Abstract
An increased volume of white matter hyperintensities (WMH) on MRI has been associated with mobility impairments in older adults. The objective of this preliminary study was to investigate the relationship between the volume of WMH and delays in auditory-cued step initiation. Eight subjects aged 75–83 y participated. The WMH volume in the corticospinal tracts and anterior thalamic radiations were summed. Subjects performed an auditory-cued stepping task that included two simple reaction time (SRT) trials and three choice reaction time (CRT) trials. SRT trials required subjects to step as quickly as possible with the right foot from a symmetric standing position to a single target position in response to an auditory stimulus. For the CRT trials, subjects stepped as quickly as possible to one of two possible locations, depending on the auditory stimulus. The time from the stimulus onset to the reaction time of the anticipatory postural adjustment (APART) and liftoff (LO) of the right foot was computed for each stimulus. The mean APART and LO were greater for the CRT steps compared with the SRT steps to the same location. Increases in WMH were significantly associated with larger APART and LO during both SRT and CRT for both target locations. These data suggest that increased volume of WMH is associated with greater central processing time during voluntary step initiation, and highlight a possible mechanism that can help to explain how damage to white matter tracts affects mobility in older adults.
Keywords: gait initiation, reaction time, posture, balance, aging
Introduction
In the past 20 years, there have been numerous reports relating the presence and severity of white matter hyperintensities (WMH) with balance dysfunction in both healthy and balance-impaired older adults. Early descriptive studies reported gait difficulties, including wide base of support, short step length, increased step variability and turning “en bloc”, in subjects with subcortical white matter lesions.(Thompson and Marsden 1987; Hennerici et al. 1994; Baloh et al. 1995; Briley et al. 1997; Camicioli et al. 1999; Ebersbach et al. 1999). In addition, case-control studies demonstrated that the severity of white matter damage was associated with greater falls risk.(Masdeu et al. 1989; Baloh et al. 1995; Kerber et al. 1998). More recent reports have detailed the relationship between severity of white matter lesions and reductions in gait speed (Tell et al. 1998; Camicioli et al. 1999; Guo et al. 2000; Longstreth et al. 2005; Rosano et al. 2005a; Rosano et al. 2005b), and reduced performance on the Short Physical Performance Battery (Guttmann et al. 2000; Benson et al. 2002; Wolfson et al. 2005) Furthermore, in longitudinal studies, the decline in gait speed and mobility function as people aged was explained partially by increases in WMH severity.(Whitman et al. 1999; Whitman et al. 2001; Baloh et al. 2003; Longstreth et al. 2005; Rosano et al. 2005a).
The white matter pathways are an integral component of frontal-subcortical (F-SC) circuits that modulate motor output for postural tasks (Alexander et al. 1986; Middleton and Strick 2001; Tekin and Cummings 2002). One of these circuits associated with motor control is the skeletomotor circuit, which originates in the supplementary motor area, premotor cortex, motor cortex, and somatosensory cortical areas. It is associated with various functions including: selection and initiation of movement, and encoding of direction, velocity and duration of movement.(Cheruel et al. 1994; Contreras-Vidal 1999; Desmurget et al. 2003; Turner et al. 2003; Ueda and Kimura 2003; Arkadir et al. 2004) Obstacle avoidance, fall recovery, and adapting to novel postural tasks all require well-functioning components of this system. There is considerable evidence that damage to white matter tracts between the motor areas in the cortex, basal ganglia, thalamus and cerebellum interfere with the motor output to the lower extremities during balance tasks.(Hennerici et al. 1994; Tell et al. 1998; Guttmann et al. 2000; Wolfson 2001; Onen et al. 2004)
The current preliminary study seeks to augment the information gained from previous work by explicitly testing the hypothesis that the degeneration of frontal-subcortical white matter pathways that are involved in motor function are related to deficits in postural performance that are served by these pathways. Consequently, in this study we used a sensory-cued choice reaction time (CRT) step task as a model for examining whether lesions in the white matter are associated with specific balance impairments (e.g. step initiation). The CRT step task requires subjects to step to different target locations based on the type of auditory stimulus that they receive. Having to choose between different step locations requires use of distributed areas of the brain that would require utlization of the white matter pathways. We hypothesize that a significant amount of the variation in the delayed step times as people age can be explained by the amount of damage that has accumulated in these frontal-subcortical white matter pathways.
Methods
Eight subjects (4 female, age 75–83 y) participated after signing informed consent. Subjects had brain MRI for the quantification of volume of WMH. Images were acquired on a 1.5 Tesla Signa Scanner (GE Medical Systems, Milwaukee, WI). The following axial series oriented parallel to the plane connecting the anterior and posterior commissures were obtained: T1-weighted (TR/TE = 500/11 ms, Nex = 1); fast fluid-attenuated inversion recovery (fast FLAIR) (TR/TE 9002/56 ms Ef; TI = 2200 ms, NEX = 1). Section thickness was 5 mm with a 1-mm inter-section gap. All axial sequences were obtained with a 24 cm field of view and a 192 × 256 pixel matrix. Additionally, 3D structural MR images were acquired at sagittal orientation using 3D Spoiled Grass (SPGR, TR/TE = 5/25 ms; flip angle = 40°; FOV = 24×18cm, slice thickness = 1.5mm, matrix = 256×192 matrix). WMHs were segmented on the FLAIR images and localized by registration to the T1-weighted images using an automated method for WMH quantification and localization, which uses a fuzzy connected algorithm to segment the WMHs and the Automated Labeling Pathway (ALP) to localize the WMHs into the anatomical space.(Wu et al. 2006) The volume of WMH was computed for the whole brain and for 10 of the specific white matter tracts specified in the Johns Hopkins University (JHU) White Matter Atlas.(Wakana et al. 2004) The primary independent variable was the sum of the volume of WMH in the left and right corticospinal tracts and anterior thalamic radiations, normalized by the total brain volume.
Subjects performed a voluntary step task that included simple reaction time (SRT) and choice reaction time (CRT) blocks. Subjects donned a harness attached to an overhead carriage in order to prevent falls from occurring. They stood with their right foot on a single forceplate. Targets were placed on the floor that represented the starting position (SP) and desired step locations. Forward step locations were placed directly in front of the subjects’ feet, and lateral step locations directly to the side of the feet. Subjects wore comfortable shoes (i.e. without raised heels).
Three SRT blocks were performed. In response to an auditory stimulus, subjects were instructed to step as quickly as possible with their right foot from the starting position (SP) to a single target position for each test. Three trial blocks of 3 min duration were performed, with the target positions located approximately a) 10 inches to the right (R1), b) 18 inches to the right (R2), and c) 10 inches forward (RF1). Immediately after taking the step, subjects returned to the starting position and waited for the next stimulus. The inter-stimulus interval was 8 ± 1 s. A different auditory stimulus was presented for each SRT location: low frequency for location R1 (560 Hz), medium for R2 (980 Hz) and high for RF1 (1715 Hz).
Subjects also performed 2 CRT blocks that required subjects to step to targets that differed in amplitude (CRT A) and direction of movement (CRT B). During CRT A, subjects stepped as quickly as possible to location R1 when they heard a 560 Hz tone and stepped to location R2 when they heard a 980 Hz tone. The addition of uncertainty in the step amplitude increases the amount of motor planning required after the cue because although the muscles needed for movement will be known a priori, their force output will not. During CRT B, subjects stepped to location R1 when they heard a 560 Hz tone, and stepped to location RF1 when they heard a 1715 Hz tone. CRT B requires greater cortical processing than CRT A because subjects need to select which muscle groups to activate to make either a forward or lateral step. Furthermore, because of the different muscle groups that are used for forward and lateral steps, the signal for the proper force output must be encoded as well. The length of the CRT blocks was 4 min, and the inter-stimulus interval was 8 ± 1 s. The order of presentation of the tones was randomized. A 3 min seated rest break was provided after each SRT and CRT step block.
For each step, the subjects were instructed to: a) bear weight equally on both feet, b) step as quickly as possible to the target footfall location after hearing the auditory stimulus and c) follow with the trailing leg until they come to a stop in the upright standing position. Subjects were instructed to move with both legs so that they generated motor responses that would be similar to those seen during gait initiation, rather than just moving one leg to a new location. Although we did not measure the accuracy of step placement, subjects were given verbal feedback and encouraged to accurately step to the location.
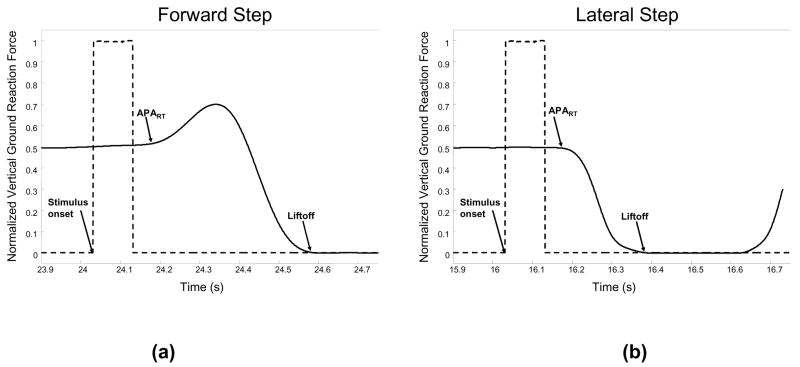
The forward and lateral step responses induced stereotypical vertical ground reaction force (VGRF) curves (Figure 1). During the forward step, the step leg first exerts a force to propel the body toward the stance leg, before unloading the stepping leg and taking the step. The first deflection of the VGRF is the reaction time of the anticipatory postural adjustment (APART). The liftoff (LO) time occurs when all weight has been shifted to the stance leg and the vertical ground reaction force goes to 0. The duration of the APA (APAdur) is equal to the difference between the LO and the APART. This pattern of propelling the body toward the stance leg is seen in only 1/3 of the lateral steps. In the other 2/3 of the lateral steps, the VGRF curve does not display a peak in the APA. For the CRT blocks, data were processed from correct step responses only, which occurred for 88% of the cues.
Figure 1.
Vertical ground reaction forces (normalized by body weight) for step leg during forward (a) and lateral (b) steps. Events include the stimulus onset, anticipatory postural adjustment reaction time (APART), and liftoff (LO) times.
Although the LO time includes peripheral muscle activation times, which may be influenced by decreased motor nerve conduction velocity, the duration of this process should be consistent across the SRT and CRT blocks. For each block, APART, APAdur, and LO times for every stimulus were visually inspected and outliers (values greater or less than 2 S.D. from the mean) were removed. The mean time of APART, APAdur, and LO for each step location during each block was then computed. For the lateral steps, we considered only those trials that did not have a peak in the APA, similar to the response shown in Figure 1.
Having to choose between targets of different amplitude and different directions not only requires different motor planning but also different APAs. Thus part of the delays observed in this experiment could be a result of the modification of the APAs required to reach different targets. Consequently, we performed a statistical analysis on each step target separately, Therefore, we focused solely on differences arising between the simple and choice reaction time conditions. Any delays observed in the choice reaction time steps relative to the simple reaction time steps should be primarily determined by delays that occur in the central processing because the conduction times in the peripheral sensory and motor systems should be consistent across tasks. Differences in the mean time between the SRT and CRT blocks were tested using paired t-tests. Subsequently, linear regression was used to quantify the strength of the correlation between the WMH volume and the dependent variables, as well as the magnitude of the slope between the dependent variables and WMH volume. Due to the skewed distribution of the WMH volume scores, the WMH volume was transformed using the logarithmic function.
Results
Table 1 shows that in most cases, there are significant increases in APART for each CRT compared with the SRT at the same step location. Although the APAdur was larger for each CRT compared with the associated SRT, this reached statistical significance only for the choice of step direction CRT for the small lateral and small forward step locations. Finally, the LO was significantly greater for both CRTs involving the small lateral step locations and the CRT for the small forward step location. To summarize, we believe that the increase in APART, APAdur, and LO time is primarily due to central conduction delays.
Table 1.
Mean (s.d.) anticipatory postural adjustment reaction time (APART), APA duration (APAdur) and liftoff (LO) times for the SRT and CRT blocks, and results from the paired t-tests comparing the CRTs with the SRTs for each step location.
| Step location | APART (ms) | APAdur (ms) | LO (ms) |
|---|---|---|---|
| Small Lateral | |||
| R1: SRT | 278 (83) | 254 (31) | 532 (97) |
| R1: CRT A (amplitude) | 406 (107) * | 292 (69) | 699 (170) * |
| R1: CRT B (direction) | 490 (187) * | 316 (88) * | 806 (195) * |
|
| |||
| Large Lateral | |||
| R2: SRT | 253 (79) | 271 (31) | 524 (81) |
| R2: CRT A (amplitude) | 367 (129) * | 348 (129) | 714 (227) |
|
| |||
| Small Forward | |||
| RF1: SRT | 151 (21) | 537 (115) | 688 (121) |
| RF1: CRT B (direction) | 218 (80)* | 637 (140)* | 828 (148)* |
indicates paired t-test is significant at p < 0.05
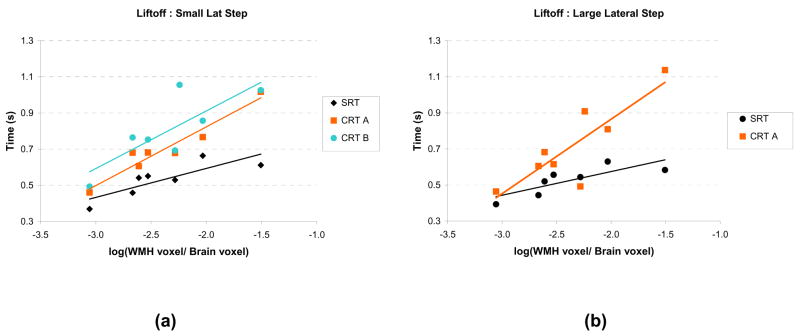
The WMH volume in the projection tracts ranged from 0.1 to 3.1% of the total brain volume. The relationship between the WMH volume and mean LO times during the SRT and CRT blocks is displayed for the small and large lateral steps in Table 2 and Figure 2. As seen in Table 2, the LO demonstrated the most consistent strong relationships with the log(WMH volume) (correlations range from 0.64 to 0.96). The correlations between log (WMH volume) and APART (range 0.38 to 0.96) and APAdur (range 0.27 to 0.90) were weaker, but the preliminary evidence is consistent with the model that disruption in the frontal-subcortical circuits is partly responsible for the delays in step initiation. In addition, in most cases, the slope obtained from linear regression of APART, APAdur and LO vs. log (WMH volume) was greater for the CRT blocks compared with the SRT blocks, although the difference between the slopes did not reach statistical significance due to the small sample size.
Table 2.
Slope and Pearson’s correlation coefficient (r) between the log10(WMH volume) and anticipatory postural adjustment reaction time (APART), APA duration (APAdur) and liftoff (LO) times for the SRT and CRT blocks.
| Step location | APART | APAdur | LO | |||
|---|---|---|---|---|---|---|
| slope | r | slope | r | slope | r | |
| Small Lateral | ||||||
| R1: SRT | 0.13 | 0.79 * | 0.03 | 0.49 | 0.16 | 0.83 * |
| R1: CRT A (amplitude) | 0.21 | 0.96 * | 0.12 | 0.87 * | 0.32 | 0.96 * |
| R1: CRT B (direction) | 0.20 | 0.52 | 0.12 | 0.69 | 0.32 | 0.81 * |
|
| ||||||
| Large Lateral | ||||||
| R2: SRT | 0.11 | 0.73 | 0.02 | 0.27 | 0.13 | 0.81 * |
| R2: CRT A (amplitude) | 0.21 | 0.76 * | 0.20 | 0.73 * | 0.41 | 0.85 * |
|
| ||||||
| Small Forward | ||||||
| RF1: SRT | 0.02 | 0.38 | 0.20 | 0.76 * | 0.21 | 0.79 * |
| RF1: CRT B (direction) | 0.12 | 0.51 | 0.27 | 0.90 * | 0.21 | 0.64 |
indicates correlation is significant at p < 0.05
Figure 2.
Liftoff times plotted as a function of the log(WMH volume) for the simple reaction time (SRT) and choice reaction time (CRT) step tasks. (a) Small lateral step location. (b) Large lateral step location. CRT A: choice between small and large amplitude step. CRT B: choice between small lateral and small forward step.
Discussion
We used a step initiation task to explore if the volume of white matter hyperintensities in motor-related white matter tracts was related to a specific balance impairment. The motivations for using this task were several. For one, impaired postural adjustments during step initiation have been described in people with diffuse white matter lesions.(Elble et al. 1996) Furthermore, older adults with balance impairments have greater step initiation times compared with older adults without balance impairments.(Medell and Alexander 2000; Lord and Fitzpatrick 2001) The time to make a step increases further when there is uncertainty about the direction of the step,(Patla et al. 1993; Lord and Fitzpatrick 2001; Luchies et al. 2002; Rogers et al. 2003) suggesting that central nervous system (i.e. skeletomotor circuit) resources are required to select an appropriate motor response.
The findings can be explained by current models of how age-related damage to the white matter subserving frontal-subcortical circuits result in posture and gait dysfunction (Pugh and Lipsitz 2002, Kuo and Lipsitz, 2004). A history of chronic hypertension in older adults induces changes in cerebral blood flow that result in hypoperfusion.(Pantoni and Garcia 1997; Roman et al. 2002) Ultimately, periods of chronic hypoperfusion result in ischemia and degeneration of the oligodendrocytes and demyelination.(Pantoni et al. 1996; Tomimoto et al. 2003) In humans, the estimated white matter volume loss is approximately 24% – 28% from the age of 20 to 80 y.(Pakkenberg and Gundersen 1997; Marner et al. 2003) On the contrary, the most recent studies have estimated only 10% loss of neurons over the human lifespan.(Pakkenberg and Gundersen 1997) The most significant consequence of the white matter damage is a disruption of timing in neuronal circuits due to reductions in nerve conduction velocity and inconsistent conduction times within a group of axons that have a similar target.(Peters 2002; Peters and Sethares 2003) In the current experiment, reduced nerve conduction velocity would be manifested as a delay in step initiation. The finding that APART and LO times from both the SRT and CRT blocks are related, in part, to the volume of WMH in frontal-subcortical tracts supports this model.
In addition, a central concept to the study design is that having to choose between different step locations requires use of distributed areas of the brain that would require extensive use of the white matter pathways. The step initiation times for type of step task will differ depending on the amount of motor planning that is required after the cue is presented. In this experiment, we have attempted to progressively increase the amount of planning needed from the SRT to the CRT:A to CRT:B. Using the model of white matter pathology, we would predict that tasks that require greater levels of central processing would result in a disparate increase in step initiation times for people with greater amount of white matter disruption. The trend of a greater slope between the APART and LO and the WMH volume for the CRT tasks supports this prediction.
One of the primary risk factors for the development of white matter hyperintensities is age.(Awad et al. 1986; Manolio et al. 1994) Although we have attempted to lessen the influence of age by testing subjects within a relatively limited age range, it is possible that the current findings can be explained by age-related mechanisms that we did not account for. Future studies should consider including a more extensive array of covariates in the model.
In the current study, the sum of the WMH in the specified motor tracts was highly correlated with the total volume of WMH as well as volume of WMH in several of the other tracts. Testing a larger group of subjects will allow us to examine the relationship between the step initiation times and spatial distribution of white matter hyperintensities in more detail.
Conclusions
These data suggest that increased volume of white matter hyperintensities in relevant white matter tracts is associated with greater central processing time during voluntary step initiation. This study highlights a possible mechanism that can help to explain how damage to white matter tracts reduces mobility in older adults.
Acknowledgments
This research was supported by funding from the National Institutes of Health (P30 AG024827, P30 DC005205) and the Eye and Ear Foundation.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Arkadir D, Morris G, Vaadia E, Bergman H. Independent coding of movement direction and reward prediction by single pallidal neurons. J Neurosci. 2004;24:10047–10056. doi: 10.1523/JNEUROSCI.2583-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad IA, Spetzler RF, Hodak JA, Awad CA, Carey R. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. I. Correlation with age and cerebrovascular risk factors. Stroke. 1986;17:1084–1089. doi: 10.1161/01.str.17.6.1084. [DOI] [PubMed] [Google Scholar]
- Baloh RW, Ying SH, Jacobson KM. A longitudinal study of gait and balance dysfunction in normal older people. Arch Neurol. 2003;60:835–839. doi: 10.1001/archneur.60.6.835. [DOI] [PubMed] [Google Scholar]
- Baloh RW, Yue Q, Socotch TM, Jacobson KM. White matter lesions and disequilibrium in older people. I. Case-control comparison. Arch Neurol. 1995;52:970–974. doi: 10.1001/archneur.1995.00540340062013. [DOI] [PubMed] [Google Scholar]
- Benson RR, Guttmann CR, Wei X, Warfield SK, Hall C, Schmidt JA, Kikinis R, Wolfson LI. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology. 2002;58:48–55. doi: 10.1212/wnl.58.1.48. [see comment] [DOI] [PubMed] [Google Scholar]
- Briley DP, Wasay M, Sergent S, Thomas S. Cerebral white matter changes (leukoaraiosis), stroke, and gait disturbance. J Am Geriatr Soc. 1997;45:1434–1438. doi: 10.1111/j.1532-5415.1997.tb03192.x. [DOI] [PubMed] [Google Scholar]
- Camicioli R, Moore MM, Sexton G, Howieson DB, Kaye JA. Age-related brain changes associated with motor function in healthy older people. J Am Geriatr Soc. 1999;47:330–334. doi: 10.1111/j.1532-5415.1999.tb02997.x. [DOI] [PubMed] [Google Scholar]
- Cheruel F, Dormont JF, Amalric M, Schmied A, Farin D. The role of putamen and pallidum in motor initiation in the cat. I. Timing of movement-related single-unit activity. Exp Brain Res. 1994;100:250–266. doi: 10.1007/BF00227195. [DOI] [PubMed] [Google Scholar]
- Contreras-Vidal JL. The gating functions of the basal ganglia in movement control. Prog Brain Res. 1999;121:261–276. doi: 10.1016/s0079-6123(08)63078-2. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton ST, Vindras P, Grea H, Turner RS. Basal ganglia network mediates the control of movement amplitude. Exp Brain Res. 2003;153:197–209. doi: 10.1007/s00221-003-1593-3. [DOI] [PubMed] [Google Scholar]
- Ebersbach G, Sojer M, Valldeoriola F, Wissel J, Muller J, Tolosa E, Poewe W. Comparative analysis of gait in Parkinson’s disease, cerebellar ataxia and subcortical arteriosclerotic encephalopathy. Brain. 1999;122:1349–1355. doi: 10.1093/brain/122.7.1349. [DOI] [PubMed] [Google Scholar]
- Elble RJ, Cousins R, Leffler K, Hughes L. Gait initiation by patients with lower-half parkinsonism. Brain. 1996;119:1705–1716. doi: 10.1093/brain/119.5.1705. [DOI] [PubMed] [Google Scholar]
- Guo X, Skoog I, Matousek M, Larsson L, Palsson S, Sundh V, Steen B. A population-based study on motor performance and white matter lesions in older women. J Am Geriatr Soc. 2000;48:967–970. doi: 10.1111/j.1532-5415.2000.tb06896.x. [DOI] [PubMed] [Google Scholar]
- Guttmann CR, Benson R, Warfield SK, Wei X, Anderson MC, Hall CB, Abu-Hasaballah K, Mugler JP, 3rd, Wolfson L. White matter abnormalities in mobility-impaired older persons. Neurology. 2000;54:1277–1283. doi: 10.1212/wnl.54.6.1277. [DOI] [PubMed] [Google Scholar]
- Hennerici MG, Oster M, Cohen S, Schwartz A, Motsch L, Daffertshofer M. Are gait disturbances and white matter degeneration early indicators of vascular dementia? Dementia. 1994;5:197–202. doi: 10.1159/000106723. [DOI] [PubMed] [Google Scholar]
- Kerber KA, Enrietto JA, Jacobson KM, Baloh RW. Disequilibrium in older people: a prospective study. Neurology. 1998;51:574–580. doi: 10.1212/wnl.51.2.574. [DOI] [PubMed] [Google Scholar]
- Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, Manolio TA, Lefkowitz D, Jungreis C, Hirsch CH, O’Leary DH, Furberg CD. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- Lord SR, Fitzpatrick RC. Choice stepping reaction time: a composite measure of falls risk in older people. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2001;56:M627–632. doi: 10.1093/gerona/56.10.m627. [DOI] [PubMed] [Google Scholar]
- Luchies CW, Schiffman J, Richards LG, Thompson MR, Bazuin D, DeYoung AJ. Effects of age, step direction, and reaction condition on the ability to step quickly. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2002;57:M246–249. doi: 10.1093/gerona/57.4.m246. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Kronmal RA, Burke GL, Poirier V, O’Leary DH, Gardin JM, Fried LP, Steinberg EP, Bryan RN. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994;25:318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462:144–152. doi: 10.1002/cne.10714. [see comment] [DOI] [PubMed] [Google Scholar]
- Masdeu JC, Wolfson L, Lantos G, Tobin JN, Grober E, Whipple R, Amerman P. Brain white-matter changes in the elderly prone to falling. Arch Neurol. 1989;46:1292–1296. doi: 10.1001/archneur.1989.00520480034016. [see comment] [DOI] [PubMed] [Google Scholar]
- Medell JL, Alexander NB. A clinical measure of maximal and rapid stepping in older women. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2000;55:M429–433. doi: 10.1093/gerona/55.8.m429. [see comment] [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Revised Neuroanatomy of Frontal-Subcortical Circuits. In: Lichter DG, Cummings JL, editors. Frontal-Subcortical Circuits in Pschiatric and Neurological Disorders. The Guilford Press; New York: 2001. pp. 44–58. [Google Scholar]
- Onen F, Feugeas MC, Baron G, De Marco G, Godon-Hardy S, Peretti II, Ravaud P, Legrain S, Moretti JL, Claeys ES. Leukoaraiosis and mobility decline: a high resolution magnetic resonance imaging study in older people with mild cognitive impairment. Neurosci Lett. 2004;355:185–188. doi: 10.1016/j.neulet.2003.10.072. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–1646. doi: 10.1161/01.str.27.9.1641. discussion 1647. [DOI] [PubMed] [Google Scholar]
- Patla AE, Frank JS, Winter DA, Rietdyk S, Prentice S, Prasad S. Age-related changes in balance control system: initiation of stepping. Clinical Biomechanics. 1993;8:179–184. doi: 10.1016/0268-0033(93)90012-7. [DOI] [PubMed] [Google Scholar]
- Peters A. Structural changes that occur during normal aging of primate cerebral hemispheres. Neuroscience & Biobehavioral Reviews. 2002;26:733–741. doi: 10.1016/s0149-7634(02)00060-x. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Is there remyelination during aging of the primate central nervous system? J Comp Neurol. 2003;460:238–254. doi: 10.1002/cne.10639. [DOI] [PubMed] [Google Scholar]
- Rogers MW, Hedman LD, Johnson ME, Martinez KM, Mille ML. Triggering of protective stepping for the control of human balance: age and contextual dependence. Cognit Brain Res. 2003;16:192–198. doi: 10.1016/s0926-6410(02)00273-2. [DOI] [PubMed] [Google Scholar]
- Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurology. 2002;1:426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT, Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005a;53:649–654. doi: 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]
- Rosano C, Simonsick EM, Harris TB, Kritchevsky SB, Brach J, Visser M, Yaffe K, Newman AB. Association between physical and cognitive function in healthy elderly: the health, aging and body composition study. Neuroepidemiology. 2005b;24:8–14. doi: 10.1159/000081043. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Tell GS, Lefkowitz DS, Diehr P, Elster AD. Relationship between balance and abnormalities in cerebral magnetic resonance imaging in older adults. Arch Neurol. 1998;55:73–79. doi: 10.1001/archneur.55.1.73. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Marsden CD. Gait disorder of subcortical arteriosclerotic encephalopathy: Binswanger’s disease. Mov Disord. 1987;2:1–8. doi: 10.1002/mds.870020101. [DOI] [PubMed] [Google Scholar]
- Tomimoto H, Ihara M, Wakita H, Ohtani R, Lin JX, Akiguchi I, Kinoshita M, Shibasaki H. Chronic cerebral hypoperfusion induces white matter lesions and loss of oligodendroglia with DNA fragmentation in the rat. Acta Neuropathol (Berl) 2003;106:527–534. doi: 10.1007/s00401-003-0749-3. [DOI] [PubMed] [Google Scholar]
- Turner RS, Desmurget M, Grethe J, Crutcher MD, Grafton ST. Motor subcircuits mediating the control of movement extent and speed. J Neurophysiol. 2003;90:3958–3966. doi: 10.1152/jn.00323.2003. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Kimura M. Encoding of direction and combination of movements by primate putamen neurons. Eur J Neurosci. 2003;18:980–994. doi: 10.1046/j.1460-9568.2003.02814.x. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Whitman GT, DiPatre PL, Lopez IA, Liu F, Noori NE, Vinters HV, Baloh RW. Neuropathology in older people with disequilibrium of unknown cause. Neurology. 1999;53:375–382. doi: 10.1212/wnl.53.2.375. [see comment] [DOI] [PubMed] [Google Scholar]
- Whitman GT, Tang Y, Lin A, Baloh RW, Tang T. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology. 2001;57:990–994. doi: 10.1212/wnl.57.6.990. [erratum appears in Neurology 2001 Nov 27;57(10):1942 Note: Tang T [corrected to Tang Y]] [DOI] [PubMed] [Google Scholar]
- Wolfson L. Gait and balance dysfunction: a model of the interaction of age and disease. Neuroscientist. 2001;7:178–183. doi: 10.1177/107385840100700212. [DOI] [PubMed] [Google Scholar]
- Wolfson L, Wei X, Hall CB, Panzer V, Wakefield D, Benson RR, Schmidt JA, Warfield SK, Guttmann CR. Accrual of MRI white matter abnormalities in elderly with normal and impaired mobility. J Neurol Sci. 2005;232:23–27. doi: 10.1016/j.jns.2004.12.017. [see comment] [DOI] [PubMed] [Google Scholar]
- Wu M, Rosano C, Butters M, Whyte E, Nable M, Crooks R, Meltzer CC, Reynolds CF, 3rd, Aizenstein HJ. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006;148:133–142. doi: 10.1016/j.pscychresns.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]