized Sst-AlstR-EGFP rats, there was a small, but significant, transient increase in ventilation in ~20 s after allatostatin administration (Supplementary Fig. 5) that is not apparent in group data (b and c). (d) Changes in the breathing pattern (tidal volume) of awake rats on allatostatin application. (e,f) Changes in the normalized frequency (e) and amplitude (f) on allatostatin application were plotted. Values are mean ± s.d. (Sst-AlstR-EGFP, n = 6; Sst-EGFP, n = 4). Vent, mechanical ventilation. Error bars represent ± s.d.
The preBötC is heterogeneous, consisting of several phenotypically distinct subpopulations of neurons, some overlapping, such as NK1R-and Sst-expressing neurons, and some exclusive, such as glutamatergic and glycinergic neurons. Perturbations of either the NK1R-or Sst-expressing subpopulations substantially disrupt normal rhythmogenesis. We cannot yet say whether this is because they have particular distinct or shared functional roles, or because they are part of a larger group of preBötC neurons that cannot generate rhythm when some threshold percentage of neurons are silenced or lesioned, as suggested by results in rats infected with Syn-AlstR AAV2 (where only a small fraction of infected neurons expressed Sst or NK1R). Regardless, essential neuronal circuitry underlying rhythmogenesis is in the preBötC.
We assume that our approach was successful in silencing preBötC neurons, but confirmation of this will require electrophysiological recordings. We suggest that the direct inhibition of preBötC Sst neurons was causal to the induction of rapid onset persistent apnea and that these neurons are important for the moment-to-moment generation of respiratory rhythmunderlying the regulation of blood gases and pH, in particular, in awake mammals. The inability of awake rats to ventilate at all in the face of relentlessly progressive hypoxia was notable, suggesting that other mechanisms that could drive breathing, such as may be associated with voluntary and/or emotional breathing control, were absent or blocked in these conditions.
Supplementary Material
ACKNOWLEDGMENTS
We thank G. Li for experimental assistance, T. Otis, L. Kruger, K. Kam and S. Pagliardini for comments on the manuscript, and N. Brecha and Y. Nie for advice and technical support. This work was supported by US National Institutes of Health grants HL37941 and HL40959 (J.L.F.).
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
References
- 1.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Nat. Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janczewski WA, Feldman JL. J. Physiol. (Lond.) 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKay LC, Janczewski WA, Feldman JL. Nat. Neurosci. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stornetta RL, et al. J. Comp. Neurol. 2003;455:499–512. doi: 10.1002/cne.10504. [DOI] [PubMed] [Google Scholar]
- 7.Pagliardini S, Ren J, Greer JJ. J. Neurosci. 2003;23:9575–9584. doi: 10.1523/JNEUROSCI.23-29-09575.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen ML, Keens TG. Paediatr. Respir. Rev. 2004;5:182–189. doi: 10.1016/j.prrv.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Birgul N, Weise C, Kreienkamp HJ, Richter D. EMBO J. 1999;18:5892–5900. doi: 10.1093/emboj/18.21.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan EM, et al. Neuron. 2006;51:157–170. doi: 10.1016/j.neuron.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Callaway EM. Trends Neurosci. 2005;28:196–201. doi: 10.1016/j.tins.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Gosgnach S, et al. Nature. 2006;440:215–219. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- 13.Lechner HA, Lein ES, Callaway EM. J. Neurosci. 2002;22:5287–5290. doi: 10.1523/JNEUROSCI.22-13-05287.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tenenbaum L, et al. J. Gene Med. 2004;6 Suppl. 1:S212–S222. doi: 10.1002/jgm.506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.