Abstract
Permanent middle cerebral artery occlusion (MCAO) causes neuronal cell death in the striatum and cortex. In rodents, estradiol treatment protects the cortex from cell death in an estrogen receptor alpha (ERα) dependent manner. ERα is only transiently expressed in the cortex during neonatal development and is very low in uninjured adult cortex. Following MCAO, ERα mRNA expression is upregulated in the cortex of female rats, but the mechanism of this increase is still unknown. It is also unknown whether a similar increase in ERα expression in seen in males. In the following studies, male and vehicle or estradiol-treated ovariectomized (OVX) female rats underwent MCAO to investigate the regulation of ERα expression after ischemia. 24 hours after surgery, mRNA or genomic DNA was collected from 1mm micropunches taken from 300µm brain sections for quantitative RT-PCR or methylation-specific (MSP) PCR, respectively. Additionally, adjacent 20µm sections were processed for ERα immunohistochemistry. In OVX females, ERα mRNA and protein were increased in the ischemic cortex, but unchanged in males. We hypothesized that this increase in ERα in females is due to a reversal of gene silencing by DNA methylation. Using MSP targeting of CpG islands within the 5’ UTR of the rat ERα gene, we found that ischemia decreased methylation in the ischemic cortex of both groups of females, but there was no change in methylation in males. Using chromatin immunoprecipitation, we found that MeCP2 associates with ERα 5’UTR corresponding with the methylation status of the promoter. These data are the first to demonstrate a difference in the regulation of ERα expression in response to MCAO between males and females and that methylation of the ERα gene corresponds with mRNA levels in the brain.
Keywords: stroke, methylation, methyl binding proteins, estrogen
Introduction
Estrogens have been shown to protect against neurodegenerative diseases and injury, including stroke. Permanent middle cerebral artery occlusion (MCAO) is a well-established model of focal ischemic stroke in rodents. Following MCAO, there is a reduction in cerebral blood flow, which leads to cell death in the striatum followed by damage to cells in the overlying cortex (Namura et al., 1998, Liu et al., 1999). In this model, gonadectomized females (Simpkins et al., 1997, Dubal et al., 1998, Rusa et al., 1999, Simpkins et al., 2005) and males (Toung et al., 1998) (Alkayed et al., 1998) have a much larger MCAO-induced cell loss than animals with higher circulating estrogen concentrations. Pre-treatment with even low doses of 17β-estradiol is sufficient to exert dramatic protection in the brains of both female (Dubal et al., 1998, Dubal and Wise, 2001) and male rats (Toung et al., 1998).
Many of estrogen’s actions occur via two nuclear receptors, ERα (Koike et al., 1987) and the more recently discovered ERβ (Kuiper et al., 1996). These receptors are important for neuroprotection by estrogen. Generalized pharmacologic blockade of estrogen receptors exacerbates ischemic injury in mice (Sawada et al., 2000) and blocks estrogen-induced neuroprotection in cultured neurons (Singer et al., 1999, Wilson et al., 2000) and in cortical explant cultures (Wilson et al., 2000). Studies using ERβ agonists in mice demonstrated that neuroprotection following global ischemia is mediated by ERβ (Carswell et al., 2004). More recently ERβ agonists were shown to have no effect following focal ischemia in rats (Farr et al., 2007). The discrepancy in the role of ERβ could be due to the type of ischemia or differences between rats and mice. Studies using ERα knockout females, however, demonstrate that neuroprotection by estradiol following focal ischemia is dependent on the presence of ERα in the cortex (Dubal et al., 2001), and that ERβ alone is not sufficient for neuroprotection in females. Another study using ERα knockout mice also suggested that in male ERα knockout mice, the absence of ERα did not increase the ischemia-induced damage, but these males were not given estradiol (Sampei et al., 2000). In both male and female rodents, ERα mRNA expression is high in the neonatal cortex, but dramatically decreased to only a few cells in the uninjured adult cortex (Miranda and Toran-Allerand, 1992, Prewitt and Wilson, 2006). Twenty-four hours after MCAO, however, ERα mRNA and protein are significantly increased in the cortex of female rats and mice (Dubal et al., 1999, Dubal et al., 2006). In OVX females, this increase in ERα expression occurs in both oil and estradiol-treated groups, but is seen earlier after injury with estradiol treatment (Dubal et al., 2006). These data suggest that in females, the ischemia-induced increase in ERα expression in the cortex is necessary for the neuroprotective effects of estradiol. In males, the injury-induced regulation of ERα and the mechanisms of estradiol action are still largely unknown.
In both male and female rodents ERα is only expressed in early postnatal development and must be upregulated following injury, thus the regulation of ERα in the cortex in both sexes following injury is critical to our understanding of estrogen’s neuroprotective mechanisms. One way to examine the ischemia-induced regulation of ERα would be to examine the way that ERα is regulated during early postnatal development. A critical process that occurs during this time of cortical organization is DNA methylation, which is essential for development and plays a vital role in the inactivation of many genes (Bienvenu and Chelly, 2006). The methylated region of DNA is associated with other methyl binding proteins, which then prevent transcription machinery from binding resulting in gene silencing (Bird and Wolffe, 1999, Klose and Bird, 2006). An example of epigenetic regulation of ERα is seen in breast cancer studies where ERα expression has been correlated with changes in methylation of the DNA. For example, MCF-7 breast cancer cells express ERα and have a demethylated ERα promoter, while MBA MD-231 cells do not express ERα and have a methylated ERα promoter (Yang et al., 2001, Sharma et al., 2005). Recent data from our laboratory suggests that ERα mRNA expression in the cortex is regulated by methylation of the ERα promoter during early postnatal development. (Prewitt, SFN abstract 55.8).
Epigenetic modification by methylation also involves the recruitment of methyl-binding proteins which contribute to the inhibition of gene expression. Methyl-CpG-binding Protein 2 (MeCP2) is a methyl binding protein associated with methylation at CpG loci (Klose and Bird, 2003, Sharma et al., 2005, Klose and Bird, 2006). MeCP2 has been implicated in Rett syndrome and mediates the transcriptional silencing of hypermethylated genes in cancer (Moretti and Zoghbi, 2006). In the present study we investigated the association of MeCP2 with the ERα promoter as a potential mechanism for the regulation of ERα gene expression by methylation in male and female rats.
Experimental Procedures
Middle Cerebral Artery Occlusion (MCAO)
Male and female Sprague Dawley rats (225–275 gm) were purchased from Zivic Laboratories, Inc (Pittsburgh, PA). Under isoflurane anesthesia, female rats were bilaterally ovariectomized (OVX) and then implanted with a 2 cm silastic capsule (Konigsberg Instruments, Pasadena, CA) containing sesame oil or 17 β-estradiol (180 µg/ml). This dose has been shown to produce estradiol levels of 20 pg/ml in rats (Dubal and Wise, 2001), which are equivalent to basal circulating levels found during the estrous cycle (Smith et al., 1975). Male rats were gonadally intact. Seven days after OVX, all animals underwent MCAO surgery. Rats were anesthetized with a mixture of ketamine (80 mg/kg i.p.) and acepromazine (0.52 mg/kg,i.p.). The right middle cerebral artery was permanently occluded using methods described previously (Dubal et al., 1998). Briefly, the common carotid and external carotid arteries and their branches were exposed and electrosurgically cut. The internal carotid was exposed and a 3/0 monofilament monofilament suture was inserted through the internal carotid artery to the base of the middle cerebral artery.
Tissue Processing
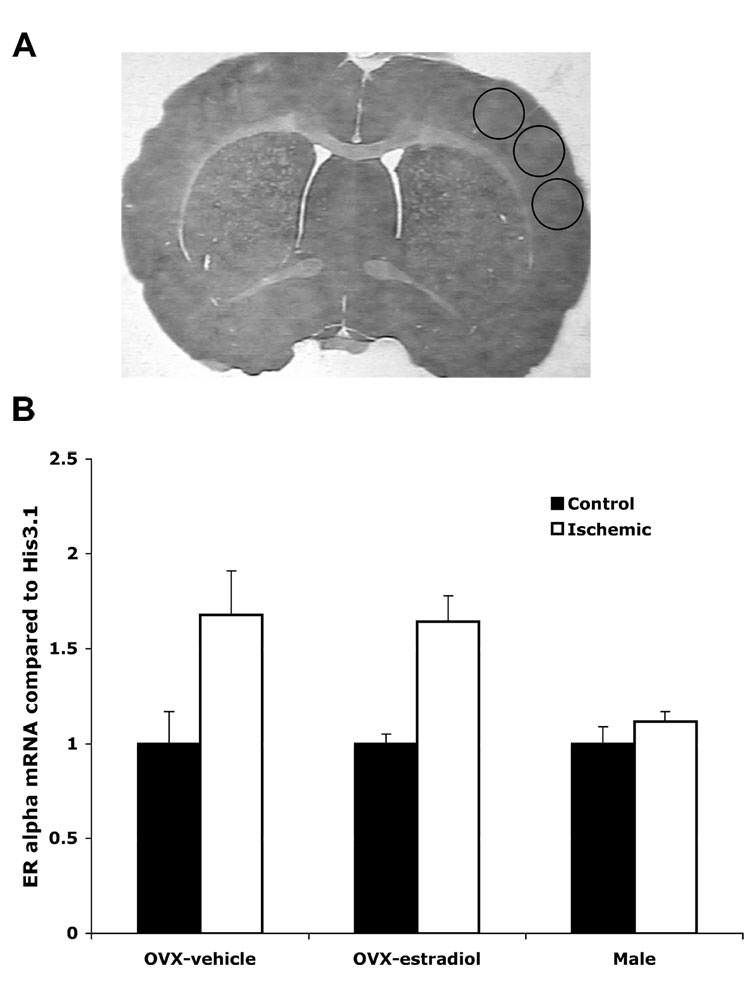
Brains were collected 24 hours after MCAO (n= 4–6 per experimental group). This time-point was chosen based earlier data showing that ERα mRNA increases during this period (Dubal et al., 2006). Brains were rapidly frozen on dry ice and stored at −80°C until use. For tissue processing, coronal sections were cut on a cryostat from approximately bregma +2.6 through bregma −3.25. This region has been shown to contain the damaged region of the cortex (Dubal et al., 1999). Three 300 µm thick sections were cut with several thinner (20µm) sections between the thick sections. The thinner sections were used to measure infarct volume and for ERα immunohistochemistry (IHC). The 300µm sections were further dissected using 1mm micropunches from the cortex. These micropunches were used to collect mRNA, genomic DNA or for the ChIP assay. The position of the micropunches is shown by circles on Figure 2.
Fig. 2.
ERα mRNA expression is increased on the ischemic cortex of vehicle and estradiol-treated OVX female, but not male rats. Real-time PCR using ERα specific primers was performed on RNA isolated from the cortex of OVX females given oil or estradiol and male rats 24 hours after MCAO. Data was normalized to Histone 3.1 and compared to the control side of the cortex. Asterisks on the graph indicate significant differences from control side of the cortex (p< 0.05, n=3). The three circles in A represent areas where micropunches were taken from the 300µm sections.
Determination of Infarct Volume
Twenty µm coronal sections were stained with cresyl violet to delineate the extent of ischemic injury. The volume of infarct was calculated by integrating the area of injury on 4 sections from each brain. Infarct tissue was considered as white enucleated tissue. Cortical infarct volume was quantified with a computer-assisted imaging system (NIH IMAGE, Version 1.6).
Immunohistochemistry
For IHC, 20µm sections were fixed on slides with 4% paraformaldehyde for 30 min. After blocking endogenous peroxidase activity with 0.30% peroxide and non-specific binding with 10% Normal Donkey Serum, sections were incubated with a rabbit anti-ERα antibody (Upstate Biochemical, Chicago, IL, C1355, 1:2000) overnight at 4°C, followed by incubation in secondary donkey anti rabbit Alexa Fluor 488 (Molecular Probes, Eugene, OR, 1:500) for 1 h at room temperature. The sections were then cover-slipped with VectaShield (Vector labs, Burlingame, CA) and visualized on a fluorescent microscope. For control experiments, either the primary antibody or the secondary antibody was excluded from the blocking buffer during incubation. We did not observe any ERα immunoreactive (-IR) cells in these control conditions. ERα -IR cells were counted in periinfarct cortex which included the region in which the most dorsal and medial extent of infarction could be found (Dubal et al., 2006). This region was marked by the edge of damaged versus undamaged tissue. ERα -IR cells were counted in 6 of the thin coronal sections adjacent to the sections used to quantify infarct volume. Cells were counting using NIH Image software.
RNA Extraction
Tissue punches were homogenized in guanidine thiocyanate and total RNA isolatd as previously described (Wilson et al., 2002). The RNA pellet was briefly air dried and suspended in 50 µl RNase-free water. The suspended RNA was incubated at 56°C for 10 minutes and stored at −80°C. 1µg of total RNA was reverse transcribed to produce cDNA in a reaction containing 1µl of Random Primers (Invitrogen, Carlsbad, CA) and 1µl of 10mM dNTPs. The samples were incubated at 65°C for 5 minutes and then chilled on ice. After this initial incubation, a master mix containing 4µl of 5X first strand buffer, 2µl 0.1M DTT, 1 µl RNasin, and 1µl Superscript RT was added to the samples and incubated at room temperature for 10 minutes, 42°C for 50 minutes and 70°C for 15 minutes.
Real time PCR
For real time PCR, each reaction contained 21.25 µl of DEPC H20, 25µ l of 2X SYBRGreen Brilliant Master Mix (Stratagene, La Jolla, CA), 250 pmol of upstream primer, 50 pmole of downstream primer, 0.75 µl of Reference Dye (diluted 1:500) (Stratagene, La Jolla, CA) and 1µl of appropriate cDNA. Primer specific concentrations were previously optimized for each gene and result in a single DNA PCR product with no primer-dimer formation (Dubal et al., 1999, Prewitt and Wilson, 2007). Each 96 well plate contained a non-template control and each sample was run in triplicate. The cycling parameters were: 1 cycle at 95°C for 10 minutes, 40 cycles of 95°C for 30 seconds, annealing temperature for 1 minute, 72°C for 30 seconds, and 1 cycle of 95°C for 1 minute and 55°C for 30 seconds. The change in threshold cycle (ΔCt) for each sample was normalized to the contralateral, unoccluded side for that gender. All data were normalized to the constitutively expressed housekeeping gene Histone 3.1 (Wellman et al., 1987, Kelley et al., 1993).
Genomic DNA Extraction and Sodium Bisulfite Modification
Genomic DNA was extracted based upon previously described methods (Nishino et al., 2004). Briefly, 6 dissected 1 mm micropunches of tissue were homogenized in 250 µl of lysis solution (10 mM Tris-HCl (pH 8.0), 150 mM EDTA, 1% SDS, 100 g/ml proteinase K) and incubated for 20 minutes at 55 °C. An RNase solution, consisting of 10 mM Tris-HCl (pH 8.0), 1 mM EDTA (TE) and 30 units of RNase was added and the mixture was incubated at 37 °C for 1 hour. The resulting mixture underwent two rounds of phenol-chloroform extraction ethanol precipitation. Sodium bisulfite modification was performed using the EZ DNA Methylation-Gold Kit (Zymo Research, Orange, CA, D5005) as per manufacturer’s directions.
Methylation-Specific PCR
For methylation-specific PCR, 100ng of bisulfite modified DNA was amplified in a 25µl reaction. Each PCR reaction contained 1X PCR Buffer (Invitrogen, Carlsbad, CA), 1.2 mM MgCl2 (Invitrogen, Carlsbad, CA), 0.1 mM dNTP’s, 2 pM forward primer, 2 pM reverse primer and 2.5 units Platinum Taq Polymerase (Invitrogen, Carlsbad, CA). The cycling conditions were: 95 °C for 2 minutes, 35 cycles of 95 °C 30 seconds, appropriate annealing temperature for 30 seconds, 72 °C for 30 seconds, subsequently followed by 72 °C for 10 minutes. The primers for MSP were designed using Methprimer (http://www-genome.wi.mit.edu/genome_software/other/primer3.html). The following primers were used CpG Island 1: TCTCAGCACACTTTGACTGCC and CAAACCGCTCAAGCTACACA and CpG Island 2: GGCCCTAGATGCCTCCTATC and AGAACCGCTATCTTCCCTGA.
Chromatin Immunoprecipitation (ChIP)
For ChIP assays, we used the Upstate Biotechnologies ChIP Assay Kit (Catalog # 17-295, Temecula, CA) and methods previously described (Martinowich et al., 2003). Briefly, 3 micropunches of tissue from each side of the cortex were minced with a clean razor blade. Proteins were cross-linked in DMEM plus 1% formaldehyde at 37°C. Cells were then lysed in cold lysis buffer containing 1% SDS, 10 mM EDTA, 50 mM Tris, pH 8.1. This lysis buffer also included Roche Complete Mini Protease Inhibitor cocktail, which contains several protease inhibitors with broad specificity. These inhibitors work to inhibit serine, cysteine, and metalloproteases (Roche Applied Science, Indianapolis, IN, Cat# 11836153001). One tablet was added for every 10 mls of buffer. Crosslinked DNA was sheared to approximately 400 to 500 basepairs with 3 sets of 5-second pulses using a Fisher Scientific Ultrasonic Dismembrator (model D100) Sonicator, 5 mm tip and set to 30% maximum power. Sonicated cell supernatant was diluted 10-fold in ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl, pH 8.1, 167 mM NaCl) with Roche Complete Mini Protease Inhibitor cocktail. A sample was removed after dilution and called the “input” sample. The remaining diluted cell supernatant was pre-cleared with Salmon Sperm DNA/Protein A agarose 50% slurry. At this point another sample was removed and used for the –antibody (−Ab) control. Primary MeCP2 antibody (Upstate Biotechnology, Lake Placid, NY, 05-764, 1:1000) was added to the remaining pre-cleared supernatant and incubated overnight. Agarose was pelleted by centrifugation to separate the beads with bound chromatin from the supernatant containing unbound chromatin. The protein A/antibody/chromatin complex was washed with Low Salt Immune Complex Wash Buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, 150 mM NaCl) and High Salt Immune Complex Wash Buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, 500 mM NaCl) followed by LiCl Complex Wash buffer (0.25 M LiCl, 1% IGEPAL-CA630, 1% deoxycholic acid), 1 mM EDTA, 10 mM Tris, pH 8.1) and TE Buffer (10 mM Tris-HCl, 1 mM EDTA, and TE buffer). The protein A agarose/antibody/chromatin complex was eluted in elution buffer (1%SDS, 0.1M NaHCO3). Cross-links were reversed by adding 20 µl of 5 M NaCl to eluates and heating for 65°C for 4 hours. DNA was recovered by phenol/chloroform extraction and ethanol precipitation. Standard PCR with 30 cycles was performed on the resulting sample as well as on the “input” and −Ab samples that did not undergo any pre-clearing or immunoprecipitation. The cycling conditions were: 95 °C for 2 minutes, 30 cycles of 95 °C 30 seconds, 60 °C for 30 seconds, 72 °C for 30 seconds, subsequently followed by 72 °C for 10 minutes. The primers for ERα promoter were: TCTCAGCACACTTTGACTGCC and CAAACCGCTCAAGCTACACA. In the initial experiments immunoprecipitation with non-specific IgG also indicated a lack of amplified band (data not shown).
Statistics
All data was analyzed by two-way analysis of variance comparing the side of the brain (Ischemic versus control) and subject type (OVX + oil, OVX + estradiol, or male). One-way ANOVA and post-hoc comparisons were made where appropriate. All differences were considered significant at p< 0.05.
Results
Estradiol treatment decreases damage in the cortex following ischemia
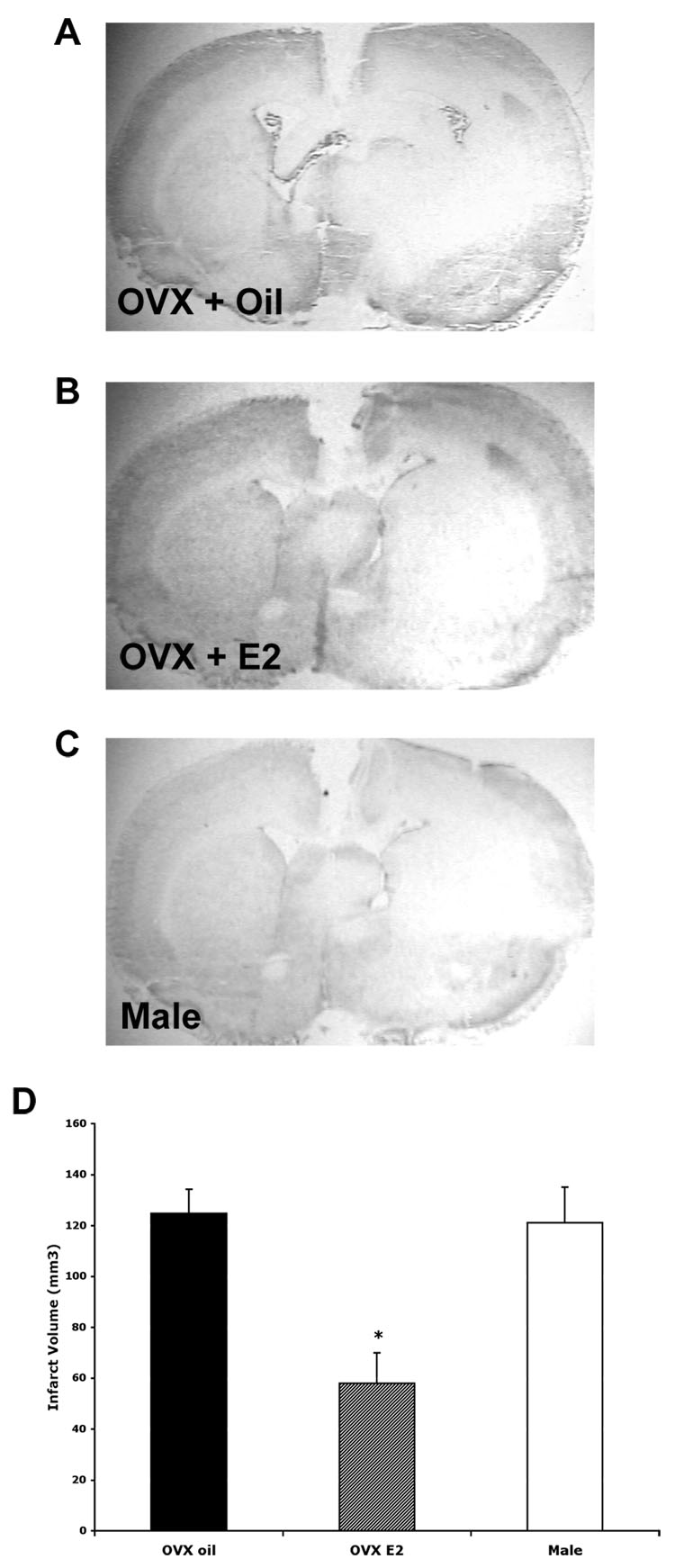
Sections were stained with cresyl violet to determine the extent of injury after permanent MCAO. Males and vehicle-treated OVX females had significant damage in both the striatum and cortex on the ischemic side compared to the control striatum and cortex. Estradiol-treated OVX females showed a decreased infarct size in the cortex and striatum. Fig. 1 shows representative low power photomicrographs from a vehicle (A) and estradiol-treated OVX (B) female and a male 24 hours following MCAO. The graph in Fig. 1 D shows the cortical infarct volume averaged from 4 sections for all three groups. The cortical infarct volume was significantly less in the estradiol-treated OVX females when compared to oil-treated OVX females and males (p< 0.01).
Fig. 1.
Cresyl violet staining of coronal sections 24 hours following MCAO. Representative photomicrographs are shown for OVX + oil (A), OVX + estradiol (B) and male (C). The vehicle-treated OVX female and the male rats had more damage in the ischemic cortex than the estradiol-treated OVX females. OVX females given estradiol had significantly less infarct volume in the cortex (D) (p< 0.01, n= 4). Photomicrographs were taken at 1.5X magnification.
Ischemia increases ERα mRNA in the cortex in female, but not male rats
To test the hypothesis that ERα mRNA expression is similar in male rats and oil-treated OVX females, we investigated the expression of ERα mRNA in the cortex 24 hours following MCAO in vehicle or estradiol-treated OVX female and male rats. RNA was collected from six micropunches taken from each side of the cortex and quantified using real time PCR. Based on previous data (Dubal et al., 2006), we predicted that ERα mRNA expression in the ischemic cortex would increase in both groups of female rats. There were very low levels of ERα mRNA in the control cortex of all groups. As predicted there was an increase in ERα mRNA expression in the ischemic cortex of both groups of females as compared to control. Surprisingly, there was no increase in ERα mRNA expression in male rats 24 hours following MCAO (Fig. 2). Data was normalized to the housekeeping gene Histone 3.1 and then presented as a percent of the control cortex. Asterisks on the graph in Fig. 2 indicate that ERα mRNA was significantly increased when compared to control (p< 0.05). These results indicate that ERα mRNA induction in the cortex following ischemic injury is gender dependent, but not dependent on estradiol treatment.
Ischemia increases ERα protein in the cortex of female, but not male rats
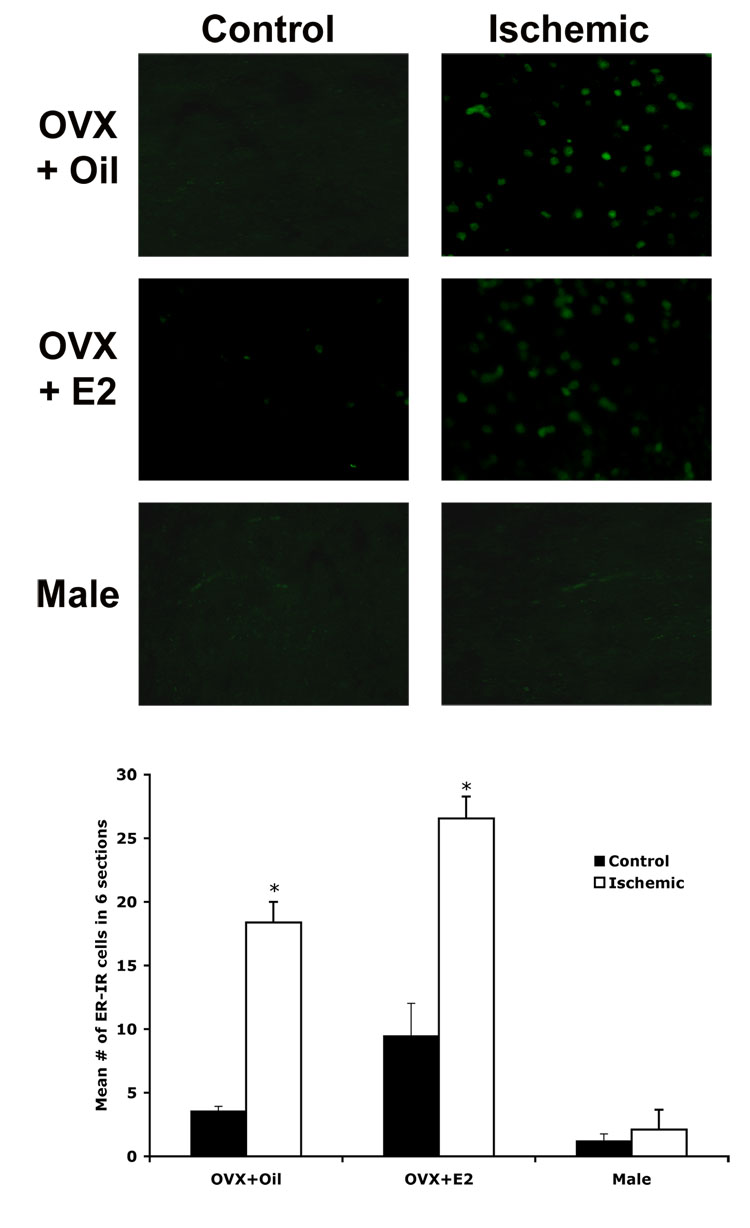
To test the hypothesis that ERα protein expression will reflect changes in mRNA expression, we used immunohistochemistry to measure the expression of ERα protein 24 hours after MCAO in vehicle or estradiol-treated female and male rats. We found that the number of ERα immunoreactive cells in the cortex was significantly increased following ischemia in both groups of females (p <0.01). There was, however, no significant difference between vehicle and estradiol-treated OVX females. There was no significant difference in ERα expression between the control and ischemic cortex in males. Figure 3 shows representative photomicrographs and a graph of the average number of ERα immunoreactive cells in 6 sections through the control (left) or damaged (right) area of cortex of vehicle-treated (top), estradiol-treated (middle) OVX female and male (bottom) rats. These data demonstrate that the gender differences in ERα mRNA expression following MCAO are also seen in ERα protein expression.
Fig. 3.
ERα protein expression is increased in the ischemic cortex (right) of vehicle (top) and estradiol-treated OVX females (middle), but not male (bottom) rats. Fluorescent immunohistochemistry (IHC) was performed on 20 µm tissue sections. A representative section from a vehicle (top) and an estradiol-treated female (middle) and a male rat (bottom) is shown. The graph shows the average number of ER-α immunoreactive cells in 6 sections through the damaged cortex and control cortex for 4 animals per group. ERα expression was significantly increased in the ischemic cortex of both groups of females (p< 0.05). There was no significant difference in ERα expression in males. The asterisks on the graph indicate significant differences from the control cortex (p< 0.05, n=4). Photomicrographs were taken at 20X magnification.
Ischemia causes demethylation of the ERα promoter in female, but not male rats
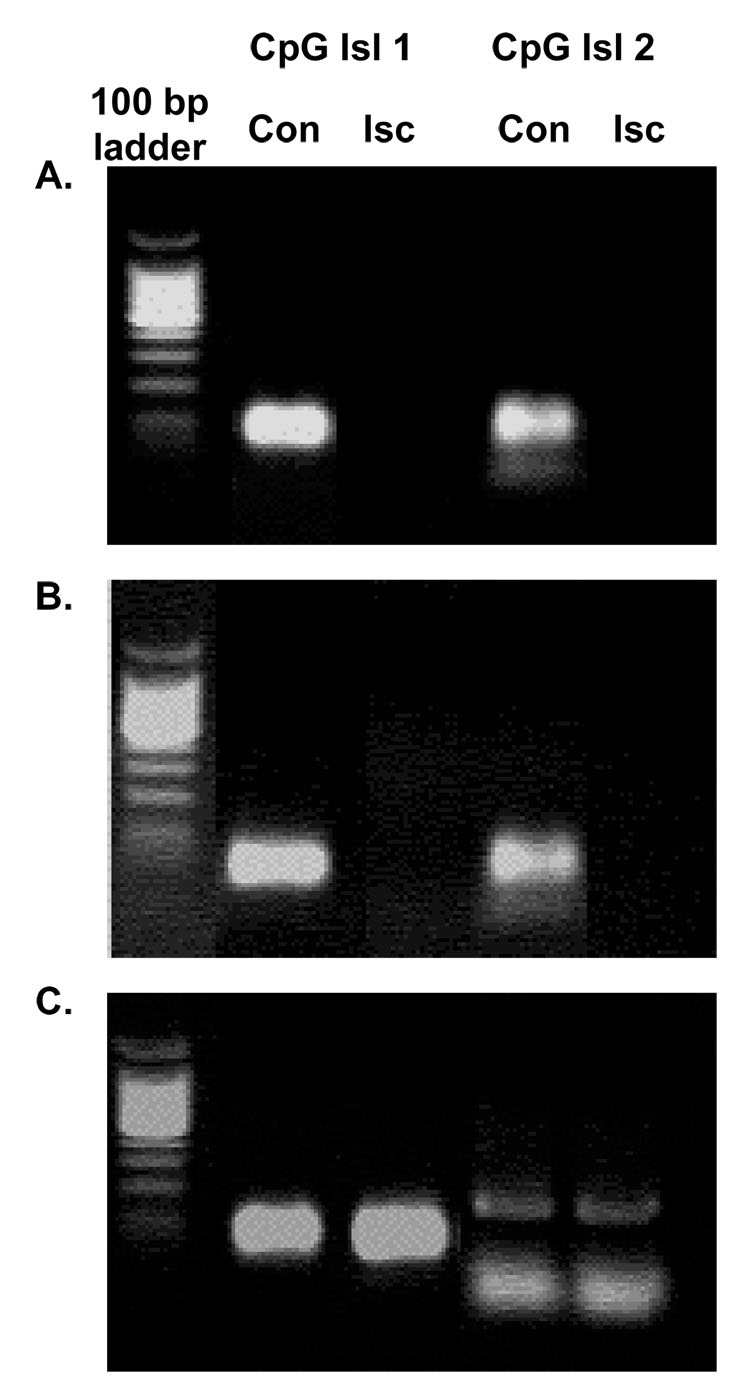
DNA methylation involves the addition of a methyl group to cytosines at CpG dinucleotides resulting in an inability of transcription machinery to bind to DNA, thus silencing transcription. To test the hypothesis that ERα gene expression is regulated by methylation following ischemia, we examined the methylation status of several CpG islands within the rat ERα 5’ untranslated region (UTR) 24 hours after MCAO in both male and female rats. We used methylation-specific PCR (MSP) to compare the methylation status of genomic DNA from both sides of the cortex. In both groups of females (Fig. 4 A and B), there was a decrease in methylation on the ischemic compared to the control side of the brain. In male rats, there was no difference in the methylation status of any of the CpG islands that we examined following MCAO (Fig. 4 C). Similar results were obtained in 3 separate repetitions of this experiment. These data suggest that methylation of the ERα promoter may account for the lack of increase in ERα mRNA expression after injury in males.
Fig. 4.
Methylation of loci of the ERα promoter changes following MCAO in vehicle-treated (A) and estradiol-treated female (B), but not male (C) rats 24 hours after MCAO. Genomic DNA was extracted from vehicle or estradiol-treated OVX female and male rats and methylation-specific PCR was conducted using primers specific for several CpG islands within the rat ER-α promoter. These studies were repeated 3 times.
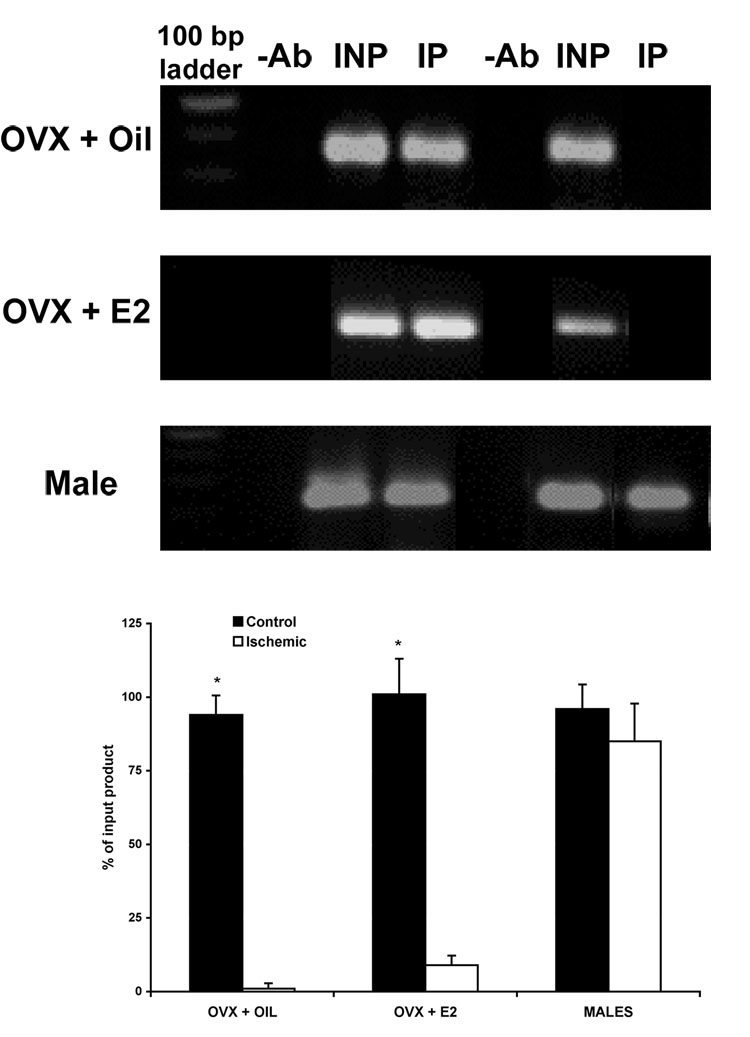
Ischemia induces dissociation of MeCP2 from ER α promoter in females, but not males
We previously demonstrated by bisulfite treatment and methylation-specific PCR the association of methylation of the ERα promoter and repression of ERα gene expression. Here we extended our study to test the hypothesis that the methyl-CpG binding protein 2 (MeCP2) interacts with the ERα gene to maintain methylation and suppress ERα gene expression. Using anti-MeCP2 antibodies in a chromatin immunoprecipitation assay (ChIP), we found that MeCP2 is associated with the ERα promoter at one locus in the control cortex of both male and female rats (Fig. 5). Following ischemia, MeCP2 was no longer associated with the ERα promoter at these same loci in either group of female rats (Fig. 5 A and B). MeCP2 was still associated with the ERα promoter in male rats even after ischemia (Fig. 5 C), suggesting that methylation and recruitment of MeCP2 could account for the changes in ERα gene expression following ischemia. The graph in Fig. 5 D shows the average intensity of PCR product compared to input (n=3).
Fig. 5.
ChIP assay using MeCP2-specific antibody on nuclear extracts from the cortex of vehicle-treated or estradiol-treated female, and male rats 24 hours after MCAO. MeCP2 is associated with a region of the ERα promoter in all groups on the control, un-occluded side of the cortex, but MeCP2 is no longer associated with the ERα promoter after ischemia in either group of females. Binding of MeCP2 to the ERα promoter was significantly decreased following injury in both groups of OVX females, but not males (n=3, p< 0.05). −Ab, no antibody immunoprecipitated cortical extracts; INP, nonimmunoprecipitated input; IP, MeCP2 antibody immunoprecipitated.
Discussion
Summary
The current studies confirm previous findings that estradiol is protective in the cortex following MCAO ( Dubal et al., 1998, Rusa et al., 1999, Hurn and Macrae, 2000, Simpkins et al., 1997). There was extensive damage in the cortex of male and vehicle-treated females after MCAO. As expected, ERα mRNA and protein levels were elevated in the cortex of both groups of female rats, regardless of estradiol pretreatment (Dubal et al., 2006). We deomonstrated for the first time, however, that there was no similar increase in ERα mRNA and protein expression in males following MCAO. In both groups of OVX females, ischemia caused demethylation at several loci of the ERα promoter along with a corresponding disassociation of the methyl binding protein MeCP2. These data suggest that the increase in ERα expression in the cortex following MCAO is due to demethylation of the ERα promoter in response to damage in the brain. In males, however, there was no change in the methylation status of the ERα promoter, and MeCP2 continued to be associated with the promoter ever after injury. These observations potentially mediate the lack of increase in ERα mRNA in the cortex of male rats following injury.
Sex differences in response to ischemia
Many studies have demonstrated that there is a sex difference in neuronal damage caused by ischemia. For example, as early at 1991, Hall demonstrated that male gerbils had a larger ischemia-induced injury than females (Hall et al., 1991). Later, Alkayed showed that female rats sustain smaller cortical and striatal infarcts after MCAO compared with age-matched males, and OVX prevented gender differences in infarct volume and blood flow (Alkayed et al., 1998). Here we show that low dose estradiol pretreatment decreases the extent of damage in the cortex 24 hours after MCAO. There was not a similar protection in vehicle-treated OVX females. These females showed the same damage as males. These data confirm previous findings that estradiol protects the cortex from the secondary cell death caused by ischemia (Dubal et al., 1998). Previous studies have also shown that estrogen-treated OVX females sustain smaller brain infarcts in the cortex than age-matched males or estrogen-deficient females (Alkayed et al., 1998, Wise et al., 2005).
Studies in male rats have shown that estradiol can reduce the extent of injury in the cortex after MCAO (Toung et al., 1998). These studies also showed that loss of testosterone did not affect the outcome following stroke and that the presence of normal levels of testosterone did not change estradiol mediated neuroprotection after ischemia in the male cortex (Toung et al., 1998). In our studies in male rats, ischemia did not increase ERα mRNA or protein in the cortex in the absence of estradiol. Thus, the protective effects of estradiol in males may take place through nongenomic signaling pathways such as non-nuclear estrogen receptors or work completely independent of ER binding (Prokai and Simpkins, 2007). Alternatively, estradiol may be required to increase ERα expression in males, which is not the case in females. Additionally, it is possible that estradiol may be working via an ERβ dependent mechanism in males. A complete analysis of this mechanism in males is warranted in future studies.
Epigenetic regulation of ERα by methylation
We extended our studies to focus on the mechanism of ERα mRNA regulation following ischemia. We found that ischemia caused demethylation of regions of the ERα promoter in female, but not male rats. This demethylation corresponded to the increase in ERα mRNA expression in both groups of OVX females regardless of treatment condition (vehicle or estradiol). In male rats, a similar demethylation or increase in ERα mRNA expression was not observed. These data suggest an intrinsic sex difference in the potential signals from injury that cause demethylation and re-expression of ERα mRNA in the cortex.
Sixty percent of primary breast cancers are ER-positive and most advanced ER-positive breast cancers respond to therapy with anti-estrogens. However, many breast cancers that initially express ERα lose ER expression during tumor progression (Yang et al., 2001). In a significant fraction of breast cancers, the absence of ERα is a result of aberrant methylation of CpG islands (Lapidus et al., 1998). In the brain, maternal behavior has been shown to modify methylation patterns of ERα in the hypothalamus (Champagne et al., 2006). The data presented here are the first to demonstrate a change in methylation status of the ERα gene mediated by an external stimulus in adult animals. These data contribute to our understanding of how ERα mRNA is regulated in the adult brain and contribute to our understanding of in a gender-specific regulation of gene expression.
MeCP2 Regulation of ERα
MeCP2 selectively recognizes and binds to methylated CpGs, subsequently preventing gene expression (Klose and Bird, 2003, Klose and Bird, 2006). Recent experiments investigating MeCP2 regulation of brain derived neurotrophic factor (BDNF) gene expression found that membrane depolarization of neurons leads to phosphorylation of MeCP2, which correlates with an increase in expression of BDNF (Chen et al., 2003). These findings suggest that regulation of MeCP2 may play an important role in regulating specific processes of activity-dependent gene transcription that are important for nervous system function. In the present study, it is possible that intracellular signals from the injured, ischemic brain could change the activity status of MeCP2, such that it no longer associates with the ERα promoter. This possibility would explain the dissociation of MeCP2, subsequent decrease in methylation and a reactivation ERα gene expression after injury.
Conclusions
While there are numerous mechanisms by which estradiol is protective, both genomic and non-genomic (Prokai and Simpkins, 2007), ERα and estradiol are both clearly needed for protection against secondary cell death in the cortex following ischemic injury. The present study identifies a novel mechanism by which ERα mRNA expression is regulated in the injured brain in a gender-specific manner. A further understanding of these mechanisms may lead to potential targets of therapeutic intervention following ischemia.
Abbreviations
- ChIP
Chromatin immunoprecipitation assay
- E2
Estradiol
- ERα
Estrogen receptor alpha
- ERβ
Estrogen receptor beta
- IHC
Immunohistochemistry
- MeCP2
Methyl CpG binding protein 2
- MSP
Methylation-specific PCR
- MCAO
Middle cerebral artery occlusion
- OVX
Ovariectomy
Footnotes
Footnote: This publication was also made possible by COBRE grant P20 RR 15592 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke; a journal of cerebral circulation. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- Bienvenu T, Chelly J. Molecular genetics of Rett syndrome: when DNA methylation goes unrecognized. Nat Rev Genet. 2006;7:415–426. doi: 10.1038/nrg1878. [DOI] [PubMed] [Google Scholar]
- Bird AP, Wolffe AP. Methylation-induced repression--belts, braces, and chromatin. Cell. 1999;99:451–454. doi: 10.1016/s0092-8674(00)81532-9. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Macrae IM, Gallagher L, Harrop E, Horsburgh KJ. Neuroprotection by a selective estrogen receptor beta agonist in a mouse model of global ischemia. American journal of physiology. 2004;287:H1501–H1504. doi: 10.1152/ajpheart.00227.2004. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Rau SW, Shughrue PJ, Zhu H, Yu J, Cashion AB, Suzuki S, Gerhold LM, Bottner MB, Dubal SB, Merchanthaler I, Kindy MS, Wise PM. Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERalpha in estradiol-mediated protection against delayed cell death. Endocrinology. 2006;147:3076–3084. doi: 10.1210/en.2005-1177. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J Neurosci. 1999;19:6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Wise PM. Neuroprotective effects of estradiol in middle-aged female rats. Endocrinology. 2001;142:43–48. doi: 10.1210/endo.142.1.7911. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr TD, Carswell HV, Gsell W, Macrae IM. Estrogen receptor beta agonist diarylpropiolnitrile (DPN) does not mediate neuroprotection in a rat model of permanent focal ischemia. Brain research. 2007;1185:275–282. doi: 10.1016/j.brainres.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Hall ED, Pazara KE, Linseman KL. Sex differences in postischemic neuronal necrosis in gerbils. J Cereb Blood Flow Metab. 1991;11:292–298. doi: 10.1038/jcbfm.1991.61. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab. 2000;20:631–652. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- Kelley MR, Jurgens JK, Tentler J, Emanuele NV, Blutt SE, Emanuele MA. Coupled reverse transcription-polymerase chain reaction (RT-PCR) technique is comparative, quantitative, and rapid: uses in alcohol research involving low abundance mRNA species such as hypothalamic LHRH and GRF. Alcohol (Fayetteville, NY) 1993;10:185–189. doi: 10.1016/0741-8329(93)90033-k. [DOI] [PubMed] [Google Scholar]
- Klose R, Bird A. Molecular biology. MeCP2 repression goes nonglobal. Science (New York, NY) 2003;302:793–795. doi: 10.1126/science.1091762. [DOI] [PubMed] [Google Scholar]
- Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Koike S, Sakai M, Muramatsu M. Molecular cloning and characterization of rat estrogen receptor cDNA. Nucleic acids research. 1987;15:2499–2513. doi: 10.1093/nar/15.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus RG, Nass SJ, Davidson NE. The loss of estrogen and progesterone receptor gene expression in human breast cancer. Journal of mammary gland biology and neoplasia. 1998;3:85–94. doi: 10.1023/a:1018778403001. [DOI] [PubMed] [Google Scholar]
- Liu D, Smith CL, Barone FC, Ellison JA, Lysko PG, Li K, Simpson IA. Astrocytic demise precedes delayed neuronal death in focal ischemic rat brain. Brain Res Mol Brain Res. 1999;68:29–41. doi: 10.1016/s0169-328x(99)00063-7. [DOI] [PubMed] [Google Scholar]
- Miranda RC, Toran-Allerand CD. Developmental expression of estrogen receptor mRNA in the rat cerebral cortex: a nonisotopic in situ hybridization histochemistry study. Cereb Cortex. 1992;2:1–15. doi: 10.1093/cercor/2.1.1. [DOI] [PubMed] [Google Scholar]
- Moretti P, Zoghbi HY. MeCP2 dysfunction in Rett syndrome and related disorders. Current opinion in genetics & development. 2006;16:276–281. doi: 10.1016/j.gde.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino K, Hattori N, Tanaka S, Shiota K. DNA methylation-mediated control of Sry gene expression in mouse gonadal development. The Journal of biological chemistry. 2004;279:22306–22313. doi: 10.1074/jbc.M309513200. [DOI] [PubMed] [Google Scholar]
- Prewitt AK, Wilson ME. Changes in estrogen receptor-alpha mRNA in the mouse cortex during develpment. Brain Res. 2006 doi: 10.1016/j.brainres.2006.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prewitt AK, Wilson ME. Changes in estrogen receptor-alpha mRNA in the mouse cortex during development. Brain research. 2007;1134:62–69. doi: 10.1016/j.brainres.2006.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokai L, Simpkins JW. Structure-nongenomic neuroprotection relationship of estrogens and estrogen-derived compounds. Pharmacol Ther. 2007;114:1–12. doi: 10.1016/j.pharmthera.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, Klaus JA, Hurn PD. 17beta-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke; a journal of cerebral circulation. 1999;30:1665–1670. doi: 10.1161/01.str.30.8.1665. [DOI] [PubMed] [Google Scholar]
- Sampei K, Goto S, Alkayed NJ, Crain BJ, Korach KS, Traystman RJ, Demas GE, Nelson RJ, Hurn PD. Stroke in estrogen receptor-alpha-deficient mice. Stroke; a journal of cerebral circulation. 2000;31:738–743. doi: 10.1161/01.str.31.3.738. discussion 744. [DOI] [PubMed] [Google Scholar]
- Sawada M, Alkayed NJ, Goto S, Crain BJ, Traystman RJ, Shaivitz A, Nelson RJ, Hurn PD. Estrogen receptor antagonist ICI182,780 exacerbates ischemic injury in female mouse. J Cereb Blood Flow Metab. 2000;20:112–118. doi: 10.1097/00004647-200001000-00015. [DOI] [PubMed] [Google Scholar]
- Sharma D, Blum J, Yang X, Beaulieu N, Macleod AR, Davidson NE. Release of methyl CpG binding proteins and histone deacetylase 1 from the Estrogen receptor alpha (ER) promoter upon reactivation in ER-negative human breast cancer cells. Molecular endocrinology (Baltimore, Md) 2005;19:1740–1751. doi: 10.1210/me.2004-0011. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. Journal of neurosurgery. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Wang J, Wang X, Perez E, Prokai L, Dykens JA. Mitochondria play a central role in estrogen-induced neuroprotection. Curr Drug Targets CNS Neurol Disord. 2005;4:69–83. doi: 10.2174/1568007053005073. [DOI] [PubMed] [Google Scholar]
- Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J Neurosci. 1999;19:2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke; a journal of cerebral circulation. 1998;29:1666–1670. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- Wellman SE, Casano PJ, Pilch DR, Marzluff WF, Sittman DB. Characterization of mouse H3.3-like histone genes. Gene. 1987;59:29–39. doi: 10.1016/0378-1119(87)90263-0. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Dubal DB, Wise PM. Estradiol protects against injury-induced cell death in cortical explant cultures: a role for estrogen receptors. Brain research. 2000;873:235–242. doi: 10.1016/s0006-8993(00)02479-3. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Liu Y, Wise PM. Estradiol enhances Akt activation in cortical explant cultures following neuronal injury. Brain Res Mol Brain Res. 2002;102:48–54. doi: 10.1016/s0169-328x(02)00181-x. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Rau SW, Brown CM, Suzuki S. Are estrogens protective or risk factors in brain injury and neurodegeneration? Reevaluation after the Women's health initiative. Endocrine reviews. 2005;26:308–312. doi: 10.1210/er.2004-0014. [DOI] [PubMed] [Google Scholar]
- Yang X, Phillips DL, Ferguson AT, Nelson WG, Herman JG, Davidson NE. Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer research. 2001;61:7025–7029. [PubMed] [Google Scholar]