Abstract
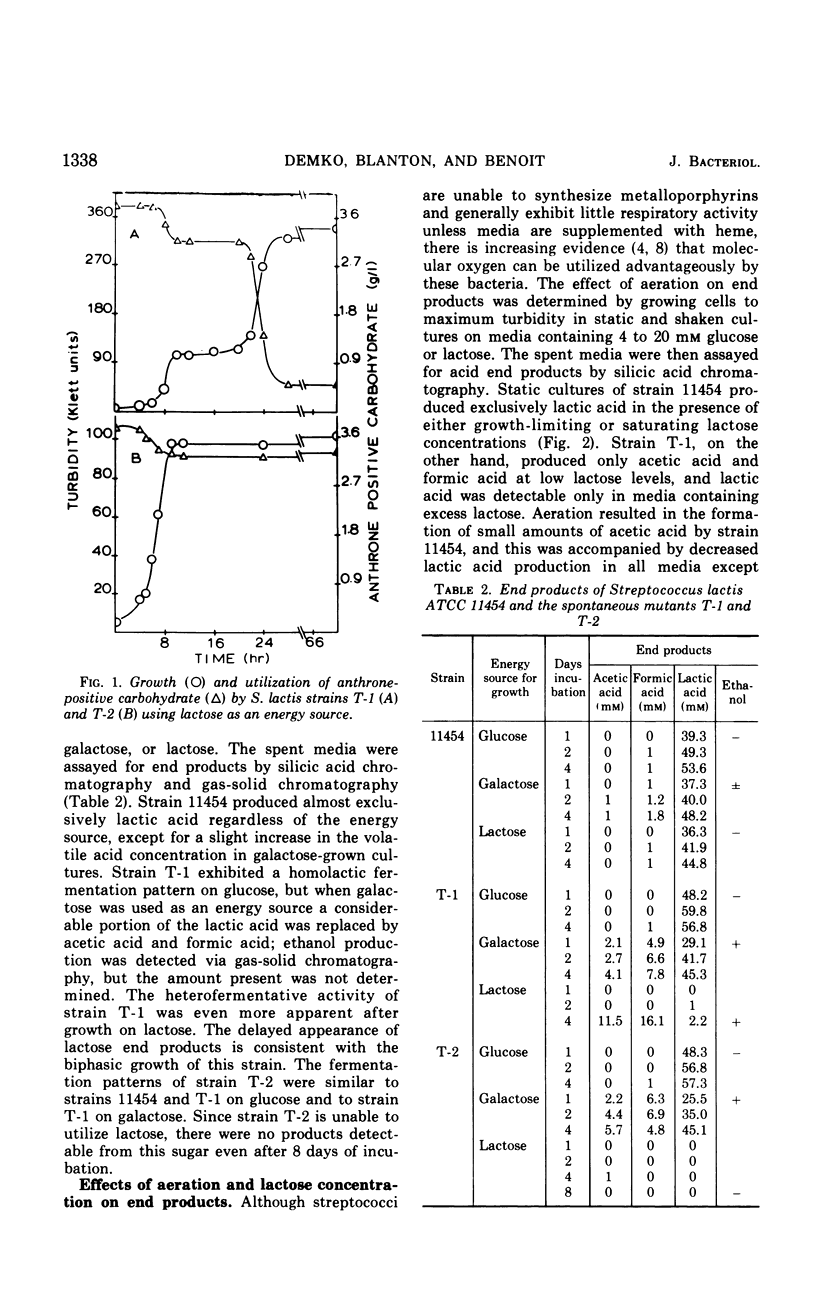
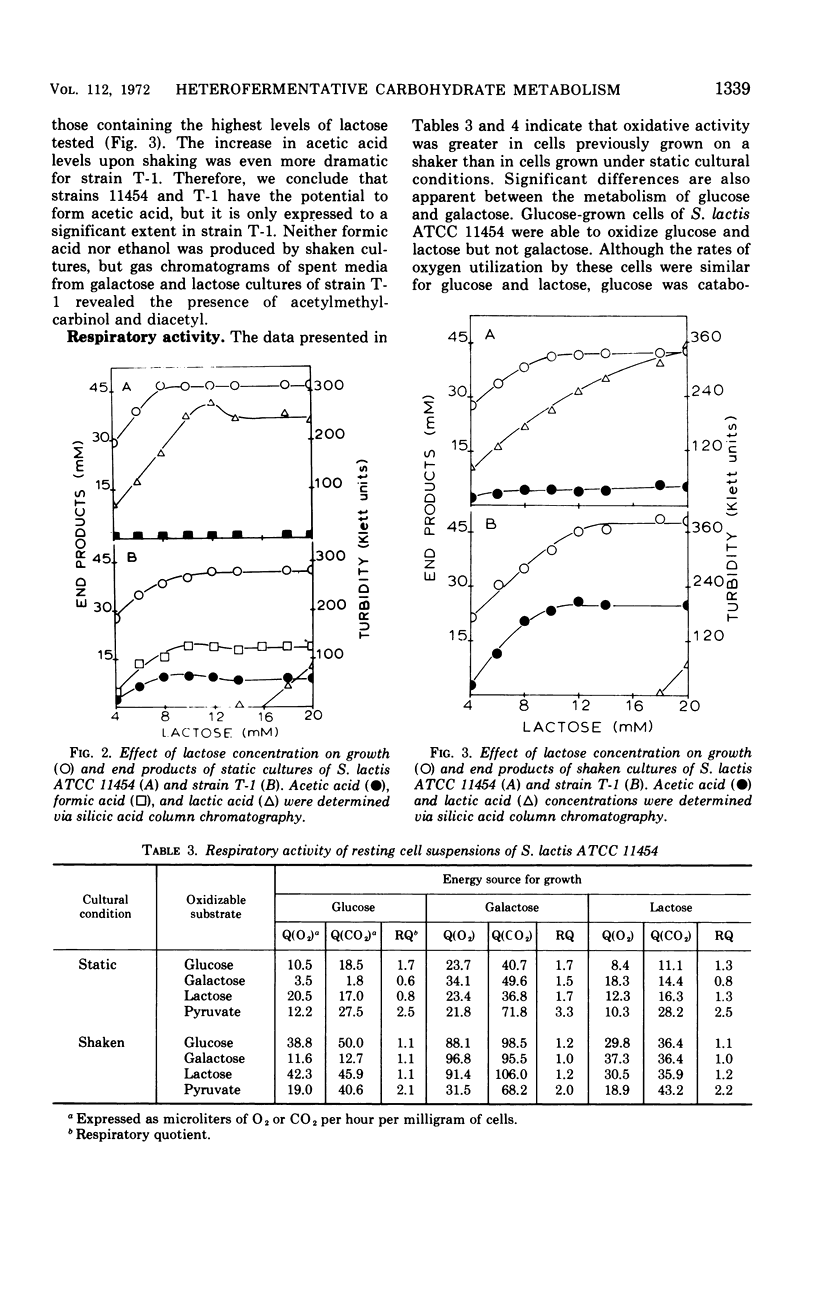
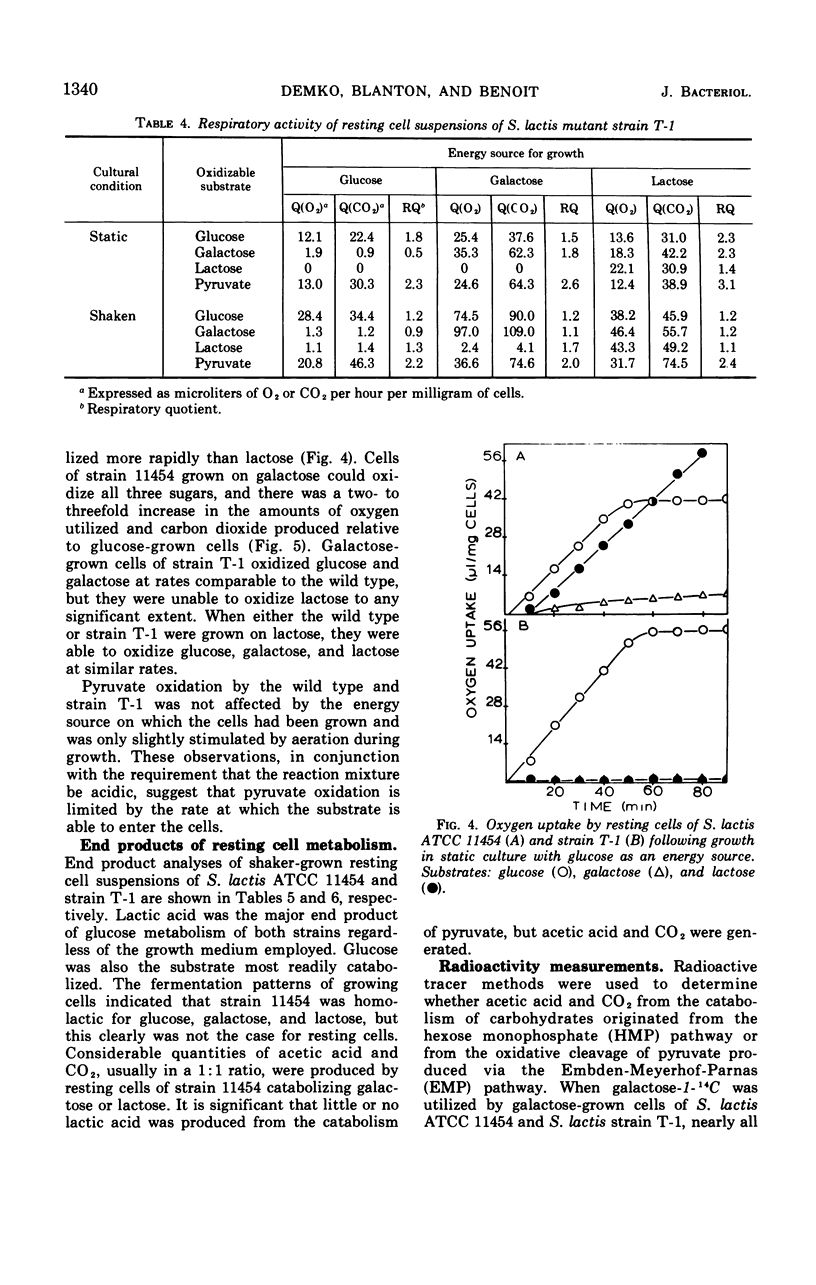
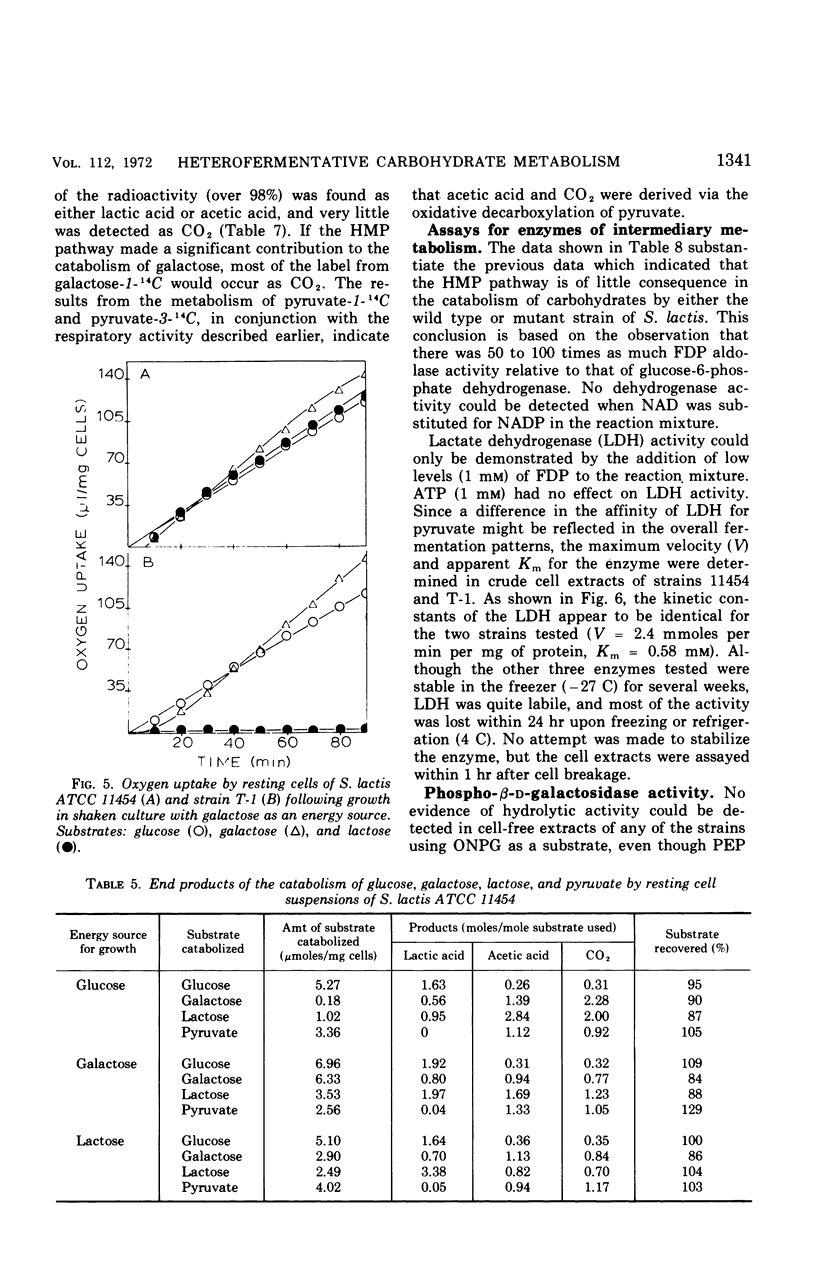
Two mutants of Streptococcus lactis ATCC 11454 have been isolated which possess an impaired lactose-fermenting capacity; galactose utilization is also affected, but to a lesser extent. Although the Embden-Meyerhof-Parnas pathway is the major, if not the sole, pathway of carbohydrate metabolism in the three strains, the fermentation end products of the mutants are dramatically different from the typical homolactic pattern of the wild type. Under conditions of low oxygen tension and growth-limiting lactose concentrations, mutant strain T-1 produces largely formic acid, acetic acid (2:1), and ethanol rather than lactic acid. Aerated cultures produce acetic acid, CO2 (1:1), acetyl-methylcarbinol, and diacetyl. When the mutants use galactose as an energy source, lactic acid is the major end product, but significant heterofermentative activity is observed. The aberrations responsible for the mutant phenotypes reside in the proteins which catalyze the transport and hydrolysis of galactosides. It is hypothesized that the impaired transport system of the mutants reduces the intracellular pool of glycolytic intermediates below that of the wild type. Since fructose-1, 6-diphosphate is an activator of lactic dehydrogenase in S. lactis, lactic acid production is reduced, and pathways leading to the formation of other products are expressed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DISCHE Z. New color reactions for determination of sugars in polysaccharides. Methods Biochem Anal. 1955;2:313–358. doi: 10.1002/9780470110188.ch11. [DOI] [PubMed] [Google Scholar]
- Faust P. J., Vandemark P. J. Phosphorylation coupled to NADH oxidation with fumarate in Streptococcus faecalis 10Cl. Arch Biochem Biophys. 1970 Apr;137(2):392–398. doi: 10.1016/0003-9861(70)90454-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McKay L. L., Walter L. A., Sandine W. E., Elliker P. R. Involvement of phosphoenolpyruvate in lactose utilization by group N streptococci. J Bacteriol. 1969 Aug;99(2):603–610. doi: 10.1128/jb.99.2.603-610.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L., Miller A., 3rd, Sandine W. E., Elliker P. R. Mechanisms of lactose utilization by lactic acid streptococci: enzymatic and genetic analyses. J Bacteriol. 1970 Jun;102(3):804–809. doi: 10.1128/jb.102.3.804-809.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelson M. N. Phosphorylation and the reduced nicotinamide adenine dinucleotide oxidase reaction in Streptococcus agalactiae. J Bacteriol. 1969 Nov;100(2):895–901. doi: 10.1128/jb.100.2.895-901.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERCE W. A., Jr Glucose and galactose metabolism in Streptococcus pyogenes. J Bacteriol. 1957 Aug;74(2):186–193. doi: 10.1128/jb.74.2.186-193.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman B., Ganesan A. K., Guzman R. Transport systems for galactose and galactosides in Escherichia coli. II. Substrate and inducer specificities. J Mol Biol. 1968 Sep 14;36(2):247–260. doi: 10.1016/0022-2836(68)90379-3. [DOI] [PubMed] [Google Scholar]
- WOLIN M. J. FRUCTOSE-1,6-DIPHOSPHATE REQUIREMENT OF STREPTOCOCCAL LACTIC DEHYDROGENASES. Science. 1964 Nov 6;146(3645):775–777. doi: 10.1126/science.146.3645.775. [DOI] [PubMed] [Google Scholar]


