Abstract
Chronic stress may lead to neuronal atrophy and functional impairments within the CNS, and increasing evidence indicates that exercise can protect the brain from these changes. Bax is a key protein of the B-cell lymphoma (Bcl) family that complexes within the mitochondrial membrane and forms pores to initiate cellular apoptosis. Herein, we measured cortical Bax levels following chronic and acute stress via immunoblotting. We reveal that chronic, but not acute, stress increases cortical levels of Bax oligomer 270, a complex revealed in previous studies to be associated with apoptosis. Several recent studies have revealed that physical exercise can protect rodents from neurochemical and/or behavioral changes occurring with stress. Previous studies have also revealed that voluntary exercise enhances the expression and activation of cellular proteins associated with enhanced neuronal survival. Herein, we reveal that three weeks of daily restraint led to increased oligomerization of Bax within the cerebral cortex, and that chronic corticosterone administration had a similar effect. Voluntary wheel running, concurrent with chronic restraint, prevented an increase in Bax oligomer 270. Analysis of subcellular fractions also revealed that the combination of exercise with chronic stress reduced the percent of total Bax localized to the mitochondria. Ours is the first study to investigate dynamic molecule complexes associated with the initiation of apoptosis with stress, and the influence of exercise upon the levels of these complexes, suggesting that exercise is an effective preventative measure that can promote neuronal survival and protect the brain against the damaging effects of chronic stress.
Keywords: neuroprotection, apoptosis, mitochondria
Introduction
Chronic stress is known to lead to atrophy and functional impairments in several key brain areas, including the cerebral cortex [21], and has been linked to the development of major depression [8]. Evidence is emerging that voluntary physical activity can protect the brain from stress-induced changes in neuronal function. Changes in rodent behavior due to chronic stress, such as decreased sucrose consumption, decreased motor activity and impaired learning have been prevented when animals were allowed voluntary access to a running wheel during or immediately following the period of stress [15, 33]. Also, neurochemical changes due to chronic stress, such as altered glucocorticoid sensitivity, were prevented with concurrent wheel running [14]. Neurodegeneration occurs with chronic stress; coupled to these changes is enhanced activation of apoptosis and decreased neuronal survival [21, 25]. Voluntary exercise, on the other hand, robustly increases growth factor and growth-associated molecule expression in the cerebral cortex and hippocampus [24, 35], and has recently been shown to enhance the activation of intracellular signal transduction pathways promoting neuronal survival [10, 34].
In this study, we investigated the effects of chronic restraint stress on the level of activation, reflected in the quaternary structure, of cortical B-cell associated X protein (Bax), a key protein of the B-cell lymphoma (Bcl) family that promotes apoptosis. Endogenous Bax is cytoplasmic in growing cells, and is drawn to the mitochondrial membrane following an apoptotic stimulus. Bax integrates into mitochondrial membranes as a multispanning membrane protein monomer that is subsequently polymerized into an oligomer in order to form pores in the membrane [3]. Previous studies have demonstrated the formation of high molecular weight oligomers in the mitochondrial membranes of apoptotic cells, with a core structure of several Bax subunits and approximate molecular weights of 260,000 and 96,000 Dal [4]. Using native (non-denaturing) Western analysis, we studied the size and concentration of Bax oligomers within cortical neurons of rats subjected to 3 weeks of restraint stress with and without access to a running wheel throughout the time of stress exposure. Total Bax localized to the mitochondrial cellular compartment vs. the cytosol was also measured using differential centrifugation and standard Western analysis. In addition to chronic restraint stress, we investigated the effects of acute immobilization stress and chronic corticosterone exposure on Bax oligomer formation in the cerebral cortex. These alternate stress paradigms have led to significant neurochemical changes in previous studies [1, 9], and the effects of acute immobilization have been prevented with voluntary exercise [1].
Materials and Methods
Experimental Subjects and Handling
Male Sprague-Dawley rats (3 months of age, 250–300 g) were obtained from Charles River (Wilmington, MA). Rats were housed singly with wood chips as bedding and fed standard rodent chow and water ad libitum. All handling procedures were in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals (1996) and our university’s Institutional Animal Care and Use Committee.
Chronic Restraint, Voluntary Exercise
Rats in the chronic restraint study were assigned randomly to four groups, at n = 7: 1) Sedentary/No Stress, 2) Sedentary/Stress, 3) Activity/No Stress and 4) Activity/Stress. Animals assigned to restraint stress were restrained for 21 consecutive days, for 6 hours/day (0900 to 1500) in polyethylene tubes (diameter = 9 cm). This is a model of chronic stress utilized in several previous studies [16, 19, 28]. Animals assigned to voluntary exercise were given free access to running wheels within their home cages (34.5 cm in diameter; Nalgene OR.) throughout the experiment. The distance traveled per 24-hr period was recorded by computer using Ratrun Software (C. Hage Associates, CA). Sedentary animals remained in polyethylene cages without running wheels. Plasma corticosterone levels were measured weekly via a corticosterone ELISA kit (Assay Designs, Ann Arbor, MI). Animals were sacrificed at 0600h following the last restraint period. Left motor cortices were extracted and stored at −80°C until analysis.
Acute Restraint
During the acute stress study, rats (n = 7) were restrained with tapered plastic film tubes (DecapiCones, Braintree Scientific, Braintree, MA; average diameter = 8 cm) for 4 hours per day × 3 days, as utilized in previous studies [1, 22]. Control animals were not restrained and remained in their home cages during the experiment.
Experimentally Elevated Serum Corticosterone Concentration
Animals assigned to chronic corticosterone treatment had three pellets (200 mg; Innovative Research of America, FL) surgically implanted subcutaneously at back of the neck one week prior to experimentation. These pellets remained for the entire 3 weeks of experimentation. The animals assigned to the placebo group had three placebo pellets surgically implanted in the same location as the corticosterone pellets, also for the same time course. Cage control animals did not undergo surgery and remained in their cages throughout the experiment. Plasma corticosterone levels were measured weekly via a corticosterone ELISA kit (Assay Designs, Ann Arbor, MI). Plasma corticosterone levels in animals receiving active pellets was 34.36 +/− 1.3 ug/dl at the time of the experiment, compared to 17.17 +/− 2.8 ug/dl in the placebo group (p < 0.01). These levels model those demonstrated during chronic mild stress [7], and are similar to corticosterone levels obtained during previous chronic hormone administration studies [9].
Differential Centrifugation for the Isolation of Specific Subcellular Fractions
Left motor cortices were thawed over ice and homogenized using a motor-driven teflon pestle in ice-cold buffer consisting of 210 mM mannitol, 70 mM sucrose, 10 mM HEPES, 1 mM EDTA, and 1 mM EGTA, containing 1 protease inhibitor tablet (Complete®, Roche Diagnostics, Mannheim, Germany) per 10 ml, pH 7.4. Samples were centrifuged at 1,200 × g at 4°C for 20 minutes and the pellet was discarded. The supernatant was then centrifuged at 10,000 × g at 4°C for 30 minutes resulting in a mitochondrial pellet and a cytosolic supernatant fraction. The mitochondrial pellet was then resuspended in sucrose buffer consisting of 395 mM sucrose, 0.4 mM EGTA, and 40 mM HEPES, pH 7.4. Protein concentrations were determined using the Lowry method [18]. Samples were stored at −80°C for Western blotting (below).
SDS-PAGE and Western Blotting
Equal amounts of protein (30 µg) were applied to each lane of an SDS-PAG, the proteins electro-transferred to nitrocellulose (Amersham-Pharmacia Biotech, Piscataway, NJ). Western blotting was performed according to the antibody manufacturer’s instructions for Bax (Cell Signaling, Beverly, MA) and immunoreactivity visualized using enhanced chemiluminescence (ECL, Amersham-Pharmacia Biotech, Piscataway, NJ). To control for inadvertent differences in protein loading, each blot was then stripped (100 mM 2-mercapto-ethanol, 2% SDS, 62.5 mM Tris-HCl, pH 6.7, 55°C, 10 min, with agitation) and re-probed with anti-GAPDH in accordance with the manufacturer’s instructions (Advanced Immunochemicals, Long Beach, CA), followed by ECL.
Quantification from film was implemented using computer-assisted densitometry (MCID Image Processing System, St. Catherine’s, Ontario, Canada). Bax optical density levels were divided by corresponding GAPDH optical density levels. Data were then statistically normalized with respect to values obtained with controls (Sedentary/No stress). A one-way ANOVA and Fisher’s Least Significant Difference post hoc Test (PLSD) were used to test for differences (p < .05) among the 4 treatment groups at n = 7 rats per treatment.
Native PAGE and Western Blotting
Native gel electrophoresis and subsequent Western blotting were identical to conventional SDS-PAGE and Western blotting, except that all denaturing conditions (e.g., boiling, SDS, 2-mercaptoethanol) were omitted, thereby allowing the proteins to maintain their native structure [13]. Thus, the level of Bax present as monomer, dimer and oligomer species were assessed.
Four proteins of known molecular weights were used as markers to calibrate a standard curve of electrophoretic mobility of native proteins: bovine serum albumin (132 kDa, dimer; 66 kDa, monomer), chicken egg albumin (45 kDa), urease, 545 kDal, hexamer; 272 kDa, trimer), and carbonic anhydrase (29 kDa) (Sigma, St. Louis, MO) were electrophoresed on non-denaturing gels. These markers were electrophoresed at the polyacrylamide concentrations of 6%, 8%, 10% and 12%. Gels were then silver-stained to determine banding pattern obtained by the separation of the protein markers. The distance traveled by the protein marker, including all isomeric forms of each protein marker, were measured relative to the tracking dye front, given by Rf = distance of protein migration/distance of tracking dye migration, where Rf is the electrophoretic mobility of the particular the protein marker. The Rf values were then used to calculate (x=100 × (log (Rf × 100), and plotted against the percent polyacrylamide concentration to produce a linear graph for each protein marker. The slope obtained for this linear graph yields a retardation coefficient (KR), whose logarithm of the negative slope for each protein marker is plotted against the logarithm of the known molecular weights of the markers, generating a linear plot, and thereby enabling the determination of the molecular weight of the unknown. (For this study: R2 = .7228, p = .0018). Once gel electrophoresis was complete as indicated by the separated molecular weight markers, the gels were silver-stained using conventional procedures [23]. Refer to figure 1 for a sample native blot, indicating the different Bax complexes measured via densitometry.
Figure 1.
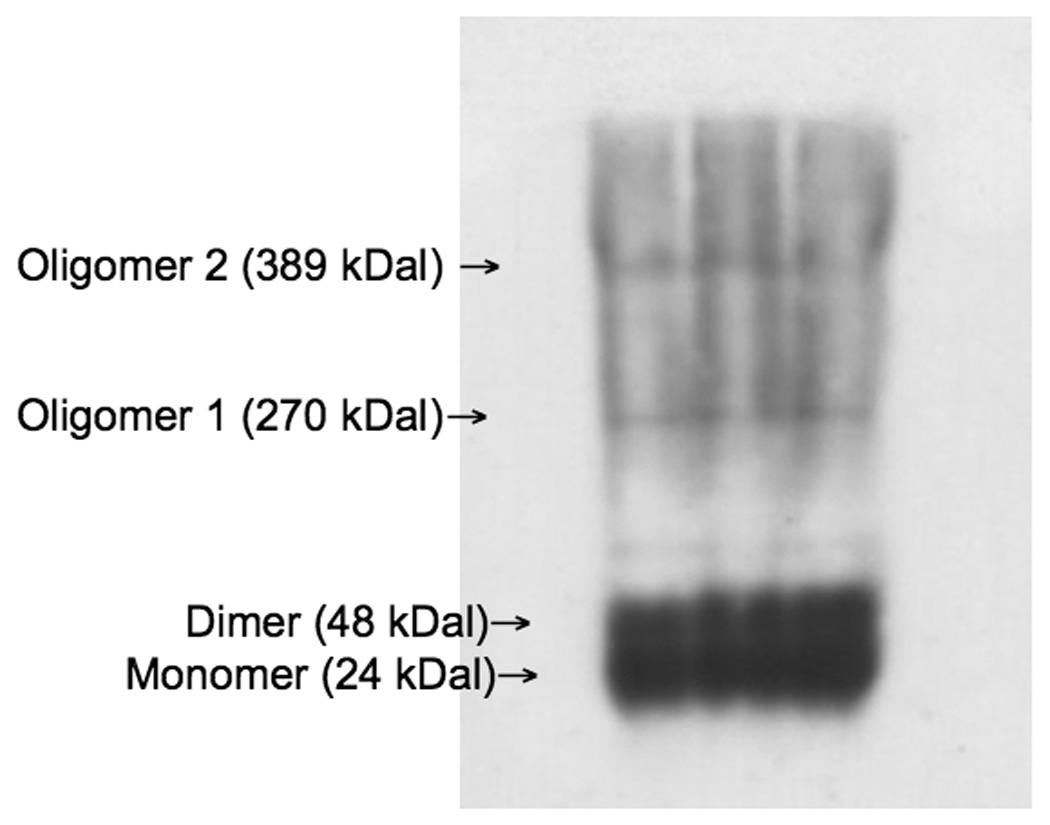
Sample native Western blot for Bax, indicating bands corresponding to the various complexes of Bax assessed in this study: monomer (24 kDa), dimer (48 kDa), oligomer 1 (270 kDa) and oligomer 2 (389 kDa).
Results
Non-denaturing Western blot of cortical homogenates revealed increased Bax oligomer (270 kDa) levels with chronic (but not acute) restraint stress
This result was duplicated when animals received corticosterone treatment via slow-release subcutaneous pellets in place of restraint. Increases in Bax oligomer 270 due to chronic restraint were prevented when animals engaged in voluntary wheel running during the period of stress. There was no difference in the level of activity between stressed (4,840 +/− 412 meters/day) and non-stressed (4,015 +/− 385 meters/day) animals. Plasma corticosterone levels were not significantly changed due to chronic restraint or exercise at any point during the experiment (data not shown).
Native Western analysis of cortical homogenates revealed no significant differences in Bax monomer (26.6 kDa), dimer (48.4 kDa), or oligomer 2 (720 kDa) levels between any of the treatment groups in our experiments (data not shown). In contrast, there was a 2-fold increase in Bax oligomer (270 kDa) formation in cortical homogenates of animals that underwent chronic (21 days) restraint stress (Figure 2; F (3,18) = 3.29, p = 0.044). This result was not evident with the combination of exercise and stress. In contrast, no changes in Bax levels were evident following acute (4 days) restraint stress, either with or without prior exercise (data not shown).
Figure 2.
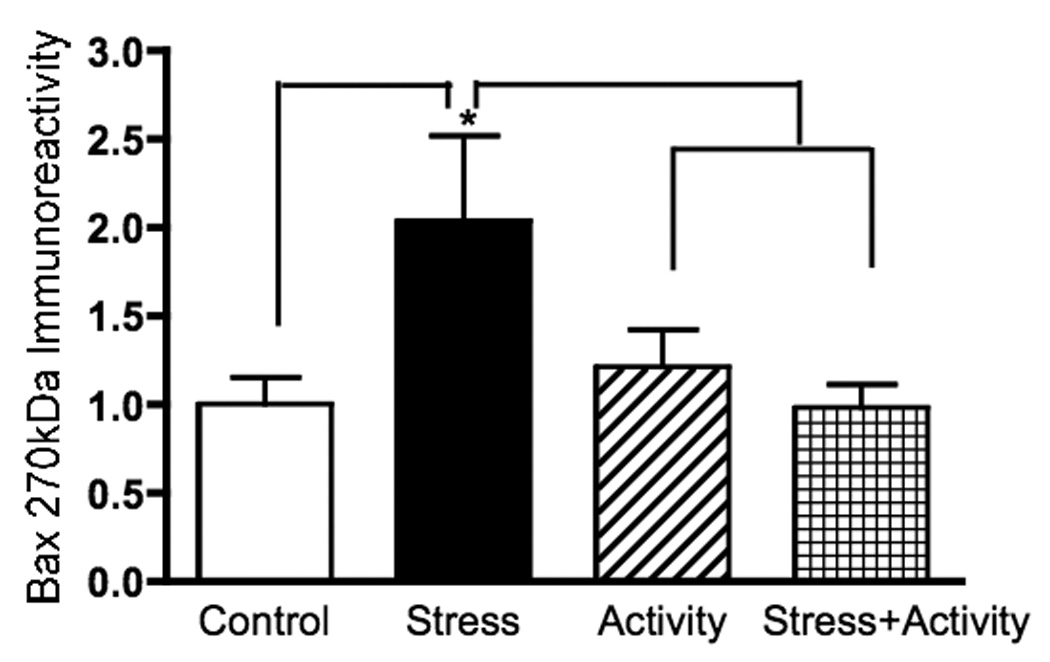
The presence of the Bax oligomer (270 kDal) was increased approximately twofold following chronic (3 weeks) restraint stress. This change was not evident when animals engaged in voluntary exercise concurrent with the period of stress. Statistical analyses entailed the use of one-way ANOVA followed by Fisher’s PLSD multiple comparisons test. The asterisk indicates a statistically significant difference from control values; brackets indicate groups that are significantly different from each other (p < 0.05).
Chronic corticosterone administration via subcutaneous pellet was followed by a 2-fold increase in Bax oligomer (270 kDa) formation in cortical homogenates as compared to control animals (Figure 3; F (2,19) = 2.16, p = 0.05). As noted in the chronic restraint study, no changes were noted in the levels of Bax monomer (26.6 kDa), dimer (48.4 kDa), or oligomer 2 (720 kDa) within the cerebral cortices of animals receiving chronic corticosterone (data not shown). There was no significant correlation between individual plasma corticosterone levels and levels of Bax oligomer 270.
Figure 3.
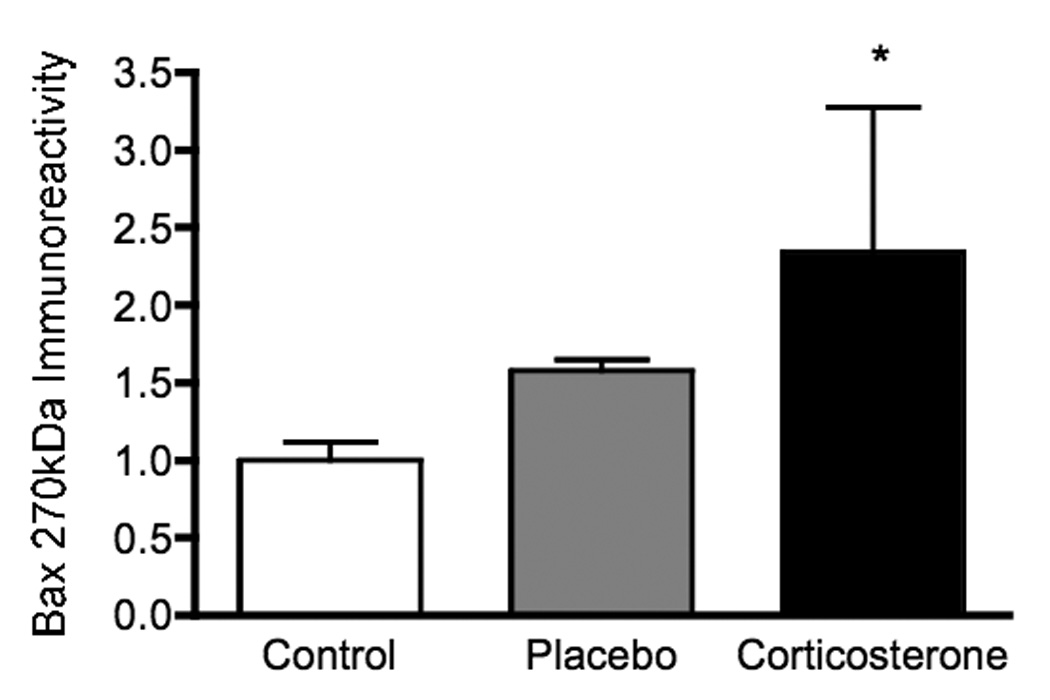
Chronic corticosterone administration via subcutaneous pellet was followed by a 2-fold increase in Bax oligomer (270 kDa) formation in cortical homogenates as compared to control animals. Statistical analyses were performed via one-way ANOVA followed by Fisher’s PLSD multiple comparisons test, and the asterisk indicates the significant increase compared to control (p < 0.05).
Subcellular fractionation of cortical homogenates revealed that the combined treatment of activity and stress significantly reduced Bax localization to the mitochondria
Subcellular fractionation followed by (standard denaturing) Western immunoblotting indicated that the combined treatment of both stress and activity significantly reduced the percent of total Bax (28 kD band) localized to the mitochondria as compared to control (p = 0.041) and animals that underwent exercise alone (Figure 4; F (3,15) = 2.32, p = 0.034).
Figure 4.

Subcellular fractionation followed by (standard denaturing) Western immunoblotting revealed that the combined treatment of both stress and activity significantly reduced the percent of total Bax localized to the mitochondria as compared to controls and animals that underwent exercise alone. Statistical analyses entailed the use of one-way ANOVA followed by Fisher’s PLSD multiple comparisons test. The asterisk indicates statistically significant difference from the control value (p < 0.05); brackets denote significant difference between other groups (p < 0.05).
Discussion
A wealth of evidence from recent years indicates that regular, repeated voluntary exercise promotes neuronal survival and growth. In animal studies, voluntary wheel running rapidly enhances the expression of the neurotrophin brain-derived neurotrophic factor (BDNF) in the cerebral cortex and hippocampus [26, 29] and enhances the activation state of a number of molecules along survival-promoting intracellular pathways [10]. Evidence also indicates that prolonged stress may inhibit growth and survival mechanisms at the cellular level, leading to neuronal atrophy and apoptosis [20, 21]. The results of our study indicate that chronic, but not acute, stress activates a key molecular complex essential for mitochondrial apoptosis, and that regular exercise during the period of stress prevents this activation.
Both human clinical and animal studies indicate that chronic stress or chronic depressive disorders may lead to neuronal atrophy within the brain. Chronic stress has been shown to induce features of neuropathology similar to those observed in major depression, such as neurite beading, dendritic branch retraction and reduced tissue volume [20, 30]. Stress-induced cellular atrophy and changes in neurotrophin expression have been observed in the frontal cortex [5, 6]. A recent study has shown that with the in vitro application of stress hormones, there is an observed up-regulation of pro-apoptotic Bax, making cells more prone to apoptosis [2].
Exercise has shown much promise as a therapeutic and/or protective intervention for stress disorders and depression. As noted earlier, exercise elevates BDNF expression and activates survival-promoting intracellular signaling cascades, such as MAPK and PI-3K [10, 31]. The activation of these pathways inhibits several pro-apoptotic molecules, thereby promoting cellular survival [12]. Exercise also reduces oxidative damage induced by stress [27]. In both rats and humans, chronic exercise improves cognitive performance and coping capabilities [11, 32].
Our experimental evidence indicates that regular voluntary exercise can prevent molecular changes that set the stage for cellular apoptosis in the cerebral cortex during chronic stress. Chronic restraint stress increased the concentration of Bax oligomer 270, which is associated with mitochondrial pore-forming activity and the activation of apoptosis [4]. This phenomenon of increased oligomer formation was also evident with chronic corticosterone administration, supporting the possibility that chronic restraint-induced Bax oligomerization may be associated with long-term secretion of corticosteroids. Nevertheless, chronic restraint did not lead to measurable changes in Cort levels, so other important factors may be involved. Also, increased oligomer formation was not evident following acute restraint, suggesting that a more long-term process is required to produce the observed cellular change. Nevertheless, it should be noted that the acute restraint procedure used in this study also varied from chronic restraint in the number of hours per day and the device used, and this could account for the difference in results. Also, only left frontal cortices were studied, and possible changes on the right side of the brain may have been missed.
Voluntary exercise concurrent with chronic stress prevented the increased concentration of Bax oligomer 270. Importantly, it was also observed that the combination of exercise with stress reduced the concentration of total Bax localized to the mitochondria, suggesting that exercise suppressed the translocation of Bax to the cellular compartment where oligomerization and pro-apoptotic activity would occur when stress was present [28]. Previous data from our laboratory indicates that exercise strongly activates the PI-3 kinase pathway [10], which, as noted above, promotes survival by influencing the activity of Bcl family molecules. Activation of the PI-3K pathway stimulates the phosphorylation of Akt (protein kinase B), which promotes Bcl-2 expression and inactivates GSK-3beta, a pro-apoptotic protein that stimulates Bax oligomerization [17]. Therefore, it is very likely that one mechanism of exercise-induced neuroprotection is the intracellular activation of the PI-3 kinase pathway, reduced translocation of Bax to the mitochondria and reduced oligomer formation. Our results suggest that exercise is an effective preventative measure that can promote neuronal survival and protect the brain against the damaging effects of chronic stress.
Acknowledgements
PHS Grant MH-59776 to A.R.N
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 2004;124:985–992. doi: 10.1016/j.neuroscience.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 2.Almawi WY, Melemedjian OK, Jaoude MM. On the link between Bcl-2 family proteins and glucocorticoid-induced apoptosis. J Leukoc Biol. 2004;76:7–14. doi: 10.1189/jlb.0903450. [DOI] [PubMed] [Google Scholar]
- 3.Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, Andrews DW. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. Embo J. 2005;24:2096–2103. doi: 10.1038/sj.emboj.7600675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou JC. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem J. 2000;345(Pt 2):271–278. [PMC free article] [PubMed] [Google Scholar]
- 5.Bland ST, Schmid MJ, Der-Avakian A, Watkins LR, Spencer RL, Maier SF. Expression of c-fos and BDNF mRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress. Brain Res. 2005;1051:90–99. doi: 10.1016/j.brainres.2005.05.065. [DOI] [PubMed] [Google Scholar]
- 6.Bogolepov NN, Koplik EV, Krivitskaya GN, Popova EN, Sudakov KV. Structural and functional characteristics of neurons in the sensorimotor cortex of rats with different resistance to emotional stress. Bull Exp Biol Med. 2001;132:715–718. doi: 10.1023/a:1013020022930. [DOI] [PubMed] [Google Scholar]
- 7.Bowman RE, Zrull MC, Luine VN. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res. 2001;904:279–289. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- 8.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Cereseto M, Reines A, Ferrero A, Sifonios L, Rubio M, Wikinski S. Chronic treatment with high doses of corticosterone decreases cytoskeletal proteins in the rat hippocampus. Eur J Neurosci. 2006;24:3354–3364. doi: 10.1111/j.1460-9568.2006.05232.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen MJ, Russo-Neustadt AA. Exercise activates the phosphatidylinositol 3-kinase pathway. Brain Res Mol Brain Res. 2005;135:181–193. doi: 10.1016/j.molbrainres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Dishman RK, Renner KJ, Youngstedt SD, Reigle TG, Bunnell BN, Burke KA, Yoo HS, Mougey EH, Meyerhoff JL. Activity wheel running reduces escape latency and alters brain monoamine levels after footshock. Brain Res Bull. 1997;42:399–406. doi: 10.1016/s0361-9230(96)00329-2. [DOI] [PubMed] [Google Scholar]
- 12.Encinas M, Iglesias M, Llecha N, Comelia JX. Extracellular-regulated kinases and phosphatidylinositol 3-kinase are involved in brain-derived neurotrophic factor-mediated survival and neuritogenesis of the neuroblastoma cell line SH-SY5Y. J Neurochem. 1999;73:1409–1421. doi: 10.1046/j.1471-4159.1999.0731409.x. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson KA. Starch-Gel Electrophoresis--Application to the Classification of Pituitary Proteins and Polypeptides. Metabolism. 1964;13 SUPPL:985–1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- 14.Filipovic D, Gavrilovic L, Dronjak S, Radojcic MB. The effect of repeated physical exercise on hippocampus and brain cortex in stressed rats. Ann N Y Acad Sci. 2007;1096:207–219. doi: 10.1196/annals.1397.087. [DOI] [PubMed] [Google Scholar]
- 15.Greenwood BN, Strong PV, Dorey AA, Fleshner M. Therapeutic effects of exercise: wheel running reverses stress-induced interference with shuttle box escape. Behav Neurosci. 2007;121:992–1000. doi: 10.1037/0735-7044.121.5.992. [DOI] [PubMed] [Google Scholar]
- 16.Gregus A, Wintink AJ, Davis AC, Kalynchuk LE. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res. 2005;156:105–114. doi: 10.1016/j.bbr.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Linseman DA, Butts DA, Precht TA, Phelps RA, Le SS, Laessig TA, Bouchard RJ, Florez-McClure ML, Heidenreich KA. Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci. 2004;24:9993–10002. doi: 10.1523/JNEUROSCI.2057-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Luine V. Sex differences in chronic stress effects on memory in rats. Stress. 2002;5:205–216. doi: 10.1080/1025389021000010549. [DOI] [PubMed] [Google Scholar]
- 20.McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci. 2006;8:367–381. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEwen BS, Magarinos AM. Stress effects on morphology and function of the hippocampus. Ann N Y Acad Sci. 1997;821:271–284. doi: 10.1111/j.1749-6632.1997.tb48286.x. [DOI] [PubMed] [Google Scholar]
- 22.McNamara RK, Lenox RH. Acute restraint stress reduces protein kinase C gamma in the hippocampus of C57BL/6 but not DBA/2 mice. Neurosci Lett. 2004;368:293–296. doi: 10.1016/j.neulet.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 23.Merril CR, Dunau ML, Goldman D. A rapid sensitive silver stain for polypeptides in polyacrylamide gels. Anal Biochem. 1981;110:201–207. doi: 10.1016/0003-2697(81)90136-6. [DOI] [PubMed] [Google Scholar]
- 24.Neeper SA, Gómez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- 25.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 26.Oliff HS, Berchtold NC, Isackson P, Cotman CW. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Brain Res Mol Brain Res. 1998;61:147–153. doi: 10.1016/s0169-328x(98)00222-8. [DOI] [PubMed] [Google Scholar]
- 27.Radak Z, Kaneko T, Tahara S, Nakamoto H, Pucsok M, Sasvari M, Nyakas C, Goto S. Regular exercise improves cognitive function and decreases oxidative damage in rat brain. Neurochem Int. 2001;38:17–23. doi: 10.1016/s0197-0186(00)00063-2. [DOI] [PubMed] [Google Scholar]
- 28.Reagan LP, Rosell DR, Wood GE, Spedding M, Munoz C, Rothstein J, McEwen BS. Chronic restraint stress up-regulates GLT-1 mRNA and protein expression in the rat hippocampus: reversal by tianeptine. Proc Natl Acad Sci U S A. 2004;101:2179–2184. doi: 10.1073/pnas.0307294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101:305–312. doi: 10.1016/s0306-4522(00)00349-3. [DOI] [PubMed] [Google Scholar]
- 30.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 31.Shen H, Tong L, Balazs R, Cotman CW. Physical activity elicits sustained activation of the cyclic AMP response element-binding protein and mitogen-activated protein kinase in the rat hippocampus. Neuroscience. 2001;107:219–229. doi: 10.1016/s0306-4522(01)00315-3. [DOI] [PubMed] [Google Scholar]
- 32.Small GW, Silverman DH, Siddarth P, Ercoli LM, Miller KJ, Lavretsky H, Wright BC, Bookheimer SY, Barrio JR, Phelps ME. Effects of a 14-day healthy longevity lifestyle program on cognition and brain function. Am J Geriatr Psychiatry. 2006;14:538–545. doi: 10.1097/01.JGP.0000219279.72210.ca. [DOI] [PubMed] [Google Scholar]
- 33.Solberg LC, Horton TH, Turek FW. Circadian rhythms and depression: effects of exercise in an animal model. Am J Physiol. 1999;276:R152–R161. doi: 10.1152/ajpregu.1999.276.1.R152. [DOI] [PubMed] [Google Scholar]
- 34.Tong L, Shen H, Perreau VM, Balazs R, Cotman CW. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiol Dis. 2001;8:1046–1056. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- 35.Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- 36.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]


