Abstract
Exposing experimental animals or human volunteers to UVA II (320–340 nm) radiation after immunization suppresses immunologic memory and the elicitation of delayed-in-time hypersensitivity reactions. Previous studies indicated that the mechanisms underlying UVA-induced immune suppression are similar to those described for UVB-induced immune suppression, i.e., transferred by T regulatory cells, overcome by repairing DNA damage, or neutralizing interleukin-10 activity, or by injecting recombinant interleukin-12. Here we continued our examination of the mechanisms involved in UVA II induced suppression. Antibodies to cis-urocanic acid blocked UVA-induced immune suppression. Treating UVA-irradiated mice with histamine receptor antagonists, calcitonin gene related peptide receptor antagonists or platelet activating receptor antagonists blocked immune suppression in UVA-irradiated mice. In light of the fact that cis-urocanic acid and calcitonin gene related peptide target mast cells, which can then release platelet activating factor and histamine, we measured UVA-induced immune suppression in mast cell deficient mice. No immune suppression was noted in UVA-irradiated mast cell deficient mice. These findings indicate that exposure to UVA II activates many of the same immune regulatory factors activated by UVB to induce immune suppression. Moreover, they indicate that mast cells play a critical role in UVA-induced suppression of secondary immune reactions.
INTRODUCTION
Exposure to ultraviolet (UV) radiation is responsible for a number of adverse health effects. The UV wavelengths in sunlight are the primary cause of non-melanoma skin cancer (1) and undoubtedly play a role in inducing melanoma (2). In addition to its carcinogenic properties, UV exposure is also immunosuppressive (3), and UV-induced immune suppression has been identified as a major risk factor for skin cancer induction (4). Moreover, acute UV exposure of both experimental animals and human volunteers suppresses cell-mediated immune reactions; such as contact and delayed type hypersensitivity (5–8).
A great majority of studies investigating photocarcinogenesis and photoimmunology employ sunlamps emitting primarily UVB (290 to 320 nm) radiation, even though approximately 95% of the solar UV radiation that reaches the biosphere is contained in the UVA (320 to 400 nm) region of the solar spectrum. Exposure to UVA radiation can be harmful. UVA exposure causes premature ageing of the skin (9) and very high rates of exposure have been associated with skin cancer induction in mice (10). The exact role of UVA in activating immune suppression has been an area full of controversy (11). Current information indicates that UVAII radiation (320 to 340 nm) suppresses secondary immune reactions (12) and immunologic memory (13). On the other hand, data generated by Reeve and colleagues (14) and latter confirmed by others (15), indicates that pre-exposure to UVAI radiation (340 to 400 nm) will block the immunosuppressive effects of subsequent exposure to UVB radiation. Thus, depending upon the sequence of events and wavelengths involved, UVA radiation can have disparate effects on the immune response.
Determining the role of UVA radiation in immune suppression has broad implications beyond its obvious interest to photobiologists. Until very recently most commercial sunscreens provided little to no protection against UVA radiation because of the perception that UVA was not as harmful as UVB. The realization that complete protection against immune suppression results only when broad spectrum sunscreens, protecting across both the UVB and the UVA region of the solar spectrum has changed the perception that UVA is not immunosuppressive (16–18). In addition, the observations that UVA exposure suppresses immunological memory suggest that excess sunlight exposure may have the potential to depress immune protection afforded by prior vaccination. These findings indicate that studies designed to determine the mechanism by which UVA radiation suppresses the immune response are important.
Previously, we reported that although the photobiological mechanisms that are involved in suppressing secondary immune reactions differ greatly from the photobiological mechanisms that suppress the induction of primary immune reactions (UVA II vs. UVB) the immunological mechanism are similar. We noted that pyrimidine dimers are found in the skin of UVA-irradiated mice, and repairing dimer formation blocks immune suppression. Antigen specific T regulatory cells were found in the spleens of UVA-irradiated mice. Cytokines are involved in transmitting the immune suppressive signal from the skin to the immune system. No suppression was noted in UVA-irradiated, anti-interleukin (IL)-10 injected mice. Similarly, treating UVA-irradiated mice with recombinant IL-12 blocked immune suppression (19).
Here we continued our examination of the mechanisms leading to UVA-induced immune suppression. Antibodies to cis-urocanic acid blocked UVA-induced immune suppression. Treating UVA-irradiated mice with histamine receptor antagonists, calcitonin gene related peptide receptor antagonists or platelet activating receptor antagonists blocked immune suppression in UVA-irradiated mice. Because cis-urocanic acid and calcitonin gene related peptide target mast cells, which can then release platelet activating factor and histamine, we measured UVA-induced immune suppression in mast cell deficient mice. No immune suppression was noted in UVA-irradiated mast cell deficient mice.
MATERIALS AND METHODS
Animals
Specific pathogen-free C3H/HeNNCr (MTV-) and C57BL/6NCr mice were obtained from the National Cancer Institute Frederick Cancer Research Facility Animal Production Area (Frederick, MD). Breeding pairs of mast cell deficient mice (Kitw-sh/Kitw-sh), backcrossed onto the C57BL/6 background were obtained from The Jackson Laboratories (Bar Harbor, ME). The animals were maintained in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International, in accordance with current regulations and standards of the National Institutes of Health. All animal procedures were reviewed and approved by MD Anderson Cancer Center Animal Care and Use Committee. Within each experiments all the mice were age and sex matched. The mice were generally 8–10 weeks old at the start of each experiment.
Antibodies and Reagents
Calcitonin gene related peptide antagonist (CGRP8–37), cyproheptadine, cimetidine and mouse IgG were purchased from Sigma-Aldrich (St Louis, MO). PCA 4248 and trans-2,5-bis (3,4,5-trimethoxyphenyl)-1,3-dioxolone (hereafter referred to as dioxolone) were purchased from Biomol (Plymouth Meeting, PA). Dr Mary Norval, University of Edinburgh, provided us with the monoclonal anti-cis-UCA antibody (20).
Radiation Source
A 1000W xenon UV solar simulator, equipped with a Schott WG-335 atmospheric attenuation filter (3 mm thick), a visible/infrared band pass blocking filter (Schott UG-11; 1 mm thick) and a dichroic mirror (Oriel, Stratford, CT) was used to provide UVA radiation, devoid of UVB. The intensity and spectral output of this light source were measured with an Optronics model OL 754 scanning spectrophotometer interfaced to a laptop computer (Optronics Laboratories, Orlando, FL), and has been published previously (12). During irradiation of the shaved dorsal skin, the mice were held individually in a specially constructed Plexiglas container with a quartz glass top. Spectrophotometric readings were taken through the quartz glass top. During the irradiation period (15 to 20 min in duration), the mice were conscious and had full range of movement.
Suppression of the elicitation of DTH by solar simulated UV radiation
The design of the experiment is outlined in Figure 1. On day 0, mice were immunized by the subcutaneous injection of 107 formalin-fixed Candida albicans into each flank. Nine days later the mice were exposed to 80 kJ/m2 of UVA radiation. The next day the mice were sedated, the thickness of each hind footpad was measured with an engineer’s micrometer (Mitutoya, Tokyo, Japan), and the mice were challenged by injecting, 50 μl of Candida antigen (Alerchek Inc, Portland, ME) into each hind footpad. Eighteen to 24 h later, the thickness of each footpad was re-measured and the mean footpad swelling for each mouse was calculated (Δ left footpad thickness + Δ right footpad thickness ÷ 2). Generally there were 5 mice per group; the mean footpad swelling ± the standard error of the mean was calculated for each group. The background footpad swelling (negative control in each experiment) was determined in a group of mice that were not immunized but were challenged. The positive control in each experiment was determined by measuring the immune response in mice that were immunized and challenged, but were not exposed to UVA radiation. Subtracting the background response from the response found in each experimental group yielded the specific footpad swelling response. Percent immune suppression was determined by the following formula: % immune suppression = (1−[specific footpad swelling of the UV-irradiated mice ÷ specific footpad swelling of the positive control] × 100. Statistical differences between each group was determined by use of a one way analysis of variance followed by the Dunn’s multiple comparison test (Prism, GraphPad Software, San Diego CA). Probabilities less than 0.05 were considered significant. Each experiment was repeated independently 2 to 3 times.
Figure 1.
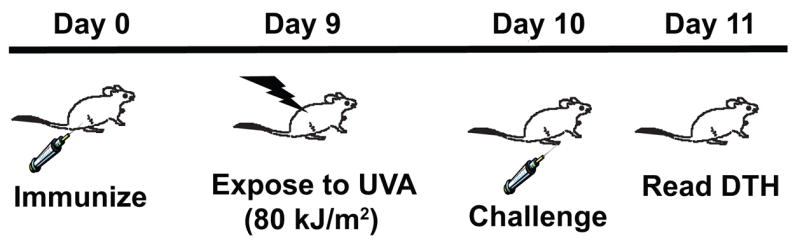
Suppressing the elicitation of DTH with UVA radiation. Mice were immunized on day 0 and then exposed to an immunosuppressive dose of UVA radiation 9 days later. On day 10 they were challenged with antigen, and DTH was measured 18 to 24 h later.
RESULTS
Is cis-UCA involved in UVA induced immune suppression?
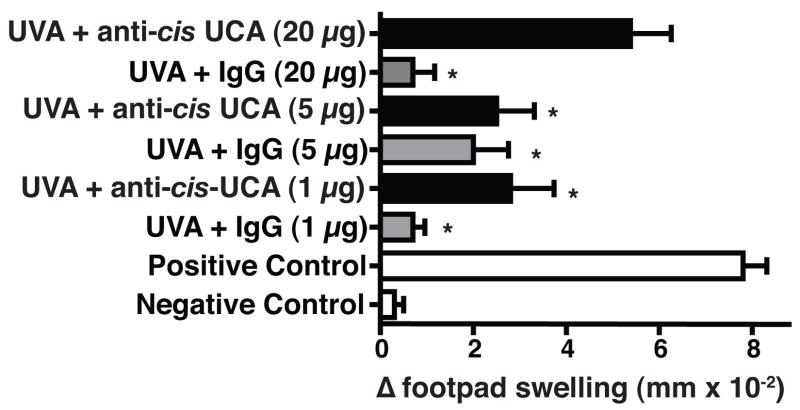
Because others have shown that trans-UCA can be converted to the cis-isomer by wavelengths in the UVA region of the solar spectrum (21), we first wanted to determine if cis-UCA contributes to UVA-induced immune suppression. UVA-irradiated mice were injected with a monoclonal antibody to cis-UCA or control IgG, and the effect this treatment had on DTH was measured (Figure 2). The DTH response in mice that were exposed to 80 kJ/m2 of UVA and injected with 1, 5, or 20 μg of control IgG was significantly suppressed (p < 0.05 vs. the positive control). Treating the UVA-irradiated mice with 1 or 5 μg of anti-cis-UCA antibody did not reverse immune suppression. When the mice were injected with 20 μg of anti-cis-UCA monoclonal antibody the immune suppression was reversed, in that the DTH reaction generated in these mice was not significantly different (p > 0.05) from the positive control. These data indicate that neutralizing cis-UCA reverses UVA-induced immune suppression.
Figure 2.
Monoclonal anti-cis-UCA antibody blocks UVA-induced immune suppression. One h prior to UVA exposure, the mice received an intraperitoneal injection of anti-cis-UCA (black bars) or control IgG (grey bars). The data are expressed as mean Δ footpad swelling ± the standard error of the mean. * indicates a statistically significant difference (p < 0.05) from the positive control.
Is Calcitonin Gene Related Peptide involved in UVA induced immune suppression?
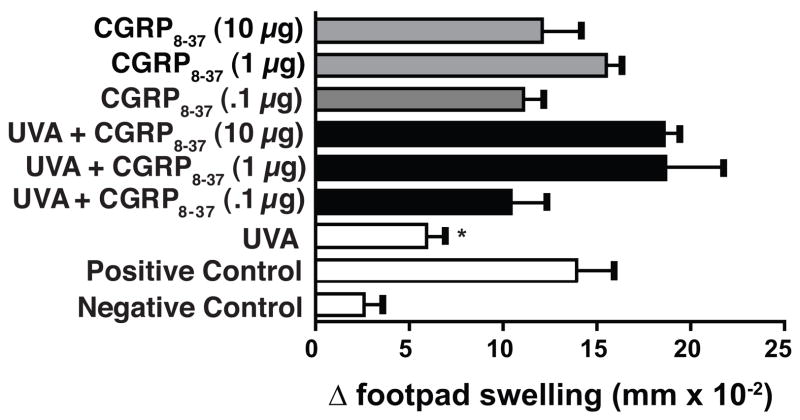
Others have demonstrated that calcitonin gene related peptide, which is released by cutaneous nerves can play a role in UV-induced immune suppression, in part by the induction of IL-10 (22). Because our previous data implicated IL-10 in UVA-induced immune suppression (19), we asked if calcitonin gene related peptide plays a role. The mice were immunized with C albicans and treated with UVA 9 days post irradiation as described above. Some animals received the calcitonin gene related peptide antagonist (GCRP8–37) one h prior to UVA treatment. Others were injected with GCRP 8–37, but were not treated with UVA. The data from this experiment (Figure 3) indicates that injecting GCRP8–37 by itself did not affect the DTH reaction, as the mice that received GCRP 8–37 without UVA generated a DTH reaction that was indistinguishable from the positive control. As expected, exposing the mice to 80 kJ/m2 of UVA radiation caused a significant decrease in the DTH reaction (67% immune suppression; p < 0.05 vs. the positive control). Injecting GCRP 8–37, into UVA-irradiated mice, at all doses tested, reversed the immune suppression. The DTH response generated in UVA-irradiated, CGRP 8–37-injected mice was not significantly different from the positive control. These data indicate that blocking CGRP activity blocks UVA-induced immune suppression.
Figure 3.
Injecting calcitonin gene related peptide antagonists into UVA-irradiated mice blocks immune suppression. One h prior to UVA exposure, the mice received an intraperitoneal injection of CGRP 8–37 (black bars). Control groups were injected with CGRP 8–37 but not exposed to UVA (grey bars). The data are expressed as mean Δ footpad swelling ± the standard error of the mean. * indicates a statistically significant difference (p < 0.05) from the positive control.
Reversal of UVA-induced immune suppression by histamine receptor antagonists
The role of histamine in UV-induced immune suppression is well recognized (23). Therefore, we decided to determine if histamine plays a role in UVA-induced immune suppression by using two well-known histamine receptor antagonists, cyproheptadine (H1 receptor antagonist) and cimetidine (H2 receptor antagonist) (Figure 4). The mice were immunized with C albicans and treated with UVA 9 days post irradiation as described above. Some animals received the 100 μg of cimetidine or 300 μg of cyproheptadine one h prior to UVA treatment. Others were injected with cimetidine or cyproheptadine, but were not treated with UVA. The doses of cimetidine and cyproheptadine used here were chosen from the literature (23). Similar to what was reported earlier when contact hypersensitivity was used as the immunological endpoint (23), injecting cimetidine or cyproheptadine into non-UV-irradiated mice did not effect the DTH reaction (p > 0.05 vs. the positive control). UVA-treatment significantly suppressed the DTH reaction (72% immune suppression, p < 0.01 vs. the positive control). Treating the mice with cimetidine or cyproheptadine prior to irradiation totally reversed UVA-induced immune suppression, as there was no significant difference between the DTH reaction generated in these mice and the positive control. These data indicate that blocking histamine from binding to either the H1 or the H2 receptor prevented UVA-induced immune suppression.
Figure 4.
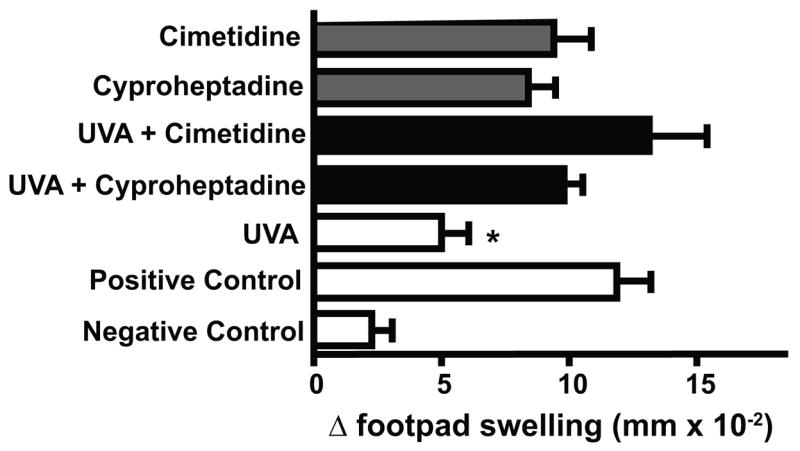
Treating UVA-irradiated mice with histamine receptor antagonists blocks UVA-induced immune suppression. One h prior to UVA exposure, the mice received an intraperitoneal injection of 100 μg of cimetidine or 300 μg of cyproheptadine (black bars). Control groups were injected with cimetidine or cyproheptadine but not exposed to UVA (grey bars). The data are expressed as mean Δ footpad swelling ± the standard error of the mean. * indicates a statistically significant difference (p < 0.01) from the positive control.
Does blocking platelet activating factor (PAF) receptor binding block UVA-induced immune suppression?
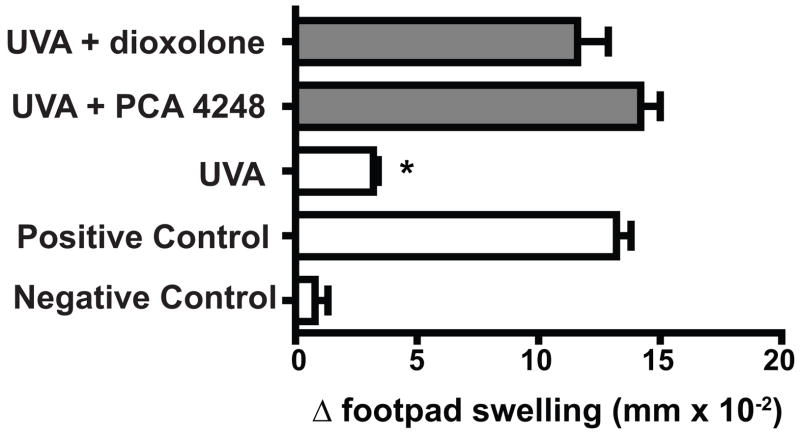
Previously we demonstrated that blocking the binding of PAF to its receptor blocked UVB-induced immune suppression (24, 25). Also, interfering with PAF receptor binding blocks the immune suppression when another dermal immunotoxin, jet fuel, was used (26). Based on these findings, we decided to test the hypothesis that blocking PAF receptor binding will block UVA-induced immune suppression. Nine days after immunization and one h before UVA exposure, the mice were injected with PAF receptor antagonists. The mice received either 500 nmol of PCA-4248, or 500 nmol of dioxolone. The doses of two PAF receptor antagonists used were chosen from the literature (24, 26). The effect that this treatment had on UVA-induced immune suppression is shown in Figure 5. Exposure to UVA radiation, one day prior to antigenic challenge induced significant immune suppression (80% immune suppression, p < 0.01 vs. the positive control). When the mice were injected with either of the PAF receptor antagonists prior to UVA-irradiation, no immune suppression was noted, as the DTH reaction in these mice was not significantly different from that found in the positive control (P > 0.05). Because we previously noted that injecting the PAF receptor antagonists into non-irradiated mice 9 days after immunization had no effect on the elicitation of DTH (26), this control was not run here in order to reduce the numbers of mice needed for this study.
Figure 5.
PAF receptor antagonists block UVA-induced immune suppression. One h prior to UVA exposure, the mice received an intraperitoneal injection of 500 pmol of PAC 4248 or dioxolone. The data are expressed as mean Δ footpad swelling ± the standard error of the mean. * indicates a statistically significant difference (p < 0.01) from the positive control.
A critical role for mast cells in UVA-induced immune suppression
It is interesting to note that many of the factors that are involved in UVA-induced immune suppression either target, or are produced by mast cells. Mast cells respond to cis-UCA (27) and CGRP (28). Mast cell express histamine and PAF receptors on their surface, and they can secrete cytokines such as IL-10 in response to activation (29). Therefore, we decided to examine the role of mast cells in UVA-induced immune suppression. We essentially repeated the experiment described in Figure 1 with one modification. One arm of the experiment employed wild-type C57Bl/6 mice, and the other arm of the experiment employed mast cell deficient mice (KitW-sh/W-sh), which have been backcrossed onto the C57Bl/6 background. The results from this experiment are found in Figure 6. As demonstrated above, exposing C albicans immunized C57Bl/6 mice to 80 kJ/m2 of UVA radiation suppressed the elicitation of DTH (86% immune suppression; p < 0.01 vs. the positive control). Treating mast cell-deficient mice with an equal dose of UVA radiation did not suppress the elicitation of DTH (p > 0.05, UVA-irradiated mast cell−/− mice vs. non-irradiated mast cell−/− positive control). These data indicate that UVA-induced immune suppression is mast cell dependent.
Figure 6.
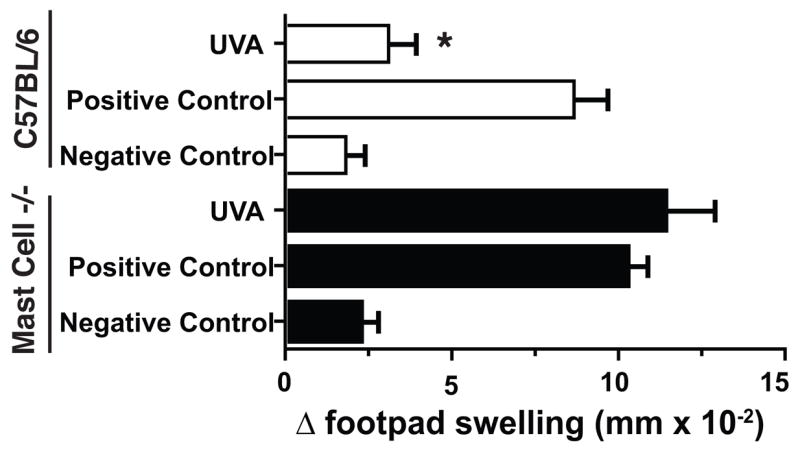
Exposing mast cell-deficient mice to UVA fails to suppress the elicitation of DTH. C57Bl/6 (black bars) and mast cell-deficient mice (open bars) were immunized with antigen on day 0 and then exposed to 80 kJ/m2 of UVA on day 9. The mice were challenged on day 10 and DTH was measured on day 11. The data are expressed as mean Δ footpad swelling ± the standard error of the mean. * indicates a statistically significant difference (p < 0.01) from the positive control.
DISCUSSION
Besides being of interest to photobiologists, we believe that studying the mechanism underlying UVA-induced suppression of established immune reactions has implications beyond photobiology. The most successful public health campaign of the 20th century was the widespread use of vaccination to reduce the morbidity and mortality due to infectious disease. Our previous data (12, 19), and those published by Halliday and colleagues (16, 17) and Moyal and co-workers (13, 18) clearly indicate that UVA radiation can suppress immunological memory, suggesting that sunlight exposure has the potential to suppress immunologic protection afforded by previous vaccination. With these findings in mind, and in light of the fact UVA radiation comprises greater than 95% of the UV radiation found in ambient sunlight, experiments designed to determine the mechanisms by which UVA radiation suppresses established immune reactions have merit. The data reported here agree with our initial report on the mechanisms involved. Although the basic photobiological mechanisms differ (12), the immunological mechanisms are remarkably similar. We report here that cis-UCA, histamine receptor binding, PAF receptor binding and CGRP receptor binding are all required for UVA-induced immune suppression. We were surprised by the close agreement between our results and those reported previously documenting immune suppression by UVB (30). The only small difference we noted was the requirement for more anti-cis-UCA monoclonal antibody to block UVA induced immune suppression. Previously we noted that 5 μg of anti-cis-UCA monoclonal antibody was sufficient to block UVB-induced immune suppression (31), here we needed to use at least 20 μg to overcome UVA-induced immune suppression. The reason why more anti-cis-UCA antibody was required to reverse UVA II-induced immune suppression remains unknown, but perhaps it reflects the fact that in these studies we measured the effect of UV radiation on the elicitation of an immune response and in previous experiments the effect of UVB on the induction of immunity were examined. The doses of CGRP 8–37, cimetidine, cyproheptadine and the PAF receptor antagonists used here to abrogate UVA-induced immune suppression were identical to those that successfully used in the past to block UVB-induced immune suppression. It is of interest to note that one of the strongest inducers of PAF is reactive oxygen species (32). Because UVA is a well-recognized generator of reactive oxygen species, it is entirely possible that UVA-induced reactive oxygen is activating PAF production in this system.
In a previous study we reported that we could suppress secondary immune reactions with UVB/UVA radiation (290 to 400 nm), and with UVA II (320 to 340 nm), but not UVA I (340 to 400 nm) (12). The fact that the immunological mechanisms involved in suppressing established immune reactions by UVA (19) and those involved in suppressing the induction of an immune response with UVB are the same (30), suggests that from an immunological point of view the skin immune system does not distinguish between UVB and UVA II. Alternatively, the results generated here and in our previous reports (12, 19) may simply reflect that fact that UVAII exposure represents the “tail-end “ of the UVB spectrum. Action spectra showing isomerization of cis-UCA by UVAII (21, 33) and immune suppression by narrow band 320 nm light (34) would support this concept.
Our data document a critical role for mast cells in UVA-induced immune suppression. These findings add to the growing appreciation of a role for mast cells in regulating adaptive immune reactions. Conventional wisdom suggests that mast cells primarily serve as effector cells in IgE-mediated allergic inflammation, in part through the release of preformed mediators stored in the cell’s cytoplasmic granules. However, immunologists now realize that mast cells regulate the adaptive immune response, in part though regulated release of cytokines and soluble factors (35). Important contributions for appreciating the role of mast cells in regulating adaptive immune reactions came from studies in photobiology. For example, Hart and colleagues failed to induce immune suppression in UV-irradiated mast cell deficient mice. Moreover, when mast cell deficient mice were reconstituted by injecting wild-type bone marrow-derived mast cells into the dermis (so called “mast cell knock-in mice”), immune suppression was restored (36). Our findings expand on these observations by showing that mast cells are also involved in the UVA-induced suppression of the elicitation of DTH. Whether this observation contributes to skin cancer induction is not known. It is important to note that increased mast cell prevalence has been associated with susceptibility to melanoma and basal cell carcinoma in humans (37, 38). Because of the well-known role of mast cells in mediating UVB-induced immune suppression, and in light of the fact that UVB-induced immune suppression is a risk factor for skin cancer development, it has generally been accepted that mast cells and the immunosuppressive products released by them are contributing to cancer development via immune suppression. Our data suggest that UVA may play also play role in this process through its effects on mast cells.
Acknowledgments
This work was supported by grants from the National Cancer Institute (CA75575 and CA112660). The animal facilities at the UT MD Anderson Cancer Center are supported in part by a Cancer Center Support Grant from the National Cancer Institute (CA16672). The authors thank Dr. Mary Norval, University of Edinburgh for the gift of the anti-cis-UCA antibody.
References
- 1.Urbach F. Ultraviolet radiation and skin cancer of humans. J Photochem Photobiol B: Biol. 1997;40:3–7. doi: 10.1016/s1011-1344(97)00029-8. [DOI] [PubMed] [Google Scholar]
- 2.Berking C, Takemoto R, Satyamoorthy K, Shirakawa T, Eskandarpour M, Hansson J, VanBelle PA, Elder DE, Herlyn M. Induction of melanoma phenotypes in human skin by growth factors and ultraviolet B. Cancer Res. 2004;64:807–811. doi: 10.1158/0008-5472.can-03-3438. [DOI] [PubMed] [Google Scholar]
- 3.Kripke ML. Antigenicity of murine skin tumors induced by ultraviolet light. J Natl Cancer Inst. 1974;53:1333–1336. doi: 10.1093/jnci/53.5.1333. [DOI] [PubMed] [Google Scholar]
- 4.Yoshikawa T, Rae V, Bruins-Slot W, Van den Berg JW, Taylor JR, Streilein JW. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol. 1990;95:530–536. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- 5.Noonan FP, De Fabo EC, Kripke ML. Suppression of contact hypersensitivity by UV radiation and its relationship to UV-induced suppression of tumor immunity. Photochem Photobiol. 1981;34:683–689. [PubMed] [Google Scholar]
- 6.Ullrich SE, Azizi E, Kripke ML. Suppression of the induction of delayed-type hypersensitivity reactions in mice by a single exposure to ultraviolet radiation. Photochem Photobiol. 1986;43:633–638. doi: 10.1111/j.1751-1097.1986.tb05639.x. [DOI] [PubMed] [Google Scholar]
- 7.Cooper KD, Oberhelman L, Hamilton TA, Baadsgaard O, Terhune M, Levee G, Anderson T, Koren H. UV exposure reduces immunization rates and promotes tolerance to epicutaneous antigens in humans: Relationship to dose, CD1a−DR+ epidermal macrophage induction, and Langerhans cell depletion. Proc Natl Acad Sci USA. 1992;89:8497–8501. doi: 10.1073/pnas.89.18.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garssen J, Goettsch W, de Gruijl F, Slob W, van Loveren H. Risk assessment of UVB effects on resistance to infectious diseases. Photochem Photobiol. 1996;64:269–274. doi: 10.1111/j.1751-1097.1996.tb02457.x. [DOI] [PubMed] [Google Scholar]
- 9.Krutmann J. The role of UVA rays in skin aging. Eur J Dermatol. 2001;11:170–171. [PubMed] [Google Scholar]
- 10.Strickland PT. Photocarcinogenesis by near-ultraviolet UVA radiation in Sencar mice. J Invest Dermatol. 1986;87:272–275. doi: 10.1111/1523-1747.ep12696669. [DOI] [PubMed] [Google Scholar]
- 11.Ullrich SE, Kim TH, Ananthaswamy HN, Kripke ML. Sunscreen effects on UV-induced immune suppression. J Invest Dermatol Symp Proc. 1999;4:65–69. doi: 10.1038/sj.jidsp.5640184. [DOI] [PubMed] [Google Scholar]
- 12.Nghiem DX, Kazimi N, Clydesdale G, Ananthaswamy HN, Kripke ML, Ullrich SE. Ultraviolet a radiation suppresses an established immune response: implications for sunscreen design. J Invest Dermatol. 2001;117:1193–1199. doi: 10.1046/j.0022-202x.2001.01503.x. [DOI] [PubMed] [Google Scholar]
- 13.Moyal D, Courbière C, Le Corre Y, de Lacharrière O, Hourseau C. Immunosuppression induced by chronic solar-simulated irradiation in humans and its prevention by sunscreens. Eur J Dermatol. 1997;7:223–225. [PubMed] [Google Scholar]
- 14.Reeve VE, Bosnic M, Boehm-Wilcox C, Nishimura N, Ley RD. Ultraviolet A radiation 320–400 nm protects hairless mice from immunosuppression induced by ultraviolet B radiation 280–320 nm or cis-urocanic acid. Int Arch Allergy Immunol. 1998;115:316–322. doi: 10.1159/000069463. [DOI] [PubMed] [Google Scholar]
- 15.Garssen J, de Gruijl F, Mol D, de Klerk A, Roholl P, Van Loveren H. UVA exposure affects UVB and cis-urocanic acid-induced systemic suppression of immune responses in Listeria monocytogenes-infected Balb/c mice. Photochem Photobiol. 2001;73:432–438. doi: 10.1562/0031-8655(2001)073<0432:UEAUAC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Damian DL, Halliday GM, Barnetson RSC. Broad-spectrum sunscreens provide greater protection against ultraviolet-radiation-induced suppression of contact hypersensitivity to a recall antigen in humans. J Invest Dermatol. 1997;109:146–151. doi: 10.1111/1523-1747.ep12319200. [DOI] [PubMed] [Google Scholar]
- 17.Poon TS, Barnetson RS, Halliday GM. Prevention of immunosuppression by sunscreens in humans is unrelated to protection from erythema and dependent on protection from ultraviolet A in the face of constant ultraviolet B protection. J Invest Dermatol. 2003;121:184–190. doi: 10.1046/j.1523-1747.2003.12317.x. [DOI] [PubMed] [Google Scholar]
- 18.Moyal DD, Fourtanier AM. Broad-Spectrum sunscreens provide better protection from the suppression of the elicitation phase of delayed-type hypersensitivity response in humans. J Invest Dermatol. 2001;117:1186–1192. doi: 10.1046/j.0022-202x.2001.01545.x. [DOI] [PubMed] [Google Scholar]
- 19.Nghiem DX, Kazimi N, Mitchell DL, Vink AA, Ananthaswamy HN, Kripke ML, Ullrich SE. Mechanisms underlying the suppression of established immune responses by ultraviolet radiation. J Invest Dermatol. 2002;119:600–608. doi: 10.1046/j.1523-1747.2002.01845.x. [DOI] [PubMed] [Google Scholar]
- 20.Moodycliffe AM, Norval M, Kimber I, Simpson TJ. Characterization of a monoclonal antibody to cis-urocanic acid: detection of cis-urocanic acid in the serum of irradiated mice by immunoassay. Immunology. 1993;79:667–672. [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbs NK, Norval M, Traynor NA, Wolf M, Johnson BE, Crosby J. Action spectra for the trans to cis photoisomerisation of urocanic acid in vitro and in mouse skin. Photochem Photobiol. 1993;57:584–590. doi: 10.1111/j.1751-1097.1993.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 22.Kitazawa T, Streilein JW. Hapten-specific tolerance promoted by calcitonin gene-related peptide. J Invest Dermatol. 2000;115:942–948. doi: 10.1046/j.1523-1747.2000.00155.x. [DOI] [PubMed] [Google Scholar]
- 23.Hart PH, Townley SL, Grimbaldeston MA, Khalil Z, Finlay-Jones JJ. Mast cells, neuropeptides, histamine, and prostaglandins in UV-induced systemic immunosuppression. Methods. 2002;28:79–89. doi: 10.1016/s1046-2023(02)00201-3. [DOI] [PubMed] [Google Scholar]
- 24.Walterscheid JP, Ullrich SE, Nghiem DX. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J Exp Med. 2002;195:171–179. doi: 10.1084/jem.20011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf P, Nghiem DX, Walterscheid JP, Byrne S, Matsumura Y, Matsumura Y, Bucana C, Ananthaswamy HN, Ullrich SE. Platelet-activating factor is crucial in psoralen and ultraviolet A-induced immune suppression, inflammation, and apoptosis. Am J Pathol. 2006;169:795–805. doi: 10.2353/ajpath.2006.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos G, Kazimi N, Nghiem DX, Walterscheid JP, Ullrich SE. Platelet activating factor receptor binding plays a critical role in jet fuel-induced immune suppression. Toxicol Appl Pharmacol. 2004;195:331–338. doi: 10.1016/j.taap.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Hart PH, Grimbaldeston MA, Swift GJ, Hosszu EK, Finlay-Jones JJ. A critical role for dermal mast cells in cis-urocanic acid-induced systemic suppression of contact hypersensitivity responses in mice. Photochem Photobiol. 1999;70:807–812. [PubMed] [Google Scholar]
- 28.Niizeki H, Alard P, Streilein JW. Cutting edge: Calcitonin gene-related peptide is necessary for ultraviolet B-impaired induction of contact hypersensitivity. J Immunol. 1997;159:5183–5186. [PubMed] [Google Scholar]
- 29.Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–799. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 30.Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutat Res. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 31.Walterscheid JP, Nghiem DX, Kazimi N, Nutt LK, McConkey DJ, Norval M, Ullrich SE. Cis-urocanic acid, a sunlight-induced immunosuppressive factor, activates immune suppression via the 5-HT2A receptor. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17420–17425. doi: 10.1073/pnas.0603119103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alappatt C, Johnson CA, Clay KL, Travers JB. Acute keratinocyte damage stimulates platelet-activating factor production. Arch Dermatol Res. 2000;292:256–259. doi: 10.1007/s004030050483. [DOI] [PubMed] [Google Scholar]
- 33.McLoone P, Simics E, Barton A, Norval M, Gibbs NK. An Action Spectrum for the Production of cis-Urocanic Acid in Human Skin In Vivo. J Invest Dermatol. 2005;124:1071–1074. doi: 10.1111/j.0022-202X.2005.23731.x. [DOI] [PubMed] [Google Scholar]
- 34.De Fabo EC, Noonan FP. Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J Exp Med. 1983;157:84–98. doi: 10.1084/jem.158.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 36.Hart PH, Grimbaldeston MA, Swift GJ, Jaksic A, Noonan FP, Finlay-Jones JJ. Dermal mast cells determine susceptibility to Ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J Exp Med. 1998;187:2045–2053. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grimbaldeston MA, Pearce AL, Robertson BO, Coventry BJ, Marshman G, Finlay-Jones JJ, Hart PH. Association between melanoma and dermal mast cell prevalence in sun-unexposed skin. Br J Dermatol. 2004;150:895–903. doi: 10.1111/j.1365-2133.2004.05966.x. [DOI] [PubMed] [Google Scholar]
- 38.Grimbaldeston MA, Skov L, Baadsgaard O, Skov BG, Marshman G, Finlay-Jones JJ, Hart PH. Communications: high dermal mast cell prevalence is a predisposing factor for basal cell carcinoma in humans. J Invest Dermatol. 2000;115:317–320. doi: 10.1046/j.1523-1747.2000.00050.x. [DOI] [PubMed] [Google Scholar]