Abstract
Background
Prostate cancer is a major health issue, and prevention of prostate cancer and/or its progression will yield benefits for men. Difluoromethylornithine (DFMO) is an antiproliferative agent, inhibiting ornithine decarboxylase, the first enzyme in the polyamine pathway, and has been studied as a therapeutic and chemopreventive agent. The prostate has high levels of tissue polyamines and has shown sensitivity to DFMO both in vitro and in vivo.
Methods
Eighty-one men participated in a 1-year randomized trial of placebo or DFMO. Prostate volume determination and biopsy of the prostate for histology and polyamine content were done at baseline and after 12 months. Other biomarker variables were assessed, including total and free prostate-specific antigen and prostate-specific antigen doubling time.
Results
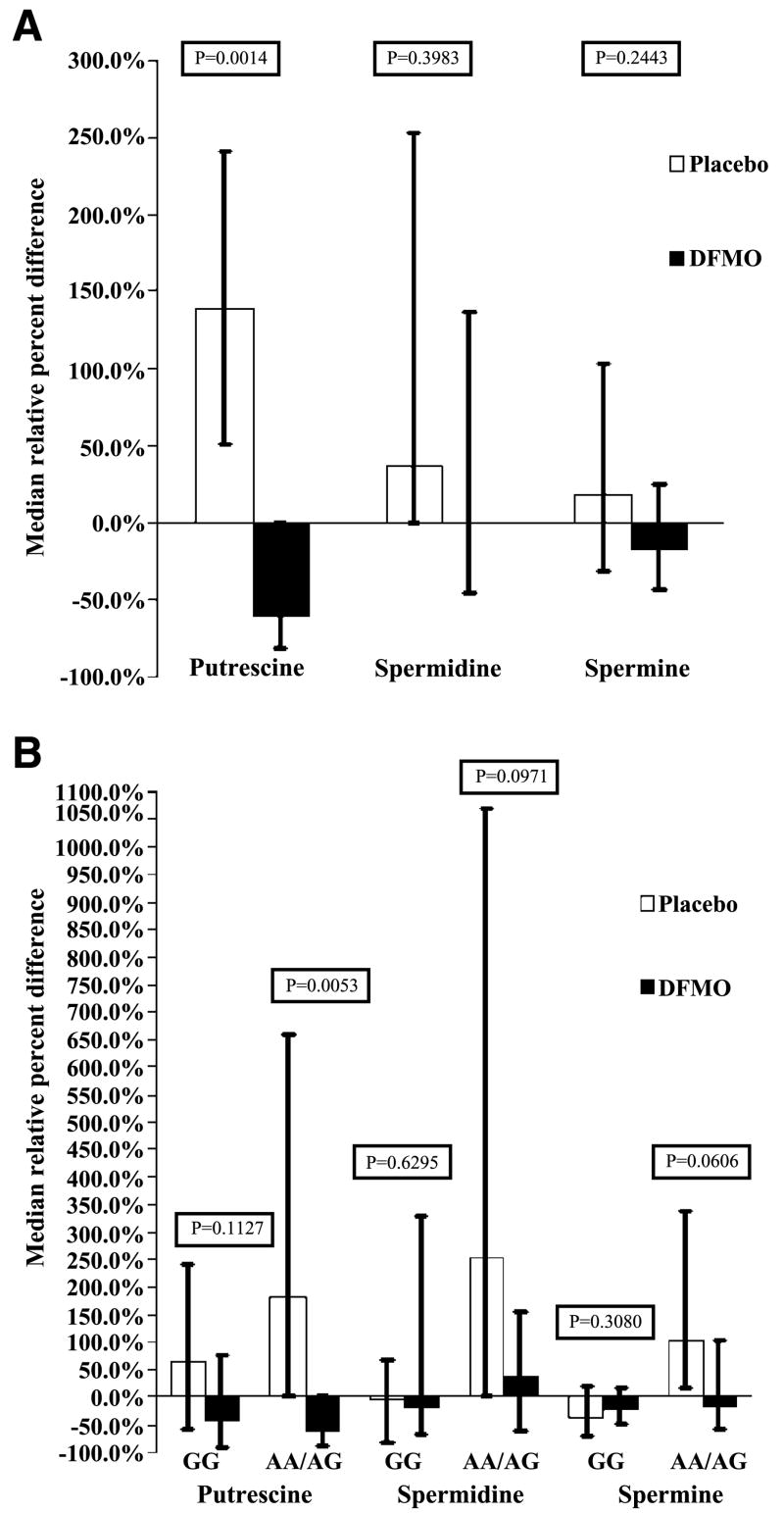
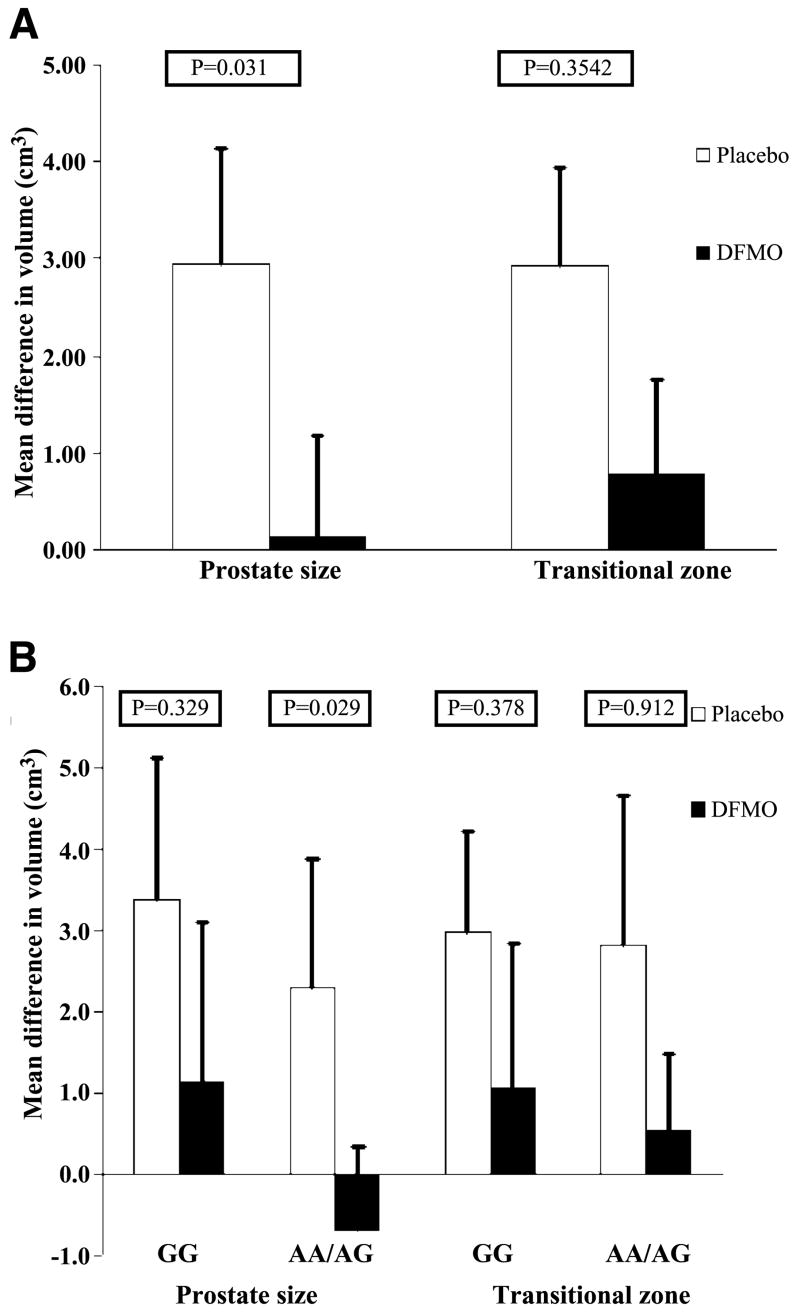
Compared with baseline, men receiving DFMO had a smaller increase in prostate volume (0.14 cm3) than those on placebo (2.95 cm3; P = 0.0301) at 1 year. In addition, DFMO caused a 60.8% reduction of prostate putrescine levels compared with a 139.5% increase in the placebo arm (P = 0.0014). Stratification by ornithine decarboxylase genotype showed that DFMO reduced prostate volume (P = 0.029) and putrescine levels (P = 0.0053) in the AA + GA group but not in the GG group. There were no grade 3 or 4 toxicities. There was no clinical ototoxicity, with one subclinical grade 2 hearing decline on audiogram.
Conclusion
In this randomized placebo-controlled trial, DFMO induced a decrease of prostate putrescine levels and rate of prostate growth. The potential of this compound for prostate cancer or hyperplasia should be further studied.
Introduction
Prostate cancer is the most common nonskin malignancy and the second leading cause of cancer deaths in men. The risk for clinical prostate cancer is linked to age, race, and family history (1). Enthusiasm for prevention of prostate cancer is based on observations of epidemiologic differences among prostate cancer mortality rates in Western countries compared with Asia (e.g., Japan and China). The etiology of prostate cancer is not completely understood, and thus there are numerous avenues of investigation under way from assessment of the genetic contribution of the androgen receptor to the effect of the oral intake of antioxidants (2). A need for prostate cancer prevention is predicated on the aging of society with estimates of a 4-fold increase in the number of people over the age of 65 years by the year 2050 (1, 3). As many men choose to monitor their prostate health, chemoprevention could be used for both prevention of prostate cancer development as well as prevention of prostate cancer progression.
Current trials for prostate cancer prevention have focused on changing the hormonal milieu [Prostate Cancer Prevention Trial with Finasteride (4) or Reduction by Dutasteride in Prostate Cancer Events (5)] or implementing dietary supplements linked to prostate cancer reduction (Selenium and Vitamin E Prevention Trial; ref. 6). We have focused our attention on an agent that is a known antiproliferative, difluoromethylornithine (DFMO). It is an irreversible inhibitor of ornithine decarboxylase (ODC), the rate-limiting step in the conversion of ornithine to putrescine and subsequently to the polyamines spermidine and spermine (7, 8). Polyamines are essential to cell proliferation and are tightly regulated by the cell. Elevated levels of polyamines have been linked to carcinogenesis (9). In vitro experiments have shown this elevation of polyamines to be closely associated and not coincidental (9-11).
The prostate has one of the highest levels of polyamines of any organ (12). The function of the prostate is to provide fluid rich in polyamines and other compounds to the ejaculate (13). In vitro and in vivo studies with DFMO in prostate models have shown its efficacy in decreasing prostate polyamine levels, tumor growth, prostate growth, and regrowth (14-17). Rodents were castrated with subsequent decrease in prostate size and polyamine content. With the return of exogenous androgens, the prostatic atrophy was readily reversed and polyamine content was normalized. DFMO markedly slowed prostatic weight gain from the androgens to half of the weight of the controls and blocked increases in putrescine and spermidine levels in the prostate (15).
Nude mouse models have shown the efficacy of DFMO in decreasing flank growth of human xenografts of prostate cancer cell lines compared with controls (14). The transgenic adenocarcinoma mouse prostate model has been used to test the efficacy of DFMO as a chemoprevention agent. Gupta et al. (18) showed marked reduction in weight and volume of the prostate as well as metastasis in the group treated with 1% DFMO. Another group reported on the manipulation of polyamines in the transgenic adenocarcinoma mouse prostate model by overexpression of spermidine/spermine N1-acetyltransferase, which regulates the catabolism and export of intracellular polyamines (19). The transgenic adenocarcinoma mouse prostate/spermidine/spermine N1-acetyltransferase animals had smaller weights of their prostates at 30 and 36 weeks and better histologic scores. The Wistar rat model, a chemically induced prostate cancer with methylnitrosamine/testosterone, showed a reduction in tumor incidence to 10% to 11%, compared with controls with 64% tumor incidence, when rats were given either oral DFMO or finasteride (20).
In humans, the genetic variability of the ODC gene, A or G at 316 (some authors label this polymorphism at 315), has been linked to altered relative risk rates in colon polyps and prostate cancer when exposed to differing environmental agents. Report of polyp formation in the Aspirin/Folate Polyp Prevention Study has shown that risk of polyps was not linked to this ODC polymorphism alone, but when combined with aspirin use, there was reduction in polyp formation and in advanced adenomatous lesions in those participants with an A allele (21). Martinez et al. (22) found similar results when they reviewed aspirin use and ODC genotype status of participants in a phase III wheat bran fiber trial to reduce colon polyp recurrence. A prostate study also showed no risk stratification for cancer based on the ODC polymorphism alone, but those with an A allele when linked with androgen receptor polymorphisms (CAG repeats <22) had an odds ratio of 2 for prostate cancer. Smoking has also been linked to prostate cancer risk in men with an A allele only (23).
We have previously shown that DFMO was able to decrease human prostate polyamine levels in the short term (1 month) with 500 mg/m2 of oral DFMO for 28 days before rebiopsy (24). Based on the association of polyamines with proliferation and carcinogenesis, the unique relationship of the prostate with polyamines, and the previous laboratory and animal studies, we set out to study longer-term use and the effect of DFMO on the prostate of men at increased risk of prostate cancer. The primary hypothesis to be tested was whether DFMO could suppress prostate tissue polyamine content. A secondary end point was to determine if DFMO affected prostate size as measured by volume and if there was a differential response to DFMO in the transitional zone compared with the total prostate.
Materials and Methods
Participants
Men ages 35 to 70 years with a family history of prostate cancer but no previous personal history of prostate cancer were recruited to a University of California, Irvine, Investigational Review Board–approved protocol for a phase II chemoprevention double-blind placebo-controlled trial. Patients were screened for family history and appropriateness for inclusion. Screening audiograms and laboratory values were done. Prerandomization biopsy and volume measurements of the prostate were done. A minimum of six cores was taken if the prostate-specific antigen (PSA) was within age-specific ranges and digital rectal exam was normal. Eight to 12 cores were taken if clinically indicated. Cores were snap frozen immediately after biopsy. After biopsy with size measurements, there was a 1-month placebo run-in period before receiving the study drug. Pill counts were done after this 1-month period for compliance. One participant refused to participate further after the run-in period secondary to the anxiety of possibly receiving an experimental drug. Participants were randomized between placebo and 500 mg/d DFMO and stratified by age (<60 or >60 years) and baseline pathology report, either benign or abnormal [i.e., prostate cancer or prostatic intraepithelial neoplasia (PIN)]. A 6-month follow-up with laboratory values, PSA, digital rectal exam, and, if indicated, biopsy was done. End of study biopsy was done at 12 months in the same manner as initial biopsy. For each man, values measured at baseline served as controls for 12-month measurements.
Early in the trial, men were unexpectantly diagnosed with prostate cancer, a function of the random tissue sampling needed for polyamine analysis. Men were given all their therapeutic options for localized prostate cancer, weighted toward treatment due to their family history. Those with a Gleason 4 component were strongly encouraged to receive definitive therapy. Due to the combination of low PSA levels, or minimal core involvement, several men elected to undergo expectant management. An amendment the second year of accrual allowed inclusion of patients diagnosed at baseline with prostate cancer who elected expectant management to continue participation in the trial. Over all of the 81 baseline biopsies, 11 men had cancer and 4 went for treatment. Two men had PIN on initial biopsy. Seven men with cancer and both men with PIN came onto the trial. The PSA ranged from 0.8 to 4.8 ng/mL. All men but one had a PSA <2.1 ng/mL. Pathology was focal to 20%, Gleason 3 + 3 in all but one man. He had Gleason 4 + 3 in 10% of one core and PSA of 2.1 ng/mL and refused definitive therapy. Five men were randomized into the DFMO arm and four onto placebo. One man with cancer randomized to DFMO did not return for end of study biopsy.
Family History Inclusion
Inclusion to the trial required two first-degree relatives (father and brother) or more than two second-degree or first cousins diagnosed with prostate cancer. Laboratory values are the following: complete blood count, chemistry 20 metabolic panel, protime, urine analysis, Hybritech PSA, and free PSA. Air conduction pure tone threshold audiograms were done at baseline and at the end of study and upon request by the participant.
Prostate Volume and Prostate Biopsy Technique
Transrectal prostate needle biopsies were done using lateral sextant template. Volumes were calculated by the software of the ultrasound machine incorporating imputed height, length, transverse length of the total prostate, and the transition zone of the prostate [π / 6 × (transverse diameter × anteroposterior diameter × cephalocaudal diameter)]. There were nine patients who did not have both (before and after treatment) transition zone measurements taken at the time of total prostate volume measurements. Lidocaine jelly was instilled into the rectal vault before probe insertion, and 1% lidocaine was injected into the periprostatic tissue after volume measurements before the biopsy. Aspirin and other anti-inflammatory medications were stopped 5 to 10 days before biopsy, as well as dietary supplements, which might affect bleeding. Six doses of ciprofloxacin, one every 12 h, were given starting the night before the procedure. A Ducolox suppository was given the morning of the procedure.
Histology
Cryostat sections were stained with H&E and basal cell–specific anti-keratin 5 antibody for diagnostic purposes. All pathology specimens were reviewed by one pathologist (R.N.) and given a Gleason grade. Those whose initial biopsy showed PIN or atypical changes were rebiopsied before randomization.
Polyamine Analysis
Three to six cores of snap-frozen prostate tissues were used for the polyamine analysis. Technical aspects have been reported previously (25-30); briefly, this included standard polyamine preparations and use of internal standards. Prostate tissue was minced in 300 μL of 9.2 N perchloric acid, homogenized vigorously with the resulting lysate stored overnight at 4°C, and rehomogenized. The acid-insoluble fraction was collected by centrifugation and the acid-soluble fraction with the polyamines was analyzed by high-performance liquid chromatography using the method of Seiler and Knodgen, normalizing to protein content. Protein levels were assayed using the bicinchoninic method.
Genetic Markers
The genotype of the ODC single nucleotide polymorphism 316 nucleotides 3′ of the transcriptional start site of the ODC gene was measured as described earlier (22). Briefly, one 7-mL Vacutainer containing EDTA of whole blood was collected for genotyping. This sample was aliquoted into two 2-mL cryovials and stored at −80°C until DNA was extracted. Genomic DNA was extracted from whole blood using the QIAamp Blood DNA Mini kits from Qiagen. Laboratory best practices, including negative and positive controls as well as duplicates, were used to generate the ODC genotypes.
Statistical Analysis
Descriptive statistics (e.g., arithmetic means) were calculated for prostate volume and transition zone volume. Characteristics of placebo and DFMO treatment groups were compared using an independent sample t test for age at baseline and the log-transformed values of percent free PSA, prostate volume, and spermine. Because of lack of normality of baseline distributions, the Wilcoxon two-sample test was applied to compare treatment groups with regard to total PSA, free PSA, transitional zone, putrescine, spermidine, and the ratio of spermidine to spermine. For putrescine, spermidine, and spermine, the relative percent difference between corresponding values measured at 12 months and at baseline was calculated as the ratio of the absolute difference to the baseline value multiplied by 100%.
Treatment groups were compared with regard to median relative percent difference by Wilcoxon two-sample tests. Stratified by ODC polymorphism, GG versus GA and AA, treatment groups were compared using nonparametric Wilcoxon two-sample tests for putrescine and spermidine and independent sample t tests for the log transform of spermine. We computed distribution-free 95% confidence intervals as described in Hahn and Meeker (31). Comparisons of the absolute 12-month difference between treatment groups were based on an independent sample t test for prostate volume and a nonparametric Wilcoxon two-sample test for transition zone volume. Mean absolute difference of 12 months to baseline was calculated for prostate volume (cm3) and transitional zone volume (cm3), stratified by ODC allele, GG versus GA and AA. For analysis of transition zone volume within the GG allele, a nonparametric Wilcoxon two-sample test was applied. Independent sample t tests were used for all other comparisons within allele subgroups. The effect of DFMO treatment on the PSA doubling time after 6 and 12 months was assessed as follows: let T = time in months, PSA1 = first PSA measurement, and PSA2 = second PSA measurement. Then, PSA doubling time was calculated as log(2) × T / [log(PSA2) − log(PSA1)]. Independent sample t tests were applied to assess differences between treatment groups.
Results
Demographics of the Participants
The trial enrolled (signed consent forms) 140 men. The median time from study entrance to baseline biopsy was 41 days. The median time from baseline biopsy to start of treatment was 35 days, which accounts for the 1-month run-in period. Eighty-one men underwent an initial biopsy, 76 men were randomized, 66 completed the study with two sets of biopsies of which 62 finished with 12 months of study drug and an end of study biopsy, and the other 4 exited the trial at 6 months when the diagnosis of cancer was made at a 6-month biopsy and treatment options were pursued. The results reported include all men with both an entrance and exit biopsy, regardless of cancer status. There were no significant differences at baseline or response to treatment, although the numbers of men with cancer (six) or PIN (two) at entrance or new cancers at exit (four) were too small to give meaningful data with substratification.
The majority (n = 57) of the participants in the clinical trial were White (75.0%), 10 (13.2%) were African-Americans, 8 (10.5%) were Hispanic, and 1 (1.3%) was Asian. Twelve of the participants (15.8%) were <45 years old on entry in the study, 23 (30.3%) were between the ages of 45 and 54 years, 29 (38.2%) were between the ages of 55 and 64 years, and 12 (15.8%) were >65 years old.
Table 1 outlines the baseline demographics of the participants randomized between the two arms. There were no statistical differences between participants in the two arms for age, race, cancer status, starting PSA, percent free PSA, prostate size, or polyamine levels. There were no statistical differences on the baseline polyamines (or log-transformed values) between the 9 randomized cancer/PIN patients and the rest of the 63 patients with normal biopsy at baseline (data not shown). Table 2 outlines the number of men in each arm with either the GG or AG/AA allele at 316 for each variable measured. There were no statistical differences between ODC allele type and treatment.
Table 1. Baseline characteristics of the 76 randomized patients by treatment arm.
| P | Placebo | DFMO | |||
|---|---|---|---|---|---|
| n | Median | n | Median | ||
| Age | 0.76* | 38 | 55.00 | 38 | 53.00 |
| Total PSA | 0.80† | 38 | 1.30 | 38 | 1.20 |
| Percent free PSA | 0.81‡ | 38 | 0.20 | 38 | 0.22 |
| Prostate volume | 0.78‡ | 37 | 30.60 | 38 | 32.60 |
| Transitional zone | 0.79† | 34 | 7.75 | 33 | 9.10 |
| Putrescine | 0.82† | 35 | 0.19 | 37 | 0.25 |
| Spermidine | 0.80† | 35 | 0.13 | 37 | 0.29 |
| Spermine | 0.84‡ | 35 | 16.95 | 37 | 18.49 |
| Spermidine/spermine ratio | 0.99† | 35 | 0.02 | 37 | 0.02 |
P values for independent sample t tests based on the actual value of the characteristic.
P values for Mann-Whitney-Wilcoxon test.
P values for independent sample t tests on the log-transformed value of the characteristic.
Table 2. The effect of placebo or DFMO on PSA, prostate volume, transitional zone, or polyamines stratified by ODC genotype.
| Placebo | DFMO | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | 12 mo | % Relative difference | P on % relative difference | n | Baseline | 12 mo | % Relative difference | |||
| Mean | Median | Mean | Median | ||||||||
| Total PSA | 33 | 1.82 | 1.85 | 1.55 | 0.00 | 0.9599 | 34 | 2.58 | 2.72 | −0.75 | 0.00 |
| Percent free PSA | 33 | 0.24 | 0.23 | 4.89 | 4.35 | 0.5149* | 34 | 0.24 | 0.26 | 10.21 | 0.00 |
| Prostate volume | |||||||||||
| All genotypes | 32 | 37.29 | 40.23 | 11.14 | 12.30 | 0.0301† | 33 | 32.54 | 32.67 | 0.94 | 0.69 |
| GG ODC genotype | 19 | 38.03 | 41.43 | 10.96 | 10.14 | 0.3295† | 15 | 32.36 | 33.50 | 3.63 | −2.07 |
| AA or AG genotype | 13 | 36.20 | 38.49 | 11.40 | 15.55 | 0.0293† | 18 | 32.69 | 31.98 | −1.30 | 0.96 |
| Transitional zone | |||||||||||
| All genotypes | 29 | 14.09 | 17.01 | 41.35 | 20.99 | 0.3542 | 27 | 10.51 | 11.30 | 18.94 | 5.13 |
| GG ODC genotype | 19 | 13.16 | 16.14 | 53.88 | 25 | 0.3775 | 13 | 10.82 | 11.87 | 18.4 | 11.54 |
| AA or AG genotype | 10 | 15.85 | 18.67 | 17.56 | 13.66 | 0.9119† | 14 | 10.22 | 10.77 | 19.45 | 1.89 |
| Putrescine | |||||||||||
| All genotypes | 27 | 0.80 | 0.99 | 221.9 | 139.47 | 0.0014 | 29 | 0.91 | 0.40 | 183.29 | −60.78 |
| GG ODC genotype | 14 | 1.13 | 0.78 | 110.98 | 62.98 | 0.1127 | 14 | 1.13 | 0.31 | 41.56 | −41.25 |
| AA or AG genotype | 13 | 0.45 | 1.23 | 341.34 | 181.3 | 0.0053 | 15 | 0.71 | 0.49 | 315.59 | −61.96 |
| Spermidine | |||||||||||
| All genotypes | 27 | 0.55 | 0.65 | 209.88 | 37.03 | 0.3983 | 29 | 0.48 | 0.42 | 127.93 | 0.00 |
| GG ODC genotype | 14 | 0.84 | 0.59 | 52.98 | −3.91 | 0.6295 | 14 | 0.61 | 0.46 | 94.82 | −18.98 |
| AA or AG genotype | 13 | 0.25 | 0.72 | 378.85 | 253.15 | 0.0971 | 15 | 0.36 | 0.39 | 158.84 | 37.09 |
| Spermine | |||||||||||
| All genotypes | 27 | 22.97 | 23.04 | 57.43 | 18.68 | 0.2443 | 29 | 21.23 | 17.49 | 12.05 | −16.58 |
| GG ODC genotype | 14 | 32.28 | 21.14 | −22.02 | −36.35 | 0.3080* | 14 | 24.18 | 16.32 | −4.75 | −22.32 |
| AA or AG genotype | 13 | 12.94 | 25.09 | 142.99 | 104.06 | 0.0606* | 15 | 18.47 | 18.59 | 27.73 | −16.05 |
NOTE: Difference refers to absolute difference of 12 mo to baseline and % relative difference refers to the ratio of the absolute difference divided by the baseline times 100. Statistical tests are based on nonparametric Mann-Whitney-Wilcoxon tests unless noted.
t tests on the log-transformed value.
t tests on the value.
Effect of DFMO on Prostate Polyamine Levels
DFMO decreased relative to baseline median putrescine levels by 60.8%, whereas the placebo group showed a relative 139.5% increase in putrescine levels (P = 0.0014; Table 2; Fig. 1A). When stratified by ODC genotype and comparing the subgroups with the corresponding subgroup in the treatment arms, the men with AA and AG alleles showed a reduction in putrescine levels with DFMO (P = 0.0053), whereas the men with the GG genotype did not show a treatment effect (P = 0.11; Table 2; Fig. 1B).
Figure 1.
A. Median relative percent difference of putrescine, spermidine, and spermine for placebo or DFMO. The relative percent difference refers to the ratio of the absolute difference divided by the baseline multiplied by 100%. Statistical tests are based on nonparametric Wilcoxon two-sample tests. DFMO lowered putrescine levels (P = 0.0014); however, the other polyamines did not show significant differences between treatment groups. B. Median relative percent difference of putrescine, spermidine, and spermine for placebo or DFMO stratified by ODC polymorphism, GG versus GA and AA. Percent relative difference is calculated as the ratio of the absolute difference divided by the baseline value multiplied by 100%. Statistical tests for differences between treatment groups include nonparametric Wilcoxon two-sample tests for putrescine and spermidine and independent sample t tests for the log transform of spermine. DFMO treatment lowered putrescine levels in the GA and AA group (P = 0.0053) but not in the GG group (P = 0.11). The other polyamine levels did not show statistical differences by treatment within polymorphism subgroup, although trends may be seen in the GA and AA group for spermine (P = 0.06) and spermidine (P = 0.09) levels for treatment with DFMO.
There was no statistically significant decrease in spermidine or spermine with 12 months of DFMO compared with placebo. However, median value of spermidine, relative to baseline, was unchanged in the DFMO arm versus an increase by 37.1% in the placebo group (P = 0.39). Median value, relative to baseline of spermine, decreased by 16.6% in the DFMO group versus an 18.7% increase in the placebo group (P = 0.24; Table 2; Fig. 1A). After being stratified by genotype, the men with the AA and AG genotype showed a trend toward relative reduction in spermidine and spermine levels with treatment (P = 0.09 and 0.06; Table 2; Fig. 1B).
Effect of DFMO on Prostate Volume
In the DFMO arm, the prostate volume was increased by a mean of 0.14 cm3 (0.94%) compared with a mean increase of 2.93 cm3 (11.14%) for the placebo arm (P = 0.030; Table 2; Fig. 2A). When evaluated by ODC genotype, DFMO reduced prostate volume in the AA + GA group (P = 0.029) but not in the GG group (P = 0.33; Table 2; Fig. 2B). When evaluating the effect on the transition zone volume, the DFMO group showed a 0.78 cm3 increase (18.9%) in transition zone volume and the placebo group showed a 2.93 cm3 (41.4%) increase in volume (P = 0.35; Table 2; Fig. 2A). Studying individual genotype groups did not show an increased efficacy in either ODC genotype (P = 0.91 for AA + GA and P = 0.38 for GG), although the sample size was smaller for this transition zone volume end point (Table 2; Fig. 2B).
Figure 2.
A. Absolute 12-mo difference in prostate and transition zone volume. Columns, mean difference of prostate volume (cm3) and transitional zone volume (cm3); bars, SE. Comparisons between treatment groups are based on an independent sample t test for prostate volume and a nonparametric Wilcoxon two-sample test for transition zone volume. Prostate volume showed a treatment effect (P = 0.03). There was no significant difference between treatment groups in mean transitional zone change (P = 0.35). B. Absolute 12-mo difference in prostate and transition zone volume stratified by ODC genotype. Columns, mean absolute difference of 12 mo to baseline of prostate volume (cm3) and transitional zone volume (cm3) stratified by ODC allele, GG versus GA and AA; bars, SE. For analysis of transition zone volume within the GG allele, a nonparametric Wilcoxon two-sample test was applied. Independent sample t tests were used for all other comparisons within allele subgroups. Prostate volume showed a treatment effect in the GA and AA grouping (P = 0.029) but not in the GG group (P = 0.33). Mean transitional zone changes were not significantly different.
Effect of DFMO on PSA Levels
The relative percent difference (before to after study) for PSA declined by a 0.75% change in the DFMO arm compared with an increase by 1.55% in the placebo arm. Free PSA and percent free PSA increased by 4.4% and 10.2% in the DFMO arm compared with an increase of 0.35% and 4.98% in the placebo arm. These changes did not achieve statistical significance (Table 2; free PSA data not shown). PSA velocity showed a different slope between the two arms. There was a PSA doubling time of 13.5 months for the placebo arm versus a decline in PSA doubling time for the DFMO arm (P = 0.13). Stratification by ODC genotype did not show differences between the treatment arms on PSA variables.
Effect of ODC Genotype on Prostate Polyamine Levels
There were no statistically significant differences of baseline putrescine (P = 0.32) values by ODC genotype, although there were statistically significant baseline differences in spermidine (P = 0.03) and spermine (P = 0.01) and total polyamine content (data not shown; P = 0.04). The likely reason for this differential response is that the spermine pool is much larger than either putrescine or spermidine in the prostate. Putrescine is likely only transiently occupied as the amines are converted to spermine.
Side Effects
There were no grade 3 or 4 toxicities in either group as assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events version 3. There were four grade 2 toxicities recorded during the trial: one in the DFMO arm (a subclinical hearing change on audiogram; see below) and three in the placebo arm (rash, chest pain, and vomiting). Men reporting grade 1 toxicities were slightly higher in the DFMO group, 20 men versus 15 in the placebo group. A grouping of grade 1 toxicities of muscular skeletal complaints (disk disease, tendonitis, etc.) showed more complaints in the DFMO group (seven participants versus two in the placebo arm), although all were recorded as unlikely related to drug. One man receiving DFMO withdrew from the study due to side effects, primarily upper gastrointestinal complaints consistent with reflux but also erectile decline. There was no difference between the arms in number of men complaining of sexual difficulties (three men versus two men) and gastrointestinal side effects (four versus four) in the DFMO versus placebo arm, respectively. The number of men with no reported side effects was similar for each group, 18 men on the DFMO and 20 men on the placebo arm.
There were six hearing-related complaints in the placebo arm (two tinnitus, two vertigo, and two hearing changes) and five hearing-related complaints in the DFMO arm (one tinnitus, three vertigo, and one hearing changes). Audiograms showed a subclinical hearing change in one participant in the DFMO arm, rated a grade 2 toxicity, secondary to the shifts of 15 dB at 2,000 and 3,000 Hz in both ears; repeat audiogram at 19 months, 7 months off study, showed the change to be stable.
Discussion
In this 1-year randomized double-blind placebo-controlled trial of the effects of DFMO on prostate polyamines, size, and PSA metrics, we were able to meet our primary objective in demonstrating a decrease in prostate putrescine level, although we were unable to show significant reduction in prostate spermidine and spermine levels as we had previously shown in a shorter study. In addition, we showed that DFMO was able to significantly modulate the rate of growth as measured by total prostate volume. In addition, total prostate volume and putrescine level changes were influenced by the ODC genotype of the participants.
Previously, we have shown in a 1-month trial that all three polyamines were suppressed. Putrescine decreased from baseline by 98% (P < 0.03), spermidine by 74% (P < 0.004), and spermine by 51% (P < 0.004; ref. 24). Compared with previous reports with DFMO with other types of tissues, the reduction in spermine levels in the prostate was marked in this short trial. In the current longer trial, we were able to show continued suppression of prostate putrescine levels. That other polyamine levels were less affected by DFMO in this trial compared with our previous report could represent the lower dose of DFMO used in this trial (500 mg/d versus 500 mg/m2/d used in the previous trial) or other factors, such as compensation, by cells over time by altering polyamine uptake or metabolism.
Stratification by ODC genotype showed that men with the AA or AG genotype showed reduction in putrescine level and trends in reduction for spermidine and spermine. In contrast, no reduction in these variables in the men with the GG genotype was seen. ODC genotyping was not done on the previous study, so genetic comparisons between the two trials cannot be done.
The decrease in prostatic growth over 1 year as measured by total prostate volume shown in the men receiving DFMO, an antiproliferative agent, is intriguing (P = 0.0301). Prostatic enlargement with age is well documented. In the Olmsted County database, an overall annual growth rate of 1.6% was seen (32), and Berry et al. (33) reported that maximal growth was seen in the men ages 31 to 50 years with a doubling time of 4.5 years. In our trial, the prostate volume in participants in the placebo arm increased 11.4% compared with the DFMO arm with an increase in size of 0.94%. The stratification of the participants into ODC genotype showed that DFMO had an effect on total prostate volume in participants with the GA and AA genotype but not the GG genotype when evaluating total prostate volume. Similar trends were seen in the transition zone, but as not all patients had both before and after transition zone measurements (nine patients), the sample size was smaller.
The relative difference in PSA over the 12 months for the placebo group was an increase of 1.55% compared with −0.75% for the DFMO arm. Free PSA and percent free PSA increased, respectively, 0.35% and 4.89% in the placebo arm versus a 4.39% and 10.21% increase in the DFMO arm (Table 2). More men had stable or declining PSA while receiving DFMO (73% versus 51%; data not shown), and PSA doubling time was altered by DFMO when compared with control (data not shown). Current PSA usage stratifies increased prostate cancer risk to higher total PSA levels, lower percent free PSA levels, and increased velocity (2). The PSA changes observed in our study were not statistically significant possibly because the sample size was too small, the baseline PSA levels were low, or DFMO does not affect PSA production. It is interesting to consider if DFMO caused a decrease in total PSA with a positive increase in free PSA and a decline in PSA doubling time; all trends if further substantiated would be associated with a decline in cancer risk as currently assessed and possibly prostate cancer (34-36).
There was no clinical ototoxicity shown during this 1-year trial of low-dose DFMO. One subclinical grade 2 hearing decline, secondary to the shifts of 15 dB at 2,000 and 3,000 Hz in both ears, was recorded in one patient. Ototoxicity has been linked to DFMO, particularly at higher doses. Doses of 9 g/m have been reported to cause reversible ototoxicity, but doses <1 g/m2 have not been consistently linked to ototoxicity (8). Love et al. (37) reported a 12.5% (three participants) incidence of reversible ototoxicity while on a 1-year trial of 0.5 g/m2/d, but Doyle et al. (38) have not shown ototoxicity at similar doses. In addition, we have followed more than 300 patients on a colon cancer prevention trial for up to 3 years on the same dose of DFMO used on the current trial and there was no difference in self-reported clinical hearing loss between patients on the treatment and placebo arms, although minimal subclinical changes in a subset of patients were evident.7
Several trials have been conducted with DFMO for cancer treatment (39-41) and prevention (25, 42-44). Many trials have lasted less than a year and focused on dosage, toxicity, and measurement of efficacy on surrogate end biomarkers (24, 25, 42, 44, 45). Results have been mixed. Promising results with actinic keratosis, with total number reduction of actinic keratosis (P = 0.001) and decreased spermine levels (P = 0.04) and decreased p53-positive cells (P = 0.04; refs. 43, 46), have been overshadowed by an absence of effect with measured biomarkers in breast and cervix trials (42, 44). A current trial with DFMO in patients with prior colon polyps but no history of colon cancer has been extended from a short-term phase II trial after showing efficacy on surrogate end biomarkers to a 3-year phase III trial in combination with sulindac in which the clinical end point of tumor/polyp reduction is being measured. Recent analysis has shown a marked effect of the DFMO/sulindac in reducing adenomas and polyamines with minimal toxicity.7 The details of the trial design are reported elsewhere (9, 47).
The Aspirin/Folate Polyp Prevention Study and the secondary analysis of aspirin use in a high-fiber cereal supplement polyp prevention trial illustrate the principle that subpopulations may be more amenable to intervention, as polyp reduction with aspirin was significantly improved in participants with an A allele at the ODC 315/316 position (21, 22). In the colon, one working hypothesis as to the effect this allelic polymorphism could have on colon cancer development is that the A allele binds more readily to gene regulators than the G allele. As adenomatous polyposis coli is lost, c-myc levels become elevated and this in turn more readily activates ODC with an A allele at position 315/316. The G alleles are less responsive to this c-myc activation (48). The mechanism for polymorphic variation in response to DFMO as reflected in prostate putrescine levels or prostate volume has yet to be elucidated.
The genetic causes of prostate cancer have been hard to determine and may reflect that prostate cancer is influenced by polymorphisms in multiple pathways (androgen receptor, DNA repair genes, steroid biosynthesis, etc.), in addition to environmental factors. Visvanathan et al. (23) studied the prostate cancer risk using two polymorphisms, CAG repeats in the androgen receptor and ODC 316, in addition to smoking status. The hypothesis is that ODC under control of the androgen receptor and stimulated by nicotine may be linked to prostate cancer. They found that an A allele and shorter CAG repeats in smokers were linked to an increased relative risk of prostate cancer. They subsequently reported racial variations in ODC 316 frequencies with the A allele most prevalent in Africans (0.415) and least prevalent in Hispanics (0.183; ref. 49).
The prostate may be a better target organ for the use of DFMO as a preventive agent (50). The function of the prostate is to provide fluid rich in polyamines to the ejaculate (13). ODC is under androgen regulation (51). The previously outlined demonstration of high ODC activity in the prostate and sensitivity to DFMO in laboratory studies supports the use of polyamine reduction in the prostate as a potential biomarker for future chemopreventive trials and a reasonable chemopreventive strategy. Our current trial shows the effectiveness of DFMO at suppressing human polyamine content and altering the volume of the prostate. This further raises the enthusiasm for DFMO as a potential chemopreventive agent targeting the prostate and provides a substantial basis for larger and definitive trials.
Acknowledgments
Grant support: U19-CA81866 and P-30 CA 62203 (F.L. Meyskens, Jr.).
Footnotes
F.L. Meyskens, Jr., unpublished data.
References
- 1.Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975-2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 2.De Marzo AM, DeWeese TL, Platz EA, et al. Pathological and molecular mechanisms of prostate carcinogenesis: implications for diagnosis, detection, prevention, and treatment. J Cell Biochem. 2004;91:459–77. doi: 10.1002/jcb.10747. [DOI] [PubMed] [Google Scholar]
- 3.Lunenfeld B. The ageing male: demographics and challenges. World J Urol. 2002;20:11–6. doi: 10.1007/s00345-002-0250-y. [DOI] [PubMed] [Google Scholar]
- 4.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 5.Andriole G, Bostwick D, Brawley O, et al. Chemoprevention of prostate cancer in men at high risk: rationale and design of the reduction by dutasteride of prostate cancer events (REDUCE) trial. J Urol. 2004;172:1314–7. doi: 10.1097/01.ju.0000139320.78673.2a. [DOI] [PubMed] [Google Scholar]
- 6.Klein EA, Thompson IM, Lippman SM, et al. SELECT: the next prostate cancer prevention trial. Selenium and Vitamin E Cancer Prevention Trial. J Urol. 2001;166:1311–5. doi: 10.1016/s0022-5347(05)65759-x. [DOI] [PubMed] [Google Scholar]
- 7.Luk GD, Casero RA., Jr Polyamines in normal and cancer cells. Adv Enzyme Regul. 1987;26:91–105. doi: 10.1016/0065-2571(87)90007-0. [DOI] [PubMed] [Google Scholar]
- 8.Verma AK. Inhibition of tumor promotion by DL-α-difluoromethylornithine, a specific irreversible inhibitor of ornithine decarboxylase. Basic Life Sci. 1990;52:195–204. doi: 10.1007/978-1-4615-9561-8_16. [DOI] [PubMed] [Google Scholar]
- 9.Gerner EW, Meyskens FL., Jr Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4:781–92. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 10.Auvinen M. Cell transformation, invasion, and angiogenesis: a regulatory role for ornithine decarboxylase and polyamines? J Natl Cancer Inst. 1997;89:533–7. doi: 10.1093/jnci/89.8.533. [DOI] [PubMed] [Google Scholar]
- 11.Gugliucci A. Polyamines as clinical laboratory tools. Clin Chim Acta. 2004;344:23–35. doi: 10.1016/j.cccn.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Dunzendorfer U, Russell DH. Altered polyamine profiles in prostatic hyperplasia and in kidney tumors. Cancer Res. 1978;38:2321–4. [PubMed] [Google Scholar]
- 13.Calandra RS, Rulli SB, Frungieri MB, et al. Polyamines in the male reproductive system. Acta Physiol Pharmacol Ther Latinoam. 1996;46:209–22. [PubMed] [Google Scholar]
- 14.Heston WD, Kadmon D, Lazan DW, Fair WR. Copenhagen rat prostatic tumor ornithine decarboxylase activity (ODC) and the effect of the ODC inhibitor α-difluoromethylornithine. Prostate. 1982;3:383–9. doi: 10.1002/pros.2990030408. [DOI] [PubMed] [Google Scholar]
- 15.Danzin C, Jung MJ, Claverie N, et al. Effects of α-difluoromethylornithine, an enzyme-activated irreversible inhibitor or ornithine decarboxylase, on testosterone-induced regeneration of prostate and seminal vesicle in castrated rats. Biochem J. 1979;180:507–13. doi: 10.1042/bj1800507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moulinoux JP, Quemener V, Cipolla B, et al. The growth of MAT-LyLu rat prostatic adenocarcinoma can be prevented in vivo by polyamine deprivation. J Urol. 1991;146:1408–12. doi: 10.1016/s0022-5347(17)38125-9. [DOI] [PubMed] [Google Scholar]
- 17.Danzin C, Jung MJ, Grove J, Bey P. Effect of α-difluoromethylornithine, an enzyme-activated irreversible inhibitor of ornithine decarboxylase, on polyamine levels in rat tissues. Life Sci. 1979;24:519–24. doi: 10.1016/0024-3205(79)90173-5. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Ahmad N, Marengo SR, et al. Chemoprevention of prostate carcinogenesis by α-difluoromethylornithine in TRAMP mice. Cancer Res. 2000;60:5125–33. [PubMed] [Google Scholar]
- 19.Kee K, Foster BA, Merali S, et al. Activated polyamine catabolism depletes acetyl-CoA pools and suppresses prostate tumor growth in TRAMP mice. J Biol Chem. 2004;279:40076–83. doi: 10.1074/jbc.M406002200. [DOI] [PubMed] [Google Scholar]
- 20.Esmat AY, Refaie FM, Shaheen MH, Said MM. Chemoprevention of prostate carcinogenesis by DFMO and/or finasteride treatment in male Wistar rats. Tumori. 2002;88:513–21. doi: 10.1177/030089160208800616. [DOI] [PubMed] [Google Scholar]
- 21.Barry EL, Baron JA, Bhat S, et al. Ornithine decarboxylase polymorphism modification of response to aspirin treatment for colorectal adenoma prevention. J Natl Cancer Inst. 2006;98:1494–500. doi: 10.1093/jnci/djj398. [DOI] [PubMed] [Google Scholar]
- 22.Martinez ME, O'Brien TG, Fultz KE, et al. Pronounced reduction in adenoma recurrence associated with aspirin use and a polymorphism in the ornithine decarboxylase gene. Proc Natl Acad Sci U S A. 2003;100:7859–64. doi: 10.1073/pnas.1332465100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visvanathan K, Helzlsouer KJ, Boorman DW, et al. Association among an ornithine decarboxylase polymorphism, androgen receptor gene (CAG) repeat length and prostate cancer risk. J Urol. 2004;171:652–5. doi: 10.1097/01.ju.0000108384.74718.73. [DOI] [PubMed] [Google Scholar]
- 24.Simoneau AR, Gerner EW, Phung M, et al. α-Difluoromethylornithine and polyamine levels in the human prostate: results of a phase IIa trial. J Natl Cancer Inst. 2001;93:57–9. doi: 10.1093/jnci/93.1.57. [DOI] [PubMed] [Google Scholar]
- 25.Meyskens FL, Jr, Gerner EW, Emerson S, et al. Effect of α-difluoromethylornithine on rectal mucosal levels of polyamines in a randomized, double-blinded trial for colon cancer prevention. J Natl Cancer Inst. 1998;90:1212–8. doi: 10.1093/jnci/90.16.1212. [DOI] [PubMed] [Google Scholar]
- 26.Meyskens FL, Jr, Emerson SS, Pelot D, et al. Dose de-escalation chemoprevention trial of α-difluoromethylornithine in patients with colon polyps. J Natl Cancer Inst. 1994;86:1122–30. doi: 10.1093/jnci/86.15.1122. [DOI] [PubMed] [Google Scholar]
- 27.Gerner EW, Garewal HS, Emerson SS, Sampliner RE. Gastrointestinal tissue polyamine contents of patients with Barrett's esophagus treated with α-difluoromethylornithine. Cancer Epidemiol Bio-markers Prev. 1994;3:325–30. [PubMed] [Google Scholar]
- 28.Hixson LJ, Garewal HS, McGee DL, et al. Ornithine decarboxylase and polyamines in colorectal neoplasia and mucosa. Cancer Epidemiol Biomarkers Prev. 1993;2:369–74. [PubMed] [Google Scholar]
- 29.Boyle JO, Meyskens FL, Jr, Garewal HS, Gerner EW. Polyamine contents in rectal and buccal mucosae in humans treated with oral difluoromethylornithine. Cancer Epidemiol Biomarkers Prev. 1992;1:131–5. [PubMed] [Google Scholar]
- 30.Seiler N, Knodgen B. High-performance liquid chromatographic procedure for the simultaneous determination of the natural polyamines and their monoacetyl derivatives. J Chromatogr. 1980;221:227–35. doi: 10.1016/s0378-4347(00)84307-8. [DOI] [PubMed] [Google Scholar]
- 31.Hahn GJ, Meeker WQ. Statistical intervals: a guide for practitioners. New York: John Wiley & Sons, Inc; 1991. [Google Scholar]
- 32.Oesterling JE, Jacobsen SJ, Chute CG, et al. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges. JAMA. 1993;270:860–4. [PubMed] [Google Scholar]
- 33.Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–9. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues NA, Chen MH, Catalona WJ, et al. Predictors of mortality after androgen-deprivation therapy in patients with rapidly rising prostate-specific antigen levels after local therapy for prostate cancer. Cancer. 2006;107:514–20. doi: 10.1002/cncr.22018. [DOI] [PubMed] [Google Scholar]
- 35.Beard C, Chen MH, Cote K, et al. Pretreatment predictors of posttreatment PSA doubling times for patients undergoing three-dimensional conformal radiotherapy for clinically localized prostate cancer. Urology. 2005;66:1020–3. doi: 10.1016/j.urology.2005.05.039. [DOI] [PubMed] [Google Scholar]
- 36.Zhou P, Chen MH, McLeod D, et al. Predictors of prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Clin Oncol. 2005;23:6992–8. doi: 10.1200/JCO.2005.01.2906. [DOI] [PubMed] [Google Scholar]
- 37.Love RR, Jacoby R, Newton MA, et al. A randomized, placebo-controlled trial of low-dose α-difluoromethylornithine in individuals at risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:989–92. [PubMed] [Google Scholar]
- 38.Doyle KJ, McLaren CE, Shanks JE, et al. Effects of difluoromethylornithine chemoprevention on audiometry thresholds and otoacoustic emissions. Arch Otolaryngol Head Neck Surg. 2001;127:553–8. doi: 10.1001/archotol.127.5.553. [DOI] [PubMed] [Google Scholar]
- 39.Levin VA, Hess KR, Choucair A, et al. Phase III randomized study of postradiotherapy chemotherapy with combination α-difluoromethylornithine-PCV versus PCV for anaplastic gliomas. Clin Cancer Res. 2003;9:981–90. [PubMed] [Google Scholar]
- 40.Levin VA, Uhm JH, Jaeckle KA, et al. Phase III randomized study of postradiotherapy chemotherapy with α-difluoromethylornithine-procarbazine, N-(2-chloroethyl)-N′-cyclohexyl-N-nitrosurea, vincristine (DFMO-PCV) versus PCV for glioblastoma multiforme. Clin Cancer Res. 2000;6:3878–84. [PubMed] [Google Scholar]
- 41.Prados MD, Wara WM, Sneed PK, et al. Phase III trial of accelerated hyperfractionation with or without difluoromethylornithine (DFMO) versus standard fractionated radiotherapy with or without DFMO for newly diagnosed patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001;49:71–7. doi: 10.1016/s0360-3016(00)01458-9. [DOI] [PubMed] [Google Scholar]
- 42.Fabian CJ, Kimler BF, Brady DA, et al. A phase II breast cancer chemoprevention trial of oral α-difluoromethylornithine: breast tissue, imaging, and serum and urine biomarkers. Clin Cancer Res. 2002;8:3105–17. [PubMed] [Google Scholar]
- 43.Alberts DS, Dorr RT, Einspahr JG, et al. Chemoprevention of human actinic keratoses by topical 2-(difluoromethyl)-dl-ornithine. Cancer Epidemiol Biomarkers Prev. 2000;9:1281–6. [PubMed] [Google Scholar]
- 44.Vlastos AT, West LA, Atkinson EN, et al. Results of a phase II double-blinded randomized clinical trial of difluoromethylornithine for cervical intraepithelial neoplasia grades 2 to 3. Clin Cancer Res. 2005;11:390–6. [PubMed] [Google Scholar]
- 45.Loprinzi CL, Messing EM, O'Fallon JR, et al. Toxicity evaluation of difluoromethylornithine: doses for chemoprevention trials. Cancer Epidemiol Biomarkers Prev. 1996;5:371–4. [PubMed] [Google Scholar]
- 46.Einspahr JG, Nelson MA, Saboda K, et al. Modulation of biologic endpoints by topical difluoromethylornithine (DFMO), in subjects at high-risk for nonmelanoma skin cancer. Clin Cancer Res. 2002;8:149–55. [PubMed] [Google Scholar]
- 47.Gerner EW, Meyskens FL, Jr, Goldschmid S, et al. Rationale for, and design of, a clinical trial targeting polyamine metabolism for colon cancer chemoprevention. Amino Acids. 2007;33:189–95. doi: 10.1007/s00726-007-0515-2. [DOI] [PubMed] [Google Scholar]
- 48.Gerner EW, Ignatenko NA, Lance P, Hurley LH. A comprehensive strategy to combat colon cancer targeting the adenomatous polyposis coli tumor suppressor gene. Ann N Y Acad Sci. 2005;1059:97–105. doi: 10.1196/annals.1339.033. [DOI] [PubMed] [Google Scholar]
- 49.O'Brien TG, Guo Y, Visvanathan K, et al. Differences in ornithine decarboxylase and androgen receptor allele frequencies among ethnic groups. Mol Carcinog. 2004;41:120–3. doi: 10.1002/mc.20047. [DOI] [PubMed] [Google Scholar]
- 50.Casero RA, Jr, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov. 2007;6:373–90. doi: 10.1038/nrd2243. [DOI] [PubMed] [Google Scholar]
- 51.Betts AM, Waite I, Neal DE, Robson CN. Androgen regulation of ornithine decarboxylase in human prostatic cells identified using differential display. FEBS Lett. 1997;405:328–32. doi: 10.1016/s0014-5793(97)00209-3. [DOI] [PubMed] [Google Scholar]