Abstract
Background
To improve methods for long-term weight management, the Weight Loss Maintenance (WLM) trial, a four-center randomized trial, was conducted to compare alternative strategies for maintaining weight loss over a 30-month period. This paper describes methods and results for the initial 6-month weight-loss program (Phase I).
Methods
Eligible adults were aged ≥25, overweight or obese (BMI=25–45 kg/m2), and on medications for hypertension and/or dyslipidemia. Anthropomorphic, demographic, and psychosocial measures were collected at baseline and 6 months. Participants (n=1685) attended 20 weekly group sessions to encourage calorie restriction, moderate-intensity physical activity, and the DASH (dietary approaches to stop hypertension) dietary pattern. Weight-loss predictors with missing data were replaced by multiple imputation.
Results
Participants were 44% African American and 67% women; 79% were obese (BMI≥30), 87% were taking anti-hypertensive medications, and 38% were taking antidyslipidemia medications. Participants attended an average of 72% of 20 group sessions. They self-reported 117 minutes of moderate-intensity physical activity per week, kept 3.7 daily food records per week, and consumed 2.9 servings of fruits and vegetables per day. The Phase-I follow-up rate was 92%. Mean (SD) weight change was −5.8 kg (4.4), and 69% lost at least 4 kg. All race–gender subgroups lost substantial weight: African-American men (−5.4 kg ± 7.7); African-American women (−4.1 kg ± 2.9); non–African-American men (−8.5 kg ± 12.9); and non–African-American women (−5.8 kg ± 6.1). Behavioral measures (e.g., diet records and physical activity) accounted for most of the weight-loss variation, although the association between behavioral measures and weight loss differed by race and gender groups.
Conclusions
The WLM behavioral intervention successfully achieved clinically significant short-term weight loss in a diverse population of high-risk patients.
Introduction
The U.S. Surgeon General’s recent call to action has highlighted the epidemic rise in obesity, as overweight and obesity affect about 65% of adults in the U.S.1,2 Obesity increases cardiovascular disease (CVD) risk factors3–5 and overall mortality.6–8 The prevalence of these risk factors—hypertension, dyslipidemia, and type 2 diabetes—is generally 1.5–2.9 times higher among overweight adults than normal-weight adults.9 Modest weight loss significantly improves CVD risk factors, including lowering blood pressure (BP)10,11 and hypertension risk12; reducing total cholesterol, low-density lipoprotein (LDL) cholesterol, and total triglycerides; and raising high-density lipoprotein (HDL) cholesterol.13 Similarly, weight loss lowers blood glucose in both diabetic and nondiabetic individuals and reduces risk of type 2 diabetes.14,15 In response to this overwhelming evidence, clinical treatment guidelines for hypertension, dyslipidemia, and type 2 diabetes include weight control as a core component.2,16–19
A combined emphasis on dietary intake and physical activity is important to both short- and long-term weight-loss goals.3,20–23 Behavioral strategies to modify these health behaviors are important components of weight-loss interventions because they emphasize the individual’s ability to monitor and regulate behavior24–26 and target the barriers to both initial weight loss20 and long-term maintenance. Weight loss is difficult, however, and in the Trials of Hypertension Prevention (TOHP-II)12 only 43% of participants lost ≥4 kg during a 6-month intensive behavioral intervention. Unfortunately, regain following weight loss is also common, and few trials have implemented interventions for longer than 18 months or have explicitly tested alternative strategies to sustain weight loss.27–30
This paper describes results from the 6-month Phase I of the Weight-Loss Maintenance (WLM) trial. This controlled trial examined three approaches for maintaining weight loss for 30 months following initial weight loss in a large, diverse adult population at high risk for CVD. Phase I of the WLM provided a nonrandomized intensive behavioral intervention10,11,31–35 to all participants to help them reduce caloric intake, promote a DASH (dietary approaches to stop hypertension) dietary pattern, and increase moderate-intensity physical activity to lose ≥4 kg of weight. Those who lost ≥4 kg during Phase I were eligible for Phase II and were randomized to a self-directed control group, a personal contact intervention providing brief monthly phone or face-to-face contacts, or an interactive technology intervention. The technology intervention provided support almost exclusively through an interactive website with self-monitoring tools, a bulletin board, problem-solving modules, up-to-date information, and other resources. Given the high prevalence of overweight in African Americans9 and the burden of CVD in that population,36 a major trial goal was to recruit about 40% African Americans. Adequate representation of both men and women was also an important recruitment goal.
Described here are the overall Phase-I weight-loss results and demographic and behavioral measures associated with weight loss in this large and diverse cohort. Race- and gender-specific weight-loss outcomes are presented, along with other demographic and behavioral modifiers of outcome.
Research Methods and Procedures
Overview of the WLM Design
The WLM trial was an investigator-initiated trial sponsored by the National Heart, Lung, and Blood Institute (NHLBI). It was reviewed and approved by IRBs at the participating institutions and by an NHLBI-appointed protocol review committee. NHLBI collaborated in the design and oversight of the trial, the analysis and interpretation of the data, and reviewed this manuscript. All participants provided written informed consent.
The overall study design for Phase I of the WLM trial is illustrated in Figure 1. During Phase I, overweight or obese adults (n=1685) were offered 20 group weight-loss sessions over about 6 months. Those who lost ≥4 kg were eligible for Phase II and were randomized to one of three 30-month maintenance interventions. This paper presents only the Phase-I methods and results. A complete description of the rationale, design, and methods for Phase II of the trial is available elsewhere.37
Figure 1.
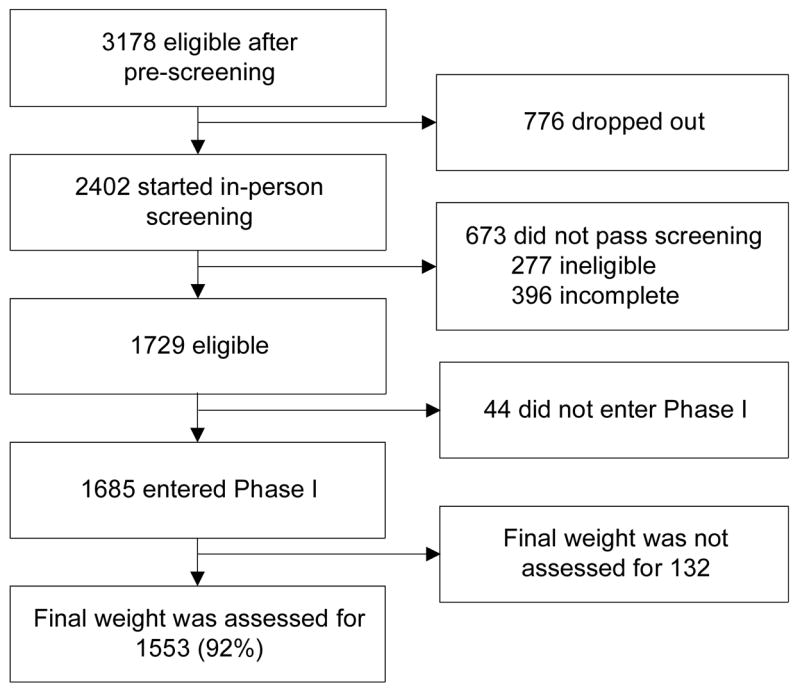
Flow diagram.
Phase-I Eligibility
Participants were eligible for Phase I if they were: aged ≥25 years; BMI 25–45 kg/m2; currently taking prescription medication for hypertension and/or dyslipidemia; willing to follow a healthy eating pattern; and willing not to use weight-loss medications for the duration of the trial. Participants also had to provide informed consent, keep a 5-day food record during screening, participate in the Phase-I weight-loss program, and try to lose at least 4 kg of weight. Because of the web- and phone-based nature of the Phase-II interventions, participants also had to have telephone and Internet access and were asked to demonstrate their ability to respond to e-mail and go to a specific website.
Participants were excluded if they had contraindications to weight loss; a cancer diagnosis (except for nonmelanoma skin cancer) in the past 2 years; medicated or poorly controlled diabetes (HbA1c≥8%); or a cardiovascular event within the past 12 months. Those with unmedicated, controlled diabetes (with a HbA1c<8%), a positive Rose angina questionnaire,38 or a prior CVD event that occurred more than 12 months before screening could participate with the permission of their physician plus a negative stress test or other diagnostic test for CVD. Other exclusion criteria were self-reported history of renal disease (other than kidney stones); psychiatric hospitalization within the last 2 years; consumption of more than 21 alcoholic drinks per week or binge drinking; and weight loss of >9.09 kg (“20 lbs” in recruiting material) in the last 3 months. Also excluded were those with histories of gastric bypass surgery or fundoplication for obesity, or scheduled surgery for this purpose; liposuction in the past 12 months or use of prescription weight-loss medications in the 3 months prior to screening; using medications for treatment of psychosis or manic–depressive illness; planning to leave the area within 3 years; currently or planning pregnancy or breast feeding; and WLM trial staff or their household members.
Recruitment and Screening
Participants were recruited at four clinical research centers (Duke University, Johns Hopkins University, Pennington Biomedical Research Center in Baton Rouge LA, and Kaiser Permanente’s Center for Health Research in Portland OR), with oversight from a Coordinating Center (also at Kaiser Permanente’s Center for Health Research) and the NHLBI. Recruitment relied primarily on mass mailings of brochures, coupons, flyers, and print media. After telephone or face-to-face prescreening of respondents to assess preliminary eligibility and interest, potential participants attended two 60-minute screening visits to confirm eligibility and to collect weight, height, accelerometry, fasting blood samples, self-reported minutes of 28 moderate-intensity physical activities per week, and a battery of questionnaires and interview items. Enrollment into Phase I occurred between August 2003 and July 2004 and final analyses were completed in 2007.
Phase-I Initial Weight-loss Intervention
The Phase-I intervention included 20, typically weekly, group sessions led by nutrition and behavioral counselors to help participants achieve initial weight loss through moderate calorie reduction and increased physical activity. The specific intervention targets for participants (Table 1) included reducing weight by at least 4 kg, engaging in 180 minutes per week of moderate-intensity physical activity (e.g., 6 × 30 minutes per day), and reducing calories while following a healthy (i.e., DASH) dietary pattern designed to reduce CVD risk factors.39
Table 1.
Phase-I goals and lifestyle guidelines given to participants
Lose weight
|
Move more! Lose more!
|
Eat fewer calories
|
Follow the DASH eating style
|
Exercise regularly
|
Eat a low-sodium diet
|
Record daily food intake and physical activity
|
Limit alcohol consumption
|
Be an active study participant
|
DASH, dietary approaches to stop hypertension
Intervention design and implementation
The approach was derived from social cognitive theory,24 and techniques of behavioral self-management25 and used the transtheoretical, or stages-of-change model,26,40 and motivational enhancement.24,41–43 The trial protocol and manual of operations is available at www.wlmtrial.org. Intervention was delivered primarily in the group sessions, although participants occasionally had individual contacts by phone or in person to help with specific behavior change needs. The group sessions were 90–120 minutes long with about 18–25 participants per group. Each center convened from 14 to 25 groups. Sessions were participatory and interactive, rather than didactic. Small-group activities fostered problem solving, social support, and program ownership. Many sessions included guided physical activity or food demonstrations. The intervention was tailored to the participants’ preferences and readiness to change. The intervention included group interactions and social support, goal setting, nutrition and physical activity information modules, skill development, and problem solving, with careful attention to cultural appropriateness for minority populations.
While the minimum weight loss needed for Phase II was 4 kg, interventionists negotiated individualized weight-loss goals with participants, encouraging as much loss as possible at a safe rate of 0.5 to 2 lbs per week. Each week, participants set reasonable short-term goals and developed specific short-term behavioral action plans to reinforce, support, and monitor their progress25 toward moderate caloric reduction (500 kcal less per day) and >180 minutes per week of moderate-intensity physical activity (3–6 METS), which was the national recommendation at the time. Participants aimed to do the following: (1) maintain daily food and physical activity records; (2) reduce portion sizes; (3) reduce foods high in calories, fat, and sodium; (4) increase consumption of fruits, vegetables, and low-fat dairy products; and (5) weigh themselves frequently and at least weekly. To promote accountability, participants weighed in at the beginning of each session and reported their minutes of physical activity and the number of daily diet records kept that week. (For analyses, those with no reports were assumed to have zero physical activity and food records for the week.) Participants worked together in small groups to identify threats to their plans and goals and to develop and rehearse coping strategies.
Intervention standardization, training, and quality control
Intervention staff received both centralized and local training throughout the study and used standardized program content, activities, materials, and data-collection forms for each session. A Minority Implementation Committee organized and conducted special trialwide training programs for all staff to highlight the cultural context for both African Americans and non–African Americans. Interventionists held monthly teleconference calls to monitor and refine intervention activities, clarify procedures, and share best practices. Local intervention directors continually monitored intervention quality, and the Coordinating Center conducted annual quality-control site visits during which intervention sessions were observed and critiqued.
Clinical Assessments
To maximize follow-up rates, staff made strong efforts to (1) select committed participants initially; (2) build a strong sense of teamwork and commitment during the groups; and (3) meet participants’ needs at follow-up, including doing home visits, if needed. Staff measured participant height once at entry to the nearest 0.1 cm using a calibrated, wall-mounted stadiometer and weight using high-quality, calibrated digital scales while participants wore light indoor clothes without shoes. BMI was calculated as the Quetelet index (kg/m2). The first of two “entry” weights taken during screening was used to compute BMI for eligibility. A second entry weight, typically measured within the 2 weeks before the Phase-I intervention started, was the starting point for measuring Phase-I weight loss. Relevant baseline measures were repeated at 6 months, although blood samples were collected only for those entering Phase II, and are not presented here. For participants randomized into Phase II, the final Phase-I weight was the Phase-II randomization weight, which was generally taken within 7 days of Phase-II randomization. For other participants, the final Phase-I weight was the last measured weight on or after the 16th group session. When no measured weight was available (8% of participants), the final Phase-I weight was imputed using the method described below.
Statistical Analysis
Missing data
A multiple imputation procedure44,45 was applied to replace the 132 missing weights at the end of Phase I and to replace other measures with missing values at entry. Five separate imputation samples were generated using Markov Chain Monte Carlo sampling in SAS® PROC MI, each with 1000 burn-in iterations and 500 iterations between data draws. Separate data analyses were then performed on each of these five complete data sets. Parameter estimates were then averaged, standard errors were adjusted with a function of the between-imputation variation, and the degrees of freedom were adjusted to obtain unbiased p-values. Most analyses were combined using SAS PROC MIANALYZE, but a SAS macro was used to implement Rubin’s rules for combining results. The resulting standard errors account for the added variability of the imputation process itself and hence are typically inflated relative to what would be seen using only a single imputation sample.
Regression model
The association of three sets of variables on weight change during Phase I was examined: (1) demographic: race, gender, and race–gender interaction; (2) behaviors: number of sessions attended, number of daily diet records kept, minutes of moderate-intensity physical activity; and (3) SES: income and education. In addition, the models were adjusted for the entry-level weight or BMI for weight change and BMI change, respectively. To simplify the modeling process, a model comparisons approach was used to evaluate the significance of second- and third-order interactions among the three sets of variables.46 This approach evaluates the significance of change in the model R2 between a starting (“full”) model and a nested (“reduced”) model using the multiple-partial F test. For instance, the cluster of 3-way interactions among variables from the three sets was dropped because it did not significantly improve the fit of the model. Two-way interactions between variables in a different cluster were then evaluated and dropped if not significant. After evaluating all interactions, nonsignificant (p>0.10) individual terms that were not in an interaction were stepped out to arrive at the final models.
Results
Baseline Characteristics
Among 1729 eligible participants, 1685 consented to the study and attended at least one Phase-I group intervention session, which constitutes the Phase-I analysis sample. Table 2 shows that, at entry, participants had a mean age of 55 years, 67% were women, and 44% were African American. Most (91%) attended at least some college, and 45% had an annual household income of <$60,000. By design, all participants were taking medications for high blood pressure (87%) or elevated lipids (38%), all were overweight, and most (79%) were obese (BMI ≥30 kg/m2).
Table 2.
Baseline characteristics
| Total | African American
|
Non–African American
|
|||
|---|---|---|---|---|---|
| Men | Women | Men | Women | ||
| n | 1685 | 196 | 540 | 355 | 594 |
| Age, mean (SD) | 54.8 (9.1) | 52.3 (10.1) | 52.3 (8.9) | 57.5 (8.9) | 56.2 (8.2) |
| Women, % | 67.3 | 0 | 100 | 0 | 100 |
| Hispanic, % | 1.4 | 0.5 | 0.4 | 2.3 | 2.0 |
| Education, % | |||||
| High school | 9.0 | 6.6 | 7.5 | 5.7 | 13.1 |
| Some college | 33.7 | 29.7 | 39.8 | 23.4 | 35.7 |
| College degree | 22.2 | 29.9 | 21.8 | 22.6 | 19.8 |
| Post college | 35.1 | 33.8 | 30.9 | 48.2 | 31.4 |
| Income, $, % | |||||
| <30,000 | 9.4 | 4.6 | 16.2 | 3.7 | 8.1 |
| 30,000-59,000 | 35.2 | 27.3 | 41.8 | 24.9 | 37.9 |
| 60,000-89,999 | 30.7 | 36.5 | 28.6 | 31.9 | 30.0 |
| ≥90K | 24.7 | 31.5 | 13.4 | 39.4 | 24.0 |
| On BP medications, % | 87.5 | 89.3 | 94.4 | 82.0 | 83.8 |
| On lipid-lowering medications, % | 38.3 | 38.3 | 23.1 | 54.4 | 42.6 |
| Weight, kg mean (SD) | 96.5 (16.5) | 107.3 (16.2) | 95.1 (15.1) | 104.7 (15.2) | 89.2 (14.5) |
| BMI, mean (SD) | 34.3 (4.8) | 34.1(4.5) | 35.6(4.9) | 33.6(4.3) | 33.6(4.9) |
| Obese (BMI≥30), % | 78.8 | 80.6 | 84.4 | 78.3 | 73.4 |
| # Alcohol beverages/day, mean (SD) | 0.2 (0.4) | 0.2 (0.3) | 0.1 (0.2) | 0.4 (0.6) | 0.2 (0.4) |
| Current tobacco use, % | 6.3 | 8.2 | 7.0 | 6.5 | 4.9 |
Attendance, Adherence, and Follow-up
Participants attended an average of 14 of the 20 possible intervention sessions (Table 3), and 61% attended 16 or more sessions. Attendance declined over time, but remained at 73% even during the last month of Phase I (data not shown). Over the 6-month intervention, participants self-reported a mean of 117 minutes of moderate-intensity physical activity per week (goal was 180) and 2.9 servings of fruits and vegetables per day (goal was 9–12). Unadjusted subgroup comparisons showed that men, compared to women, reported more physical activity per week (p<0.0001) and more food records per week (p<0.0038). Non–African Americans, relative to African Americans, attended more sessions, reported more physical activity, and kept more food records (p<0.0001 for each test).
Table 3.
Attendance, adherence, and weight loss by race and gender
| Total | African American
|
Non–African American
|
|||
|---|---|---|---|---|---|
| Men | Women | Men | Women | ||
| n | 1685 | 196 | 540 | 355 | 594 |
| Number of sessions attended, mean (SD) | 14.4 (5.8) | 13.8 (6.4) | 13.5 (6.0) | 15.4 (5.3) | 15.0 (5.4) |
| Number of sessions attended, % | |||||
| < 11 | 24 | 29 | 30 | 16 | 21 |
| 11–15 | 15 | 14 | 19 | 15 | 12 |
| 16–20 | 61 | 57 | 51 | 69 | 67 |
| Moderate physical activity/week, minutes, mean (SD) | 117.1 (111.7) | 112.9 (115.4) | 90.0 (95.8) | 159.4 (135.1) | 117.7 (100.4) |
| Number of food records/week, mean (SD) | 3.7 (2.3) | 3.4 (2.4) | 3.1 (2.2) | 4.2 (2.2) | 3.9 (2.1) |
| Fruit and vegetable servings/day, mean (SD) | 2.9 (2.7 | 2.5 (2.6) | 2.1 (2.3) | 3.6 (3.1) | 3.3 (2.6) |
| Weight change,a kg, mean (SD) | −5.8 (4.4) | −5.4 (7.7) | −4.1 (2.9) | −8.5 (12.9) | −5.8 (6.1) |
| BMI change,a mean (SD) | −2.0 (1.5) | −1.7 (2.4) | −1.5 (1.2) | −2.7 (4.0) | −2.2 (2.1) |
| Percent weight change,a mean (SD) | −6.0 (4.1) | −5.1 (7.2) | −4.4 (3.1) | −8.1 (11.8) | −6.5 (6.9) |
| Weight loss ≥ 4 kg,a N (%) | 1167 (69) | 135 (69) | 316 (59) | 281 (79) | 435 (73) |
| Randomized to Phase II (%) | 1032 (61) | 121 (62) | 267 (49) | 257 (72) | 387 (65) |
With missing data imputed using multiple imputation procedures.
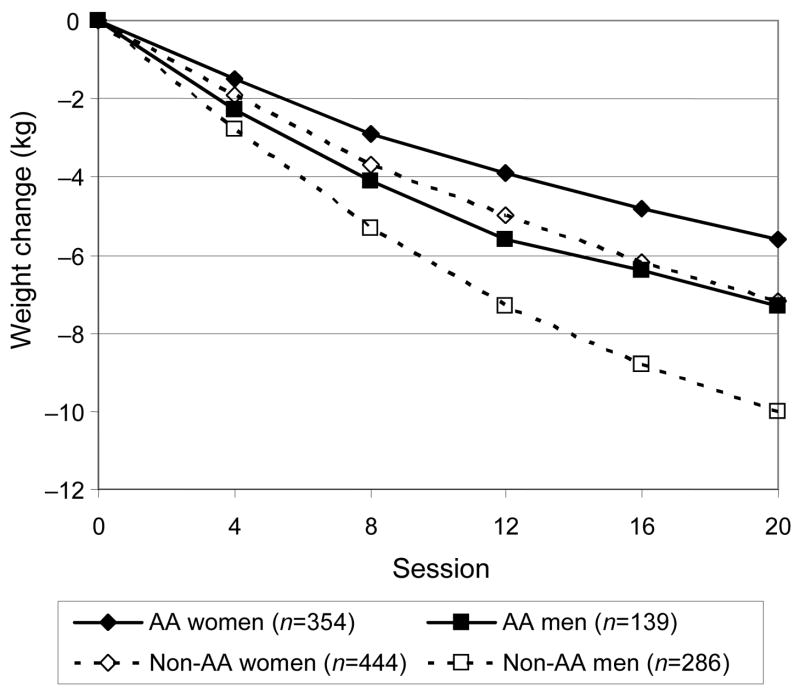
Weight Change
Among participants with at least one weight measurement in each month, mean weight decreased steadily over time in all race–gender subgroups, and was still on a downward trajectory at the end of Phase I (Figure 2). In all 1685 participants, with imputed missing data, mean (SD) weight decreased by 5.8 kg (4.4) during Phase I, and 69% of participants lost at least 4 kg (Table 3). Among the 1553 with follow-up data (i.e., no imputation), mean (SD) weight loss was 6.2 kg (5.1). This weight loss corresponded to a mean (SD) change in BMI of −2.0 (1.5) kg/m2 and a mean percentage decrease in initial weight of 6.0% (4.1).
Figure 2.
Mean weight loss during Phase I by race–gender subgroups for participants with at least one weight measurement in every month.
Predictors of Phase-I Weight Loss
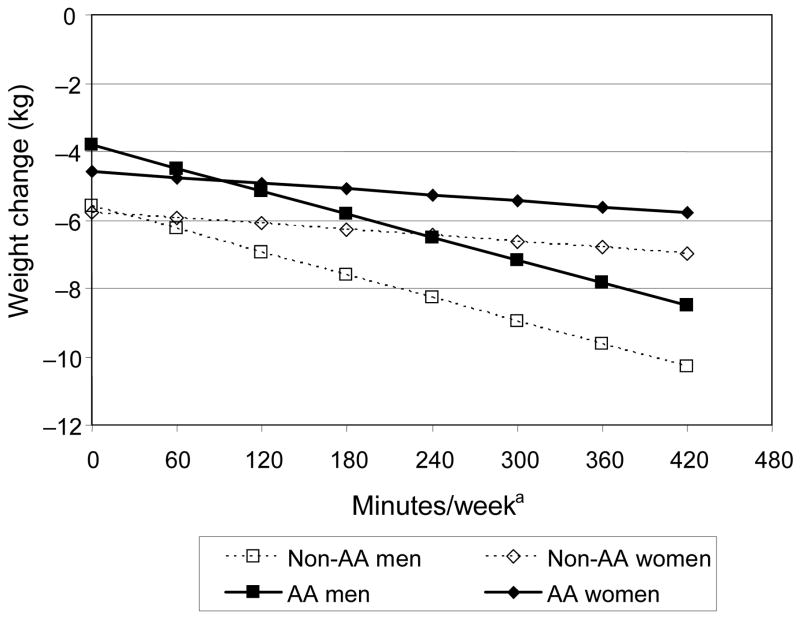
Table 4 shows the results for final multiple regression models on absolute weight change that include both entry characteristics and measures of intervention adherence. Significant predictors included higher initial weight, more sessions attended, more food records kept per week, and more minutes of reported moderate-intensity physical activity per week. Model 1 (R2=0.48) shows the direct effect of race, gender, race-by-gender interaction, and behavioral measures (food records and physical activities) with weight loss. Model 2 (R2=0.49) is the outcome of model comparisons evaluating interactions among race, gender, and behavioral measures. In contrast to Model 1, race and gender moderated the effects of food records and physical activities on weight loss (i.e., race by number of food records kept per week and gender by minutes of exercise per week). The difference in R2 between these models is significant (F[2,1673]=18.19, p<0.0001). Figure 3 illustrates how, based on Model 2, a given increase in physical activity per week is estimated to have a greater effect on weight loss for men than for women, regardless of race. A sensitivity analysis that dropped outlying activity values (>420 min, n=27) gave virtually identical results. Similarly, Figure 4 illustrates the greater estimated impact of keeping more food records on weight loss for non–African Americans than for African Americans, regardless of gender. Other potential predictors, including income, education, and other 2- and 3-way interactions, were considered but rejected as nonsignificant from the final model. The same model comparisons approach was used for BMI change and percent weight change with similar results.
Table 4.
Baseline measures and adherence indicators as predictors of change in weight
| Model 1 without behavior interactions | Model 2 with behavior interactions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effect | Parameter estimatea | SE | 95% | CIs | p< | Parameter estimatea | SE | 95% | CIs | p< |
| Intercept | 6.71 | 0.78 | 5.17 | 8.25 | <0.0001 | 7.84 | 0.81 | 6.23 | 9.44 | <0.0001 |
| Weight at entry (kg) | −0.07 | 0.01 | −0.08 | −0.06 | <0.0001 | −0.07 | 0.01 | −0.08 | −0.05 | <0.0001 |
| African American race (AA=1) | 2.02 | 0.37 | 1.30 | 2.74 | <0.0001 | 0.79 | 0.54 | −0.28 | 1.87 | 0.1447 |
| Gender (female=1) | 0.98 | 0.29 | 0.42 | 1.54 | 0.0006 | −0.17 | 0.38 | −0.92 | 0.57 | 0.6479 |
| Racea × gender | −0.98 | 0.43 | −1.83 | −0.13 | 0.0240 | −0.59 | 0.43 | −1.44 | 0.25 | 0.1699 |
| Sessions attended | −0.29 | 0.03 | −0.35 | −0.24 | <0.0001 | −0.30 | 0.03 | −0.35 | −0.25 | <0.0001 |
| Diet records per week | −0.52 | 0.08 | −0.67 | −0.37 | <0.0001 | −0.69 | 0.09 | −0.86 | −0.52 | <0.0001 |
| Moderate-intensity physical activity (MPA) per week (100 min) | −0.76 | 0.12 | −1.00 | −0.51 | <0.0001 | −1.12 | 0.15 | −1.41 | −0.83 | <0.0001 |
| Race × diet records per week | 0.27 | 0.09 | 0.08 | 0.45 | 0.0052 | |||||
| Gender × MPA per week (100 min) | 0.83 | 0.18 | 0.48 | 1.18 | <0.0001 | |||||
A negative parameter estimate indicates that increase in the measure predicts greater weight loss (weight loss is a negative change score)
Figure 3. Estimated effect of physical activity on weight change, by gender and race.
a Model evaluated at the overall mean of 3.7 food records per week.
Figure 4. Estimated effect of number of food records kept per week on weight change, by gender and race.
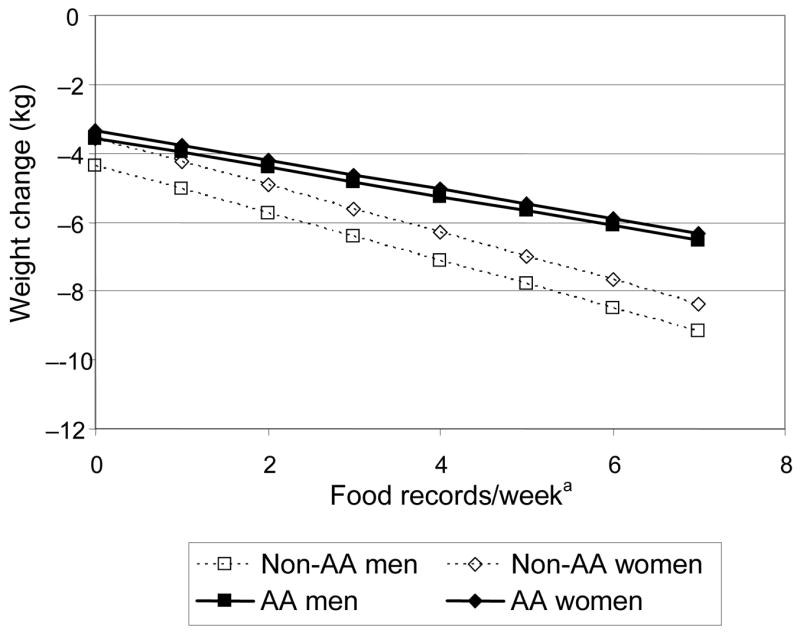
a Evaluated at the overall mean of 117 minutes per week of moderate-intensity physical activity.
Discussion
The WLM trial’s Phase-I behavioral weight-loss program resulted in a substantial 5.8 kg mean weight loss (BMI decrease of 2.0 kg/m2) over about 20 weeks in a population of overweight and obese adults who were being treated for cardiovascular risk factors. Two thirds (69%) of all participants who started the program lost a clinically relevant12,47,48 4 kg or more of weight by the end of Phase I. While other studies have shown similar amounts of weight loss,32,33,47 these findings are particularly noteworthy given the highly diverse nature of the participants, which included 44% African Americans. This group has been generally under-represented in weight-control studies and has typically achieved less weight loss.12,49 In this study, a majority of African American men (69%) and women (59%) lost at least 4 kg of weight. While African Americans lost somewhat less weight than non–African Americans on average, racial differences in weight loss in WLM were less pronounced than in previous studies. This improvement may be attributable to the extensive trialwide efforts to make the intervention culturally appropriate. African Americans were also well represented among investigators, interventionists, and other staff. The higher proportion of African-American participants in the intervention group meetings may also have created a more comfortable and supportive environment.
After adjusting for race, gender, and initial weight, greater weight loss was associated with more frequent attendance at the group sessions, number of food records kept per week, and minutes of moderate-intensity physical activity per week. These findings provide additional evidence50 that these standard behavioral strategies are key for successful weight loss. Further, even more weight loss would be expected if more participants had achieved the recommended minimum of 180 minutes of exercise per week. Race and gender, however, also affected the associations among weight loss, reported activity, and food record adherence. The adjusted association between activity and weight loss was stronger for men than for women. On average, men weigh more and have greater muscle mass and might therefore burn more calories in a given period of exercise. Several previous studies in both humans and animals have shown that a given increase in physical activity leads to greater weight loss in males than females, and this relationship may have a biological basis.51–53 Another possibility is that WLM men may have exercised at higher intensity levels than did women. It is less clear why the association between the number of food records kept per week and weight loss was greater for non–African Americans than for African Americans, regardless of gender. More research is needed on this differential effect of food records to refine intervention approaches and possibly develop alternative methods for tracking dietary intake in African Americans.
Even though the 20-session intervention in this study was a bit shorter than the 6-month series of weekly meetings commonly recommended,54,55 a greater percentage of WLM’s racially diverse participants lost at least 4 kg during the first 6 months than in the TOHP-II trial (69% vs 43%).12 Weights were still declining at meeting 20 and, had the intervention continued for another 6 weeks or longer, it is likely that the mean weight loss and the proportion of participants with at least 4 kg weight loss would have been even greater.
Even a modest weight loss of at least 4 kg has been associated with health benefits in studies of the prevention and/or treatment of hypertension,10–12,33,56 diabetes,14,15,47,57,58 and lipid management.13 These health benefits occur contemporaneously with weight loss and persist as long as weight loss is maintained.59,60 For example, Stevens and colleagues12 reported that a net mean weight loss of only 2 kg led to a 20% relative risk reduction in incident hypertension. Meta-analyses by Neter and colleagues61 and Staessen and colleagues48 suggest that systolic BP decreases 1.0–2.4 mmHg per kg of weight loss. This reduction in systolic BP, in turn, has been estimated to reduce stroke mortality by 6%–8% and CHD mortality by 4%–5%.62 Based on these meta-analyses, the mean weight loss in Phase I of WLM (5.8 kg) would be predicted to reduce systolic BP by 5.8–13.9 mmHg. The weight loss is comparable to the effect of a single antihypertensive medication.63 Because the amount of achieved weight loss varies by initial weight, the preceding discussion may over-simplify the likely observed effects.64
A limitation of Phase I of WLM was that it was an uncontrolled observational study. Compared to other large weight-loss studies, however, a higher proportion of both African-American and non–African American participants achieved clinically important weight loss. Another limitation is the use of self-reported adherence measures of physical activity and diet. Finally, Phase I was relatively short and, in the absence of additional intervention, regain would be expected over longer periods of time.50 Research specifically comparing various maintenance strategies is limited50,65,66 and badly needed. Phase II of the WLM trial will help address this need.37
In summary, as part of a study comparing alternative strategies for maintaining weight loss, the WLM trial enrolled a large and heterogeneous sample into an intensive 6-month behavioral intervention to achieve weight loss. Two thirds of all participants achieved clinically significant weight loss of 4 kg or more. Although some racial and gender differences were noted, all subgroups achieved clinically meaningful weight loss. Differences in attendance, food records adherence, and physical activity accounted for most of the weight-loss variation by race and gender groups. These results suggest that the WLM Phase-I intervention successfully achieved clinically significant short-term weight loss in an unusually diverse high-risk population, including 44% African Americans. Long-term maintenance results are presented elsewhere.67
Acknowledgments
This work was supported by National Heart, Lung, Blood Institute grants 5-U01 HL68920, 5-U01 HL68734, 5-U01 HL68676, 5-U01 HL68790 and 5-HL68955. We also wish to thank the WLM Data and Safety Monitoring Board and special thanks go to the WLM participants that made this study possible.
In addition, we would like to thank the following individuals at each of the participating sites:
From Duke University Medical Center, Durham NC: Kathleen Aicher, Joel Bronstein, W Blondeaner Brown, William Fan, MD, Jeanne Gresko, Tovon Hamilton, Aidan Hysjulien, Keely Kelly, Martis King, Lillian Lien, MD, Velda Martin, Heather McGuire, MD, Tonya Milligan, LaVerne Pruden, LaChanda Reams, Patrice Reams, Fran Rukenbrod, RD, LDN, Sonia Steele, MPH, RD, LDN, Edward Van Williamson.
From Johns Hopkins Pro Health, Baltimore MD: Andrea Booth, Cassie Brode, BA, Jeanne Charleston, RN, Janelle Coughlin, PhD, Berkley C. Curtis, Jr., MS, PA-S, Arlene T. Dalcin, RDI, Maura K. Deeley, Thomas Erlinger MD, MPH, Debra J. Gayles BS, Tara Harrison, Gerald J Jerome, PhD, Gloria Lawrence, Adriana E. Matos, BA, Karen McCully, MSc, LDN, Denise Monnett, Dana Owens, BS, Theresa L. Panichello, Mark E. Poisall, MS, CSCS, Ryan Rinke, Joy Q. Saunders, Amber C. Summers, MHS, RD, Karen White MS, RD.
From Kaiser Permanente Center for Health Research Clinical Site, Portland OR: Kristina Booker, BS, Leah Brooks, Pam Evans, MS, RD, Adrianne C. Feldstein, MD, MS, Craig Fleming, MD, Renee Giroux, RD, LD, CDE, Cheryl A. Johnson, EdM, Thomas Knapp, Ann MacFarlane, Leah Puderbaugh, Debbie Reck, Jodi Reed Kathy Schwab, MPH, RD, Nina Scott, LSWC, Dana R. Sturtevant, MS, RD, Marti Summer Judy Wick, RN, MAT, Catherine Willeford, MS, RD, Carol L. Young, DTR.
From Pennington Biomedical Research Center, Baton Rouge LA: Calynn Bunol, Lindsay Coates, Laura Decuir, RD, LDN, Frank Greenway, MD, David Harsha, PhD, Betty Kennedy, PhD, Katherine Lastor, LDN, RD, Lekeisha Lee, Erma Levy, MPH, RD, LDN, Jennifer Perault, Dawn Rachal, Anne Schulte, Patti Smith, Liz Tucker, Dana Vieselmeyer.
From the Coordinating Center at Kaiser Permanente Center for Health Research, Portland OR: Michael Allison, BS, Alan Bauck, BS, Chuhe Chen, PhD, Luanna Diller, Judy Donald, MS, Kristy Funk, MS, RD, Fran Heinith, RN, BSN, Clifton Hindmarsh, MS, Njeri Karanja, PhD, RD, Nidhi Kochar, MS, Teresa Kimes, MS, Carrie Meeks, Richard Meenan, PhD, Parker Pettus, MS, Rina Prasad, BA, PgDipLGA, Gayle Meltesen, MS,
From the NHLBI Project Office: Catherine Loria, PhD, MS, MA, Susan M. Czajkowski, PhD, Jungnam Joo, PhD, Eva Obarzanek, PhD, MPH, RD, Charlotte Pratt, PhD, RD
Footnotes
Human Participant Protection
This study was approved by the following IRBs: Kaiser Permanente Northwest Committee for the Protection of Human Subjects; Duke University Health System’s IRB for Clinical Investigations; The Johns Hopkins Medicine IRBs; and the Pennington Biomedical Research Center IRB.
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.USDHHS. The surgeon general’s call to action to prevent and decrease overweight and obesity. Rockville MD: USDHHS, Public Health Service, Office of the Surgeon General; 2001. [PubMed] [Google Scholar]
- 2.National Institutes of Health. NHLBI clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults, executive summary. USDHHS; 1998. [Google Scholar]
- 3.Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993;119(7 Pt 2):655–60. doi: 10.7326/0003-4819-119-7_part_2-199310011-00006. [DOI] [PubMed] [Google Scholar]
- 4.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–9. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 5.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–6. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293(15):1861–7. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 7.Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 8.Muennig P, Lubetkin E, Jia H, Franks P. Gender and the burden of disease attributable to obesity. Am J Public Health. 2006;96(9):1662–8. doi: 10.2105/AJPH.2005.068874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991–1998. JAMA. 1999;282(16):1519–22. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 10.Elmer PJ, Grimm R, Jr, Laing B, et al. Lifestyle intervention: results of the Treatment of Mild Hypertension Study (TOMHS) Prev Med. 1995;24(4):378–88. doi: 10.1006/pmed.1995.1062. [DOI] [PubMed] [Google Scholar]
- 11.Whelton PK, Appel LJ, Espeland MA, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. 1998;279(11):839–46. doi: 10.1001/jama.279.11.839. [DOI] [PubMed] [Google Scholar]
- 12.Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, Phase II. Ann Intern Med. 2001;134(1):1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 13.Wood PD, Stefanick ML, Dreon DM, et al. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med. 1988;319(18):1173–9. doi: 10.1056/NEJM198811033191801. [DOI] [PubMed] [Google Scholar]
- 14.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 15.Knowler WC, Barrett-Connor E, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institutes of Health. NHLBI task force report on research in prevention of cardiovascular disease. USDHHS; 2001. Available at: www.nhlbi.nih.gov/resources/docs/cvdrpt.htm. [Google Scholar]
- 17.National Institutes of Health. The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157(21):2413–46. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 18.National Institutes of Health. The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V) Arch Intern Med. 1993;153(2):154–83. [PubMed] [Google Scholar]
- 19.National Institutes of Health. NHLBI report of the conference on socioeconomic status and cardiovascular health and disease. USDHHS; 1995. Available at: www.nhlbi.nih.gov/resources/docs/sesintro.htm. [Google Scholar]
- 20.Fujioka K. Management of obesity as a chronic disease: nonpharmacologic, pharmacologic, and surgical options. Obes Res. 2002;10(2S):S116–23. doi: 10.1038/oby.2002.204. [DOI] [PubMed] [Google Scholar]
- 21.Wing RR. Behavioral approaches to the treatment of obesity. 2000:855–73. [PubMed] [Google Scholar]
- 22.McGuire MTWR, Klem ML, Lang W, Hill JO. What predicts weight regain among a group of successful weight losers? J Consult Clin Psychol. 1999;67(2):177–85. doi: 10.1037//0022-006x.67.2.177. [DOI] [PubMed] [Google Scholar]
- 23.Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr. 1997;66(2):239–46. doi: 10.1093/ajcn/66.2.239. [DOI] [PubMed] [Google Scholar]
- 24.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs; Prentice Hall: 1986. [Google Scholar]
- 25.Watson D, Tharp R. Self-Directed Behavior: Self-Modification for Personal Adjustment. 5. Pacific Grove CA; Brooks/Cole: 1989. [Google Scholar]
- 26.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390–5. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 27.Phelan S, Wing RR. Prevalence of successful weight loss. Arch Intern Med. 2005;165(20):2430. doi: 10.1001/archinte.165.20.2430-a. [DOI] [PubMed] [Google Scholar]
- 28.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132(6):2226–38. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 29.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355(15):1563–71. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 30.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL, Machin J. STOP regain: are there negative effects of daily weighing? J Consult Clin Psychol. 2007 Aug;75(4):652–6. doi: 10.1037/0022-006X.75.4.652. [DOI] [PubMed] [Google Scholar]
- 31.Jeffery RW, Gillum R, Gerber WM, Jacobs D, Elmer PJ, Prineas RJ. Weight and sodium reduction for the prevention of hypertension: a comparison of group treatment and individual counseling. Am J Public Health. 1983;73(6):691–3. doi: 10.2105/ajph.73.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.TOHP Research Group. The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels: results of the Trials of Hypertension Prevention, Phase I. JAMA. 1992;267(9):1213–20. doi: 10.1001/jama.1992.03480090061028. [DOI] [PubMed] [Google Scholar]
- 33.The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure: the Trials of Hypertension Prevention, phase II. Arch Intern Med. 1997;157(6):657–67. [PubMed] [Google Scholar]
- 34.Neaton JD, Grimm RH, Jr, Prineas RJ, et al. Treatment of Mild Hypertension Study: final results. Treatment of Mild Hypertension Study Research Group. JAMA. 1993;270(6):713–24. [PubMed] [Google Scholar]
- 35.King AC, Haskell WL, Young DR, Oka RK, Stefanick ML. Long-term effects of varying intensities and formats of physical activity on participation rates, fitness, and lipoproteins in men and women aged 50 to 65 years. Circulation. 1995;91(10):2596–604. doi: 10.1161/01.cir.91.10.2596. [DOI] [PubMed] [Google Scholar]
- 36.Cooper RS. Health and the social status of blacks in the United States. Ann Epidemiol. 1993;3(2):137–44. doi: 10.1016/1047-2797(93)90126-o. [DOI] [PubMed] [Google Scholar]
- 37.Brantley PJ, Appel LJ, Hollis JF, et al. Weight Loss Maintenance (WLM): Design and Rationale of a Multi-Center Trial to Sustain Weight Loss. Journal of the Society of Clinical Trials. doi: 10.1177/1740774508096315. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rose G, McCartney P, Reid DD. Self-administration of a questionnaire on chest pain and intermittent claudication. Br J Prev Soc Med. 1977;31(1):42–8. doi: 10.1136/jech.31.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–24. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 40.Bock BC, Marcus BH, Pinto BM, Forsyth LH. Maintenance of physical activity following an individualized motivationally tailored intervention. Ann Behav Med. 2001;23(2):79–87. doi: 10.1207/S15324796ABM2302_2. [DOI] [PubMed] [Google Scholar]
- 41.Miller W, Rollnick S. Motivational interviewing: preparing people to change addictive behavior. New York: Guilford Press; 1991. [Google Scholar]
- 42.Rollnick S, Mason P, Butler C. Health behavior change: a guide for practitioners. New York: Churchill Livingstone; 1999. [Google Scholar]
- 43.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 44.Schafer JL. Analysis of incomplete multivariate data. Boca Raton: Chapman & Hall/CRC; 1997. [Google Scholar]
- 45.Collins LM, Schafer JL, Kam CM. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Methods. 2001;6(4):330–51. [PubMed] [Google Scholar]
- 46.Kleinbaum DG, Kupper LL, Muller KE. Applied regression analysis and other multivariable methods. Boston: PWS-Kent; 1988. p. 718. [Google Scholar]
- 47.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006 Sep;29(9):2102–7. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Staessen J, Fagard R, Amery A. The relationship between body weight and blood pressure. J Hum Hypertens. 1988;2(4):207–17. [PubMed] [Google Scholar]
- 49.Kumanyika SK, Obarzanek E, Stevens VJ, Hebert PR, Whelton PK. Weight-loss experience of black and white participants in NHLBI-sponsored clinical trials. Am J Clin Nutr. 1991;53(6S):S1631–8. doi: 10.1093/ajcn/53.6.1631S. [DOI] [PubMed] [Google Scholar]
- 50.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004;12(S):S151–62. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 51.Donnelly JE, Hill JO, Jacobsen DJ, et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the Midwest Exercise Trial. Arch Intern Med. 2003;163(11):1343–50. doi: 10.1001/archinte.163.11.1343. [DOI] [PubMed] [Google Scholar]
- 52.Dunn CL, Hannan PJ, Jeffery RW, Sherwood NE, Pronk NP, Boyle R. The comparative and cumulative effects of a dietary restriction and exercise on weight loss. Int J Obes (Lond) 2006;30(1):112–21. doi: 10.1038/sj.ijo.0803046. [DOI] [PubMed] [Google Scholar]
- 53.Williams PT, Wood PD. The effects of changing exercise levels on weight and age-related weight gain. Int J Obes (Lond) 2006;30(3):543–51. doi: 10.1038/sj.ijo.0803172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeffery RW, Drewnowski A, Epstein LH, et al. Long-term maintenance of weight loss: current status. Health Psychol. 2000;19(1S):5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- 55.Wadden TA, Crerand CE, Brock J. Behavioral treatment of obesity. Psychiatr Clin North Am. 2005;28(1):151–70. doi: 10.1016/j.psc.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 56.Moore LL, Visioni AJ, Qureshi MM, Bradlee ML, Ellison RC, D’Agostino R. Weight loss in overweight adults and the long-term risk of hypertension: the Framingham study. Arch Intern Med. 2005;165(11):1298–1303. doi: 10.1001/archinte.165.11.1298. [DOI] [PubMed] [Google Scholar]
- 57.Wing RR, Hamman RF, Bray GA, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12(9):1426–34. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. NIH Publication No. 98-4083, 2005.
- 59.Stevens VJ, Corrigan SA, Obarzanek E, et al. Weight loss intervention in phase 1 of the Trials of Hypertension Prevention. The TOHP Collaborative Research Group. Arch Intern Med. 1993;153(7):849–58. [PubMed] [Google Scholar]
- 60.Diabetes Prevention Program Research Group. The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22(4):623–34. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42(5):878–84. doi: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- 62.Stamler R. Implications of the INTERSALT study. Hypertension. 1991;17(1S):I16–I20. doi: 10.1161/01.hyp.17.1_suppl.i16. [DOI] [PubMed] [Google Scholar]
- 63.Materson BJ, Reda DJ, Cushman WC, et al. Single-drug therapy for hypertension in men: a comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med. 1993;328(13):914–21. doi: 10.1056/NEJM199304013281303. [DOI] [PubMed] [Google Scholar]
- 64.Erlinger TP, Vollmer WM, Svetkey LP, Appel LJ. The potential impact of nonpharmacologic population-wide blood pressure reduction on coronary heart disease events: pronounced benefits in African Americans and hypertensives. Prev Med. 2003;37(4):327–33. doi: 10.1016/s0091-7435(03)00140-3. [DOI] [PubMed] [Google Scholar]
- 65.Hill JO, Wyatt H, Phelan S, Wing R. The National Weight Control Registry: is it useful in helping deal with our obesity epidemic? J Nutr Educ Behav. 2005;37(4):206–10. doi: 10.1016/s1499-4046(06)60248-0. [DOI] [PubMed] [Google Scholar]
- 66.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82(1S):S222–5. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 67.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299(10):1139–48. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]