Summary
The emergence of neurites from a symmetrical cell body is an essential feature of nervous system development. Neurites are the precursors of axons and dendrites and are tipped by growth cones, motile structures that guide elongating axons in the developing nervous system. Growth cones steer the axon along a defined path to its appropriate target in response to guidance cues. This navigation involves the dynamic extension and withdrawal of actin-filled finger-like protrusions called filopodia that continuously sample their environment. Ena/VASP proteins, a conserved family of actin regulatory proteins, are critical for filopodia formation and function downstream of several guidance cues. Here we review recent findings into Ena/VASP function in neurite initiation, axon outgrowth and guidance.
Introduction
Growth cones, specialized fan-shaped structures at the ends of developing axons, steer growing axons to their targets. As an axon extends through the complex extracellular environment in vivo, its growth cone explores the local environment and responds to a variety of short- and long-range guidance cues. Although progress has been made in identifying the guidance cues and receptors [1], less is known about how environmental signals are converted into changes in the direction and rate of axonal outgrowth. Growth cone morphology and motility are determined by local dynamic changes in the actin and microtubule (MT) cytoskeleton, which are regulated by complex interactions downstream of second messenger signaling pathways [2]. Filopodia, rod-like projections composed of parallel F-actin bundles, extend beyond the dense actin meshwork of the lamellipodial veils at the growth cone periphery. Filopodia are characteristic for cells displaying exploratory behavior and are thought to sense guidance cues and steer the growth cone [3,4]. Ena/VASP proteins are implicated in integrating guidance signals into appropriate changes in cytoskeletal dynamics and are key regulators of filopodia formation and dynamics.
Ena/VASP proteins are a conserved family of actin-regulatory proteins concentrated at areas of dynamic actin remodeling and have a well-established role in filopodia formation and elongation [3,5]. The Ena/VASP family, which is conserved from Dictyostelium to vertebrates, has a domain structure consisting of an N-terminal Ena/VASP homology 1 (EVH1) domain, a central proline-rich region and a C-terminal EVH2 domain (Figure 1). Binding of the EVH1 domain to proteins that contain a proline-rich motif with the consensus (D/E)FPPPPX(D/E)(D/E) (abbreviated “FPPPP”) regulates subcellular localization of Ena/VASP proteins and mediates formation of complexes with receptors and signaling molecules. EVH1-binding proteins include the axon guidance receptor Robo and Lamellipodin (Lpd), the mammalian orthologue of the C.elegans protein MIG-10 [6,7]. Exogenous expression of this FPPPP motif fused to a mitochondrial-targeting motif (“FPPPP-Mito”) has been used in functional studies to neutralize Ena/VASP activity by sequestering them away from their subcellular sites of function [8]. This sequestration approach mimics the deletion of Ena/VASP in isolated cortical neurons [9] and phenocopies zygotic Ena mutations in the fly [7,10]. Conversely, directing Ena/VASP localization to the plasma membrane by fusing the FPPPP motif to a membrane targeting motif enhances Ena/VASP activity [11].
Figure 1.
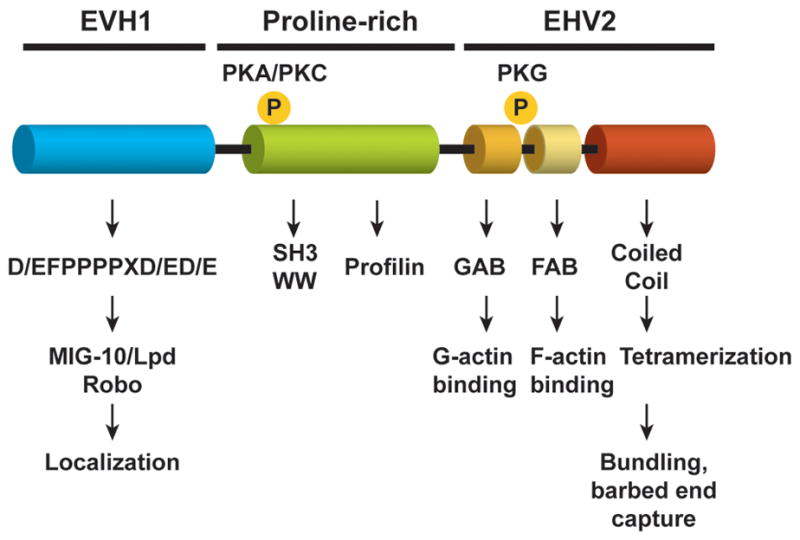
Organization of Ena/VASP proteins. The domains and protein interaction sites are shown in the schematic. The EVH-1 binding partners Lpd and Robo are shown; other ligands not known to function in axon outgrowth and guidance are not indicated. Phosphorylation sites shared between Mena and VASP are also indicated. The amino terminal site can be phosphorylated by either PKA or PKC. The carboxy-terminal PKG site is not conserved in EVL. Note that the invertebrate Ena/VASP proteins lack these phosphorylation sites.
Ena/VASP proteins regulate cellular protrusions by modulating the geometry of actin filament network assembly [8]. Motifs in the EVH2 domain mediate direct binding to both monomeric (G-) and filamentous (F-) actin and, because they are tetramers, they can bundle actin filaments [12–14]. Ultrastructural analysis of actin filaments at the leading edge of fibroblasts and neuronal growth cones provided important clues to how Ena/VASP proteins influence cytoskeletal architecture. Depletion of Ena/VASP proteins from the cell edge in fibroblasts or growth cones promote formation of dense actin networks with short, highly branched filaments. In contrast, enrichment of Ena/VASP proteins at the plasma membrane results in sparse networks containing primarily long, unbranched filaments, which in growth cones coalesce into filopodia [8,15].
Ena/VASP promotes filopodia formation by 1) binding and clustering actin filaments barbed ends, 2) shielding elongating filaments from capping protein (3) and decreasing filament branching. Ena/VASP proteins are stably anchored within filopodial tips [16] through interactions with elongating actin filaments and binding to EVH-1 ligands such as Lpd [6]. In vitro actin polymerization assays and, more recently, direct visualization of growing fluorescently-labeled actin filaments show that Ena/VASP proteins capture filament barbed ends, antagonize barbed end capping and enhance filament elongation; this anti-capping activity is enhanced by direct binding to profilin-actin complexes [12,17]. However, the mechanism underlying Ena/VASP function in decreasing filament branching remains to be elucidated.
Ena/VASP proteins are likely to function during multiple steps in nervous system development. Roles for Ena/VASP in neurulation [18,19], neuronal migration [20–22], dendritic morphology [23,24], and synapse formation [25,26] have been demonstrated. In addition, recent evidence suggests that both Ena/VASP and MIG-10/Lpd play a role in axon regeneration in C.elegans [27]. Here we focus on two aspects of nervous system development that require Ena/VASP: neuritogenesis and axon guidance.
Ena/VASP proteins in Neurite Initiation
Recent analysis of the cortex of mice lacking all three vertebrate paralogs (Mena, EVL and VASP) reveal an unexpected requirement for Ena/VASP proteins in neurite initiation. Complete loss of Ena/VASP blocks axon fiber tract formation in the cortex in a cell autonomous manner. Analysis of cultured cortical neurons indicates that this defect results from the failure of cortical neurons to produce neurites [22]. This neuritogenesis defect arises from a failure to form actin bundles and filopodia. Neuritogenesis in Ena/VASP deficient neurons can be rescued by ectopic expression of mDia2 and myosinX [9], factors that can induce filopodia independently of Ena/VASP [28–30]. Dynamic MTs are also required to form neurites [9]. MT behavior is altered in the absence of Ena/VASP as a result of a lack of bundled F-actin to help guide MTs into filopodia. This is consistent with observations that F-actin bundles underlying filopodia act as guides for MTs [31] in growth cones. During axon outgrowth, MTs invade and dynamically explore filopodia [32,33], where they stabilize elongating axons and likely support transport of proteins and membrane out to the tip; it is likely that MT: F-actin interactions function in a similar manner to support neurite initiation.
Interestingly, although Ena/VASP proteins were required for neuritogenesis within the cortex, cortical neurons that had aberrantly migrated outside of the pial membrane in Ena/VASP deficient mice formed axons, as did other neuronal types, such as retinal ganglia, hippocampus and dorsal root ganglia. This suggests that signals absent from the cortex but present in structures that form axons promote Ena/VASP-independent neurite initiation. One such factor is the extracellular matrix protein laminin which is largely absent from the cortex but found in the areas where Ena/VASP-independent neuritogenesis occurs. Neuritogenisis is rescued by plating Ena/VASP deficient primary cortical neurons on laminin but not fibronectin or collagen [9]. The existence of extrinsic (such as laminin) or intrinsic (such as mDia2 and myosinX) mechanisms that bypass the requirement for Ena/VASP in neuritogenesis may also explain why this defect is not observed in mutants of the invertebrate Ena/VASP orthologues.
Ena/VASP proteins in Axon Guidance
Drosophila and C.elegans each have a single Ena/VASP ortholog, Enabled (Ena) and UNC-34, respectively. Loss of Ena/VASP function in invertebrates leads to subtle defects in axon guidance. These phenotypes are primarily observed in sensitized genetic backgrounds. Interestingly, Ena/VASP appears to function downstream of both attractive and repulsive guidance cues, sometimes within the same cell. In worms, for example, UNC-34 functions downstream of UNC-40/DCC and UNC-5, the two Netrin receptors in C.elegans [34,35]. Loss of UNC-34 partially suppresses the morphological phenotypes induced by a gain-of-function mutation in UNC-40/DCC [35], as well as axon repulsion induced by ectopic expression of UNC-5 [34]. Genetic evidence also implicates Drosphila Ena in Netrin mediated guidance [36]. In addition, Ena functions downstream of the repulsive guidance receptor Robo/Sax3 and can bind directly to Robo [7,37,38].
Ena mutations in Drosophila also result in defects in motor axon pathways in which the ISNb motor axons fail to branch and instead bypass their muscle target [39]. Deletions of the receptor tyrosine phosphatase Dlar cause a similar phenotype, and both Dlar and Ena antagonize the function of the tyrosine kinase D-Abl in this pathway. Ena suppresses D-Abl dependent phenotypes, at least in part by reducing formation of ectopic F-actin spikes formed in D-Abl mutants [40]. Since Drosophila Ena is a direct target of both Dlar and D-Abl [41], these three proteins may define a tyrosine phosphorylation state-dependent switch controlling growth cone behavior. Interestingly, however, a role for Abl in axon guidance has not been observed in vertebrates [42].
The broad, overlapping expression patterns of the three vertebrate Ena/VASP paralogs requires combined deletions or inhibition of two or all three to reveal their functions in cell and developmental biology. Mice lacking Mena exhibit subtle defects in forebrain commissure [18] and optic nerve formation [19]. Mena/VASP double mutants display defects in the formation of several axon fiber tracts in the central and peripheral nervous systems, including the defects in all of the major forebrain commissures [19].
Development of the optic nerves is perturbed in the absence of Ena/VASP function. However, different phenotypes have been reported in mice and Xenopus [9,19,43]. In mice, Mena/VASP/EVL triple mutants exhibit stunted optic nerves that extend into the brain but fail to form the optic chiasm [9]. In contrast, inhibition of Ena/VASP function in Xenopus retina by transfection of the FPPPP-Mito construct did not affect axon guidance, but did reduce elongation rates and terminal arborization [43]. Differences in the severity of the phenotypes may reflect variations in the experimental approaches. While growth cones expressing FPPPP-Mito show an expected reduction or absence of filopodia, not all axons express the construct. Therefore, some pioneer axons are preserved and, because they elongate at a faster rate, extend in front of the axons with reduced Ena/VASP function and could therefore guide them by adhesive mechanisms. Additionally, accumulation of sufficient levels of the transfected FPPPP-Mito construct to inhibit Ena/VASP may only occur after axon outgrowth initiation.
Many of the axonal defects seen in Ena/VASP knockout mice involve midline guidance decisions, a major choice point in the developing nervous system, where axons must choose to cross to the contralateral side or remain ipsilateral. Formation of the forebrain commissures and the optic nerves is orchestrated by an array of short and long range guidance cues, including Slit and Netrin [44,45], suggesting that Ena/VASP functions downstream of receptors for these guidance factors as is the case in invertebrates. It is important to note that since Mena and VASP are expressed in glial cells structures required for commissure formation [19], it is also possible that non-cell autonomous defects contribute to the observed guidance phenotypes. A more thorough understanding of Ena/VASP function in axon guidance in vertebrates will require use of conditional knockouts to distinguish roles intrinsic to axons as well as to bypass the neuritogenesis defect in the cortex, which precludes analysis of complete Ena/VASP deficiency in axon guidance in the developing brain.
Growth Cone Filopodia in Guidance Sensing
Growth cone filopodia function as sensors for guidance signals and can initiate the growth cone turning response [46,47]. DCC is enriched in filopodial tips on growth cones, supporting the idea that filopodia act as remote sensors for guidance cues [48]. Netrin stimulation of dissociated hippocampal neurons in culture drives rapid increases the number and length of filopodia in an Ena/VASP-mediated manner [15]. Elimination of growth cone filopodia by cytochalasin B treatment, a drug that blocks actin polymerization, increases pathfinding defects [49] and eliminates growth cone turning responses [50,51]. Thus, Ena/VASP-deficient neurons may fail to respond to guidance cues because they have lost their filopodia. Therefore their ability to sample the environment is compromised. When presented with an attractant stimulus, growth cone filopodia re-orient towards the guidance cue [51,52]. Not all axon guidance decisions require filopodia. For example, unc-34 mutants form axonal growth cones that lack filopodia but still migrate towards Netrin, apparently through lamellipodial guidance [35]. The requirement for filopodia (and Ena/VASP) in guidance responses might be species or system specific. For instance, filopodia may be much more important in vertebrate guidance since axons must extend much longer distances and navigate through much more complicated environments than their counterparts in C.elegans or Drosophila.
Signaling Downstream of Guidance Receptors
In invertebrates, genetic studies indicate that Ena/VASP proteins are targets of signaling cascades downstream of guidance receptors and that their activity is modulated by second messenger signaling pathways. In addition to the D-Abl/D-Lar pathways, Ena mutants also interact genetically with Trio, which contains two guanine-nucleotide exchange domains specific for Rac and Rho [53]. Genetic and biochemical evidence links Ena/VASP to MIG-10/Lpd function [54,55]. MIG-10/Lpd in turn interact with phosphoinositides and Ras superfamily proteins [6,54], suggesting that these signaling pathways could also modulate Ena/VASP function. However, it is clear that MIG-10/Lpd and Ena/VASP also have independent functions since mig-10/unc-34 double mutants have a more severe phenotype than either single mutant [54,55]. Interestingly, MIG-10 is implicated in the very earliest steps of neuronal polarization towards Netrin [55,56].
In vertebrates, signaling through various guidance factors can be modulated by cyclic nucleotides and the ratio of cAMP to cGMP present in a given cell can convert them from attraction to repulsion and vice versa [57]. Vertebrate Ena/VASP proteins are targets of both the cyclic-nucleotide activated kinases PKA and PKG [58-60]. Since they are known to function downstream of attractive and repulsive guidance factors, Ena/VASP proteins are perfectly positioned to relay this switch into cytoskeletal and migratory changes (Figure 2). Indeed, Netrin stimulation or pharmacological activation of PKA both cause a rapid increase in filopodia number and length in an Ena/VASP dependent manner along with a concomitant increase in Mena phosphorylation [15]. Though it is not known whether repulsive guidance signaling affects the phosphorylation status of Ena/VASP proteins in neurons, in vitro phosphorylation at the preferred PKG site decreases VASP’s affinity for actin filaments and abolishes its anti-capping and filament bundling activities [12]. Thus, modulation of Ena/VASP activity by PKA might promote axon turning by supporting filopodia formation and elongation towards a guidance cue. Conversely, repulsive guidance cues might inhibit Ena/VASP function locally, thereby suppressing filopodia formation in the direction of the cue in favor of movement in the opposite direction. It is important to note, however, that while PKA activity can regulate the sensitivity of axons to Netrin-1, a direct role for PKA in guidance conversion has not been observed [61]. In addition, Netrin-1 stimulation does not activate PKA [62]. It seems likely that PKA phosphorylation of Ena/VASP may be triggered by other cues, for example changes in the extracellular matrix [63]. It is well established that growth cone responses to guidance factors changes depending on environmental context; PKA- and PKG mediated phosphorylation of Ena/VASP may facilitate such context-dependent guidance conversion in concert with other cyclic-nucleotide mediated signaling events [64]. Interestingly, this PKA/PKG regulation of Ena/VASP is not conserved between vertebrates and invertebrates, since the invertebrate orthologs lack the corresponding phosphorylation sites. How phosphorylation modulates Ena/VASP activity in vertebrate neurons, and how this contributes to cytoskeletal reorganization during axon guidance remains to be elucidated.
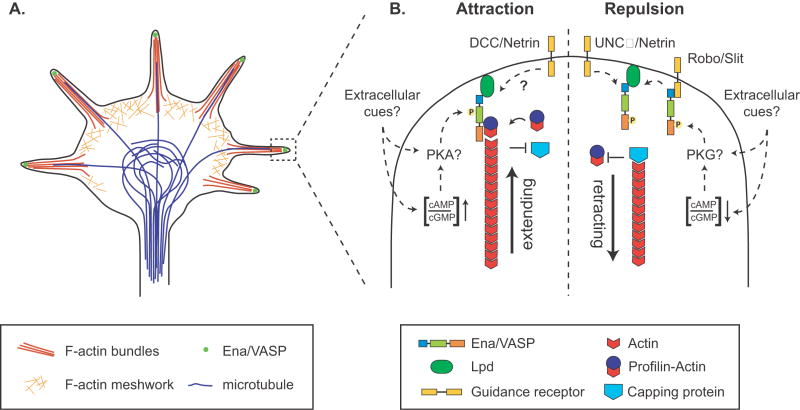
Figure 2.
A. Localization of Ena/VASP proteins in growth cones. Growth cones contain a meshwork of actin filaments in the lamellipodium, actin filament bundles that penetrate filopodia, bundled stable microtubules in the center, and dynamic microtubules that extend into the periphery, associating with actin filaments. Ena/VASP proteins are clustered at the tips of filopodia. B. Model for regulation of Ena/VASP activity by guidance cues. Activation of Ena/VASP proteins downstream of attractive guidance cues and/or their phosphorylation by PKA might result in the protection of actin filaments from capping protein and promote addition of actin-profilin and filament elongation. In contrast, repulsive guidance cues might inhibit Ena/VASP function, potentially through phosphorylation by PKG, leading to filament capping and retraction of filopodia. It is also possible that Ena/VASP-dependent filopodial dynamics are important to sense repulsive cues initially; subsequent inhibition/inactivation of Ena/VASP may then contribute to filopodial retraction.
Conclusions
Since their discovery in Drosophila, Ena/VASP proteins have been implicated in the proper development of the nervous system [65]. In vertebrates, Ena/VASP plays a pivotal role in the initial establishment of cortical neuronal morphology and is required elsewhere for proper axon guidance; both neuritogenesis and guidance depend on filopodia, highlighting the importance of understanding the mechanisms that govern filopodial dynamics. However, Ena/VASP proteins appear to be involved in different aspects of neuronal development depending on the organisms, experimental conditions and cell types studied. This variability indicates that while Ena/VASP proteins universally play an important role in the developing nervous system, different organisms and neuronal cell types have evolved distinct mechanisms utilizing Ena/VASP proteins for outgrowth and guidance. Although progress towards understanding the mechanism underlying Ena/VASP regulation of actin dynamics and filopodia formation has been made, many questions remain. A major challenge will be to understand how Ena/VASP is regulated during axon guidance. Further characterization of filopodial tip complexes, monitoring of growth cones with altered Ena/VASP activity in vitro, and biochemical studies to determine the formation of differential protein complexes in response to guidance cues will help elucidate how Ena/VASP protein facilitate accurate axon guidance.
Acknowledgments
We thank S. Gupton and E. Pinheiro for critical reading of the manuscript. This work was supported by NIH grant GM58801 (F.B.G). F.D. is a Damon Runyon Fellow supported by the Damon Runyon Cancer Research Foundation (DRG-1920-06).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Suggested Reading
- 1.Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- 2.Kalil K, Dent EW. Touch and go: guidance cues signal to the growth cone cytoskeleton. Curr Opin Neurobiol. 2005;15:521–526. doi: 10.1016/j.conb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Gupton SL, Gertler FB. Filopodia: the fingers that do the walking. Sci STKE. 2007;2007:re5. doi: 10.1126/stke.4002007re5. [DOI] [PubMed] [Google Scholar]
- 4.Koleske AJ. Do filopodia enable the growth cone to find its way? Sci STKE. 2003;2003:pe20. doi: 10.1126/stke.2003.183.pe20. [DOI] [PubMed] [Google Scholar]
- 5.Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 6.Krause M, Leslie JD, Stewart M, Lafuente EM, Valderrama F, Jagannathan R, Strasser GA, Rubinson DA, Liu H, Way M, et al. Lamellipodin, an Ena/VASP ligand, is implicated in the regulation of lamellipodial dynamics. Dev Cell. 2004;7:571–583. doi: 10.1016/j.devcel.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Bashaw GJ, Kidd T, Murray D, Pawson T, Goodman CS. Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell. 2000;101:703–715. doi: 10.1016/s0092-8674(00)80883-1. [DOI] [PubMed] [Google Scholar]
- 8.Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly IV, Chaga OY, Cooper JA, Borisy GG, et al. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109:509–521. doi: 10.1016/s0092-8674(02)00731-6. [DOI] [PubMed] [Google Scholar]
- ••9.Dent EW, Kwiatkowski AV, Mebane LM, Philippar U, Barzik M, Rubinson DA, Gupton S, Van Veen JE, Furman C, Zhang J, et al. Filopodia are required for cortical neurite initiation. Nat Cell Biol. 2007;9:1347–1359. doi: 10.1038/ncb1654. This study demonstrates that filopodia are necessary for neurite formation. The neuritogenesis defect observed in Ena/VASP-deficient cortical neurons can be rescued through intrinsic, such as mDia2, or extrinsic, such as laminin, factors that induce filpodia formation. Interestingly, though filopodia are necessary, they are not sufficient for neuritogensis. Dynamic microtubules are also required to form neurites. [DOI] [PubMed] [Google Scholar]
- 10.Gates J, Mahaffey JP, Rogers SL, Emerson M, Rogers EM, Sottile SL, Van Vactor D, Gertler FB, Peifer M. Enabled plays key roles in embryonic epithelial morphogenesis in Drosophila. Development. 2007;134:2027–2039. doi: 10.1242/dev.02849. [DOI] [PubMed] [Google Scholar]
- 11.Bear JE, Loureiro JJ, Libova I, Fassler R, Wehland J, Gertler FB. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell. 2000;101:717–728. doi: 10.1016/s0092-8674(00)80884-3. [DOI] [PubMed] [Google Scholar]
- 12.Barzik M, Kotova TI, Higgs HN, Hazelwood L, Hanein D, Gertler FB, Schafer DA. Ena/VASP proteins enhance actin polymerization in the presence of barbed end capping proteins. J Biol Chem. 2005;280:28653–28662. doi: 10.1074/jbc.M503957200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachmann C, Fischer L, Walter U, Reinhard M. The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J Biol Chem. 1999;274:23549–23557. doi: 10.1074/jbc.274.33.23549. [DOI] [PubMed] [Google Scholar]
- 14.Walders-Harbeck B, Khaitlina SY, Hinssen H, Jockusch BM, Illenberger S. The vasodilator-stimulated phosphoprotein promotes actin polymerisation through direct binding to monomeric actin. FEBS Lett. 2002;529:275–280. doi: 10.1016/s0014-5793(02)03356-2. [DOI] [PubMed] [Google Scholar]
- 15.Lebrand C, Dent EW, Strasser GA, Lanier LM, Krause M, Svitkina TM, Borisy GG, Gertler FB. Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron. 2004;42:37–49. doi: 10.1016/s0896-6273(04)00108-4. [DOI] [PubMed] [Google Scholar]
- •16.Applewhite DA, Barzik M, Kojima S, Svitkina TM, Gertler FB, Borisy GG. Ena/VASP proteins have an anti-capping independent function in filopodia formation. Mol Biol Cell. 2007;18:2579–2591. doi: 10.1091/mbc.E06-11-0990. This study uses cell spreading in combination with depletion of capping protein as a novel assay to monitor filopodia formation. Expression of mutant proteins in Ena/VASP-deficient cells was used to demonstrate that F- and G-actin binding along with tetramerization are required for Ena/VASP to support filopodia formation. FRAP studies demonstrated that VASP is stably localized to filopodial tips through a mechanism that requires its G-actin binding motif. Interestingly, this motif is similar to WH2 domains that have recently been shown to bind barbed ends. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •17.Pasic L, Kotova TI, Schafer DA. Ena/VASP proteins capture actin filament barbed ends. J Biol Chem. 2008 doi: 10.1074/jbc.M710475200. In this elegant study, direct visualization of growing fluorescently-labeled actin filaments by TIRF microscopy shows that Ena/VASP proteins promote the formation of actin filaments by transiently capturing filament barbed ends, antagonizing barbed end capping and enhancing filament elongation in the presence of profiling, though Ena/VASP proteins do not remain stably associated with the ends of elongating filaments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanier LM, Gates MA, Witke W, Menzies AS, Wehman AM, Macklis JD, Kwiatkowski D, Soriano P, Gertler FB. Mena is required for neurulation and commissure formation. Neuron. 1999;22:313–325. doi: 10.1016/s0896-6273(00)81092-2. [DOI] [PubMed] [Google Scholar]
- 19.Menzies AS, Aszodi A, Williams SE, Pfeifer A, Wehman AM, Goh KL, Mason CA, Fassler R, Gertler FB. Mena and vasodilator-stimulated phosphoprotein are required for multiple actin-dependent processes that shape the vertebrate nervous system. J Neurosci. 2004;24:8029–8038. doi: 10.1523/JNEUROSCI.1057-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goh KL, Cai L, Cepko CL, Gertler FB. Ena/VASP proteins regulate cortical neuronal positioning. Curr Biol. 2002;12:565–569. doi: 10.1016/s0960-9822(02)00725-x. [DOI] [PubMed] [Google Scholar]
- 21.Forrester WC, Garriga G. Genes necessary for C. elegans cell and growth cone migrations. Development. 1997;124:1831–1843. doi: 10.1242/dev.124.9.1831. [DOI] [PubMed] [Google Scholar]
- ••22.Kwiatkowski AV, Rubinson DA, Dent EW, Edward van Veen J, Leslie JD, Zhang J, Mebane LM, Philippar U, Pinheiro EM, Burds AA, et al. Ena/VASP Is Required for neuritogenesis in the developing cortex. Neuron. 2007;56:441–455. doi: 10.1016/j.neuron.2007.09.008. Analysis of mice lacking all three paralogs of Ena/VASP reveals an unexpected requirement for these proteins in neuritogenesis in the cortex, leading to a block of cortical axon fiber tract formation. This defect was shown to arise from a failure of Ena/VASP-deficient cortical neurons to form filopodia. [DOI] [PubMed] [Google Scholar]
- 23.Gao FB, Brenman JE, Jan LY, Jan YN. Genes regulating dendritic outgrowth, branching, and routing in Drosophila. Genes Dev. 1999;13:2549–2561. doi: 10.1101/gad.13.19.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li W, Li Y, Gao FB. Abelson, enabled, and p120 catenin exert distinct effects on dendritic morphogenesis in Drosophila. Dev Dyn. 2005;234:512–522. doi: 10.1002/dvdy.20496. [DOI] [PubMed] [Google Scholar]
- 25.Lin YL, Lei YT, Hong CJ, Hsueh YP. Syndecan-2 induces filopodia and dendritic spine formation via the neurofibromin-PKA-Ena/VASP pathway. J Cell Biol. 2007;177:829–841. doi: 10.1083/jcb.200608121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rostaing P, Real E, Siksou L, Lechaire JP, Boudier T, Boeckers TM, Gertler F, Gundelfinger ED, Triller A, Marty S. Analysis of synaptic ultrastructure without fixative using high-pressure freezing and tomography. Eur J Neurosci. 2006;24:3463–3474. doi: 10.1111/j.1460-9568.2006.05234.x. [DOI] [PubMed] [Google Scholar]
- •27.Gabel CV, Antonie F, Chuang CF, Samuel AD, Chang C. Distinct cellular and molecular mechanisms mediate initial axon development and adult-stage axon regeneration in C. elegans. Development. 2008;135:1129–1136. doi: 10.1242/dev.013995. This elegant study reveals a role for UNC34/Ena and MIG-10/Lpd in adult stage axon regeneration in C.elegans after laser axotomy downstream of Netrin and Slit guidance cues. Interestingly, Unc34/Ena appears to be required for adult stage axonal re-growth, while Mig-10/LPD is involved in regenerative axon guidance. [DOI] [PubMed] [Google Scholar]
- 28.Pellegrin S, Mellor H. The Rho family GTPase Rif induces filopodia through mDia2. Curr Biol. 2005;15:129–133. doi: 10.1016/j.cub.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Schirenbeck A, Bretschneider T, Arasada R, Schleicher M, Faix J. The Diaphanous-related formin dDia2 is required for the formation and maintenance of filopodia. Nat Cell Biol. 2005;7:619–625. doi: 10.1038/ncb1266. [DOI] [PubMed] [Google Scholar]
- 30.Bohil AB, Robertson BW, Cheney RE. Myosin-X is a molecular motor that functions in filopodia formation. Proc Natl Acad Sci U S A. 2006;103:12411–12416. doi: 10.1073/pnas.0602443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaefer AW, Kabir N, Forscher P. Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. J Cell Biol. 2002;158:139–152. doi: 10.1083/jcb.200203038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dent EW, Kalil K. Axon branching requires interactions between dynamic microtubules and actin filaments. J Neurosci. 2001;21:9757–9769. doi: 10.1523/JNEUROSCI.21-24-09757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schober JM, Komarova YA, Chaga OY, Akhmanova A, Borisy GG. Microtubule-targeting-dependent reorganization of filopodia. J Cell Sci. 2007;120:1235–1244. doi: 10.1242/jcs.003913. [DOI] [PubMed] [Google Scholar]
- 34.Colavita A, Culotti JG. Suppressors of ectopic UNC-5 growth cone steering identify eight genes involved in axon guidance in Caenorhabditis elegans. Dev Biol. 1998;194:72–85. doi: 10.1006/dbio.1997.8790. [DOI] [PubMed] [Google Scholar]
- 35.Gitai Z, Yu TW, Lundquist EA, Tessier-Lavigne M, Bargmann CI. The netrin receptor UNC-40/DCC stimulates axon attraction and outgrowth through enabled and, in parallel, Rac and UNC-115/AbLIM. Neuron. 2003;37:53–65. doi: 10.1016/s0896-6273(02)01149-2. [DOI] [PubMed] [Google Scholar]
- 36.Forsthoefel DJ, Liebl EC, Kolodziej PA, Seeger MA. The Abelson tyrosine kinase, the Trio GEF and Enabled interact with the Netrin receptor Frazzled in Drosophila. Development. 2005;132:1983–1994. doi: 10.1242/dev.01736. [DOI] [PubMed] [Google Scholar]
- 37.Yu TW, Hao JC, Lim W, Tessier-Lavigne M, Bargmann CI. Shared receptors in axon guidance: SAX-3/Robo signals via UNC-34/Enabled and a Netrin-independent UNC-40/DCC function. Nat Neurosci. 2002;5:1147–1154. doi: 10.1038/nn956. [DOI] [PubMed] [Google Scholar]
- 38.Wills Z, Emerson M, Rusch J, Bikoff J, Baum B, Perrimon N, Van Vactor D. A Drosophila homolog of cyclase-associated proteins collaborates with the Abl tyrosine kinase to control midline axon pathfinding. Neuron. 2002;36:611–622. doi: 10.1016/s0896-6273(02)01022-x. [DOI] [PubMed] [Google Scholar]
- 39.Wills Z, Marr L, Zinn K, Goodman CS, Van Vactor D. Profilin and the Abl tyrosine kinase are required for motor axon outgrowth in the Drosophila embryo. Neuron. 1999;22:291–299. doi: 10.1016/s0896-6273(00)81090-9. [DOI] [PubMed] [Google Scholar]
- 40.Grevengoed EE, Fox DT, Gates J, Peifer M. Balancing different types of actin polymerization at distinct sites: roles for Abelson kinase and Enabled. J Cell Biol. 2003;163:1267–1279. doi: 10.1083/jcb.200307026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wills Z, Bateman J, Korey CA, Comer A, Van Vactor D. The tyrosine kinase Abl and its substrate enabled collaborate with the receptor phosphatase Dlar to control motor axon guidance. Neuron. 1999;22:301–312. doi: 10.1016/s0896-6273(00)81091-0. [DOI] [PubMed] [Google Scholar]
- 42.Moresco EM, Donaldson S, Williamson A, Koleske AJ. Integrin-mediated dendrite branch maintenance requires Abelson (Abl) family kinases. J Neurosci. 2005;25:6105–6118. doi: 10.1523/JNEUROSCI.1432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••43.Dwivedy A, Gertler FB, Miller J, Holt CE, Lebrand C. Ena/VASP function in retinal axons is required for terminal arborization but not pathway navigation. Development. 2007;134:2137–2146. doi: 10.1242/dev.002345. To test if filopodia are required for growth cone navigation in the developing visual path in Xenopus, the authors neutralized Ena/VASP function in retinal ganglion cells. Inhibition of Ena/VASP induced a loss of growth cone filopodia in vivo. However, the lack of filopodia caused by depletion of Xena/XVASP did not affect retinal pathfinding, indicating that filopodia are not required for axon guidance in the optic pathway. Instead, the authors observed slower growth rate and a striking decrease in terminal arborization of axons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erskine L, Herrera E. The retinal ganglion cell axon’s journey: insights into molecular mechanisms of axon guidance. Dev Biol. 2007;308:1–14. doi: 10.1016/j.ydbio.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Lindwall C, Fothergill T, Richards LJ. Commissure formation in the mammalian forebrain. Curr Opin Neurobiol. 2007;17:3–14. doi: 10.1016/j.conb.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Davenport RW, Dou P, Rehder V, Kater SB. A sensory role for neuronal growth cone filopodia. Nature. 1993;361:721–724. doi: 10.1038/361721a0. [DOI] [PubMed] [Google Scholar]
- 47.Dent EW, Tang F, Kalil K. Axon guidance by growth cones and branches: common cytoskeletal and signaling mechanisms. Neuroscientist. 2003;9:343–353. doi: 10.1177/1073858403252683. [DOI] [PubMed] [Google Scholar]
- ••48.Zhu XJ, Wang CZ, Dai PG, Xie Y, Song NN, Liu Y, Du QS, Mei L, Ding YQ, Xiong WC. Myosin X regulates netrin receptors and functions in axonal pathfinding. Nat Cell Biol. 2007;9:184–192. doi: 10.1038/ncb1535. This study reports that DCC interacts directly with the unconventional myosin MyoX. MyoX can drive filopodia formation and exhibits intrafilopodial movement. The authors show that DCC localizes to filopodial tips through a MyoX-dependent mechanism. Furthermore, through RNAi knockdown and expression of motorless MyoX, the authors demonstrate that MyoX is required for Netrin mediated axon outgrowth. [DOI] [PubMed] [Google Scholar]
- 49.Bentley D, Toroian-Raymond A. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature. 1986;323:712–715. doi: 10.1038/323712a0. [DOI] [PubMed] [Google Scholar]
- 50.Kaufmann N, Wills ZP, Van Vactor D. Drosophila Rac1 controls motor axon guidance. Development. 1998;125:453–461. doi: 10.1242/dev.125.3.453. [DOI] [PubMed] [Google Scholar]
- 51.Zheng JQ, Wan JJ, Poo MM. Essential role of filopodia in chemotropic turning of nerve growth cone induced by a glutamate gradient. J Neurosci. 1996;16:1140–1149. doi: 10.1523/JNEUROSCI.16-03-01140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng JQ, Felder M, Connor JA, Poo MM. Turning of nerve growth cones induced by neurotransmitters. Nature. 1994;368:140–144. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]
- 53.Liebl EC, Forsthoefel DJ, Franco LS, Sample SH, Hess JE, Cowger JA, Chandler MP, Shupert AM, Seeger MA. Dosage-sensitive, reciprocal genetic interactions between the Abl tyrosine kinase and the putative GEF trio reveal trio’s role in axon pathfinding. Neuron. 2000;26:107–118. doi: 10.1016/s0896-6273(00)81142-3. [DOI] [PubMed] [Google Scholar]
- •54.Chang C, Adler CE, Krause M, Clark SG, Gertler FB, Tessier-Lavigne M, Bargmann CI. MIG-10/lamellipodin and AGE-1/PI3K promote axon guidance and outgrowth in response to slit and netrin. Curr Biol. 2006;16:854–862. doi: 10.1016/j.cub.2006.03.083. [DOI] [PubMed] [Google Scholar]
- •55.Quinn CC, Pfeil DS, Chen E, Stovall EL, Harden MV, Gavin MK, Forrester WC, Ryder EF, Soto MC, Wadsworth WG. UNC-6/netrin and SLT-1/slit guidance cues orient axon outgrowth mediated by MIG-10/RIAM/lamellipodin. Curr Biol. 2006;16:845–853. doi: 10.1016/j.cub.2006.03.025. [DOI] [PubMed] [Google Scholar]
- •56.Adler CE, Fetter RD, Bargmann CI. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat Neurosci. 2006;9:511–518. doi: 10.1038/nn1666. Chang et al., and Quinn et al. demonstrate that MIG-10/Lpd and UNC-34/Ena cooperate during neuronal development to guide axons toward UNC-6/Netrin and away from SLT-1/Slit. In axons that are guided towards Netrin, UNC-34/Ena is required for growth cone filopodia formation, whereas MIG-10/Lpd increases the number of filopodia. Furthermore, MIG-10 facilitates directed outgrowth in response to UNC-6 and SLT-1. Quinn et al. and Adler et al. demonstrate that MIG-10 is an early marker for polarization towards a Netrin cue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishiyama M, Hoshino A, Tsai L, Henley JR, Goshima Y, Tessier-Lavigne M, Poo MM, Hong K. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;423:990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]
- 58.Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem. 1994;269:14509–14517. [PubMed] [Google Scholar]
- 59.Gertler FB, Niebuhr K, Reinhard M, Wehland J, Soriano P. Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell. 1996;87:227–239. doi: 10.1016/s0092-8674(00)81341-0. [DOI] [PubMed] [Google Scholar]
- 60.Lambrechts A, Kwiatkowski AV, Lanier LM, Bear JE, Vandekerckhove J, Ampe C, Gertler FB. cAMP-dependent protein kinase phosphorylation of EVL, a Mena/VASP relative, regulates its interaction with actin and SH3 domains. J Biol Chem. 2000;275:36143–36151. doi: 10.1074/jbc.M006274200. [DOI] [PubMed] [Google Scholar]
- 61.Moore SW, Kennedy TE. Protein kinase A regulates the sensitivity of spinal commissural axon turning to netrin-1 but does not switch between chemoattraction and chemorepulsion. J Neurosci. 2006;26:2419–2423. doi: 10.1523/JNEUROSCI.5419-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore SW, Sun KL, Xie F, Barker PA, Conti M, Kennedy TE. Soluble adenylyl cyclase is not required for axon guidance to netrin-1. J Neurosci. 2008;28:3920–3924. doi: 10.1523/JNEUROSCI.0547-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hopker VH, Shewan D, Tessier-Lavigne M, Poo M, Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- 64.Piper M, van Horck F, Holt C. The role of cyclic nucleotides in axon guidance. Adv Exp Med Biol. 2007;621:134–143. doi: 10.1007/978-0-387-76715-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gertler FB, Comer AR, Juang JL, Ahern SM, Clark MJ, Liebl EC, Hoffmann FM. enabled, a dosage-sensitive suppressor of mutations in the Drosophila Abl tyrosine kinase, encodes an Abl substrate with SH3 domain-binding properties. Genes Dev. 1995;9:521–533. doi: 10.1101/gad.9.5.521. [DOI] [PubMed] [Google Scholar]