Abstract
Cervical cancer ranks among the top three cancer diagnoses in women worldwide. In the United States, the SEER Cancer Statistics Review identified cervical cancer as the third leading cause (following childhood cancers and testicular cancer) of average years of life lost per person dying of cancer for all races and both genders. Approximately one-third of cervical cancer patients develop disease recurrence and the majority of these recurrences occur within the first 2 years after completion of therapy. Predictors of disease recurrence include stage and lymph node status at the time of initial diagnosis. The initial diagnosis and staging of cervical cancer has traditionally been achieved by history and physical examination and by use of selected imaging studies. Accurate staging is important both for selecting appropriate therapy and for prognosis. Computed tomography (CT) has been the most widely used imaging method for assessment of nodal involvement and detection of distant metastatic disease. Positron emission tomography (PET) has become an established imaging tool for cervical cancer. The functional information about regional glucose metabolism provided by fluorodeoxyglucose (FDG)-PET provides for greater sensitivity and specificity in most cancer imaging applications by comparison with CT and other anatomic imaging methods. PET is superior to conventional imaging modalities for evaluating patients with cervical cancer.
Keywords: Cervix cancer, radiation, FDG-PET/CT, diagnosis, prognosis
Introduction
Cervical cancer ranks among the top three cancer diagnoses in women worldwide[1]. In the United States, the SEER Cancer Statistics Review identified cervical cancer as the third leading cause (following childhood cancers and testicular cancer) of average years of life lost per person dying of cancer for all races and both genders[2]. Recent strategies to reduce the incidence of cervical cancer have focused on the development of a human papilloma virus (HPV) vaccine. While HPV vaccine has the potential to significantly reduce de novo HPV infection in women less than 26 years of age, a significant population of women (older than 26 years and unvaccinated) is currently at risk for future development of cervical cancer. Even assuming 100% compliance with vaccination, a recent study estimated that the impact of HPV vaccination would not be appreciated clinically until after 2040[3]. In the coming years, clinicians will continue to face the challenges associated with the treatment and follow-up of patients with cervical cancer.
Approximately one-third of cervical cancer patients develop disease recurrence and the majority of these recurrences occur within the first 2 years after completion of therapy. Predictors of disease recurrence include stage and lymph node status at the time of initial diagnosis.
Initial diagnosis
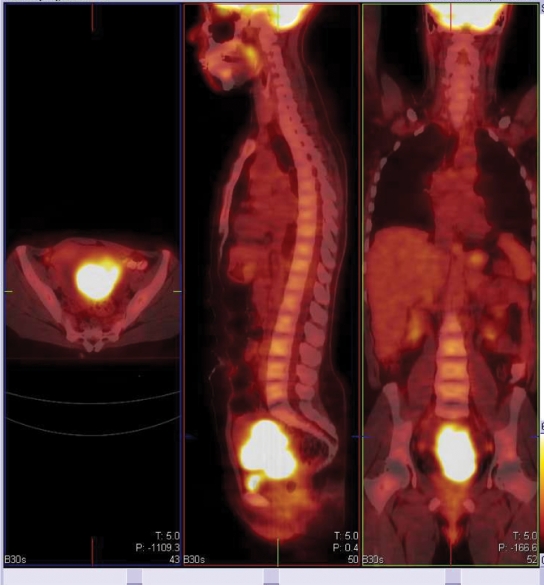
The initial diagnosis and staging of cervical cancer has traditionally been achieved by history and physical examination and by use of selected imaging studies. Accurate staging is important both for selecting appropriate therapy and for prognosis. Cervical cancer initially spreads regionally and then through lymphatic channels before hematogenous dissemination to distant organs. With locally advanced disease, the status of pelvic and para-aortic lymph nodes is an important determinant of prognosis and guides treatment planning decisions. Computed tomography (CT) has been the most widely used imaging method for assessment of nodal involvement and detection of distant metastatic disease. Despite its high resolution and excellent depiction of anatomy, CT is limited by its inability to detect small-volume metastatic involvement in normal-size lymph nodes and to determine whether enlarged nodes represent metastasis or reactive hyperplasia. PET has become an established imaging tool for cervical cancer (Fig. 1). The functional information about regional glucose metabolism provided by fluorodeoxyglucose (FDG)-positron emission tomography (PET) provides for greater sensitivity and specificity in most cancer imaging applications by comparison with CT and other anatomic imaging methods. PET is superior to conventional imaging modalities for evaluating patients with cervical cancer. The development and rapid dissemination of integrated PET/CT scanners that allow functional and anatomical information to be obtained in a single examination represents an important advance in PET imaging technology, resulting in a synergistic improvement in the accuracy of interpretation of both PET and CT images.
Figure 1.
Large primary cervical cancer at diagnosis.
A number of studies have shown that FDG-PET is superior to conventional imaging methods for detecting metastatic disease, particularly lymph node metastasis[4,5]. Havrilesky and associates[6] recently reported a systematic review of the published literature up through 2003. They included only those studies involving 12 or more subjects who had PET performed with a dedicated scanner with specified resolution, and with clinical follow-up ≥6 months or histopathology as the reference standards. In patients with newly diagnosed cervical cancer, the pooled sensitivity of PET was 79% (95% CI 65–90%), and the pooled specificity was 99% (96–99%) for detection of pelvic lymph nodes metastasis[5,7–9]. Two studies were identified that each compared PET to magnetic resonance imaging (MRI) and CT[4,5]. MRI had a pooled sensitivity of 72% (53–87%) and pooled specificity of 96% (92–98%), whereas CT had a pooled sensitivity of 47% (21–73%) (there were insufficient data to calculate a pooled specificity). In four prospective studies in which histology after para-aortic lymphadenectomy was used as the reference standard, the pooled sensitivity of PET for the detection of para-aortic nodal metastasis was 84% (95% CI 68–94%) and the pooled specificity was 95% (89–98%)[5,7,8, 10]. In three of these studies, the inclusion criteria for study entry included a negative CT or MRI of the abdomen[7,9,10]. Thus, the accuracy of conventional imaging could not be calculated. Reinhardt and colleagues[5] did not require a negative abdominal imaging study prior to surgery. The sensitivity and specificity of MRI in the 12 patients who underwent aortic node sampling were 67% and 100%, respectively.
Our own studies have shown that FDG-PET is superior to CT and lymphangiography in showing unsuspected sites of metastasis in pelvic lymph nodes, extrapelvic lymph nodes, and visceral organs in patients with newly diagnosed advanced cervical cancer[11]. FDG-PET showed abnormalities consistent with metastasis more often than did CT in pelvic lymph nodes (67% vs. 20%) and in para-aortic lymph nodes (21% vs. 7%). PET also showed disease in supraclavicular lymph nodes in 8%[12]. These initial results have been sustained in subsequent evaluations of data from our prospective registry that now includes over 600 patients[13].
Based on the results in the literature to date, the United States Center for Medicare and Medicaid Services in January 2005 approved coverage for use of FDG-PET in initial staging of patients with cervical cancer who have no evidence of extrapelvic metastatic disease on CT or MRI[14].
Prognostic factors
Several prognostic factors have been identified for patients with carcinoma of the cervix. These include patient age, tumor histology, tumor stage, tumor size, lymph node metastasis, and tumor hypoxia[15,16]. In a study of 101 patients with newly diagnosed cervical cancer, Grigsby and colleagues[12] demonstrated that the lymph node status determined by FDG-PET is the most significant independent pre-treatment predictor of progression-free and overall survival in patients with cervical cancer. The 2-year, disease-free survival was better predicted by PET evidence of lymph node involvement than by CT findings. Based on the imaging findings in the pelvic lymph nodes, the 2-year, disease-free survival was 84% for CT–/PET– patients, 64% for CT–/PET + patients, and 48% for CT + /PET + patients (p = 0.05). Based on the imaging findings in the para-aortic nodes, the 2-year, disease-free survival was 78% in CT–/PET– patients, 31% for CT–/PET+ patients, and 14% for CT + /PET + patients (p ≤ 0.0001). No patients with PET+ supraclavicular lymph nodes survived 2 years. The PET-determined status of the para-aortic nodes was the strongest predictor of survival in a multivariate logistic regression analysis. These results suggest an opportunity to cure patients with para-aortic nodal metastasis defined by PET that were not detected by CT. In a recent review of data from 256 patients in our registry, we also found that the extent of lymph node involvement is inversely correlated with survival[17]. We have also found that FDG-PET demonstrated metastatic involvement in the left supraclavicular lymph nodes in 8% of our patient population[18]. This finding had a positive predictive value of 100% and indicates a dismal prognosis, despite aggressive therapy. Similarly, we found that the cause-specific survival for patients with FIGO stage IIIb carcinoma is highly dependent upon the extent of lymph node metastasis demonstrated by whole-body FDG-PET at initial presentation[19]. The three-year estimates of cause-specific survival were 73% for those with no lymph node metastasis, 58% for those with only pelvic lymph node metastasis, 29% for those with pelvic and para-aortic lymph node metastasis, and 0% for those with pelvic, para-aortic, and supraclavicular lymph node metastasis (p = 0.0005). Extent of regional lymph node metastases was also found by Unger and colleagues to be a significant prognostic factor[20].
Miller and Grigsby[21] evaluated the usefulness of tumor volume measurement with FDG-PET in 57 patients with cervical cancer. Tumor volume and lymph node status determined by PET and FIGO stage determined by clinical examination were predictive of progression-free survival; tumor volume and lymph node involvement by PET predicted overall survival[21]. The avidity of FDG uptake in the primary cervical tumor is a predictor of survival outcome. Patient tumors that have a high maximum standardized uptake value (SUVmax) have a worse survival outcome than those with a low SUVmax[22].
Approximately 30% of cervical cancer patients with advanced stage disease will ultimately fail after definitive treatment[23]. Clinical and radiological techniques have been used for early detection of recurrent disease. FDG-PET has been shown to have a role in the post-treatment monitoring of patients with cervical cancer. In a large retrospective study by Ryu and associates[24], 249 women with previously treated cervical cancer without overt evidence of recurrence underwent FDG-PET as part of their routine follow-up. Eighty patients (32%) were found to have abnormal FDG uptake; 28 (11%) had clinically or histologically confirmed recurrent disease. The sensitivity and specificity of FDG-PET for detection of recurrent disease were 90% and 76%, respectively. The positive and negative predictive values were 35% and 98%, respectively. There was a high false-positive rate associated with FDG uptake in the pulmonary hila, lungs, neck, inguinal, and axillary regions. The majority of the recurrences were detected within 6–18 months after diagnosis. In another series by Unger and associates[25], FDG-PET detected recurrences in 31% of asymptomatic patients and recurrences in 67% of symptomatic patients. In symptomatic patients, the sensitivity of FDG-PET was 100%, the specificity was 86%, and the positive and negative predictive values were 93% and 100%, respectively. By comparison, in asymptomatic patients, the sensitivity of FDG-PET was 80%, the specificity was 100%, and the positive and negative predictive values were 100% and 100%, respectively. In a study by Grigsby and associates[26], 152 patients previously treated with radiotherapy with or without concurrent chemotherapy who were free of FDG-avid sites on PET obtained an average of 3 months post-therapy, had 5-year cause-specific and overall survival of 80% and 92%, respectively. Persistent abnormal uptake in the cervix or lymph nodes was found in 20 patients, and their cause-specific survival was 32%. New areas of increased FDG uptake in previously unirradiated regions were found in 18 patients, none of whom was alive at 5 years. Post-treatment PET abnormalities were found to be the most significant predictor of death from cervical cancer in this study. Together these results point to a significant impact of FDG-PET findings on treatment strategy after primary therapy.
Glucose metabolism
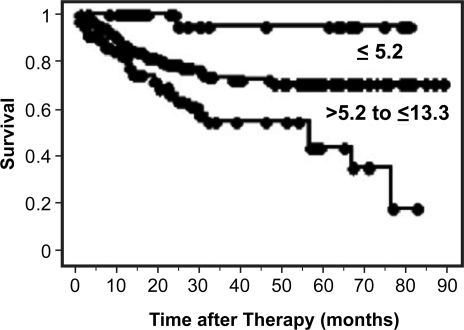
The basis of FDG-PET imaging of tumors is increased glucose metabolism of tumor tissue compared to normal tissue. Historically, patient- and tumor-related factors such as age, histology, tumor volume and stage have been critical attributes for predicting patient outcome and overall survival for cervical cancer. Studies for lung and head and neck cancers have suggested that a higher SUV correlates with worse prognosis[27–30]. Other studies have also shown that patients with a primary tumor SUV greater than the median tended to have poorer local control and disease-free survival. We have evaluated SUV in patients with cervical cancer and found that primary tumor SUVmax at diagnosis is correlated with the presence of lymph node involvement at diagnosis, how well the primary tumor responds to treatment, the likelihood of disease recurrence, and overall survival. We analyzed data from 287 patients with cervical cancer who underwent FDG-PET and found that the mean SUVmax was 11.4 (range 1.0–50.4)[31]. This is much higher than the mean value reported for other epithelial tumors. There was no relationship between tumor volume and SUVmax (correlation coefficient R2 = 0.01). Three prognostic groups were established using SUVmax. The cause-specific survivals at 5 years were 95% for SUVmax ≤5.2, 70% for SUVmax >5.2 and ≤ 13.3, and 44% for SUVmax > 13.3 (p < 0.0001). Increasing SUVmax was associated with persistent abnormal FDG uptake in the cervix on the 3-month FDG-PET in 238 patients treated with curative chemoradiation (p = 0.04). SUVmax of the cervical tumor at diagnosis is a sensitive biomarker of treatment response and prognosis (Fig. 2).
Figure 2.
Cause-specific survival based on pre-treatment cervix tumor SUVmax[31].
In our study, we showed that primary tumor SUVmax at diagnosis is predictive of lymph node involvement. For lung cancer, others have also found an association between primary tumor SUV and presence of lymph node involvement[28]. Primary tumor SUVmax predicting lymph node involvement in cervical cancer is significant because our group has previously shown that lymph node status is significantly related to disease-free and overall survivals. This suggests that SUVmax might correlate with disease aggression.
We also found that high primary tumor SUVmax at diagnosis was predictive of subsequent biopsy-proven local recurrence and correlates with increased risk of persistent cervical disease, in particular as shown by persistent disease on the 3-month post-treatment PET scan. Previous groups have found that lack of cervical tumor regression is associated with an inferior outcome. Achieving local control is critical to prognosis and overall survival.
As our data show that SUV of the primary tumor is an important predictor of prognosis, treatment response, and overall survival, this leads to the question of how glucose metabolism varies among cervical tumors and how that correlates with SUV and patient outcome. In an attempt to understand the biologic mechanism causing increased FDG uptake in tumors, others have looked at glucose transporter gene expression and had mixed results. For example, with breast cancer, one group found a correlation between Glut-1 expression and FDG uptake; another study did not find an association[32]. With regard to cervical cancer, Mendez et al.[33] found a correlation between Glut-1 expression and tumor grade, but Airley et al.[34] did not find an association between Glut-1 expression and disease-free or recurrence-free survival. Yen et al.[35] found a correlation between Glut-1 expression and SUV in cervical cancer, but Tian et al.[36] did not find a relationship between FDG SUV and Glut-1 or Glut-3 expression for oral squamous cell carcinoma. As more groups investigate this topic, more types of glucose transporters are being discovered and different methods of measuring expression are also being used, suggesting that this is a complex issue that might involve multiple factors. Additional research is needed to investigate the biologic mechanism leading to varying degrees of increased glucose uptake in tumors.
In summary, our research has clearly demonstrated the prognostic significance of maximum FDG uptake in patients with cervical cancer. However, can this information be utilized to guide individual clinical decision making? Our unpublished preliminary data suggest that patients with a high SUVmax have an excellent clinical outcome if they are treated with either surgery or radiation alone. If these patients receive concurrent chemoradiation (weekly cisplatin) then their survival is much worse than their survival when treated with either single modality therapy alone. These intriguing findings, if validated, may provide a guide to individualizing therapy for these patients.
Tumor heterogeneity
It is understood that, on a microscopic level, tumors are heterogeneous.[37–39] Evaluation of tumor microenvironments has demonstrated heterogeneity relating to variation in tumor responsiveness to treatment,[40,41] degree of vascularity,[42–44] hypoxia,[37,43,45] proliferation rates,[43] energy metabolites, and gene expression.[44,46–50] Although tumor heterogeneity has been shown within these tumor microenvironments, intra-tumoral heterogeneity across the entire volume of primary tumors in humans has not been quantified or analyzed for its association with outcome measures. FDG-PET imaging presents the opportunity to do so.
Cervical cancer, in particular, is a tumor that has been suggested to have heterogeneity relating to hypoxia, variation in response to treatment, risk of metastatic spread, and gene expression. Additionally, the response of primary cervical cancer to treatment has been shown to be a much more complex issue than simply relating outcome to clinical stage, tumor volume, or tumor hypoxia. Specifically, our previous research has shown that primary cervix tumor maximal standardized uptake value (SUVmax) on FDG-PET is predictive of disease prognosis and outcome irrespective of tumor stage or tumor volume[31,51]. We have observed that this primary cervix tumor glucose metabolism from the FDG-PET image can vary greatly across the volume of individual cervical tumors[52].
The variation in tumor microenvironment with small changes in local heterogeneity has been demonstrated. Others have demonstrated heterogeneity of FDG uptake for tumors[43,46,53]. However, in terms of evaluating the clinical significance of intra-tumoral heterogeneity and outcome measures, only a limited amount of small studies have been published. Our preliminary data suggests that cervix tumors with high levels of heterogeneity have a worse clinical outcome than do tumors that are less heterogeneous[54]. [18F]misonidazole (MISO) PET is an agent utilized to discriminate areas of hypoxia within tumors. For head and neck and non-small cell lung cancer, some preliminary data suggests radiation treatment outcome can be associated with kinetic behavior of [18F]MISO PET[30]. O'Sullivan et al. noticed that FDG heterogeneity of sarcoma tumors was associated with time to patient death[53,55], but no one has investigated the prognostic significance of cervical intra-tumor heterogeneity on FDG-PET.
Supporting this argument that tumor heterogeneity is related to hypoxia, Walenta and colleagues have shown that cervical cancers with higher lactate concentrations are more likely to have metastatic spread[56], just as our study showed that tumors with greater heterogeneity were more likely to have metastatic spread to lymph nodes. Animal modeling studies by Walenta and associates have demonstrated the presence of oxygen gradients in R3230AC tumors grown in window chambers[57]. They found that lactate content, hypoxic fraction, ATP, glucose, redox potential, and vessel density vary across the tumor in their model. This suggests the variation in tumor microenvironment and possible causes of the intra-tumoral heterogeneity. For head and neck cancers, it has also been shown that high lactate levels correlated with worse survival and increased risk of metastasis[58]. It has also been demonstrated that tissue lactate content in head and neck squamous cell carcinoma correlates with radiosensitivity[59] just as tumors with greater differential metabolic heterogeneity in our study were more likely to have an incomplete metabolic response on the 3-month post-treatment PET. For head and neck cancers, high lactate levels correlated with worse survival and increased risk of metastasis[58]. At the same time, some studies have shown a lack of correlation between FDG uptake and hypoxia, as evidenced on FMISO scans[60,61]. Therefore, more investigation is needed to determine the biologic basis for cervical tumor differential heterogeneity as evidenced on FDG-PET.
Gerlee and Anderson have explored an evolutionary hybrid cellular automation model of solid tumor growth. The results of their modeling study show that with a low tissue oxygen concentration and a switch to anaerobic glycolysis (high glucose utilization) that tumors develop with a more aggressive phenotype with a low apoptotic potential compared to tumors with high oxygen concentrations, aerobic glycolysis and containing less aggressive phenotypes. Perhaps it can be argued that large tumors develop because of the dynamics of clonal evolution of cells in response to their microenvironmental supply of the nutrients oxygen and glucose[62].
Bentzen and Thames have reviewed the data regarding the general notion that there is a relationship between increasing tumor volume and a decreasing probability of tumor control. They conclude from their review of published clinical data that, “because of heterogeneity in patient and tumor characteristics, the volume effect is less pronounced than would be expected from a simple proportionality between number of clonogens and volume.” Their hypothesis is supported by our previously published data and data from other institutions for patients with cervical cancer. We have demonstrated a radiation dose–response relationship for patients with cervical cancer by tumor stage (tumor volume)[63]. However, this dose–response relationship plateaus at about 85 Gy irrespective of tumor volume implying that the radiation dose–response curve is not uniquely affected by the number of tumor cells but also by mechanisms that are as yet unexplained. It appears intra-tumoral heterogeneity provides additional information beyond volume that helps clarify tumor behavior.
Metabolic imaging response to therapy
The concept of utilizing FDG-PET to assess tumor response to therapy is based on in vitro studies that associate decreases in tumor cell glucose uptake with decreases in the fraction of viable tumor cells[64]. Clinically, the association between decreased tumor glucose uptake and treatment response has been documented in several small series for tumors of the breast, head and neck, gastrointestinal tract, and lymphoma[65–71]. There are two settings in which FDG-PET can be used to evaluate treatment response. FDG-PET can be utilized after the completion of therapy to evaluate the tumor response to a given treatment regimen utilizing the endpoint of complete versus incomplete metabolic response. In this setting, a complete metabolic response implies that no further therapy is indicated and an incomplete metabolic response implies that further therapy is warranted. The other setting is to utilize FDG-PET after a partial course of therapy to evaluate the effectiveness of that therapy and to change therapy if the tumor is unresponsive to the initial treatment. In the majority of these studies, FDG-PET was performed after 1–2 cycles of chemotherapy rather than after completion of the entire course of planned therapy. Most of these studies have linked initial response to chemotherapy, as measured by FDG-PET, to clinical outcome. However, few studies have specifically addressed the impact of FDG-PET response on patient management in the post-therapy setting[72].
In the post-treatment setting, FDG-PET has been used to identify response to a complete course of therapy (chemotherapy or chemoradiotherapy) for lymphoma[73–76]. The goal for this is to identify patients for appropriate immediate salvage therapy (e.g., more intensive chemotherapy or stem cell transplant). Notably, the International Working Group response criteria in lymphoma have recently been modified to include routine use of post-therapy FDG-PET for assessing response in patients with these tumors[77]. In these guidelines, visual assessment alone of the FDG-PET images was sufficient to determine therapeutic response[78].
FDG-PET has been used in a more limited fashion to assess response to a complete course of therapy for malignant disease in other sites, including lung and head and neck cancer[79–83]. The hesitation to use FDG-PET to evaluate treatment response is based on the assumption that local inflammation due to radiation may result in false-positive PET scans. On the contrary, recent evidence has shown that post-radiation normal tissue FDG uptake does not interfere with the prognostic information provided by the FDG response in the tumor itself. In fact, normal tissue FDG uptake has been positively correlated with tumor metabolic response and superior survival outcomes after radiation therapy for lung cancer[84].
The rationale for using post-therapy FDG-PET in cervical cancer is twofold. First, the post-therapy FDG-PET provides information that may affect the approach to salvage therapy. Historically, reported outcomes from salvage therapy for cervical carcinoma were poor[85]. Locally recurrent cervical cancer was most often detected as the presence of gross tumor on pelvic examination. Total pelvic exenteration, while potentially curative, was associated with significant patient morbidity and limited long-term survival (16% in one study)[85]. For patients with distant failures, the results were even more dismal. These patients were often undiagnosed until they developed symptoms related to disease recurrence. Not surprisingly, this resulted in poor rates of success for salvage therapy. In 1994, Grigsby and colleagues reported no survivors at 2 years for patients with isolated recurrences in the para-aortic lymph node chain[86].
The current treatment strategy for locally advanced cervical cancer (definitive radiation with concurrently administered cisplatin chemotherapy) achieves local control in approximately 75% of patients[87]. However, as is the case with malignant diseases in other sites, some tumors do not respond completely to standard therapy. Clinicians are then faced with the challenge of early identification of non-responders to decrease treatment failures and avoid the toxicity of futile treatment.
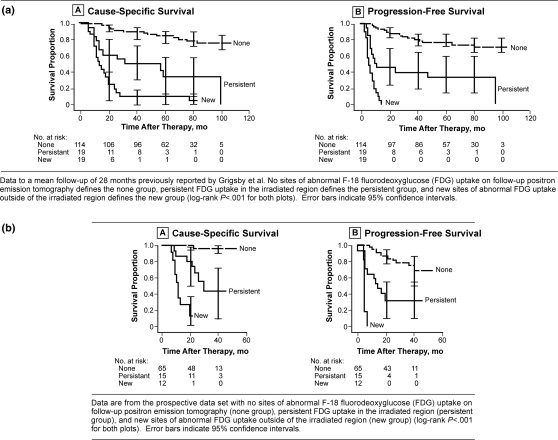
Our initial use of FDG-PET for patients with cervical cancer was focused on the use of the initial diagnostic FDG-PET image to identify sites of disease and to evaluate prognostic factors for treatment outcome. We then began performing post-therapy FDG-PET on our patients with cervical cancer at 3 months after the completion of their therapy. Initially we found on our diagnostic studies that lymph node stage (region of lymph node involvement identified by FDG-PET at the time of diagnosis) was more predictive of outcome than traditional prognostic factors such as FIGO stage, patient age, or tumor histology[12]. In our studies of 3-month post-therapy FDG-PET imaging we have found that progressive metastatic disease and an incomplete metabolic response by post-therapy FDG-PET are more predictive of clinical outcome than the pretreatment tumor characteristics including clinical stage and pretreatment lymph node status and the treatment-related variables, overall radiation treatment time and number of cycles of chemotherapy. We have also demonstrated that the post-therapy FDG-PET in cervical cancer provides valuable long-term prognostic information only 3 months after the completion of therapy (Fig. 3a). We have prospectively validated (Fig. 3b) the use of post-therapy FDG-PET as a metabolic biomarker of tumor response in cervical cancer[88]. Complete metabolic response is associated with excellent survival outcome (3-year cause-specific survival 100%). Partial metabolic response is associated with intermediate survival outcome (3-year cause-specific survival 51%) and decreased progression-free survival (3-year progression-free survival 35%). New sites of metabolic activity on post-therapy FDG-PET are associated with very poor survival outcome (3-year cause-specific survival 17%).
Figure 3.
(a) Long-term survival based on 3-months post-therapy FDG-PET, (b) prospective study of survival based on 3-month post-therapy FDG-PET/CT.
Summary
Over the last decade, positron emission tomography with the glucose analogue FDG has become an established oncological imaging tool for many forms of cancers. The functional information about regional glucose metabolism provided by FDG-PET provides for greater sensitivity and specificity in most cancer imaging applications by comparison with CT and other anatomic imaging methods. The role of PET in gynecological cancers is evolving, but the current literature suggests that PET is superior to conventional imaging modalities for evaluating patients with cervical and ovarian cancers. The role of PET in other gynecological cancers is less well defined. The recent development and rapid dissemination of integrated PET/CT scanners that allow functional and anatomical information to be obtained in a single examination represents an important advance in PET imaging technology, resulting in a synergistic improvement in the accuracy of interpretation of both PET and CT images.
FDG-PET/CT performed in patients with cervical cancer provides much important information. The diagnostic FDG-PET/CT determines the extent of disease at the time of diagnosis which is used to direct therapy. Prognostic information from the diagnostic FDG-PET/CT derives from the extent of the disease and from metabolic information such as degree of glucose uptake. The FDG response to therapy permits an accurate prediction of patient survival outcome. Routine screening of patients with FDG-PET/CT will allow for early diagnosis of recurrent disease and guide therapy.
References
- [1].Ellenson LH, Wu TC. Focus on endometrial and cervical cancer. Cancer Cell. 2004;5:533–8. doi: 10.1016/j.ccr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- [2]. http://seer.cancer.gov/csr/1975_2002/
- [3].Plummer M, Franceschi S. Strategies for HPV prevention. Virus Res. 2002;89:285–93. doi: 10.1016/s0168-1702(02)00197-1. [DOI] [PubMed] [Google Scholar]
- [4].Belhocine T, Thille A, Fridman V, et al. Contribution of whole-body 18FDG PET imaging in the management of cervical cancer. Gynecol Oncol. 2002;87:90–7. doi: 10.1006/gyno.2002.6769. [DOI] [PubMed] [Google Scholar]
- [5].Reinhardt MJ, Ehritt-Braun C, Vogelgesang D, et al. Metastatic lymph nodes in patients with cervical cancer: detection with MR imaging and FDG PET. Radiology. 2001;218:776–82. doi: 10.1148/radiology.218.3.r01mr19776. [DOI] [PubMed] [Google Scholar]
- [6].Havrilesky LJ, Kulasingam SL, Matchar DB, Myers ER. FDG-PET for management of cervical and ovarian cancer. Gynecol Oncol. 2005;97:183–91. doi: 10.1016/j.ygyno.2004.12.007. [DOI] [PubMed] [Google Scholar]
- [7].Rose PG, Adler LP, Rodriguez M, Faulhaber PF, Abdul-Karim FW, Miraldi F. Positron emission tomography for evaluating para-aortic nodal metastasis in locally advanced cervical cancer before surgical staging: a surgicopathologic study. J Clin Oncol. 1999;17:41–5. doi: 10.1200/JCO.1999.17.1.41. [DOI] [PubMed] [Google Scholar]
- [8].Yeh LS, Hung YC, Shen YY, Kao CH, Lin CC, Lee CC. Detecting para-aortic lymph nodal metastasis by positron emission tomography of 18F-fluorodeoxyglucose in advanced cervical cancer with negative magnetic resonance imaging findings. Oncol Rep. 2002;9:1289–92. [PubMed] [Google Scholar]
- [9].Sugawara Y, Eisbruch A, Kosuda S, Recker BE, Kison PV, Wahl RL. Evaluation of FDG PET in patients with cervical cancer. J Nucl Med. 1999;40:1125–31. [PubMed] [Google Scholar]
- [10].Lin WC, Hung YC, Yeh LS, Kao CH, Yen RF, Shen YY. Usefulness of (18)F-fluorodeoxyglucose positron emission tomography to detect para-aortic lymph nodal metastasis in advanced cervical cancer with negative computed tomography findings. Gynecol Oncol. 2003;89:73–6. doi: 10.1016/s0090-8258(03)00058-1. [DOI] [PubMed] [Google Scholar]
- [11].Grigsby PW, Dehdashti F, Siegel BA. FDG-PET evaluation of carcinoma of the cervix. Clin Positron Imaging. 1999;2:105–9. doi: 10.1016/s1095-0397(99)00008-4. [DOI] [PubMed] [Google Scholar]
- [12].Grigsby PW, Siegel BA, Dehdashti F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol. 2001;19:3745–9. doi: 10.1200/JCO.2001.19.17.3745. [DOI] [PubMed] [Google Scholar]
- [13].Grigsby PW. The contribution of new imaging techniques in staging cervical cancer. Gynecol Oncol. 2007;107:S10–12. doi: 10.1016/j.ygyno.2007.07.035. [DOI] [PubMed] [Google Scholar]
- [14].Carey PE, Coleman RE, Grigsby PW, Siegel BA. Medicare coverage of PET for cervical cancer. J Am Coll Radiol. 2006;3:19–22. doi: 10.1016/j.jacr.2005.08.018. [DOI] [PubMed] [Google Scholar]
- [15].Stehman F, Bundy B, DiSaia P, Keys HM, Larson JE, Fowler WC. Carcinoma of the cervix treated with irradiation therapy. I. A multivariate analysis of prognostic variables in the Gynecologic Oncology Group. Cancer. 1991;67:2776–85. doi: 10.1002/1097-0142(19910601)67:11<2776::aid-cncr2820671111>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- [16].Dehdashti F, Grigsby PW, Mintun MA, Lewis JS, Siegel BA, Welch MJ. Assessing tumor hypoxia in cervical cancer by positron emission tomography with 60Cu-ATSM: Relationship to therapeutic response – a preliminary report. Int J Radiat Oncol Biol Phys. 2003;55:1233–8. doi: 10.1016/s0360-3016(02)04477-2. [DOI] [PubMed] [Google Scholar]
- [17].Grigsby PW. 4th International Cervical Cancer Conference: update on PET and cervical cancer. Gynecol Oncol. 2005;99:S173–5. doi: 10.1016/j.ygyno.2005.07.076. [DOI] [PubMed] [Google Scholar]
- [18].Tran BN, Grigsby PW, Dehdashti F, Herzog TJ, Siegel BA. Occult supraclavicular lymph node metastasis identified by FDG-PET in patients with carcinoma of the uterine cervix. Gynecol Oncol. 2003;90:572–6. doi: 10.1016/s0090-8258(03)00402-5. [DOI] [PubMed] [Google Scholar]
- [19].Singh AK, Grigsby PW, Dehdashti F, Herzog TJ, Siegel BA. FDG-PET lymph node staging and survival of patients with FIGO stage IIIB cervical carcinoma. Int J Radiat Oncol Biol Phys. 2003;56:489–93. doi: 10.1016/s0360-3016(02)04521-2. [DOI] [PubMed] [Google Scholar]
- [20].Unger JB, Lilien DL, Caldito G, Ivy JJ, Charrier A, Bellaire B. The prognostic value of pretreatment 2-[18F]-fluoro-2-deoxy-d-glucose positron emission tomography scan in women with cervical cancer. Int J Gynecol Cancer. 2007;17:1062–7. doi: 10.1111/j.1525-1438.2007.00902.x. [DOI] [PubMed] [Google Scholar]
- [21].Miller TR, Grigsby PW. Measurement of tumor volume by PET to evaluate prognosis in patients with advanced cervical cancer treated by radiation therapy. Int J Radiat Oncol Biol Phys. 2002;53:353–9. doi: 10.1016/s0360-3016(02)02705-0. [DOI] [PubMed] [Google Scholar]
- [22].Xue F, Lin LL, Dehdashti F, Miller TR, Siegel BA, Grigsby PW. F-18 fluorodeoxyglucose uptake in primary cervical cancer as an indicator of prognosis after radiation therapy. Gynecol Oncol. 2006;101:147–51. doi: 10.1016/j.ygyno.2005.10.005. [DOI] [PubMed] [Google Scholar]
- [23].DiSaia PJ, Creasman WT. Clinical gynecologic oncology. 6th. St. Louis, MO: Saunders; 2001. [Google Scholar]
- [24].Ryu SY, Kim MH, Choi SC, Choi CW, Lee KH. Detection of early recurrence with 18F-FDG PET in patients with cervical cancer. J Nucl Med. 2003;44:347–52. [PubMed] [Google Scholar]
- [25].Unger JB, Ivy JJ, Connor P, et al. Detection of recurrent cervical cancer by whole-body FDG PET scan in asymptomatic and symptomatic women. Gynecol Oncol. 2004;94:212–6. doi: 10.1016/j.ygyno.2004.04.021. [DOI] [PubMed] [Google Scholar]
- [26].Grigsby PW, Siegel BA, Dehdashti F, Rader J, Zoberi I. Post-therapy [18F]fluorodeoxyglucose positron emission tomography in carcinoma of the cervix: response and outcome. J Clin Oncol. 2004;22:2167–71. doi: 10.1200/JCO.2004.09.035. [DOI] [PubMed] [Google Scholar]
- [27].Allal AS, Slosman DO, Kebdani T, Allaoua M, Lehmann W, Dulguerov P. Prediction of outcome in head-and-neck cancer patients using the standardized uptake value of 2-[18F]fluoro-2-deoxy-D-glucose. Int J Radiat Oncol Biol Phys. 2004;59:1295–300. doi: 10.1016/j.ijrobp.2003.12.039. [DOI] [PubMed] [Google Scholar]
- [28].Borst GR, Belderbos JS, Boellaard R, et al. Standardised FDG uptake: a prognostic factor for inoperable non-small cell lung cancer. Eur J Cancer. 2005;41:1533–41. doi: 10.1016/j.ejca.2005.03.026. [DOI] [PubMed] [Google Scholar]
- [29].Downey RJ, Akhurst T, Gonen M, et al. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol. 2004;22:3255–60. doi: 10.1200/JCO.2004.11.109. [DOI] [PubMed] [Google Scholar]
- [30].Eschmann SM, Friedel G, Paulsen F, et al. Is standardised (18)F-FDG uptake value an outcome predictor in patients with stage III non-small cell lung cancer? Eur J Nucl Med Mol Imaging. 2006;33:263–9. doi: 10.1007/s00259-005-1953-2. [DOI] [PubMed] [Google Scholar]
- [31].Kidd EA, Siegel BA, Dehdashti F, Grigsby PW. The standardized uptake value for F-18 fluorodeoxyglucose is a sensitive predictive biomarker for cervical cancer treatment response and survival. Cancer. 2007;110:1738–44. doi: 10.1002/cncr.22974. [DOI] [PubMed] [Google Scholar]
- [32].Brown RS, Goodman TM, Zasadny KR, Greenson JK, Wahl RL. Expression of hexokinase II and Glut-1 in untreated human breast cancer. Nucl Med Biol. 2002;29:443–53. doi: 10.1016/s0969-8051(02)00288-3. [DOI] [PubMed] [Google Scholar]
- [33].Mendez LE, Manci N, Cantuaria G, et al. Expression of glucose transporter-1 in cervical cancer and its precursors. Gynecol Oncol. 2002;86:138–43. doi: 10.1006/gyno.2002.6745. [DOI] [PubMed] [Google Scholar]
- [34].Airley R, Loncaster J, Davidson S, et al. Glucose transporter glut-1 expression correlates with tumor hypoxia and predicts metastasis-free survival in advanced carcinoma of the cervix. Clin Cancer Res. 2001;7:928–34. [PubMed] [Google Scholar]
- [35].Yen T-C, See L-C, Lai C-H, et al. 18F-FDG uptake in squamous cell carcinoma of the cervix is correlated with glucose transporter 1 expression. J Nucl Med. 2004;45:22–9. [PubMed] [Google Scholar]
- [36].Tian M, Zhang H, Nakasone Y, Mogi K, Endo K. Expression of Glut-1 and Glut-3 in untreated oral squamous cell carcinoma compared with FDG accumulation in a PET study. Eur J Nucl Med Mol Imaging. 2004;31:5–12. doi: 10.1007/s00259-003-1316-9. [DOI] [PubMed] [Google Scholar]
- [37].Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955;9:539–49. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Heppner GH. Tumor heterogeneity. Cancer Res. 1984;44:2259–65. [PubMed] [Google Scholar]
- [39].Alexandrova R. Tumour heterogeneity. Exp Pathol Parasitol. 2001;4/6:57–67. [Google Scholar]
- [40].Britten RA, Evans AJ, Allalunis-Turner MJ, Franko AJ, Pearcey RG. Intratumoral heterogeneity as a confounding factor in clonogenic assays for tumour radioresponsiveness. Radiother Oncol. 1996;39:145–53. doi: 10.1016/0167-8140(96)01719-7. [DOI] [PubMed] [Google Scholar]
- [41].Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–15. [PubMed] [Google Scholar]
- [42].Delorme S, Knopp MV. Non-invasive vascular imaging: assessing tumour vascularity. Eur Radiol. 1998;8:517–27. doi: 10.1007/s003300050428. [DOI] [PubMed] [Google Scholar]
- [43].Pugachev A, Ruan S, Carlin S, et al. Dependence of FDG uptake on tumor microenvironment. Int J Radiat Oncol Biol Phys. 2005;62:545–53. doi: 10.1016/j.ijrobp.2005.02.009. [DOI] [PubMed] [Google Scholar]
- [44].Choi JY, Jang KT, Shim YM, et al. Prognostic significance of vascular endothelial growth factor expression and microvessel density in esophageal squamous cell carcinoma: comparison with positron emission tomography. Ann Surg Oncol. 2006;13:1054–62. doi: 10.1245/ASO.2006.08.012. [DOI] [PubMed] [Google Scholar]
- [45].Thorwarth D, Eschmann SM, Paulsen F, Alber M. A model of reoxygenation dynamics of head-and-neck tumors based on serial 18F-fluoromisonidazole positron emission tomography investigations. Int J Radiat Oncol Biol Phys. 2007;68:515–21. doi: 10.1016/j.ijrobp.2006.12.037. [DOI] [PubMed] [Google Scholar]
- [46].Zhao S, Kuge Y, Mochizuki T, et al. Biologic correlates of intratumoral heterogeneity in 18F-FDG distribution with regional expression of glucose transporters and hexokinase-II in experimental tumor. J Nucl Med. 2005;46:675–82. [PubMed] [Google Scholar]
- [47].Bachtiary B, Boutros PC, Pintilie M, et al. Gene expression profiling in cervical cancer: an exploration of intratumor heterogeneity. Clin Cancer Res. 2006;12:5632–40. doi: 10.1158/1078-0432.CCR-06-0357. [DOI] [PubMed] [Google Scholar]
- [48].Schwarz JK, Rader JS, Huettner PC, Watson MA, Grigsby PW. Molecular characterization of FDG-PET metabolic response in cervical cancer. Int J Radiat Oncol Biol Phys. 2007;69:S115. [Google Scholar]
- [49].Grigsby PW, Watson M, Powell MA, Zhang Z, Rader JS. Gene expression patterns in advanced human cervical cancer. Int J Gynecol Cancer. 2006;16:562–7. doi: 10.1111/j.1525-1438.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- [50].Grigsby PW, Malyapa RS, Higashikubo R, et al. Comparison of molecular markers of hypoxia and imaging with (60)Cu-ATSM in cancer of the uterine cervix. Mol Imaging Biol. 2007;9:278–83. doi: 10.1007/s11307-007-0095-2. [DOI] [PubMed] [Google Scholar]
- [51].Xue F, Lin LL, Dehdashti F, Miller TR, Siegel BA, Grigsby PW. F-18 fluorodeoxyglucose uptake in primary cervical cancer as an indicator of prognosis after radiation therapy. Gynecol Oncol. 2006;101:147–51. doi: 10.1016/j.ygyno.2005.10.005. [DOI] [PubMed] [Google Scholar]
- [52].Miller TR, Pinkus E, Dehdashti F, Grigsby PW. Improved prognostic value of 18F-FDG PET using a simple visual analysis of tumor characteristics in patients with cervical cancer. J Nucl Med. 2003;44:192–7. [PubMed] [Google Scholar]
- [53].O'Sullivan F, Roy S, Eary J. A statistical measure of tissue heterogeneity with application to 3D PET sarcoma data. Biostatistics. 2003;4:433–48. doi: 10.1093/biostatistics/4.3.433. [DOI] [PubMed] [Google Scholar]
- [54].Kidd EA, El Naqa IM, Deasy JO, Grigsby PW. FDG metabolic heterogeneity of cervical cancer. Int J Radiat Oncol Biol Phys. 2007;69:S115–16. [Google Scholar]
- [55].O'Sullivan F, Roy S, O'Sullivan J, Vernon C, Eary J. Incorporation of tumor shape into an assessment of spatial heterogeneity for human sarcomas imaged with FDG-PET. Biostatistics. 2005;6:293–301. doi: 10.1093/biostatistics/kxi010. [DOI] [PubMed] [Google Scholar]
- [56].Walenta S, Wetterling M, Lehrke M, et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000;60:916–21. [PubMed] [Google Scholar]
- [57].Walenta S, Snyder S, Haroon ZA, et al. Tissue gradients of energy metabolites mirror oxygen tension gradients in a rat mammary carcinoma model. Int J Radiat Oncol Biol Phys. 2001;51:840–8. doi: 10.1016/s0360-3016(01)01700-x. [DOI] [PubMed] [Google Scholar]
- [58].Brizel DM, Schroeder T, Scher RL, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51:349–53. doi: 10.1016/s0360-3016(01)01630-3. [DOI] [PubMed] [Google Scholar]
- [59].Quennet V, Yaromina A, Zips D, et al. Tumor lactate content predicts for response to fractionated irradiation of human squamous cell carcinomas in nude mice. Radiother Oncol. 2006;81:130–5. doi: 10.1016/j.radonc.2006.08.012. [DOI] [PubMed] [Google Scholar]
- [60].Rajendran JG, Mankoff DA, O'Sullivan F, et al. Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res. 2004;10:2245–52. doi: 10.1158/1078-0432.ccr-0688-3. [DOI] [PubMed] [Google Scholar]
- [61].Cherk MH, Foo SS, Poon AM, et al. Lack of correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in non-small cell lung cancer assessed by 18F-fluoromisonidazole and 18F-FDG PET. J Nucl Med. 2006;47:1921–6. [PubMed] [Google Scholar]
- [62].Gerlee P, Anderson AR. An evolutionary hybrid cellular automaton model of solid tumour growth. J Theor Biol. 2007;246:583–603. doi: 10.1016/j.jtbi.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Perez CA, Grigsby PW, Chao KS, Mutch DG, Lockett MA. Tumor size, irradiation dose, and long-term outcome of carcinoma of uterine cervix. Int J Radiat Oncol Biol Phys. 1998;41:307–17. doi: 10.1016/s0360-3016(98)00067-4. [DOI] [PubMed] [Google Scholar]
- [64].Spaepen K, Stroobants S, Dupont P, et al. [(18)F]FDG PET monitoring of tumour response to chemotherapy: does [(18)F]FDG uptake correlate with the viable tumour cell fraction? Eur J Nucl Med Mol Imaging. 2003;30:682–8. doi: 10.1007/s00259-003-1120-6. [DOI] [PubMed] [Google Scholar]
- [65].Schelling M, Avril N, Nahrig J, et al. Positron emission tomography using [(18)F]fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol. 2000;18:689–95. doi: 10.1200/JCO.2000.18.8.1689. [DOI] [PubMed] [Google Scholar]
- [66].Smith IC, Welch AE, Hutcheon AW, et al. Positron emission tomography using [(18)F]-fluorodeoxy-D-glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol. 2000;18:1676–88. doi: 10.1200/JCO.2000.18.8.1676. [DOI] [PubMed] [Google Scholar]
- [67].Weber WA, Ott K, Becker K, et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol. 2001;19:3058–65. doi: 10.1200/JCO.2001.19.12.3058. [DOI] [PubMed] [Google Scholar]
- [68].Ott K, Fink U, Becker K, et al. Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol. 2003;21:4604–10. doi: 10.1200/JCO.2003.06.574. [DOI] [PubMed] [Google Scholar]
- [69].Weber WA, Petersen V, Schmidt B, et al. Positron emission tomography in non-small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol. 2003;21:2651–7. doi: 10.1200/JCO.2003.12.004. [DOI] [PubMed] [Google Scholar]
- [70].Brun E, Kjellen E, Tennvall J, et al. FDG PET studies during treatment: prediction of therapy outcome in head and neck squamous cell carcinoma. Head Neck. 2002;24:127–35. doi: 10.1002/hed.10037. [DOI] [PubMed] [Google Scholar]
- [71].Kostakoglu L, Coleman M, Leonard JP, Kuji I, Zoe H, Goldsmith SJ. PET predicts prognosis after 1 cycle of chemotherapy in aggressive lymphoma and Hodgkin's disease. J Nucl Med. 2002;43:1018–27. [PubMed] [Google Scholar]
- [72].Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med. 2006;354:496–507. doi: 10.1056/NEJMra050276. [DOI] [PubMed] [Google Scholar]
- [73].Spaepen K, Stroobants S, Dupont P, et al. Prognostic value of positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose ([18F]FDG) after first-line chemotherapy in non-Hodgkin's lymphoma: is [18F]FDG-PET a valid alternative to conventional diagnostic methods? J Clin Oncol. 2001;19:414–9. doi: 10.1200/JCO.2001.19.2.414. [DOI] [PubMed] [Google Scholar]
- [74].Jerusalem G, Beguin Y, Fassotte MF, et al. Whole-body positron emission tomography using 18F-fluorodeoxyglucose for posttreatment evaluation in Hodgkin's disease and non-Hodgkin's lymphoma has higher diagnostic and prognostic value than classical computed tomography scan imaging. Blood. 1999;94:429–33. [PubMed] [Google Scholar]
- [75].Weihrauch MR, Re D, Scheidhauer K, et al. Thoracic positron emission tomography using 18F-fluorodeoxyglucose for the evaluation of residual mediastinal Hodgkin disease. Blood. 2001;98:2930–4. doi: 10.1182/blood.v98.10.2930. [DOI] [PubMed] [Google Scholar]
- [76].Naumann R, Vaic A, Beuthien-Baumann B, et al. Prognostic value of positron emission tomography in the evaluation of post-treatment residual mass in patients with Hodgkin's disease and non-Hodgkin's lymphoma. Br J Haematol. 2001;115:793–800. doi: 10.1046/j.1365-2141.2001.03147.x. [DOI] [PubMed] [Google Scholar]
- [77].Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- [78].Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–8. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- [79].MacManus MP, Hicks RJ, Matthews JP, et al. Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2003;21:1285–92. doi: 10.1200/JCO.2003.07.054. [DOI] [PubMed] [Google Scholar]
- [80].MacManus MP, Hicks RJ, Matthews JP, Wirth A, Rischin D, Ball DL. Metabolic (FDG-PET) response after radical radiotherapy/chemoradiotherapy for non-small cell lung cancer correlates with patterns of failure. Lung Cancer. 2005;49:95–108. doi: 10.1016/j.lungcan.2004.11.024. [DOI] [PubMed] [Google Scholar]
- [81].Greven KM, Williams 3rd DW, McGuirt Sr WF, et al. Serial positron emission tomography scans following radiation therapy of patients with head and neck cancer. Head Neck. 2001;23:942–6. doi: 10.1002/hed.1136. [DOI] [PubMed] [Google Scholar]
- [82].Porceddu SV, Jarmolowski E, Hicks RJ, et al. Utility of positron emission tomography for the detection of disease in residual neck nodes after (chemo)radiotherapy in head and neck cancer. Head Neck. 2005;27:175–81. doi: 10.1002/hed.20130. [DOI] [PubMed] [Google Scholar]
- [83].Yao M, Graham MM, Smith RB, et al. Value of FDG PET in assessment of treatment response and surveillance in head-and-neck cancer patients after intensity modulated radiation treatment: a preliminary report. Int J Radiat Oncol Biol Phys. 2004;60:1410–8. doi: 10.1016/j.ijrobp.2004.05.058. [DOI] [PubMed] [Google Scholar]
- [84].Hicks RJ, Mac Manus MP, Matthews JP, et al. Early FDG-PET imaging after radical radiotherapy for non-small-cell lung cancer: inflammatory changes in normal tissues correlate with tumor response and do not confound therapeutic response evaluation. Int J Radiat Oncol Biol Phys. 2004;60:412–8. doi: 10.1016/j.ijrobp.2004.03.036. [DOI] [PubMed] [Google Scholar]
- [85].Sommers G, Grigsby PW, Perez CA, et al. Outcome of recurrent cervical carcinoma following definitive irradiation. Gynecol Oncol. 1989;35:150–5. doi: 10.1016/0090-8258(89)90033-4. [DOI] [PubMed] [Google Scholar]
- [86].Grigsby P, Vest M, Perez C. Recurrent carcinoma of the cervix exclusively in the para-aortic nodes following radiation therapy. Int J Radiat Oncol Biol Phys. 1994;28:451–5. doi: 10.1016/0360-3016(94)90070-1. [DOI] [PubMed] [Google Scholar]
- [87].Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90–01. J Clin Oncol. 2004;22:872–80. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- [88].Schwarz JK, Siegel BA, Dehdashti F, Grigsby PW. Association of post-therapy positron emission tomography with tumor response and survival in cervical carcinoma. JAMA. 2007;298:2289–95. doi: 10.1001/jama.298.19.2289. [DOI] [PubMed] [Google Scholar]