Abstract
The yeast Saccharomyces cerevisiae contains two genes, PDE1 and PDE2, which respectively encode a low-affinity and a high-affinity cAMP phosphodiesterase. The physiological function of the low-affinity enzyme Pde1 is unclear. We show that deletion of PDE1, but not PDE2, results in a much higher cAMP accumulation upon addition of glucose or upon intracellular acidification. Overexpression of PDE1, but not PDE2, abolished the agonist-induced cAMP increases. These results indicate a specific role for Pde1 in controlling glucose and intracellular acidification-induced cAMP signaling. Elimination of a putative protein kinase A (PKA) phosphorylation site by mutagenesis of serine252 into alanine resulted in a Pde1ala252 allele that apparently had reduced activity in vivo. Its presence in a wild-type strain partially enhanced the agonist-induced cAMP increases compared with pde1Δ. The difference between the Pde1ala252 allele and wild-type Pde1 was strongly dependent on PKA activity. In a RAS2val19 pde2Δ background, the Pde1ala252 allele caused nearly the same hyperaccumulation of cAMP as pde1Δ, while its expression in a PKA-attenuated strain caused the same reduction in cAMP hyperaccumulation as wild-type Pde1. These results suggest that serine252 might be the first target site for feedback inhibition of cAMP accumulation by PKA. We show that Pde1 is rapidly phosphorylated in vivo upon addition of glucose to glycerol-grown cells, and this activation is absent in the Pde1ala252 mutant. Pde1 belongs to a separate class of phosphodiesterases and is the first member shown to be phosphorylated. However, in vitro the Pde1ala252 enzyme had the same catalytic activity as wild-type Pde1, both in crude extracts and after extensive purification. This indicates that the effects of the S252A mutation are not caused by simple inactivation of the enzyme. In vitro phosphorylation of Pde1 resulted in a modest and variable increase in activity, but only in crude extracts. This was absent in Pde1ala252, and phosphate incorporation was strongly reduced. Apparently, phosphorylation of Pde1 does not change its intrinsic activity or affinity for cAMP but appears to be important in vivo for protein-protein interaction or for targeting Pde1 to a specific subcellular location. The PKA recognition site is conserved in the corresponding region of the Schizosaccharomyces pombe and Candida albicans Pde1 homologues, possibly indicating a similar control by phosphorylation.
INTRODUCTION
The budding yeast Saccharomyces cerevisiae contains two cAMP phosphodiesterases, Pde1 and Pde2, that are unrelated in primary sequence (Fujimoto et al., 1974; Londesborough, 1974; Suoranta and Londesborough, 1984; Sass et al., 1986; Nikawa et al., 1987b). The high affinity Michaelis-Menten constant ([Km] = 170 nM) cAMP phosphodiesterase Pde2 belongs to a well studied class of phosphodiesterases of which representatives have been found in many species, including mammals (Suoranta and Londesborough, 1984; Charbonneau et al., 1986). Several enzymes of this class are known to be regulated by phosphorylation and are involved in control of agonist-induced cAMP responses (Conti et al., 1995). On the other hand, only four homologues of S. cerevisiae Pde1 are currently known (Wera et al., 1997): they have been identified in Vibrio fischeri (Dunlap and Callahan, 1993), Dictyostelium discoideum (Lacombe et al., 1986), Schizosaccharomyces pombe (DeVoti et al., 1991), and Candida albicans (Hoyer et al., 1994). Up to now there is no evidence for regulation of any one of these enzymes by phosphorylation. This report provides the first evidence that a member of this family is involved in controlling agonist-induced cAMP signaling and suggests that the enzyme is regulated in vivo by phosphorylation.
Pde1 displays a low affinity for cAMP with a Km value that varies between 20 and 250 μM, depending on the assay conditions (Fujimoto et al., 1974; Londesborough and Lukkari, 1980). Londesborough and Lukkari (1980) calculated that at 10 μM cAMP (the upper limit of the cAMP level they estimated to occur in yeast), at 30°C and pH 6.4, Pde1 can degrade 27 nmol cAMP/min/g. They suggested that, in spite of its high Km, Pde1 might contribute significantly to the degradation of the high cAMP concentration that occurs in yeast cells after addition of glucose. This proposed function for Pde1, however, has never been supported by experimental evidence.
Addition of glucose to yeast cells grown on a nonfermentable carbon source results in a rapid and transient increase in intracellular cAMP (van der Plaat, 1974; Thevelein et al., 1987b). A higher and longer-lasting cAMP spike occurs after intracellular acidification induced by protonophores such as 2,4-dinitrophenol (Trevillyan and Pall, 1979; Caspani et al., 1985; Thevelein et al., 1987a). cAMP synthesis in yeast cells is controlled by an elaborate pathway (reviewed by Broach and Deschenes, 1990; Thevelein, 1991, 1992; and Tatchell, 1993). Adenylate cyclase activity is largely dependent on the Ras proteins, the activity of which is controlled by the guanine nucleotide exchange proteins, Cdc25 and Sdc25, and the GTPase-activating proteins, Ira1 and Ira2. Recent work has shown that intracellular acidification, but not glucose, leads to a rapid increase in the ratio of GTP/GDP bound to the Ras proteins. On the other hand, for glucose activation of cAMP synthesis, another G protein, Gpa2, is required (Colombo et al., 1998).
It is known that cAMP accumulation in yeast is strongly inhibited by protein kinase A (PKA)1, since mutants with reduced activity of the protein kinase display hyperaccumulation of cAMP, while mutants with unbridled PKA activity display a reduced cAMP level (Nikawa et al., 1987a). Also, for the glucose-induced cAMP signal, a close, inverse correlation is observed between the amplitude and duration of the cAMP spike and the activity of PKA (Mbonyi et al., 1990). In strains lacking the two phosphodiesterases, the basal cAMP level is only elevated two- to threefold compared with the level in a wild-type strain, indicating that most of the feedback-inhibition on cAMP accumulation is independent of the phosphodiesterases, or at least that their effect can be mimicked by other mechanisms in such a genetic background (Nikawa et al., 1987b). Several targets for this feedback-inhibition mechanism have been proposed: Cdc25 (Munder and Küntzel, 1989), Ras (Resnick and Racker, 1988), Ira (Tanaka et al., 1989, 1990), and adenylate cyclase itself (De Vendittis et al., 1986). Recent work has shown that the feedback inhibition apparently does not act through a mechanism influencing the ratio of GTP/GDP on the Ras proteins (Colombo et al., 1998).
Although the transient nature of the glucose-induced cAMP signal correlates inversely with the activity of PKA and therefore with the intensity of the feedback-inhibition mechanism, the rapid decrease in cAMP levels after the initial increase appears to be more consistent with PKA-mediated activation of phosphodiesterase activity. Previous results have also indicated that phosphodiesterase activity in yeast might be activated by PKA-mediated phosphorylation. As opposed to a RAS2val19 strain, a RAS2val19 pde1Δ pde2Δ strain displays a very high cAMP level. This indicates that in a RAS2val19 strain the phosphodiesterases are able to prevent hyperaccumulation of cAMP. However, in a strain with reduced PKA activity, the phosphodiesterases are apparently unable to prevent cAMP hyperaccumulation (Nikawa et al., 1987a). This is consistent with stimulation of phosphodiesterase activity by PKA (Thevelein, 1992).
It has been unclear up to now which phosphodiesterase, Pde1 or Pde2, or both, is responsible for the degradation of the elevated cAMP levels in yeast cells after stimulation with glucose or intracellular acidification and how the activity of this phosphodiesterase is controlled. In the present article we show that Pde1 is specifically involved in control of agonist-induced cAMP signaling and that it is most likely regulated by reversible phosphorylation. Our results identify serine252 of Pde1 as a possible target site of the feedback-inhibition mechanism involved in control of agonist-induced cAMP signaling.
MATERIALS AND METHODS
Strains, Media, and Growth Conditions
S. cerevisiae strains used in this work are shown in Table 1. All results shown were obtained with the strains in the W303–1A background except where otherwise stated. Composition of the growth media was as follows. Rich media contained 2% bacto-peptone, 1% yeast extract, and 2% glucose (YPD). Synthetic media contained 0.67% yeast nitrogen base without amino acids (Difco, Detroit, MI) and 2% glucose (SDglucose) or 3% glycerol (SDglycerol), supplemented with the appropriate auxotrophic requirements. The cells were grown at 30°C in the appropriate medium (as specified in the figure legends).
Table 1.
Saccharomyces cerevisiae strains used in this work
| Strain | Genetic background | Relevant genotype | Complete genotype | Source and/or reference |
|---|---|---|---|---|
| W303-1A | W303-1A | wild type | MATa leu2-3, 112 ura3-1 trp1-92 his3-11, 15ade2-1 can1-100 GAL SUC mal | Thomas and Rothstein (1989) |
| PM941 | W303-1A | pde1Δ | MATa leu2-3, 112 ura3-1 trp1-92 his3-11, 15 ade2-1 can1-100 GAL SUC mal pde1∷URA3 | This work |
| PM942 | W303-1A | pde2Δ | MATa leu2-3, 112 ura3-1 trp 1-92 his3-11, 15 ade2-1 can 1-100 GAL SUC mal pde2∷URA3 | This work |
| PM943 | W303-1A | pde1Δ pde2Δ | MATa leu2-3, 112 ura3-1 trp1-92 his3-11, 15 ade2-1 can1-100 GAL SUC mal pde1∷TRP1 pde2∷URA3 | This work |
| PM850 | W303-1A | wild type + YEplac195 | MATa leu2-3, 112 ura3-1 trp1-92 his3-11, 15 ade2-1 can1-100 GAL SUC mal + YEplac195 (URA3) | This work |
| PM851 | W303-1A | wild type + YEpPDE1 | MATa leu2-3, 112 ura3-1 trp1-92 his3-11, 15 ade2-1 can1-100 GAL SUC mal + YEpPDE1 (URA3) | This work |
| PM852 | W303-1A | wild type + YEpPDE2 | MATa leu2-3, 112 ura3-1 trp1-92 his3-11, 15 ade2-1 can1-100 GAL SUC mal + YEpPDE2 (URA3) | This work |
| PM581 | W303-1A | pde1ala252 | MATa leu2-3, 112 ura3-1 trp1-92 his3-11, 15 ade2-1 can1-100 GAL SUC mal pde1ala252 | This work |
| PM582 | W303-1A | pde1ala252 pde2Δ | MATa leu2-3, 112 ura3-1 trp1-92 his3-11, 15 ade2-1 can1-100 GAL SUC mal pde1ala252 pde2∷URA3 | This work |
| PM541 | W303-1A | pde1Δ pde2Δ + YCpPDE1 | MATa leu2-3, 112 ura3-1 trp1-92 his3-11, 15 ade2-1 can1-100 GAL SUC mal pde1∷TRP1 pde2∷URA3 + YCpL-PDE1 | This work |
| PM542 | W303-1A | pde1Δ pde2Δ + YCppde1ala252 | MATa leu2-3, 112 ura3-1 trp1-92 his3-11, 15 ade2-1 can1-100 GAL SUC mal pde1∷TRP1 pde2∷URA3 + YCpL-pde1ala252 | This work |
| TK161-R2V | SP1 | RAS2val19 | MATa leu2 his3 trp1 ade8 can1 ura3 RAS2val19 | Broek et al. (1985) |
| DC124 | SP1 | wild type | Matα his4 leu2 ura3 trp1 ade8 can1 | M. Wigler (Cold Spring Harbor) |
| PM944 | SP1 | RAS2val19pde1Δ | MATa leu2 his3 trp1 ade8 can1 ura3 RAS2val19 pde1∷URA3 | This work |
| PM945 | SP1 | RAS2val19pde2Δ | MATa leu2 his3 trp1 ade8 can1 ura3 RAS2val19 pde2∷URA3 | This work |
| PM946 | SP1 | RAS2val19pde1Δ pde2Δ | MATa leu2 his3 trp1 ade8 can1 ura3 RAS2val19 pde1∷TRP1 pde2∷URA3 | This work |
| PM584 | SP1 | RAS2val19pde1ala252 | MATa leu2 his3 trp1 ade8 can1 ura3 RAS2val19 pde1ala252 | This work |
| PM586 | SP1 | RAS2val19pde1ala252pde2Δ | MATa leu2 his3 trp1 ade8 can1 ura3 RAS2val19 pde1ala252pde2∷URA3 | This work |
| J105 | SP1 | pde1Δ | MATa leu2 his3 ura3 trp1 ade8 can1 pde1∷LEU2 | Nikawa et al. (1987b) |
| J104 | SP1 | pde2Δ | MATa leu2 his3 ura3 trp1 ade8 can1 pde2∷HIS3 | Sass et al. (1986) |
| J106 | SP1 | pde1Δ pde2Δ | MATa leu2 his3 ura3 trp1 ade8 can1 pde1∷URA3 pde2∷HIS3 | Nikawa et al. (1987b) |
| PM975 | SP1 | tpk1w1tpk2 tpk3 bcy1 + | MATa his3 leu2 ura3 trp1 ade8 tpk1w1 tpk2∷HIS3 tpk3∷TRP1 | Nikawa et al. (1987a) |
| YCplac33 | bcy1∷LEU2 (RS13-58A-1) + YCplac33(URA3) | This work | ||
| PM976 | SP1 | tpk1w1tpk2 tpk3 bcy1 + YCpPDE1 | MATa his3 leu2 ura3 trp1 ade8 tpk1w1 tpk2∷HIS3 tpk3∷TRP1 bcy1∷LEU2 (RS13-58A-1) + YCpU-PDE1 | This work |
| PM977 | SP1 | tpk1w1tpk2 tpk3 bcy1 + YCppde1ala252 | MATa his3 leu2 ura3 trp1 ade8 tpk1w1 tpk2∷HIS3 tpk3∷TRP1 bcy1∷LEU2 (RS13-58A-1) + YCpU-pde1ala252 | This work |
| PM978 | SP1 | tpk1w1tpk2 tpk3 bcy1 + YCpPDE2 | MATa his3 leu2 ura3 trp1 ade8 tpk1w1 tpk2∷HIS3 tpk3∷TRP1 bcy1∷LEU2 (RS13-58A-1) + YCpPDE2 (URA3) | This work |
| PM979 | SP1 | tpk1w1tpk2 tpk3 bcy1 + YCppde1asp252 | MATa his3 leu2 ura3 trp1 ade8 tpk1w1 tpk2∷HIS3 tpk3∷TRP1 bcy1∷LEU2 (RS13-58A-1) + YCpU-pde1asp252 | This work |
| PM545 | W303-1A | PDE1-HA | MATa leu2-3, 112 ura3-1 trp1-92 his3-11, 15 ade2-1 can1-100 GAL SUC mal-PDE1-HA | This work |
| PM546 | W303-1A | PDE1S252A-HA | MATa leu2-3, 112 ura3-1 trp1-92 his3-11, 15 ade2-1 can1-100 GAL SUC mal pde1ala252-HA | This work |
Plasmid and Strain Constructions
The vectors YCplac33, YCplac111, and YEplac195 (Gietz and Sugino, 1988) were used for the construction of new plasmids. The plasmids pJJ242 and pJJ246 (Jones and Prakash, 1990) were used as source of the marker genes for disruption of PDE1 and PDE2. Plasmids pYT20 (Nikawa et al., 1987b) and pYEpPDE2-2 (Sass et al., 1986) were generous gifts of Michael Wigler (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY). Plasmids YCpU-PDE1, YCpL-PDE1, YEpPDE1, and pUCPDE1 were constructed by subcloning the XbaI–SmaI fragment of pYT20 containing PDE1 in between the corresponding sites of YCplac33, YCplac111, YEplac195, and pUC18. Plasmid ppde1::URA3 was constructed by inserting the BamHI--PvuII fragment of pJJ242 containing the yeast URA3 gene in between the BamHI--BalI sites of pUCPDE1. The XmnI–SnaBI fragment of ppde1::URA3 was used for the disruption of the PDE1 genomic locus by homologous recombination. ppde1::TRP1 was constructed by the same method as described for ppde1::URA3, except that the BamHI–PvuII fragment with TRP1 from pJJ246 was used. Plasmids YCpPDE2, YEpPDE2, and pUCPDE2 were constructed by subcloning the BamHI–SpeI fragment of YEpPDE2-2 in between the BamHI–XbaI site of YCplac33, YEplac195, and pUC19, respectively. The SphI–PvuII fragment of pJJ242 containing the yeast URA3 gene was inserted in between the SphI–HpaI sites of pUCPDE2 generating ppde2::URA3. This plasmid was linearized by SspI for disruption of the PDE2 genomic locus by homologous recombination.
Construction of the Pde1ala252, Pde1asp252 Alleles and the Corresponding Yeast Strains
The Pde1ala252 and Pde1asp252 alleles were constructed by Megaprimer PCR-mediated site-directed mutagenesis (Sarkar and Sommer, 1990) using the outer primers, 5′-GTTCATCATGGGATAGGC-3′ and 5′-CGAGTATGGTTAGTCTTGG-3′, and the following mutagenic primers, respectively (mutation in bold), 5′-GATTCTTCAGCTTCTCTGCG-3′ and 5′-GATTCTTCATCTTCTCTGCG-3′. The resulting PCR products were digested by MfeI and BssHII and cloned in between the corresponding sites of YCpU-PDE1, YCpL-PDE1, and YEpPDE1 creating the YCpU-pde1ala252, YCpL-pde1ala252, YEppde1ala252 and YCpU-pde1asp252, YCpL-pde1asp252, YEppde1asp252 alleles. The entire cloned PCR fragments were sequenced confirming the nucleotide changes causing the ser252ala and ser252asp mutations, respectively, as the only nucleotide change. The HindIII–HincII fragments of YCppde1ala252 and YCppde1asp252 were inserted in between the corresponding sites of pUC19. The resulting constructs were digested with BamHI and HincII and ligated with the BamHI-SmaI fragment of pJJ242 containing the yeast URA3 gene. These constructs were then cut by EcoRI and SmaI, and the EcoRI-BalI fragments of YCppde1ala252 and YCppde1asp252 were inserted, creating plasmids pPAI3 and pPAS3, respectively. Yeast strains were transformed to ura+ with the SmaI–HaeII fragment of pPAI3 or pPAS3 to replace the wild-type PDE1 allele, which was confirmed by Southern hybridization. The ura+ transformants were grown on rich medium (YPD) until stationary phase (2 d) and then plated on 5′-FOA plates to select for ura− colonies in which the URA3 gene was lost again by homologous recombination of the overlapping pde1ala252 or pde1asp252 sequences flanking the URA3 gene (Boeke et al., 1984). Genomic DNA was isolated from the resulting strains for Southern hybridization, PCR, and sequence analysis to confirm that they carried the proper pde1 mutant allele.
Epitope-Tagging of the Pde1 and Pde1ala252 Alleles
For epitope tagging of the Pde1 and Pde1ala252 alleles at the C terminus, the sequence from +643 to +1107 (ATG start codon = +1) was amplified by PCR using the following primers: 5′-gaattcATAGGCGTCAAGACTGGCGCG-3′ and 5′-cccgggTAGAAACAAAGTGTGGCCTTC-3′. The resulting PCR product was digested with EcoRI and SmaI and cloned in the corresponding sites of PYX012 (RD Systems, Minneapolis, MN) containing an hemagglutinin (HA)-epitope tag following the SmaI site. The plasmid was digested with MfeI and integrated at the PDE1 locus of a wild-type strain and a strain carrying a Pde1ala252 allele.
Determination of cAMP and Phosphodiesterase Activity
For determination of the cAMP responses, exponentially growing cells (OD600 = 1.5) were harvested by centrifugation at 4°C, washed with ice-cold SD-complete medium without carbon source, and resuspended in the same medium. This cell suspension was preincubated at 30°C for 10 min. Subsequently, 100 mM glucose or 2 mM 2,4-dinitrophenol (from a stock solution of 80 mM in ethanol) was added as indicated. Samples containing 75 mg cells were used for determination of cAMP as described previously (Thevelein et al., 1987a). The activity of Pde1 was measured as described by Wera et al. (1997) by following the time-dependent degradation of cAMP. Samples and controls were incubated in 50 mM Tris-HCl (pH 8), 0.1 mM EDTA, and 500 μM cAMP at 30°C. The reaction was stopped by heating, and cAMP was measured using the cAMP [3H] assay system (Amersham, Arlington Heights, IL).
Determination of Heat Shock Resistance
Yeast strains were pregrown either in rich medium (YPD) or in SDglucose-uracil medium (for plasmid maintenance) until OD600 = 2.0–2.5. The density of the cultures was adjusted to OD600 = 2 with the same medium before the heat shock was performed. Heat shocks were done for the indicated periods of time in a water bath at 50°C. A series of dilutions of treated and untreated cells was spotted on YPD plates, incubated for 2 d at 30°C, and then scored for growth and photographed.
Phosphodiesterase Pde1 Purification, Gel Electrophoresis, Western Blotting, and Protein Determination
Pde1 was purified from pde1 pde2 cells overexpressing Pde1 or Pde1ala252. Cells in the exponential phase of growth were lysed in buffer A (50 mM Tris-HCl, pH 8, 0.1 mM EDTA, 0.3 mM PMSF). After a high-speed centrifugation the extract was loaded on a mono Q (Pharmacia, Piscataway, NJ) ion-exchange column and eluted with a linear gradient from 0 to 500 mM NaCl in buffer A. An equal volume of 4 M (NH4)2SO4 in buffer A was added to the Pde1-containing samples, and the mixture was immediately loaded on a Phenyl Resource column (Pharmacia) that was eluted with a linear gradient from 2 to 0 M (NH4)2SO4 in buffer A. Pde1-containing samples were concentrated using a Vivaspin 10000 concentrator (Vivascience, Binbrook Lincoln, United Kingdom) and loaded on a Pharmacia Superdex75 column equilibrated in buffer A containing 100 mM NaCl. Purified Pde1 was concentrated as before and stored at −20°C. The final preparation displayed two bands after denaturing gel electrophoresis and silver staining: a 42.6-kDa band corresponding to Pde1 (as confirmed by Western blotting with a specific antibody: see below) and a 70-kDa band. Specific activities typically amounted to 13.8 nmol/min/mg, 140 nmol/min/mg, 620 nmol/min/mg, and 9900 nmol/min/mg in the crude extract and after mono Q, Phenyl Resource, and Superdex chromatography, respectively.
Western blotting was performed using an antibody raised against the synthetic peptide ‘CKSTPAKRDPRLTILE’ (Eurogentec, Liège, Belgium), corresponding to residues 328–342 of Pde1 plus an additional N-terminal cysteine. Specificity of the antibody was confirmed by Western blotting of extracts from pde1Δ- and Pde1-overexpressing cells with preimmune and immune sera. Protein was determined using the Lowry method (Lowry et al., 1951).
Phosphorylation of Pde1
In Vitro.
For phosphorylation of Pde1 in vitro, samples were incubated at 30°C for 30 min in the presence of 2 mM magnesium acetate, 0.1 mM ATP, and the catalytic subunit of bovine PKA (Sigma Chemical, St. Louis, MO). For labeling experiments 0.3 μCi/ml [γ32P]-labeled ATP (Amersham) was included. For determination of the stoichiometry of phosphate incorporation, the purity of Pde1 was estimated at 50%.
In Vivo.
Cells, grown overnight to exponential phase in YP medium containing 2% glycerol, were harvested by centrifugation, washed once in water, and resuspended to OD600 = 5 in low-phosphate medium (Bio-101) containing 0.1% glucose and 2% glycerol. 32P was added to a final concentration of 50–150 μCi/ml, and incubation was continued for another hour. 32P incorporation was measured and was typically higher than 99%. Aliquots of 5 ml of cell suspension were prepared, and 2% glucose was added where appropriate. After 3 min, cells were harvested and extracts were prepared and immunoprecipitated with anti-HA antibodies (Boehringer Mannheim, Indianapolis, IN) and protein A Sepharose (Sigma) as described.
RESULTS
Pde1 Plays a Specific Role in Agonist-induced cAMP Signaling
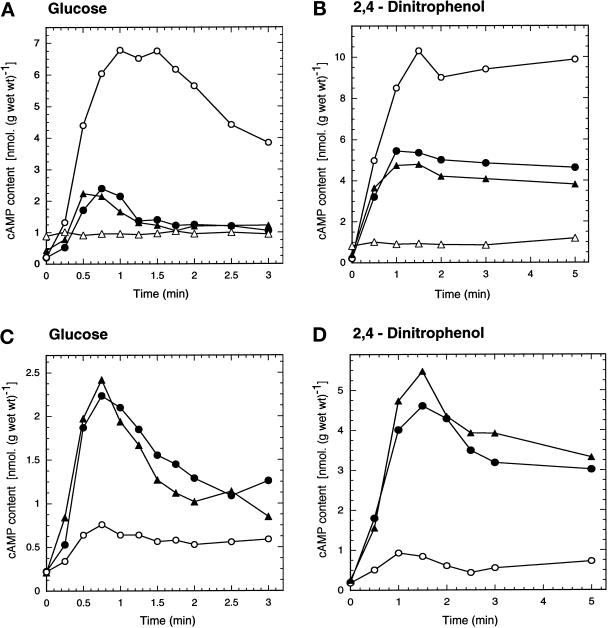
When glucose is added to yeast cells grown on a nonfermentable carbon source, such as glycerol, a transient spike in the cAMP level is observed within ∼1–2 min (Figure 1A). In a pde1Δ mutant, lacking the low-affinity cAMP phosphodiesterase, this cAMP signal was much higher (approximately threefold) and also longer-lived (Figure 1A). In the pde2Δ mutant, which lacks the high-affinity cAMP phosphodiesterase, the cAMP signal was not significantly changed (Figure 1A) or partially reduced (in the SP1 background, our unpublished results). In the pde1Δ pde2Δ strain the cAMP signal was virtually absent (Figure 1A). The latter strain always displayed a significantly higher (approximately twofold) basal cAMP level, as has been reported previously (Nikawa et al., 1987b). Similar results were obtained with pdeΔ mutants in the SP1 background (our unpublished results). The reduction or disappearance of the cAMP signal in the pde2Δ and pde1Δ pde2Δ strains seems at first sight contradictory, but can be explained by enhanced feedback inhibition of PKA on cAMP synthesis (see DISCUSSION).
Figure 1.
Intracellular cAMP level as a function of time after addition of 100 mM glucose (A and C) or 2 mM 2,4-dinitrophenol (B and D). (A and B) Phosphodiesterase-deficient strains: wild-type strain (W303–1A) (•); pde1Δ strain (PM941) (○); pde2Δ strain (PM942) (▴); and pde1Δ pde2Δ strain (PM943) (▵). (C and D) Strains with overexpression of PDE1 or PDE2: wild-type strain + YEplac195 (PM850) (•); wild-type strain + YEpPDE1 (PM851) (○); wild-type strain + YEpPDE2 (PM852) (▴).
Intracellular acidification triggered by addition of the protonophore 2,4-dinitrophenol at an extracellular pH of 6 to wild-type cells causes a higher and longer-lived increase in the cAMP level than glucose addition (Figure 1B). This intracellular acidification-induced cAMP increase was enhanced (approximately twofold) in the pde1Δ strain compared with the wild-type strain (Figure 1B). It was reduced to a variable extent in the pde2Δ strain and eliminated in the pde1Δ pde2Δ strain (Figure 1B). Similar results were obtained with pdeΔ mutants in the SP1 background. The effects of the single pde1Δ and pde2Δ mutations were even somewhat more pronounced (our unpublished results).
Since these results pointed to a possible specific role of the Pde1 low-affinity phosphodiesterase in controlling agonist-induced cAMP signaling, we investigated glucose- and 2,4-dinitrophenol–induced cAMP stimulation in strains overexpressing Pde1 or Pde2. The overexpression of PDE1 and PDE2 was confirmed by Northern blotting. At least a tenfold higher level of PDE1 or PDE2 transcripts was detected (our unpublshed results). Figure 1C shows that the glucose-induced cAMP signal was not affected by overexpression of Pde2, but it was largely eliminated by overexpression of Pde1. Figure 1D shows that the same is true for the cAMP increase triggered by intracellular acidification.
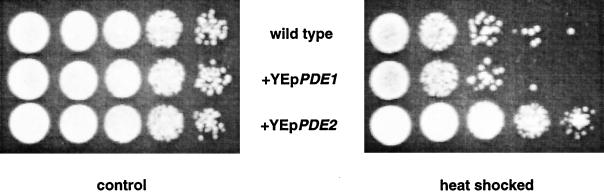
To check whether overexpression of PDE1 or PDE2 affects the basal cAMP level significantly in vivo, we compared the heat resistance of the overexpression strains with that of the wild-type strain. This is a more sensitive in vivo assay than determination of the basal cAMP level, since the latter only changes slightly upon modification of the PDE genes separately (Nikawa et al., 1987b). It is known that yeast strains with reduced activity of the cAMP pathway show enhanced heat resistance (Iida and Yahara, 1984; Shin et al., 1987). Figure 2 shows that the strain with the multicopy PDE2 plasmid displayed an enhanced heat resistance compared with the control strain (W303–1A). This most likely indicates that overexpression of PDE2 reduces the basal cAMP level in vivo and also confirms that the Pde2–overexpression construct is functional. Interestingly, the strain with overexpression of the Pde1 enzyme did not show a significant change in heat resistance (Figure 2), indicating, most likely, that overexpression of this enzyme does not significantly affect the basal cAMP level during growth. These results show that Pde2, rather than Pde1, controls the basal cAMP level during growth. Similar results were obtained with yeast strains of the SP1 and M5 background expressing either Pde1 or Pde2 from the same plasmid.
Figure 2.
Cells of a wild-type strain (W303–1A) transformed with either YEpPDE1 (PM851), YEpPDE2 (PM852), or the empty vector YEplac195 (PM850) were grown in SD glucose-uracil medium until OD600 = 2.0–2.5, after which the OD was adjusted to 2 with the same medium. The cell suspension was heat shocked for 30 min at 50°C in a water bath. Treated and untreated cell suspensions were spotted on YPD plates and incubated at 30°C for 2 d. Serial dilutions were made with a factor of 10.
Serine252, a Putative PKA Phosphorylation Site, Is Important for Pde1 Activity In Vivo
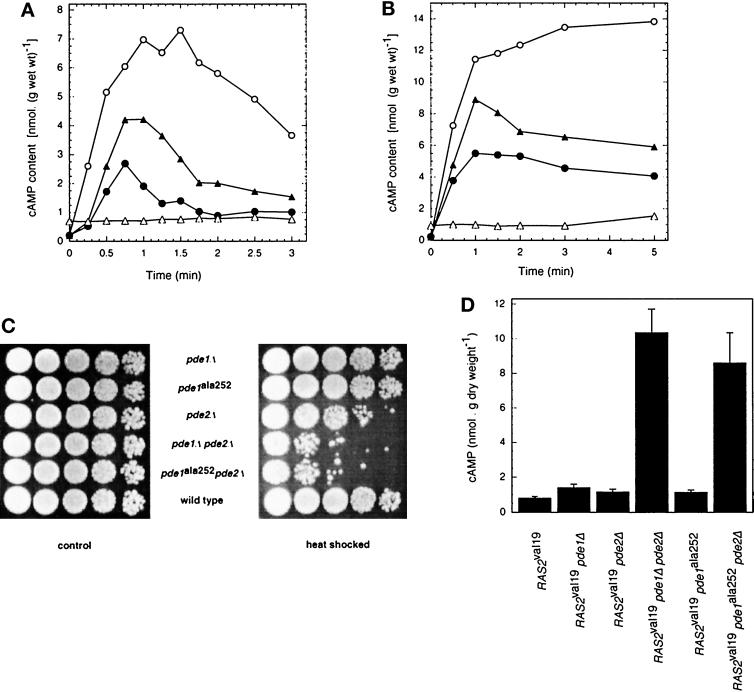
Since previous data in the literature indicated a possible role of PKA-regulated phosphodiesterase activity in the control of cAMP levels in yeast (see INTRODUCTION), we have scanned the Pde1 sequence for putative PKA phosphorylation sites. At amino acid positions 249–252, an RRXS sequence was found, which is an ideal consensus site for phosphorylation by PKA. We have changed serine252 by site-directed mutagenesis into alanine (see MATERIALS AND METHODS). A strain with the wild-type Pde1 allele replaced by the mutant Pde1ala252 allele displayed an enhanced (approximately twofold) and also longer-lived glucose-induced cAMP signal compared with the wild-type strain (Figure 3A). However, the increase was not as high as in the pde1Δ strain. This seems to indicate that the pde1ala252 allele apparently displays a partial activity in vivo with respect to the control of glucose-induced cAMP accumulation. In a pde1ala252 pde2Δ strain, the glucose-induced cAMP signal was eliminated as was seen in the pde1Δ pde2Δ strain. Deletion of Pde2 combined with partial inactivation of Pde1 (at least as judged from the effect in vivo on cAMP accumulation) causes the same elimination of the cAMP signal as double deletion of Pde1 and Pde2.
Figure 3.
Intracellular cAMP level as a function of time after addition of 100 mM glucose (A) or 2 mM 2,4-dinitrophenol (B) in strains in which PDE1 has been replaced by pde1ala252: wild-type strain (W303–1A) (•); pde1Δ strain (PM941) (○); pde1ala252 strain (PM581) (▴); and pde1ala252 pde2Δ strain (PM582) (▵). (C) Heat shock resistance of strains carrying different alleles of the PDE genes. Cells were grown in YPD medium until OD600 = 2.0–2.5, after which the OD was adjusted to 2 with the same medium. The cell suspension was heat shocked for 20 min at 50°C in a water bath. Treated and untreated cell suspensions were spotted on YPD plates and incubated at 30°C for 2 d. Serial dilutions were made with a factor of 10. (D) Basal cAMP level during exponential growth on YPD medium in RAS2val19 strains with deletion and/or modification of the phosphodiesterase genes.
The results obtained for the acidification-induced cAMP increase in the strains with the Pde1ala252 mutant allele were very similar to those obtained for the glucose-induced cAMP signal. The strain in which the wild-type Pde1 allele was replaced by the mutant Pde1ala252 allele showed a higher 2,4-dinitrophenol–induced cAMP increase, while additional deletion of PDE2 in this strain practically eliminated the cAMP increase (Figure 3B). Reintroduction on a single-copy plasmid of the wild-type Pde1 allele, but not of the mutant Pde1ala252 allele, in the pde1Δ pde2Δ strain reduced the basal cAMP level and restored the glucose- and acidification-induced cAMP increases practically up to the level observed in the wild-type strain (our unpublished results).
We have also investigated whether substitution of serine252 by an aspartate residue might result in a phenotype indicating a constitutively activated Pde1 enzyme. We constructed a strain in which the wild-type Pde1 allele was replaced by the Pde1asp252 allele. The glucose- and acidification-induced cAMP increases were enhanced in a similar way in this strain as in a strain where Pde1 was replaced by Pde1ala252 (our unpublished results). This shows that Pde1asp252 does not display higher catalytic activity and that apparently its activity in vivo is reduced to a similar extent as in Pde1ala252 because of the loss of the putative phosphorylation site.
All previous experiments have been performed with cells grown in the absence of glucose. In glucose-repressed cells, glucose is unable to trigger rapid cAMP accumulation. However, intracellular acidification produces a similar increase in the cAMP level in glucose-repressed and -derepressed wild- type cells (Beullens et al., 1988; Argüelles et al., 1990). In glucose-repressed cells we observed the same effects of the PDE1 mutations as in derepressed cells. This means that in glucose-repressed cells of both the pde1Δ strain and the strain in which the wild-type PDE1 gene has been replaced by the pde1ala252 allele, similar enhancements of the cAMP level upon intracellular acidification were observed compared with the level in the wild- type strain as were observed in glucose-derepressed cells (our unpublished results).
We have also investigated the effect of a pde1 null allelle and pde1ala252 on the heat shock resistance of the cells. Figure 3C shows that pde1ala252 in combination with pde2Δ has the same effect on heat shock resistance as pde1Δ combined with pde2Δ. In the presence of a wild-type PDE2 allele, however, there is no significant difference in heat shock resistance between a strain carrying PDE1, pde1Δ, or pde1ala252 (Figure 3C). This indicates again that Pde1 itself has only little control over the basal cAMP level of the cells. Only in the absence of PDE2 is there a clear effect of deletion of PDE1, and in this background the pde1ala252 allele behaves as an inactive allele in vivo.
To gain further evidence for the importance of the putative serine252 phosphorylation site in controlling Pde1 activity in vivo, we have also introduced the pde1ala252 allele in RAS2val19 strains. A RAS2val19 strain displays only a 2- to 3-fold higher cAMP level during growth on YPD medium compared with the wild-type strain (Broek et al., 1985; Toda et al., 1985). However, when the two PDE genes are deleted in a RAS2val19 strain, the cAMP level increases to very high values, similar to those observed in PKA-attenuated strains (Nikawa et al., 1987a) (Figure 3D). The basal cAMP level during growth on YPD medium was only slightly higher (approximately twofold) in a RAS2val19 strain in which either PDE1 or PDE2 has been deleted compared with the level in the RAS2val19 strain (Figure 3D). This indicates that both phosphodiesterases are able to hydrolyze efficiently the very high cAMP level that accumulates in their absence in the RAS2val19 pde1Δ pde2Δ strain (Figure 3D). However, when the pde1ala252 allele was present in a RAS2val19 pde2Δ strain instead of the wild-type PDE1 allele, a very high cAMP level was observed, barely lower compared with the level in the RAS2val19 pde1Δ pde2Δ strain (Figure 3D). This indicates that the putative serine252 phosphorylation site in Pde1 is in some way essential for efficient hydrolysis of the very high cAMP level in the RAS2val19 pde1Δ pde2Δ strain.
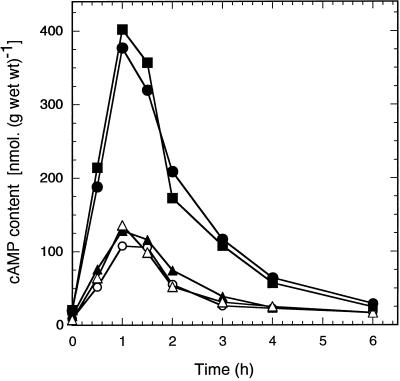
We have also expressed the wild-type Pde1 allele and the Pde1ala252 and Pde1asp252 mutant alleles from the centromeric plasmid YCplac33 in a PKA-attenuated strain (tpk1w1 tpk2Δ tpk3Δ bcy1Δ) that displays an elevated basal cAMP level and a very high cAMP increase after addition of glucose (Nikawa et al., 1987a; Mbonyi et al., 1990). Figure 4 shows that expression of the wild-type Pde1 allele and the Pde1ala252 and Pde1asp252 mutant alleles resulted in the same reduction of the glucose-induced cAMP spike in the PKA-attenuated strain. This confirms that the Pde1asp252 allele does not display more activity than the other two alleles. Interestingly, in this strain the wild-type and Pde1ala252 displayed the same reduction in the cAMP spike, further supporting the idea that lack of PKA-mediated phosphorylation of the serine252 site in Pde1 lowers its activity in vivo to the same extent as substitution of serine252 by alanine. A control experiment in which Pde2 was expressed from the same plasmid in this PKA-attenuated strain further confirmed that only Pde1 is able to down-regulate agonist-induced cAMP signaling (Figure 4).
Figure 4.
Intracellular cAMP level as a function of time after addition of 100 mM glucose to cells of a PKA-attenuated strain (tpk1w1 tpk2Δ tpk3Δ bcy1Δ) expressing an additional copy of distinct Pde1 alleles or Pde2 from the centromeric plasmid YCplac33. The cells were pregrown into exponential phase on SDglucose medium and resuspended after washing with water in the same medium without glucose. Control strain: empty YCplac33 (PM975) (•); YCplac33 + PDE1 (PM976) (○); YCplac33 + pde1ala252 (PM977) (▴); YCplac33 + pde1asp252 (PM 979) (▵); YCplac33 + PDE2 (PM978) (▪).
The Serine252 Site Is Not Important for Pde1 Activity In Vitro
The results mentioned above demonstrate that Pde1 is involved in the feedback inhibition of cAMP accumulation and that serine252 of Pde1 is crucial for this phenomenon. A likely possibility to explain our results would hence be an activation of Pde1 through direct phosphorylation of serine252 by PKA. Serine252 is localized in a perfect PKA recognition site (Kennelly and Krebs, 1991). However, additional potential PKA phosphorylation sites are present in the Pde1 sequence (such as threonine154). On the other hand, an alternative explanation for our results is that the serine252 residue is essential for catalytic activity of Pde1 and that its replacement by an alanine residue simply generates a completely or partially inactive enzyme.
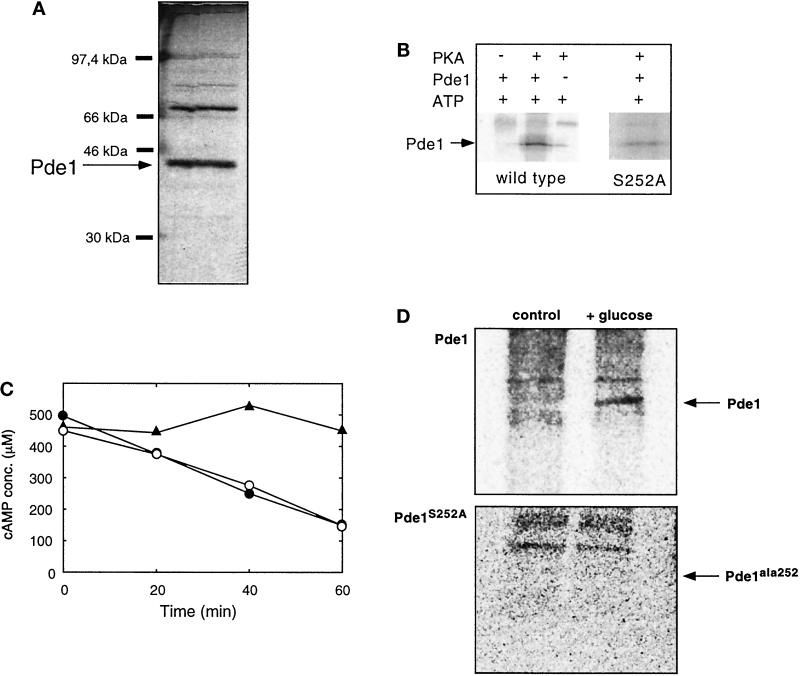
To distinguish between these possibilities, we have purified wild-type Pde1 enzyme and the Pde1ala252 mutant enzyme from cells overexpressing one of the two types to near homogeneity using ion-exchange chromatography, hydrophobic-interaction chromatography, and gel filtration as described in the MATERIALS AND METHODS. A purified preparation separated with SDS-PAGE and visualized with silver staining is shown in Figure 5A. Both wild-type and mutant preparations behaved identically on denaturing gel electrophoresis (our unpublished results). The 42.6-kDa band could be identified as Pde1 on the basis of its recognition by specific Pde1 antibodies (see MATERIALS AND METHODS).
Figure 5.
Purification and phosphorylation of Pde1. (A) SDS-PAGE and protein silver staining of highly purified Pde1 preparation. Pde1 has a molecular mass of 42.6 kDa. (B) Phosphorylation of wild-type Pde1 (250 ng) with PKA and [γ32P]-labeled ATP results in incorporation of label into a band with the same molecular mass as Pde1. In control incubations, either the kinase or Pde1 was omitted. Samples were analyzed by SDS-PAGE and autoradiography. The arrow denotes the migration position of Pde1 (42.6 kDa). The right panel shows phosphorylation of Pde1ala252, which results in much weaker incorporation of label at the same position (see RESULTS). This panel was exposed about 2.5 times longer than the panel of the wild-type strain. (C) A purified preparation of Pde1 was incubated with ATPMg and PKA as described in MATERIALS AND METHODS. Both samples and a negative control (buffer) were subsequently incubated in 100 μl buffer A containing 50 nmol (500 μM) cAMP. At the indicated time points aliquots were taken and assayed for cAMP. Purified Pde1 not treated with PKA (•) or treated with PKA (○); negative control, absence of purified Pde1 (▴). A typical result is shown. (D) HA-tagged Pde1 was immunoprecipitated from glycerol-grown 32P-labeled cells of a wild-type strain (W303–1A) and a Pde1ala252 strain, before or after addition of 2% glucose, and analyzed by electrophoresis and autoradiography. The arrow denotes the migration position of Pde1 or Pde1ala252; background signals correspond to unspecifically labeled protein A.
Purified preparations of the wild-type Pde1 enzyme and the Pde1ala252 mutant form displayed a very similar specific phosphodiesterase activity (7 ± 1.8 μmol cAMP/min/mg for wild-type Pde1 and 7 ± 0.2 μmol cAMP/min/mg for Pde1ala252). This implies that serine252 is not essential for catalytic activity of Pde1 and that our results cannot be explained simply by loss of Pde1 catalytic activity.
Hence, it is most likely that serine252 is important because it is a phosphorylation site of Pde1 and that its phosphorylation, possibly by PKA, is crucial for feedback inhibition. To check whether Pde1 is a substrate of PKA, we incubated a purified preparation (cf. Figure 5A) of wild-type Pde1 with a commercial preparation of bovine heart PKA and [γ32P]-labeled ATP. As shown in Figure 5B, this led to incorporation of radioactive label in a band corresponding to the position of Pde1. Incubation of Pde1 and labeled ATP alone or PKA and labeled ATP alone did not lead to incorporation of label in the 42.6-kDa band (Figure 5B). We can thus conclude that Pde1 is indeed a substrate for PKA. This phosphorylation had, however, no effect on the phosphodiesterase activity of the purified preparation as shown in Figure 5C. On average, the activity of phosphorylated Pde1 was 100.2 ± 6.1% of the control activity (n = 3). Since this Pde1 assay is performed at a high (500 μM) cAMP concentration, we also checked whether phosphorylation had any effect on the phosphodiesterase activity at the much lower concentration of 10 μM. Under these more physiological conditions, however, phosphorylation of purified Pde1 also remained without significant effect on the activity (our unpublished results).
Incubation of the mutant form Pde1ala252 with bovine heart PKA and [γ32P]-labeled ATP still resulted in incorporation of label (Figure 5B). This shows that Pde1 has at least one other in vitro phosphorylation site in addition to serine252. However, determination of the stoichiometry of phosphate incorporation (see MATERIALS AND METHODS) showed that it was reduced from 1.25 mol/mol in the wild-type strain to 0.4 mol/mol for the Pde1ala252 allele. This indicates that mutation of the serine252 residue eliminates a major part of the phosphorylation of Pde1.
We then investigated whether Pde1 was phosphorylated in vivo upon addition of glucose, a physiological condition known to stimulate PKA activity. To do so we labeled wild-type yeast cells, in which the original PDE1 gene was replaced with either an HA-tagged version of this gene or with an HA-tagged version of pde1ala252, with 32phosphate in YP medium containing glycerol as carbon source. Cell extracts were prepared before and 2 min after addition of 2% glucose. Pde1 was subsequently isolated using anti-HA antibodies and analyzed by electrophoresis, blotting, and autoradiography. Addition of glucose resulted in the incorporation of radioactive label in wild-type Pde1, but not in the Pde1ala252 allele (Figure 5D). Further characterization of the labeled residue was hampered by the very low concentration of Pde1 in yeast cells. Based on the purification data reported by Fujimoto et al. (1974) and Londesborough (1974), we calculated that 10 g of yeast cells contains only 11–13 μg (± 0.55 nmol) of Pde1. The in vivo labeling experiments could only be performed with a strain without overexpression of Pde1, since overexpression of this enzyme abolishes the cAMP signal (see above) necessary for PKA activation. Nevertheless, the observation that wild-type Pde1, but not Pde1ala252, was phosphorylated in vivo strongly supports that serine252 represents an in vivo phosphorylation site.
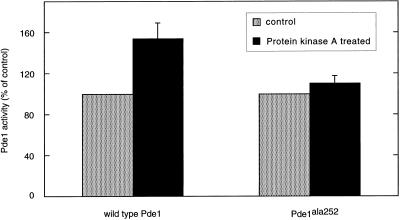
In view of the importance of serine252 for feedback inhibition of cAMP accumulation, we are bound to conclude that, although the phosphorylation of Pde1 by PKA has no direct effect on phosphodiesterase activity of the purified preparation in vitro, it should have an indirect effect in vivo. This could be exerted, for instance, by targeting the phosphorylated form of Pde1 to a specific subcellular location where cAMP degradation preferentially takes place, or by interaction of phosphorylated Pde1 with an activator of phosphodiesterase activity. With respect to the latter possibility, it is noteworthy that incubation of crude extracts from yeast cells overexpressing wild-type Pde1 with MgATP and PKA led to a moderate and highly variable (154 ± 15% of unphosphorylated control, n = 6) increase in phosphodiesterase activity (Figure 6). This increase was not observed after incubation of crude extracts from yeast cells overexpressing the mutant Pde1ala252 form (110 ± 8% of unphosphorylated control, n = 3) (Figure 6), indicating the importance of serine252 for this activation. The activation of wild-type Pde1 by PKA treatment was no longer observed after the first purification step (mono Q chromatography), suggesting that an interacting protein important for the activation was lost during this step.
Figure 6.
Effect of PKA treatment on Pde1 activity in total crude cell extracts from a wild-type strain (W303–1A) and a strain in which PDE1 has been replaced by pde1ala252 (PM581).
DISCUSSION
A Specific Function for Pde1 in Controlling Agonist-induced cAMP Signaling
All previous studies on the two yeast phosphodiesterases, Pde1 and Pde2, indicated a much more prominent role for the high-affinity cAMP phosphodiesterase, Pde2, compared with the low-affinity cAMP phosphodiesterase, Pde1. For all phenotypic characteristics controlled by PKA investigated, deletion of PDE2 always caused strong effects while deletion of PDE1 had small-to-negligible effects (Sass et al., 1986; Nikawa et al., 1987b). The function of Pde1 has always been enigmatic because of its very low affinity for cAMP, at least as measured in vitro. The Km of Pde1 in vitro is one order of magnitude higher than the estimated basal cAMP concentration in vivo (Londesborough, 1974; Londesborough and Lukkari, 1980). However, it was proposed that this low-affinity phosphodiesterase could be involved in degrading the high cAMP levels that transiently occur in yeast cells after stimulation with glucose (Londesborough and Lukkari, 1980). We have now obtained experimental evidence for a specific role of Pde1, as opposed to Pde2, in controlling glucose- and also acidification-induced stimulation of cAMP accumulation. The following results are indicative for such a function: 1) deletion of Pde1, but not of Pde2, results in much higher glucose- and acidification-induced cAMP accumulation (Figure 1, A and B); 2) overexpression of Pde1, but not Pde2, abolishes glucose- and acidification-induced cAMP accumulation (Figure 1, C and D); and 3) overexpression of Pde2, but not of Pde1, enhanced the basal heat resistance of the cells (Figure 2), which is indicative of a lower basal cAMP level (Iida and Yahara, 1984; Shin et al., 1987). Hence, Pde1 appears to have a specific role in down-regulating agonist-induced cAMP increases (in transient, adaptation conditions), while Pde2 specifically controls the basal cAMP level in the cell (in stable conditions, i.e., during growth and in stationary phase).
Pde1 Activity Is Most Likely Controlled by Phosphorylation In Vivo
In addition to providing experimental evidence that Pde1 is specifically involved in controlling agonist-induced cAMP accumulation, our data suggest that its activity is controlled by phosphorylation. Previous results have pointed to the possibility that phosphodiesterase activity in yeast might be controlled by PKA-mediated phosphorylation. Deletion of the two phosphodiesterase genes in a RAS2val19 strain caused a very high increase in the cAMP level, but in a strain with attenuated PKA activity there is a similar very high cAMP level in spite of the presence of the phosphodiesterases (Nikawa et al., 1987a). This indicates that high PKA activity in some way is required for efficient breakdown of cAMP by the phosphodiesterases (Thevelein, 1992). In the present article we show that this is true for both Pde1 and Pde2 since the presence of either Pde1 or Pde2 in a RAS2val19 strain is sufficient to lower the cAMP level to about the wild-type level (Figure 3D). We have also demonstrated previously that the feedback inhibition on cAMP accumulation plays a role in down-regulating the glucose-induced cAMP signal. In yeast strains with a different activity level of PKA, the glucose-induced cAMP signal was inversely correlated with PKA activity (Mbonyi et al., 1990). This suggests that agonist-induced cAMP accumulation is down-regulated by cAMP itself through PKA-mediated stimulation of phosphodiesterase activity.
In the present article we have provided several arguments for regulation of Pde1 by PKA-mediated phosphorylation. 1) Mutagenesis of a putative PKA phosphorylation site (serine252) in Pde1 causes dramatic effects on cAMP accumulation in vivo (Figure 3). The most striking quantitative difference between wild-type Pde1 and the Pde1ala252 allele was observed in a RAS2val19 pde2Δ background. The presence of Pde1 in such a background resulted in about the same cAMP level as in wild-type or RAS2val19 strains, whereas the presence of Pde1ala252 in the same background resulted in a similar very high cAMP level as observed in the RAS2val19 pde1Δ pde2Δ strain (Figure 3D). This result is in agreement with the conclusion that phosphorylation of the serine252 site influences the in vivo cAMP phosphodiesterase activity of Pde1 to such an extent that it is able to hydrolyze very efficiently the huge cAMP levels that acumulate in a RAS2val19 strain. 2) In a PKA-attenuated strain (tpk1w1 tpk2Δ tpk3Δ bcy1Δ) there was no difference between wild-type Pde1 and the Pde1ala252 allele in their capacity to reduce the cAMP level (Figure 4). This corroborates that PKA-mediated phosphorylation of the serine252 site is required for the difference in activity in vivo between the wild-type Pde1 and mutant Pde1ala252 allele. 3) The Pde1 enzyme can be phosphorylated in vitro with PKA (Figure 5B) and in vivo by addition of glucose to derepressed cells (Figure 5D). Phosphorylation of the Pde1ala252 allele was strongly reduced in vitro (Figure 5B) while it was not phosphorylated at all in vivo under the same experimental conditions as the wild-type allele (Figure 5D). The activity of Pde1 in vitro was not affected by site-directed mutagenesis of serine252 nor did PKA treatment of purified Pde1 affect its activity. The value of 1.25 mol/mol obtained for the stoichiometry of phosphate incorporation in vitro into the wild-type Pde1 allele indicates that insufficient phosphate incorporation cannot be the cause of the absence of effect of phosphorylation on the enzymatic activity. Moreover, in the Pde1ala252 allele the stoichiometry was reduced to 0.4 mol/mol indicating a major effect of serine252 mutagenesis on the phosphorylation of the enzyme. The fact that the wild-type Pde1 and mutant Pde1ala252 alleles displayed the same enzymatic activity in vitro is interesting because it clearly demonstrates that site-directed mutagenesis of serine252 does not simply abolish or reduce the activity of the enzyme, which would have made this manipulation similar to a deletion or partial inactivation. It shows that serine252 is important for another reason, presumably as a phosphorylation site. The absence of in vivo phosphorylation of the Pde1ala252 allele is consistent with the latter. In crude extracts a modest increase in activity of Pde1 could be observed upon PKA treatment, suggesting that phosphorylation of Pde1 could lead to interaction with an activating protein (Figure 6). Taken together, our results indicate that serine252 of Pde1 is not essential for catalytic activity, but most likely represents a PKA phosphorylation site of which the phosphorylation promotes interaction of Pde1 with protein(s) that in some way enhances its cAMP phosphodiesterase activity or at least renders it much more efficient in hydrolyzing cAMP in vivo. Interaction with such an activating protein in vivo might explain why Pde1, in spite of its high, supraphysiological Km in vitro, exerts an important effect on agonist-induced cAMP signaling. At present we cannot exclude, however, that in vivo a PKA-induced kinase (rather than PKA itself) phosphorylates serine252. Such an indirect effect of PKA would offer an alternative explanation why in vitro phosphorylation of Pde1 with PKA remains without effect.
Interference with Feedback Inhibition of cAMP Synthesis
Our results on the effect of PDE deletion on agonist-induced cAMP signaling illustrate that interpretation of the effects observed is not straightforward. Normally one would expect reduction of phosphodiesterase activity to result in higher cAMP increases. However, this is only observed for PDE1 deletion (Figure 1, A and B). Deletion of PDE2 causes either a slight decrease or no significant effect, while more strikingly double deletion of PDE1 and PDE2 causes a complete elimination of the agonist-induced cAMP increases (Figure 1, A and B). The basal cAMP level was clearly enhanced in the pde1Δ pde2Δ strain and in the pde1ala252 pde2Δ strain (Figures 1 and 3), and pde1Δ pde2Δ strains are well known to display a phenotype indicative of elevated PKA activity (Sass et al., 1986; Nikawa et al., 1987b). Therefore, a likely explanation for the absence of the increases of cAMP in these strains is that the elevated PKA activity causes constitutively high feedback inhibition of cAMP synthesis. As a result, agonist-induced cAMP signaling is constitutively down-regulated. A similar elimination of the agonist-induced cAMP increases has been observed in bcy1Δ strains, which also display constitutively high PKA activity (Colombo et al., 1998).
The Pde1 Class of Phosphodiesterases
At present, only four phosphodiesterases with homology to Pde1 have been identified (Wera et al., 1997). The enzymes of this class seem to have rather versatile functions, but interestingly both the C. albicans (Hoyer et al., 1994) and S. pombe (DeVoti et al., 1991) Pde1 homologues contain a PKA recognition site at a similar location C-terminal of the conserved catalytic domain, serine238 and serine251, respectively. This part of the Pde1 homologues is otherwise not well conserved. The presence of the conserved PKA recognition site might indicate that the C. albicans and S. pombe Pde1 enzymes are also involved in controlling agonist-induced cAMP signaling. The existence of a specific cAMP phosphodiesterase for control of this process in S. cerevisiae underscores the physiological importance of rapid cAMP signaling. A proper, precisely modulated response to sudden changes in the nutrient supply might provide a selective advantage, not only in yeasts but also in other microorganisms.
ACKNOWLEDGMENTS
We thank W. Verheyden for excellent technical assistance and J. Morren for help with the figures. This work was supported by fellowships from the Katholieke Universiteit Leuven to P.M., the Fund for Scientific Research-Flanders (Senior research assistant) to S.W., and by grants from the Fund for Scientific Research-Flanders and the Research Fund of the Katholieke Universiteit Leuven (Concerted Research Actions).
Abbreviation used:
- PKA
protein kinase A
REFERENCES
- Argüelles JC, Mbonyi K, Van Aelst L, Vanhalewyn M, Jans AWH, Thevelein JM. Absence of glucose-induced cAMP signaling in the Saccharomyces cerevisiae mutants cat1 and cat3 which are deficient in derepression of glucose-repressible proteins. Arch Microbiol. 1990;154:199–205. doi: 10.1007/BF00423333. [DOI] [PubMed] [Google Scholar]
- Beullens M, Mbonyi K, Geerts L, Gladines D, Detremerie K, Jans AWH, Thevelein JM. Studies on the mechanism of the glucose-induced cAMP signal in glycolysis and glucose repression mutants of the yeast Saccharomyces cerevisiae. Eur J Biochem. 1988;172:227–231. doi: 10.1111/j.1432-1033.1988.tb13877.x. [DOI] [PubMed] [Google Scholar]
- Boeke JD, Lacroute F, Fink GR. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5′-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Broach JR, Deschenes RJ. The function of RAS genes in Saccharomyces cerevisiae. Adv Cancer Res. 1990;54:79–139. doi: 10.1016/s0065-230x(08)60809-x. [DOI] [PubMed] [Google Scholar]
- Broek D, Samiy N, Fasano O, Fujiyama A, Tamanoi F, Northup J, Wigler M. Differential activation of yeast adenylate cyclase by wild type and mutant Ras proteins. Cell. 1985;41:763–769. doi: 10.1016/s0092-8674(85)80057-x. [DOI] [PubMed] [Google Scholar]
- Caspani G, Tortora P, Hanozet GM, Guerritore A. Glucose-stimulated cAMP increase may be mediated by intracellular acidification in Saccharomyces cerevisiae. FEBS Lett. 1985;186:75–79. [Google Scholar]
- Charbonneau H, Beier N, Walsh KA, Beavo JA. Identification of a conserved domain among cyclic nucleotide phosphodiesterases from diverse species. Proc Natl Acad Sci USA. 1986;83:9308–9312. doi: 10.1073/pnas.83.24.9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo S, Ma P, Cauwenberg L, Winderickx J, Teunissen A, Nauwelaers D, de Winde JH, Gorwa M-F, Colavizza D, Thevelein JM. Involvement of distinct G-proteins, Gpa2 and Ras, in glucose- and intracellular acidification-induced cAMP signalling in the yeast Saccharomyces cerevisiae. EMBO J. 1998;17:3326–3341. doi: 10.1093/emboj/17.12.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M, Nemoz G, Sette C, Vicini E. Recent progress in understanding the hormonal regulation of phosphodiesterases. Endocr Rev. 1995;16:370–389. doi: 10.1210/edrv-16-3-370. [DOI] [PubMed] [Google Scholar]
- De Vendittis E, Vitelli A, Zahn R, Fasano O. Suppression of defective RAS1 and RAS2 functions in yeast by an adenylate cyclase activated by a single aminoacid change. EMBO J. 1986;5:3657–3663. doi: 10.1002/j.1460-2075.1986.tb04696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoti J, Seydoux G, Beach D, McLeod M. Interaction between ran1+ protein kinase and cAMP dependent protein kinase as negative regulators of fission yeast meiosis. EMBO J. 1991;10:3759–3768. doi: 10.1002/j.1460-2075.1991.tb04945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap PV, Callahan SM. Characterization of a periplasmic 3′:5′-cyclic nucleotide phosphodiesterase gene, cpdP, from the marine symbiotic bacterium Vibrio fischeri. J Bacteriol. 1993;175:4615–4624. doi: 10.1128/jb.175.15.4615-4624.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Ichikawa A, Tomita K. Purification and properties of adenosine 3′,5′-monophosphate phosphodiesterase from Baker’s yeast. Arch Biochem Biophys. 1974;161:54–63. [Google Scholar]
- Gietz RD, Sugino A. New-yeast Escherichia coli shuttle vectors constructed with in vitro mutagenized genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Hoyer LL, Cieslinski LB, McLaughlin MM, Torphy TJ, Shatzman AR, Livi GP. A Candida albicans cyclic nucleotide phosphodiesterase: cloning and expression in Saccharomyces cerevisiae and biochemical characterization of the recombinant enzyme. Microbiology. 1994;140:1533–1542. doi: 10.1099/13500872-140-7-1533. [DOI] [PubMed] [Google Scholar]
- Iida H, Yahara I. A heat shock-resistant mutant of Saccharomyces cerevisiae shows constitutive synthesis of two heat shock proteins and altered growth. J Cell Biol. 1984;99:1441–1450. doi: 10.1083/jcb.99.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JS, Prakash L. Yeast Saccharomyces cerevisiae selectable markers in pUC18 polylinkers. Yeast. 1990;6:363–366. doi: 10.1002/yea.320060502. [DOI] [PubMed] [Google Scholar]
- Kennelly PJ, Krebs EG. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991;266:15555–15558. [PubMed] [Google Scholar]
- Lacombe ML, Podgorski GJ, Franke J, Kessin RH. Molecular cloning and developmental expression of the cyclic nucleotide phosphodiesterase gene of Dictyostelium discoideum. J Biol Chem. 1986;261:16811–16817. [PubMed] [Google Scholar]
- Londesborough J. Partial purification and characterization of an adenosine 3′,5′-cyclic monophosphate phosphodiesterase from Saccharomyces cerevisiae. Biochem Soc Trans. 1974;2:398–400. [Google Scholar]
- Londesborough J, Lukkari TM. The pH and temperature dependence of the activity of the high Km cyclic nucleotide phosphodiesterase of baker’s yeast. J Biol Chem. 1980;255:9262–9267. [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mbonyi K, Van Aelst L, Argüelles JC, Jans AWH, Thevelein JM. Glucose-induced hyperaccumulation of cyclic AMP and defective glucose repression in yeast strains with reduced activity of cyclic AMP-dependent protein kinase. Mol Cell Biol. 1990;10:4518–4523. doi: 10.1128/mcb.10.9.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munder T, Küntzel H. Glucose-induced cAMP signaling in Saccharomyces cerevisiae is mediated by the CDC25 protein. FEBS Lett. 1989;242:341–345. doi: 10.1016/0014-5793(89)80498-3. [DOI] [PubMed] [Google Scholar]
- Nikawa J, Cameron S, Toda T, Ferguson KW, Wigler M. Rigorous feedback control of cAMP levels in Saccharomyces cerevisiae. Genes Dev. 1987a;1:931–937. doi: 10.1101/gad.1.9.931. [DOI] [PubMed] [Google Scholar]
- Nikawa J, Sass P, Wigler M. Cloning and characterization of the low-affinity cyclic AMP phosphodiesterase gene of Saccharomyces cerevisiae. Mol Cell Biol. 1987b;7:3629–3636. doi: 10.1128/mcb.7.10.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick RJ, Racker E. Phosphorylation of the RAS2 gene product by kinase A inhibits the activation of yeast adenylyl cyclase. Proc Natl Acad Sci USA. 1988;85:2474–2478. doi: 10.1073/pnas.85.8.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar G, Sommer SS. The “Megaprimer” method of site-directed mutagenesis. BioTechniques. 1990;8:404–407. [PubMed] [Google Scholar]
- Sass P, Field J, Nikawa J, Toda T, Wigler M. Cloning and characterization of the high-affinity cAMP phosphodiesterase of S. cerevisiae. Proc Natl Acad Sci USA. 1986;83:9303–9307. doi: 10.1073/pnas.83.24.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D-Y, Matsumoto K, Iida H, Uno I, Ishikawa T. Heat shock response of Saccharomyces cerevisiae altered in cyclic AMP-dependent protein phosphorylation. Mol Cell Biol. 1987;7:244–250. doi: 10.1128/mcb.7.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suoranta K, Londesborough J. Purification of intact and nicked forms of a zinc-containing, Mg+-dependent, low Km cyclic AMP phosphodiesterase from baker’s yeast. J Biol Chem. 1984;259:6964–6971. [PubMed] [Google Scholar]
- Tanaka K, Matsumoto K, Toh-e A. Ira1, an inhibitory regulator of the RAS-cyclic AMP pathway in Saccharomyces cerevisiae. Mol Cell Biol. 1989;9:757–768. doi: 10.1128/mcb.9.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Nakafuku M, Tamanoi F, Kaziro Y, Matsumoto K, Toh-e A. IRA2, a second gene in Saccharomyces cerevisiae that encodes a protein with a domain homologous to mammalian Ras GTP-ase activating protein. Mol Cell Biol. 1990;10:4303–4313. doi: 10.1128/mcb.10.8.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatchell K. RAS genes in the budding yeast Saccharomyces cerevisiae. In: Kurjan J, Taylor BJ, editors. RAS Genes in the Budding Yeast Saccharomyces cerevisiae. San Diego, CA: Academic Press; 1993. pp. 147–188. [Google Scholar]
- Thevelein JM. Fermentable sugars and intracellular acidification as specific activators of the Ras-adenylate cyclase signalling pathway in yeast: the relationship to nutrient-induced cell cycle control. Mol Microbiol. 1991;5:1301–1307. doi: 10.1111/j.1365-2958.1991.tb00776.x. [DOI] [PubMed] [Google Scholar]
- Thevelein JM. The RAS-adenylate cyclase pathway and cell cycle control in Saccharomyces cerevisiae Antonie Leeuwenhoek, J. Microbiol. 1992;62:109–130. doi: 10.1007/BF00584466. [DOI] [PubMed] [Google Scholar]
- Thevelein JM, Beullens M, Honshoven F, Hoebeeck G, Detremerie K, den Hollander JA, Jans AWH. Regulation of the cAMP level in the yeast Saccharomyces cerevisiae: intracellular pH and the effect of membrane depolarizing compounds. J Gen Microbiol. 1987a;133:2191–2196. doi: 10.1099/00221287-133-8-2191. [DOI] [PubMed] [Google Scholar]
- Thevelein JM, Beullens M, Honshoven F, Hoebeeck G, Detremerie K, Griewel B, den Hollander JA, Jans AWH. Regulation of the cAMP level in the yeast Saccharomyces cerevisiae: the glucose-induced cAMP signal is not mediated by a transient drop in the intracellular pH. J Gen Microbiol. 1987b;133:2197–2205. doi: 10.1099/00221287-133-8-2197. [DOI] [PubMed] [Google Scholar]
- Thomas BJ, Rothstein RJ. Elevated recombination rates in transcriptionally active DNA. Cell. 1989;56:619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- Toda T, Uno I, Ishikawa T, Powers S, Kataoka T, Broek D, Cameron S, Broach J, Matsumoto K, Wigler M. In yeast, Ras proteins are controlling elements of adenylate cyclase. Cell. 1985;40:27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Trevillyan JM, Pall ML. Control of cyclic adenosine 3′,5′-monophosphate levels by depolarizing agents in fungi. J Bacteriol. 1979;138:397–403. doi: 10.1128/jb.138.2.397-403.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plaat JB. Cyclic 3′,5′-adenosine monophosphate stimulates trehalose degradation in baker’s yeast. Biochem Biophys Res Commun. 1974;56:580–587. doi: 10.1016/0006-291x(74)90643-3. [DOI] [PubMed] [Google Scholar]
- Wera S, Ma P, Thevelein JM. Glucose exerts opposite effects on mRNA versus protein and activity levels of Pde1, the low-affinity cAMP phosphodiesterase from budding yeast Saccharomyces cerevisiae. FEBS Lett. 1997;420:147–150. doi: 10.1016/s0014-5793(97)01508-1. [DOI] [PubMed] [Google Scholar]