Summary
Interest in the diverse biology of protein tyrosine phosphatases that are encoded by more than 100 genes in the human genome continues to grow at an accelerated pace. In particular, two cytoplasmic protein tyrosine phosphatases composed of two Src homology 2 (SH2) NH2-terminal domains and a C-terminal protein-tyrosine phosphatase domain referred to as SHP-1 and SHP-2 are known to govern a host of cellular functions. SHP-1 and SHP-2 modulate progenitor cell development, cellular growth, tissue inflammation, and cellular chemotaxis, but more recently the role of SHP-1 and SHP-2 to directly control cell survival involving oxidative stress pathways has come to light. SHP-1 and SHP-2 are fundamental for the function of several growth factor and metabolic pathways yielding far reaching implications for disease pathways and disorders such as diabetes, neurodegeneration, and cancer. Although SHP-1 and SHP-2 can employ similar or parallel cellular pathways, these proteins also clearly exert opposing effects upon downstream cellular cascades that affect early and late apoptotic programs. SHP-1 and SHP-2 modulate cellular signals that involve phosphatidylinositol 3-kinase, Akt, Janus kinase 2, signal transducer and activator of transcription proteins, mitogen-activating protein kinases, extracellular signal-related kinases, c-Jun-amino terminal kinases, and nuclear factor-κB. Our progressive understanding of the impact of SHP-1 and SHP-2 upon multiple cellular environments and organ systems should continue to facilitate the targeted development of treatments for a variety of disease entities.
Keywords: Akt, Alzheimer’s, Apoptosis, Cancer, diabetes, ERK, erythropoietin, Gab1, Inflammation, Jak2, JNK, MAP kinase, Nuclear factor-κB, Oxidative stress, Stem cells, SHP1, SHP2, Src homology, STAT, Tyrosine phosphatases
Introduction
Protein tyrosine phosphorylation plays a variety of significant roles in cell signaling transduction, physiological functions, and pathological processes. At least 107 human protein-tyrosine phosphatases (PTPs) have been identified and form a family of receptor-like and cytosolic enzymes (Alonso et al., 2004). The PTPs can be divided into four groups based on their amino acid sequence in the catalytic domain (Schaapveld et al., 1997). Class I, II, and III are cysteine-based PTPs and Class IV is an aspartate-based PTP. Among Class I PTPs, 38 members have been identified in the human genome and some of these members have been linked to type 2 diabetes mellitus and clinical cancer susceptibility (Andersen et al., 2004). The classical PTPs are strictly tyrosine specific. They are subdivided into the receptor-like PTPs (RPTPs) and the non-receptor-like PTPs (NRPTPs) or cytosolic PTPs. The RPTPs exist in plasma membranes while the NRPTPs occupy intracellular compartments (Alonso et al., 2004; Stoker, 2005).
The emerging roles of SHP-1 and SHP-2
In the PTP family, a subgroup of cytoplasmic PTPs characterized by containing two Src homology 2 (SH2) NH2-terminal domains and a C-terminal protein-tyrosine phosphatase domain are referred to as SHP. They are intimately involved in several cellular activities, such as cytoskeletal maintenance, cell division, and cell differentiation (Feng et al., 1994; Bialy and Waldmann, 2005). Of these, two particular SHP proteins known as SHP-1 (SH-PTP1, PTP1C, HCP, and SHP) and SHP-2 (SH-PTP2, PTP1D, Syp, PTP2C, and SH-PTP3) have been linked to trophic factor signaling (Yamauchi et al., 1995; You and Zhao, 1997), cell growth (Yi et al., 1993), mitogen-activated protein kinase (MAPK) activity (Pani et al., 1995), and chemotactic responses (Kim et al., 1999). Previously, SHP-1 was believed to play a negative role in modulating cell function, whereas SHP-2 was believed to up-regulate a variety of cell signal transduction processes. However, present realization of the increasing complex functions of SHP-1 and SHP-2 has matured into a less simplistic view of the influence of these proteins upon cell signaling pathways.
In regards to the distribution and expression of SHP-1 and SHP-2, SHP-1 is predominantly present in hematopoietic cells (Yi et al., 1992). Of particular interest is the observation that both SHP-1 and SHP-2 are expressed in the nervous system in the brain cortex, cerebellum, and the hippocampus (Suzuki et al., 1995; Horvat et al., 2001). SHP-1 expression also has been found in astrocytes (Lurie et al., 2000; Horvat et al., 2001), oligodendrocytes (Massa et al., 2000), and microglia (Kim et al., 2006). In addition, SHP-1 expression has been identified in the epithelial cells of the prostate (Valencia et al., 1997). In contrast, SHP-2 is ubiquitously distributed and is expressed in the nervous system in glial cells, the central nervous system (Servidei et al., 1998; Chong et al., 2003e), and sympathetic neurons (Chen et al., 2002).
SHP-1 and SHP-2 regulation of cellular development and demise
Cellular development
High expression of SHP-1 and SHP-2 in the hematopoietic system suggests that these two tyrosine phosphatases play important roles in hematopoietic cell function. SHP-1 generally exerts a negative effect upon the hematopoietic differentiation of embryonic stem cells and can act at different stages of embryonic stem cell differentiation (Paling and Welham, 2005). Expression of dominant-negative forms of SHP-1 in embryonic stem cells illustrates that SHP-1 acts at multiple stages of hematopoietic differentiation to alter cell lineage and progenitor cell number. Yet, there instances in which SHP-1 can function as a positive regulator to promote oligodendrocyte function for the formation of myelin sheaths (Massa et al., 2004).
SHP-2 has been shown to be required for hemangioblast, primitive, and progenitor hematopoietic cell development (Chan and Yoder, 2004). Reduction of SHP-2 expression through SHP-2 siRNA transfection significantly decreases the formation of hematopoietic progenitor formation and also blocks fibroblast growth factor induced hemangioblast formation (Zou et al., 2006a). In addition, blastocysts with a SHP-2 null mutation may initially develop normally, but eventually are unable to yield trophoblast stem cell lines, illustrating that SHP-2 can control trophoblast stem cell survival (Yang et al., 2006). Development of embryonic cells with a homozygous SHP-2 mutation also leads to the suppression of hematopoietic cell differentiation, but appears to have more severe ramifications with the impaired development of cardiac muscle cells (Qu and Feng, 1998). Consistent with these studies, haploinsufficiency of SHP-2 results in a reduction in hematopoietic stem cells after transplantation into lethally irradiated recipients (Chan et al., 2006). Yet, some investigations suggest that the role of SHP-2 during hematopoietic cell survival is not entirely clear. For example, through pathways that can dephosphorylate signal transducer and activator of transcription 5 (STAT5), overexpression of SHP-2 has been shown to depress the ability of primary bone marrow hematopoietic progenitor cells to differentiate and proliferate leading to a negative regulatory effect upon hematopoietic cell survival (Chen et al., 2004).
In the nervous system, SHP-2 can play a role in brain development. Expression of SHP-2 is present in both mitotic and post-mitotic neurons during rodent development. In addition, SHP-2 expression can become more pronounced in non-neuronal cells during cell injury (Servidei et al., 1998). Mutation of SHP-2 function also can result in the inhibition of sympathetic neurite outgrowth during nerve growth factor stimulation (Chen et al., 2002). Interestingly, SHP-2 function also may be vital for energy metabolism during neuronal development as well as during the function of adult neuronal cells. Deletion of SHP-2 in post-mitotic forebrain neurons through the crossing of CaMKIIalpha-Cre transgenic mice with a conditional SHP-2 mutant strain resulted in down-regulation of signal transducer and activator of transcription 3 (STAT3) and the development of early-onset obesity with elevated leptin and triglycerides (Zhang et al., 2004).
Promotion of cell survival during oxidative stress and inflammation
Oxidative stress represents an important pathway for the apoptotic destruction of cells (Chong et al., 2005e; Li et al., 2006a). Oxidative stress occurs during the excess generation of free radicals, such as reactive oxygen species. Reactive oxygen species consist of oxygen free radicals and associated entities that include superoxide free radicals, hydrogen peroxide, singlet oxygen, nitric oxide (NO), and peroxynitrite (Chong et al., 2005d). The production of reactive oxygen species can lead to cell injury through cell membrane lipid destruction and cleavage of DNA (Vincent and Maiese, 1999; Wang et al., 2003). Reactive oxygen species generation can lead to the peroxidation of cellular membrane lipids (Siu and To, 2002), peroxidation of docosahexaenoic acid, a precursor of neuroprotective docosanoids (Greco and Minghetti, 2004), and the inhibition of complex enzymes in the electron transport chain of mitochondria resulting in the blockade of mitochondrial respiration (Yamamoto et al., 2002).
Once the pathways responsible for oxidative stress are set in motion, cell injury through apoptosis may ensue. Apoptosis contributes to a variety of diseases such as stroke, Alzheimer’s disease, and trauma (Chong et al., 2004b; Doonan and Cotter, 2004; Ferretti, 2004; Koyama and Ikegaya, 2004; Li et al., 2004) as well as cardiovascular injury, cancer (Chong et al., 2002a, 2004b; Maiese et al., 2005b), and diabetes (Li et al., 2006a; Maiese et al., 2007). The cleavage of genomic DNA into fragments (Maiese et al., 1999; Maiese and Vincent, 2000a,b) is considered to be a later event during apoptotic injury (Dombroski et al., 2000; Maiese and Vincent, 2000b; Jessel et al., 2002; Kang et al., 2003b). Endonucleases lead to DNA degradation and have been differentiated based on their ionic sensitivities to zinc (Torriglia et al., 1997), magnesium (Sun and Cohen, 1994), and calcium (Maiese et al., 1999), an important regulator that can independently impair cell survival (Weber, 2004). In the nervous system, three separate endonuclease activities are present. These include a constitutive acidic cation-independent endonuclease, a constitutive calcium/magnesium-dependent endonuclease, and an inducible magnesium dependent endonuclease (Vincent and Maiese, 1999; Vincent et al., 1999; Chong et al., 2005e).
In contrast to DNA degradation, apoptotic cellular membrane phosphatidylserine (PS) externalization can become a signal for early injury mechanisms (Mari et al., 2004) and the phagocytosis of cells (Hong et al., 2004). During inflammation, microglial cells require the activation of intracellular cytoprotective pathways (Li et al., 2006b; Chong et al., 2007b) to proliferate and remove injured cells (Li et al., 2005; Mallat et al., 2005). Yet, microglia also may lead to cellular damage through the generation of reactive oxygen species (Sankarapandi et al., 1998; Maiese and Chong, 2004) and through the production of cytokines (Benzing et al., 1999; Mehlhorn et al., 2000). The translocation of membrane PS residues from the inner cellular membrane to the outer surface is a necessary component under most conditions for the removal of apoptotic cells (Maiese and Vincent, 2000b; Maiese, 2002; Chong et al., 2005a). The loss of membrane phospholipid asymmetry leads to the externalization of membrane PS residues and assists microglia to target cells for phagocytosis (Hoffmann et al., 2001; Chong et al., 2003c; Kang et al., 2003b; Maiese and Chong, 2003). This process occurs with the expression of the phosphatidylserine receptor (PSR) on microglia during oxidative stress (Li et al., 2006a; Li et al., 2006c), since blockade of PSR function in microglia prevents the activation of microglia (Chong et al., 2003b; Kang et al., 2003a).
The function of SHP-1 and SHP-2 during cell injury and inflammation are not easily defined, since these PTPs appear to possess dual roles to either enhance or inhibit cell survival under a variety of environmental conditions. Yet, both SHP-1 and SHP-2 protein expression can be decreased during cell injury and can be inactivated during oxidative stress in the presence of reactive oxygen species (Lee et al., 1998) and nitric oxide by-products (Touyz, 2004), suggesting that the loss of these proteins and the cellular pathways that they control may coincide with cell injury (Aoki et al., 2000; Horvat et al., 2001). For example, it has been shown that the SHPS-1 receptor-type glycoprotein that can bind and activate SHP-1 and SHP-2 contributes to the survival of circulating platelets and reduces red blood cell destruction from peritoneal macrophages (Yamao et al., 2002). In particular, SHP-1 has been shown to influence inflammatory cell function by promoting the survival of macrophages that is independent of growth factors, such as colony-stimulating factor-1 (Berg et al., 1999). In addition, SHP-1 can reduce inflammation through the regulation of chemokine gene expression (Forget et al., 2005) and the modulation of interferon regulatory factor-1 (Massa and Wu, 1996). SHP-1 is responsible for some of the effects of estrogen to protect against B cell mediated apoptosis (Grimaldi et al., 2002) and peripheral T cell death during the induction of antigen receptor-induced apoptosis (Zhang et al., 1999). Furthermore, SHP-1 may be responsible for the support of photoreceptors and the protection against retinal degeneration (Lyons et al., 2006). Although the mechanisms for these observations are not entirely known, SHP-1 can inhibit the generation of NO and potentially reduce the ill effects of reactive oxygen species generation (Forget et al., 2006).
On the converse side, SHP-1 also may lead to cellular demise. SHP-1 expression in the bone marrow of patients with myelodysplastic syndrome suggests a more rapid disease progression when compared to patients without SHP-1 expression (Mena-Duran et al., 2005). In addition, experimental rat models reveal that following an acute myocardial infarction, SHP-1 expression is increased and may contribute to infarct size (Sugano et al., 2005). Transfection of a dominant-negative SHP-1 in rat fetal vascular smooth muscle cells leads to the attenuation of apoptosis during angiotensin II type 2 receptor activation (Cui et al., 2001). SHP-1 also may diminish sympathetic neuronal cell number through inhibitory control of nerve growth factor TrkA receptor (Marsh et al., 2003). Furthermore, SHP-1 has been shown to limit neutrophil survival through death-receptor stimulation (Daigle et al., 2002). Other mechanisms that may be responsible for the detrimental effects of SHP-1 on cell survival include caspase 8 activation, cellular acidification (Liu et al., 2000), and inhibition of interleukin-3 signal transduction (Paling and Welham, 2002). In this particular instance, it is of interest to note that SHP-2 has been reported to employ interleukin-3 (Wheadon et al., 2003; Yu et al., 2003) as well as interleukin-5 (Pazdrak et al., 1997) and interleukin-6 (Chauhan et al., 2000) to block apoptosis.
In a number of studies, SHP-2 has been demonstrated to be beneficial to cell survival. SHP-2 has been linked to the protective capacity of vascular endothelial growth factor receptor-3 (Wang et al., 2004). SHP-2 also promotes cell survival by platelet endothelial cell adhesion molecule-1 (Maas et al., 2003) during oxidative stress injury. In other models of oxidative stress, loss of SHP-2 function can increase infarct size (Aoki et al., 2000) and lead to hippocampal neuronal cell apoptotic DNA fragmentation and elevated neuronal membrane PS exposure (Chong et al., 2003e) (Fig. 1, Fig. 2). Other work suggests that SHP-2 can promote cell survival during the application of brain-derived neurotrophic factor (Takai et al., 2002; Easton et al., 2006) as well as during injury paradigms that include serum starvation (Chen et al., 2007), intercellular adhesion molecule-1 (Pluskota et al., 2000), and cytokine signaling that involves interferon (You et al., 1999). Cellular protection through SHP-2 may be controlled by several pathways potentially critical for post-mitotic cell survival (Chong et al., 2006a) that involve the activation of protein kinase B (Akt) (Hakak et al., 2000; Ivins Zito et al., 2004) and the inhibition of caspase activity, such as caspase 1 (Chong et al., 2003e) and caspase 3 (Chong et al., 2003e; Ivins Zito et al., 2004).
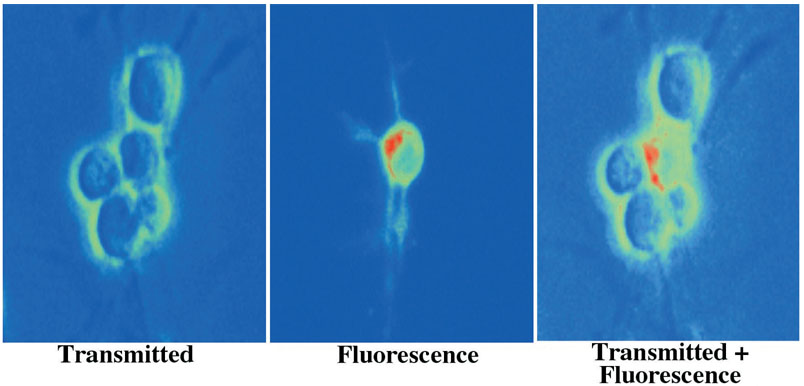
Fig. 1.
Oxidative stress leads to significant externalization of phosphatidylserine (PS) residues in SHP-2 mutant neurons. Murine neurons lacking SHP-2 function were labeled with annexin V phycoerythrin 5 hours following exposure to oxidative stress with a nitric oxide (NO) agent (NOC-9, 300 mM). Imaging uses transmitted light, corresponding fluorescence light, and superimposed transmitted and fluorescent images of the same microscopy field at 490 nm excitation and 585 nm emission wavelengths to locate the annexin V phycoerythrin label (red-green).
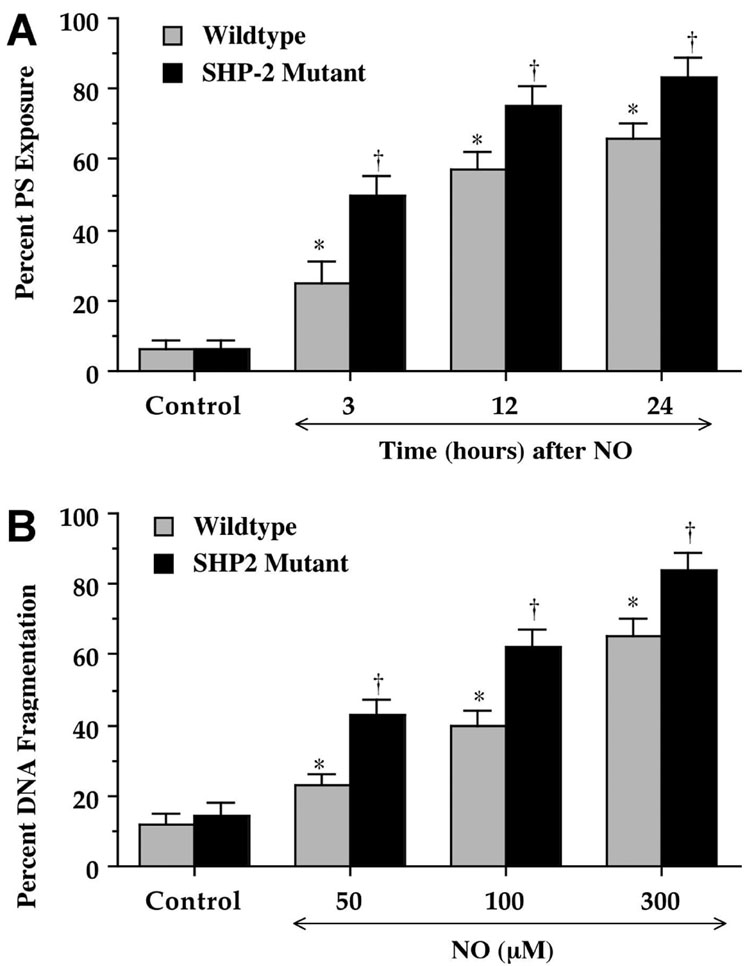
Fig. 2.
Absence of SHP-2 function in neurons leads to increased phosphatidylserine (PS) exposure and DNA fragmentation during oxidative stress. (A) Wildtype murine neurons or neurons lacking SHP-2 function were labeled with annexin V phycoerythrin 3, 12, and 24 hours following exposure to oxidative stress with a nitric oxide (NO) agent (NOC-9, 300 mM) and the percentage of membrane PS exposure was determined at each time point. The percentage of PS exposure progressively increased to a greater extent in neurons lacking SHP-2 cells over a 24 hour period, suggesting a protective role for SHP-2 against apoptotic early PS externalization (*p<0.01 versus untreated control; *p<0.01 versus corresponding concentration of NO treated wildtype neurons). Each data point represents the mean and SEM. (B) Increasing concentrations of NO donor (NOC-9, 50–300 mM) to lead to oxidative stress was applied to wildtype murine neurons or neurons lacking SHP-2 function and DNA fragmentation was determined 24 hours later using the terminal deoxynucleotidyl transferase nick end labeling assay. Exposure to NO resulted in a greater increase in DNA fragmentation in neurons lacking SHP-2, suggesting a protective role for SHP-2 against apoptotic DNA injury (*p<0.01 versus untreated control; †p<0.01 versus corresponding concentration of NO treated wildtype neurons). Each data point represents the mean and SEM.
Despite the evidence for SHP-2 to enhance cell survival, other investigations point to a darker side for SHP-2 in relation to cell survival and injury. For example, it has been suggested that enhanced expression of SHP-2 and its promotion of vascular smooth muscle cell growth during balloon-induced injury may accelerate atherosclerosis (Seki et al., 2002). SHP-2 also has been associated with the negative regulation and inhibition of platelet-derived growth factor (Vantler et al., 2006). During oxidative stress injury, SHP-2 can bind to the Gab1 docking protein to decrease cell survival (Holgado-Madruga and Wong, 2003) and inhibit the neurotrophin TrkB receptor during excitotoxicity to sensitize neurons to injury (Rusanescu et al., 2005). Even during conditions of cell injury that involve chemotherapeutic agents such as cisplatin-induced apoptosis or growth factor withdrawal-induced cell injury, SHP-2 can accelerate the induction of apoptosis (Yuan et al., 2005) through the dephosphorylation of STAT5 (Chen et al., 2004).
Although both SHP-1 and SHP-2 rely upon the signaling pathways of several cytokines and growth factors to regulate cell survival (Rakesh and Agrawal, 2005), the erythropoietin (EPO) protein has a strong link to SHP-1 and SHP-2. EPO is currently approved by the Food and Drug Administration for the treatment of anemia, but recent investigations have suggested that EPO modulates cellular pathways in organs and tissues outside of the liver and the kidney, such as the brain and heart. As a result, EPO has been identified as a possible candidate for disorders that involve both cardiac and nervous system diseases (Maiese et al., 2004, 2005c) and has a significant clinical relevance for multiple disorders that can involve cerebral ischemia, inflammation, and Alzheimer’s disease (Chong et al., 2005d,f; Li et al., 2005). For example, EPO not only can preserve microglial integrity (Li et al., 2006b), but it also can prevent microglial cell activation and proliferation to block phagocytosis of injured cells through pathways that involve cellular membrane PS exposure (Maiese et al., 2004, 2005c). EPO works through a number of pathways to exert cytoprotection. Activation of the phosphatidylinositol 3-kinase (PI 3-K)/Akt pathway is necessary for EPO protection during injury models that include excitotoxicity (Dzietko et al., 2004), cardiomyocyte ischemia (Parsa et al., 2003), hypoxia (Chong et al., 2002b), and oxidative stress (Chong et al., 2003a,b,d). EPO uses the Akt pathway to prevent apoptosis by maintaining mitochondrial membrane potential (ΔΨm ), preventing the cellular release of cytochrome c (Chong et al., 2003a,b,d), and modulating caspase activity (Chong et al., 2002b, 2003a,b). EPO modulation of pro-inflammatory cytokines (Chong et al., 2002a,c; Genc et al., 2004), forkhead transcription factors (Maiese et al., 2004; Chong and Maiese, 2007), STAT3, STAT5, ERK1/2 (Menon et al., 2006a; Um and Lodish, 2006; Chong and Maiese, 2007), glycogen synthase kinase-3β (Sharples et al., 2005; Li et al., 2006b; Nishihara et al., 2006), nuclear factor-κB (NF-κB) (Bittorf et al., 2001; Chong et al., 2005c; Li et al., 2006b), and caspases (Chong et al., 2002b, 2003a) also prevents the apoptotic destruction of cells.
In clinical investigations, hemodialysis patients that are hyporesponsive to the administration of recombinant human EPO has been attributed to active SHP-1 expression that leads to the dephosphorylation of STAT5 to impair EPO signaling (Akagi et al., 2004). Furthermore, primary familial and congential polycythemia, disorders that are characterized by elevated erythrocyte numbers with increased EPO-responsiveness of erythroid progenitor cells, have been shown to be a result of impaired modulation of the EPO receptor as a result of diminished expression and recruitment of SHP-1 (Furukawa et al., 1997; Wickrema et al., 1999a; Motohashi et al., 2001). Under normal conditions, SHP-1 can modulate and reduce EPO signaling through STAT5, the Jak2 kinase, and the inhibitory protein suppressor of cytokine signaling-1 (Minoo et al., 2004). Interestingly, SHP-2 also is linked to EPO signaling. EPO can lead to the tyrosine phosphorylation of the SH2/SH3-containing adapter protein CrkL that can associate with SHP-2 (Chin et al., 1997). EPO also relies upon the insulin receptor substrate-related proteins Gab1 and GAb2 to lead to the activation of SHP-2 and phosphatidylinositol 3-kinase (PI 3-K) pathways that also can result in Akt activation (Wickrema et al., 1999b).
Pathways that regulate SHP-1 and SHP-2 function
SHP and phosphatidylinositol 3-kinase (PI 3-K)/Akt
The phosphatidylinositol 3-kinase (PI 3-K) pathway that ultimately leads to the activation of Akt plays a prominent role for cellular protection in a number of disorders that can involve ischemia, hypoxia, and free radical induced oxidative stress (Chong et al., 2002b, 2003b; Bahlmann et al., 2004; Li et al., 2006b; Miki et al., 2006). PI 3-K is composed of a catalytic p110 subunit and a regulatory p85 subunit and phosphorylates membrane glycerophospholipid phosphatidylinositol 4,5-bisphosphate [PI (4,5)P2] resulting in production of phosphatidylinositol 3,4,5 trisphosphate (PIP3) and phosphatidylinositol 3,4 trisphosphate (PIP2). Once activated through the PI 3-K pathway, Akt can promote cellular integrity and survival during free radical injury (Chong et al., 2004a; Wang and MacNaughton, 2005), oxidative stress (Kang et al., 2003a,b), tumor invasion (Hardee et al., 2006), cell longevity (Li et al., 2006a), and amyloid toxicity (Nakagami et al., 2002; Du et al., 2004; Chong et al., 2005c). In addition, the role of Akt in other signal transduction system is receiving greater appreciation such as during the Wnt family of cysteine-rich glycosylated proteins (Li et al., 2005; Li et al., 2006c; Chong et al., 2007a). Three family members of Akt are now known to exist known as PKBa or Akt1, PKBb or Akt2, and PKBg or Akt3 (Staal, 1987; Staal et al., 1988; Chong et al., 2005b).
Of the three Akt isoforms, Akt1 is the most highly expressed. Significant expression of Akt2 occurs in insulin-responsive tissues, such as skeletal muscle, liver, heart, kidney, and adipose tissue (Altomare et al., 1995). In the nervous system, expression of Akt1 and Akt2 is initially weak with an increased expression during cell injury (Owada et al., 1997; Kang et al., 2003b; Chong et al., 2004a), suggesting that the increased expression of Akt may represent a reparative response by injured or phagocytized cells (Chong et al., 2006b). In contrast to Akt1 and Akt2, Akt3 is expressed only in a limited number of tissues, such as in the brain and testes, with lower expression evident in skeletal muscle, pancreas, heart, and kidney (Nakatani et al., 1999).
In some cellular systems, SHP-1 and SHP-2 have been closely aligned with the Akt pathway. For example, the ability of SHP-1 to potentially contribute to myocardial infarction in rodents also has been tied to the loss of Akt activity (Sugano et al., 2005). In addition, mice with functionally deficient SHP-1 protein have been found to be both glucose tolerant and insulin sensitive when compared to control mice. The SHP-1 deficient mice demonstrated enhanced activation of PI 3-K/Akt pathway in skeletal muscle and liver (Dubois et al., 2006) (Fig. 3). Under normal conditions, the p85 regulatory subunit of PI 3-K serves to both stabilize and inactivate the p110 catalytic subunit. This inhibitory activity of p85 is relieved by occupancy of the NH(2)-terminal SH2 domain of p85 by Src family kinases. This process is reversed by SHP-1 and its association with the regulatory p85 subunit of PI 3-K (Yu et al., 1998) that serves to inhibit PI 3-K activity (Cuevas et al., 2001). In the absence of SHP-1, insulin sensitivity is increased through the 85 kDa regulatory subunit of PI 3-K to allow the 110 kDa catalytic subunit of PI 3-K to become active and lead to the phosphorylation of Akt. This work suggests that PI 3-K may be an important component by which SHP-1 influences insulin signaling during glucose metabolism.
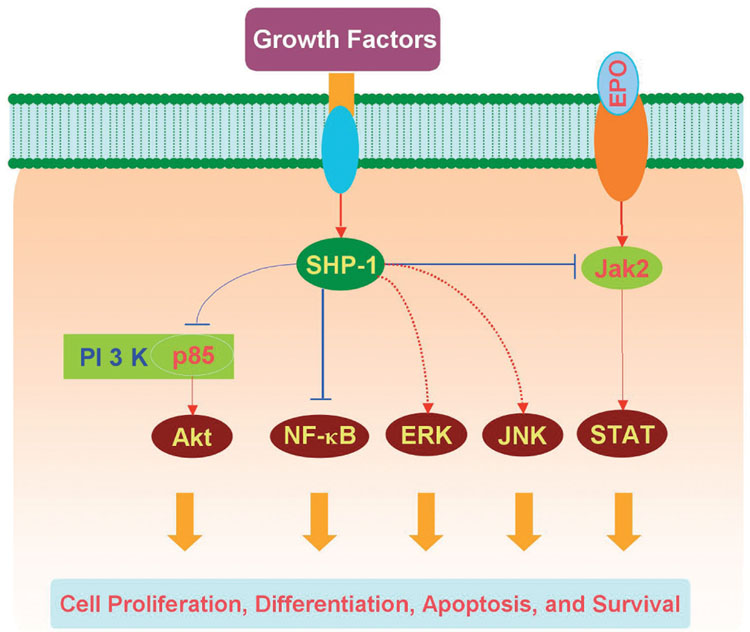
Fig. 3.
Illustration of potential signaling pathways controlled by Src homology 2 (SH2) domain-containing protein tyrosine phosphatase 1 (SHP-1). SHP-1 plays an important role in the regulation of growth factor and cytokine signal transduction to modulate cell proliferation, differentiation, survival, and apoptosis. SHP-1 can regulate growth factor induced activation of phosphatidylinositol 3-kinase (PI 3-K)/Akt and nuclear factor-kappa B (NF-κB). SHP-1 may either negatively or positively regulate the activation of the extracellular signal-related kinases (ERKs) and the c-Jun-amino terminal kinases (JNKs). In addition, SHP-1 can bind to the erythropoietin (EPO) receptor via its SH2 domain and modulate EPO activation of the Janus kinase 2 (Jak2)/signal transducer and activator of transcription (STAT).
SHP-1 can alter the activity of Akt through other cellular pathways that may not directly depend upon PI 3-K. SHP-1 can selectively bind and dephosphorylate the PTEN tumor suppressor that can subsequently modulate signal transduction in the Akt pathway (Lu et al., 2003). Angiotensin II subtype 2 receptor-mediated activation of SHP-1 leads to insulin receptor substrate-2 inhibition and the eventual blockade of Akt phosphorylation and activity (Cui et al., 2002). In endothelial cells, the ability of SHP-1 to inhibit PI 3-K may offer the capacity to reduce endothelial superoxide formation, since SHP-1 prevents endothelial NAD(P)H-oxidase activity through the inhibition of PI 3-K-dependent Rac1 activation (Krotz et al., 2005).
As previously noted, SHP-2 also employs the PI 3-K/Akt pathway, especially to prevent cellular injury (Hakak et al., 2000; Ivins Zito et al., 2004) (Fig. 4). SHP-2 also may utilize activation of Akt for the maintenance of cellular physiology during periods of increased metabolic demands that can affect cellular respiratory function and longevity (Maiese and Chong, 2003; Li et al., 2006a; Maiese et al., 2007). In a clinical study that examined the effects of exercise during insulin stimulation in healthy individuals, cytosolic SHP-2 was markedly increased in conjunction with Akt phosphorylation, suggesting that SHP-2 through Akt activation may assist with cellular respiratory function (Wadley et al., 2006). SHP-2 also has been identified as a necessary component to activate Akt for endothelial cell migration during vascular endothelial growth factor stimulation (Laramee et al., 2006) and to initiate cellular transformation through the fibroblast growth factor receptor 3 (Burks and Agazie, 2006). Tissue regeneration, such in hepatocytes, also may require SHP-2 linked to several pathways that include enhanced Akt activity (Bard-Chapeau et al., 2006).
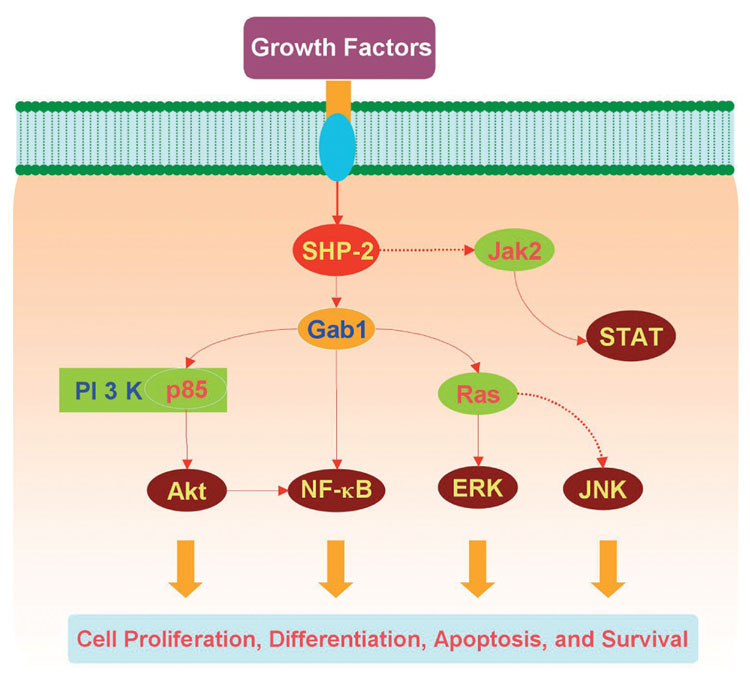
Fig. 4.
Illustration of potential signaling pathways controlled by Src homology 2 (SH2) domain-containing protein tyrosine phosphatase 2 (SHP-2). Similar to SHP-1, SHP-2 has a critical role in a host of cellular signal transduction pathways that involve cell proliferation, differentiation, survival, and apoptosis. Through the association with Grb2-associated binder-1 (Gab1), a docking protein containing an N-terminal pleckstrin homology domain and several proline-rich SH3 domain-binding sequences, SHP-2 promotes growth factor induced activation of phosphatidylinositol 3-kinase (PI 3-K)/Akt, the extracellular signal-related kinases (ERKs), and nuclear factor-kappa B (NF-κB). SHP-2 can either negatively or positively regulate the activation of Janus kinase 2 (Jak2)/signal transducer and activator of transcription (STAT) and the c-Jun-amino terminal kinases (JNKs) depending on different circumstances.
Although in several circumstances SHP-2 has been shown to associate with the p85 component of PI 3-K to promote Akt activation (Kwon et al., 2006), other work points to the ability of SHP-2 to prevent activation of Akt in different cellular environments. Resveratrol has been reported to require SHP-2 to block Akt activity in vascular smooth muscle cells (Haider et al., 2005). Other studies that show decreased neuronal survival with during excitotoxicity (Rusanescu et al., 2005) or pathways that involve epidermal growth factor stimulation of the PI 3-K pathway suggest involvement of SHP-2 that inhibits Akt activation (Zhang et al., 2002; Mattoon et al., 2004).
SHP, Janus-tyrosine Kinase 2 (Jak2), and Signal Transducer and Activator of Transcription (STAT) Pathways
For many growth factors and cytokines, initial cellular signaling can involve phosphorylation and activation of the Janus-tyrosine kinase 2 (Jak2). Jak2 is a member of a family of Janus-type protein-tyrosine kinases including Jak1, Jak2, Jak3, and Tyk2 that are characterized by a kinase domain in the carboxyl portion, a kinase-like domain, and a large amino-terminal domain. Jak2 and activation of the signal transducer and activator of transcription (STAT) proteins are essential to mediate cell growth, differentiation, survival, and death. Six primary mammalian STAT genes, STAT1, 2, 3, 4, 5, and 6 have been identified and are phosphorylated on tyrosine residues through Jak kinases and on serine residues by different serine/threonine kinases (Wilks et al., 1991; Grote et al., 2005; Ferrajoli et al., 2006). The STATs subsequently dimerize, undergo nuclear translocation, and bind to specific DNA sequences in the promoter regions of responsive genes to lead to gene transcription.
Jak2 activation can be a critical component for cytoprotection. For example, EPO prevents apoptotic injury through its dependence upon Jak2 phosphorylation (Kawakami et al., 2001). In addition, cytokines such as EPO have been shown to lead not only to the activation of STAT 3 (Parsa et al., 2003) and STAT 5 (Menon et al., 2006b; Um and Lodish, 2006), but also to possibly rely upon these pathways for cell development and cell protection. Interestingly, SHP-1 with Jak2 plays a role in the EPO signal transduction pathways. SHP-1 can bind to the tyrosine phosphorylated EPO receptor through the SH2 domain of SHP-1 (Yi et al., 1995) (Fig. 3). SHP-1 can associate with the tyrosine-phosphorylated EPO receptor via its SH2 domains by binding to the tyrosine residue Y429 in the cytoplasmic domain of the EPO receptor. This action prevents EPO from activating Jak2, illustrating a potential mechanism to regulate EPO signal transduction (Klingmuller et al., 1995) as well as offer another mechanism for the ability of SHP-1 to decrease the responsiveness of EPO during clinical treatments (Akagi et al., 2004). SHP-1 also has been shown to constitutively associate with Jak2 in EPO-dependent human leukemia cells. However, this association with SHP-1 involves a dephosphorylated Jak2 through the N terminus of SHP-1 that is independent of the SH2 domain (Wu et al., 2000) and not essential for SHP-1-mediated dephosphorylation of Jak2, suggesting that SHP-1 regulates Jak2 phosphorylation through mechanisms that are SH2-independent (Jiao et al., 1996). More recent work also demonstrates the capacity of SHP-1 to block Jak2 activation during NO production (Forget et al., 2006), during cytokine receptor signaling with inhibitory protein suppressor of cytokine signaling-1 (SOCS-1) that targets EPO signaling (Minoo et al., 2004), and during hyperinsulinemia-associated leptin resistance (Kellerer et al., 2001). In addition, loss of Jak2 activity though SHP-1 can be accompanied as expected by STAT inactivation, such as STAT3 (Bousquet et al., 1999).
SHP-2 also is intimately associated with Jak2/STAT signaling pathways (Fig. 4). SHP-2 can block the cytotoxic actions of cytokines, such as interferon (IFN), by preventing STAT activation. In mouse fibroblast cells lacking a functional SHP-2, IFN-α or IFN-γ decrease cell viability, increase STAT proteins bound to DNA, and increase the tyrosine phosphorylation of STAT1. These effects were reversed by SHP-2 reintroduction (You et al., 1999). SHP-2 also may be required to maintain a basal steady activity of STAT5 (Yu et al., 2000). SHP-2 can have diverse effects upon the Jak/STAT pathways under different environmental conditions. In a series of experiments involving the selective deletion of SHP-2 in the mouse forebrain, SHP-2 was found to down-regulate Jak2 and STAT3 (Zhang et al., 2004), but in mouse mammary glands SHP-2 may inversely regulate STAT3 and STAT5 activities (Ke et al., 2006). Furthermore, SHP-1 and SHP-2 may have opposing roles in the Jak/STAT pathways. Angiotensin II in vascular smooth muscle cells modulates the activation of the Jak2/STAT pathway by association of Jak2 with the angiotensin II receptor. SHP-1 dephosphorylates Jak2 to block the angiotensin II-induced Jak2/STAT cascade, while SHP-2 appears to result in Jak2 phosphorylation and initiation of the angiotensin II controlled Jak/STAT cascade leading to cell proliferation in vascular smooth muscle cells (Marrero et al., 1998). Additional work indicates that the N-terminal SH2 domain of SHP-2 is essential for the recruitment of Jak2 to the angiotensin II type AT1 receptor to facilitate angiotensin II signaling, since SHP2 mutant cells lacking the N-terminal SH2 domain are unable to facilitate AT1 receptor/Jak2 association, STAT activation, and subsequent angiotensin II mediated gene transcription (Godeny et al., 2006). The tyrosine 201 residue in the N-terminal SH2 domain has been suggested to be predominantly responsible for SHP-2 to bind Jak2 and to facilitate the recruitment of Jak2 to AT1 receptor (Godeny et al., 2006). Additionally, a dominant negative mutant of SHP-2 has been found to inhibit the induction of tyrosine phosphorylation and DNA-binding activity of STAT5, suggesting that SHP-2 is required for Jak2 activity and transcriptional induction of STAT5 (Berchtold et al., 1998).
SHP and mitogen-activated protein kinases
Another series of pathways that can oversee SHP-1 and SHP-2 function involves the stress activated family of mitogen-activated protein kinases (MAPK). The family of MAPKs consists of the three major subgroups (Maiese et al., 2005a; Li et al., 2006a). One subgroup includes the MAPK family members ERK1 and ERK2. The second subgroup is termed the JNKs, since they can activate the Jun transcription factor through phosphorylating two residues near its N-terminus. The third subgroup is termed p38 MAPKs named after the first representative in this subgroup with a molecular weight of 38 kDa (Haddad, 2004). The members of both JNK and p38 pathways are also classified as stress-activated protein kinases. They are activated in response to a variety of stress factors including osmotic shock, ultraviolet irradiation, inflammatory cytokines, and other stressful conditions such as oxidative stress (Haddad, 2004). The MAPKs are activated by phosphorylation and have an important function during cell differentiation, growth, and death (Chong et al., 2003e). Activation of p38 and JNK is present in both neurons and endothelial cells during oxidative stress (Lin et al., 2000, 2001; Maiese and Chong, 2003; Chong et al., 2005d).
Through work that has examined the effects of SHP-1 and SHP-2 on pathways such as Akt and the Jak/STAT pathway, it should come as no surprise that SHP-1 and SHP-2 may regulate MAPK pathways differently despite the shared sequence identity between these proteins. Yet, studies that examine these proteins together suggest that they may cooperate to promote cell growth signaling, such as with epidermal growth factor (Wang et al., 2006) and EPO through the activation of ERK1/2 (Bullard et al., 2005; Chong and Maiese, 2007). However, the effect of SHP-1 on ERK activation is not always positive to increase activity. For example, SHP-1 can dephosphorylate ERK in response to vascular endothelial growth factor administration in endothelial cells (Cai et al., 2006) (Fig. 3).
SHP-1 also has been shown to regulate angiotensin II receptor AT2 mediated JNK inactivation resulting in a decrease in the expression of c-Jun (Matsubara et al., 2001) (Fig. 3). Angiotensin II receptor isoform AT1 can activate JNK through calcium-sensitive tyrosine kinase Pyk2. In vascular smooth muscle cells, AT2 stimulation enhanced the activity of SHP-1 and overexpression of SHP-1 dominant negative mutant completely abolished AT2-mediated inhibition of JNK activation, suggesting that AT2 inhibits JNK activity in a SHP-1-dependent manner (Matsubara et al., 2001). SHP-1 also dephosphorylates SH2-domain containing leukocyte protein of 76 kDa (SLP-76) and subsequently regulates B cell receptor induced JNK activity (Mizuno et al., 2005). SLP-76 plays an important role in the development and activation of T cells, nature killer cells, mast cells, and macrophages, and also is found to function in B cells. Upon dephosphorylation by SHP-1, SLP-76 is recruited to CD22 to downregulate JNK activation in B cells and enhances apoptosis, suggesting that SHP-1 inhibits JNK activation in B cells through a SLP-76 dependent pathway. It should be noted that in other cell systems, SHP-1 can activate JNK. Overexpression of wild-type SHP-1 increased the FceRI aggregation-induced JNK activation whereas the dominant negative SHP-1 enhanced dephosphorylation of JNK (Xie et al., 2000), illustrating that SHP-1 may play a role in allergy and inflammation by regulating mast cell activity.
Similar to SHP-1, SHP-2 also can enhance the activity of ERK (Fig. 4). SHP-2 mediates ERK activation in several growth factor signaling pathways. The tyrosine phosphatase activity of SHP-2 is required for ERK activation with insulin (Milarski and Saltiel, 1994), insulin growth factor (Shi et al., 1998), and fibroblast growth factor (Araki et al., 2003), since absence or reduction of SHP-2 function leads to a decrease in ERK activity that is usually enhanced by these trophic factors. In addition, activation of ERK is regulated by SHP-2 in conjunction with the epidermal growth factor receptor in glioblastoma cells (Zhan and O’Rourke, 2004). In regards to epidermal growth factor activation of ERK, SHP-2 may require the Grb2-associated binder-1 (Gab1) binding partner. Phosphorylation of both the tyrosine 627 and the tyrosine 659 of Gab1 is necessary for SHP-2 to attach to Gab1 and subsequently activate SHP-2 and ERK (Cunnick et al., 2001). SHP-2 mediated ERK activation also may be necessary for interleukins-1 (IL-1) to associate with focal adhesions. IL-1b has been demonstrated to enhance the maturation of focal adhesions in human gingival fibroblasts (Herrera Abreu et al., 2006). IL-1b results in ERK activation and the recruitment of active phospho-ERK to focal complexes/adhesions to result in the maturation of focal adhesions.
SHP-2 also serves as a regulator of leptin signaling through ERK activation (Bjorbaek et al., 2001). Leptin is a 16-kDa hormone that functions to control food intake and energy metabolism (White and Tartaglia, 1996). A catalytically inactive mutant of SHP-2 can block leptin-stimulated ERK phosphorylation by the long leptin receptor, ObRb. Analysis of signaling by ObRb lacking intracellular tyrosine residues or by the short leptin receptor, ObRa, has revealed that two pathways are critical for ERK activation. One pathway does not require the intracellular domain of ObRb, whereas the other pathway requires the tyrosine residue 985 of ObRb, both of which depend upon the phosphatase activity of SHP-2 (Bjorbaek et al., 2001). Through the activation of ERK and p38, SHP-2 also regulates other pathways that involve matrix metalloproteinases. SHP-2 modulates concanavalin A induced secretion and activation of matrix metalloproteinase-2 (Ruhul Amin et al., 2003). Matrix metalloproteinase-2 is initially secreted as an inactive zymogen and subsequently activated through proteolytic cleavage that results in extracellular matrix destruction. Work has demonstrated that concanavalin A fails to regulate matrix metalloproteinase-2 and cannot activate ERK or p38 in cells expressing mutant SHP-2 with deletion of 65 amino acids in the N-terminal of SH2 domain. In addition, inhibition of ERK and p38 prevents concanavalin A-dependent secretion and activation of matrix metalloproteinase-2 while overexpression of active ERK in SHP-2 mutant cells doe not result in matrix metalloproteinase-2 activation. These studies suggest that SHP-2 regulates concanavalin A-dependent activation of matrix metalloproteinase-2 secretion through ERK and p38 signaling (Ruhul Amin et al., 2003).
In regards to the JNK pathway, SHP-2 appears to be ineffective in bringing about a significant change in the activity of JNK in some cell systems. Yet, in other cell types, SHP-2 may either inhibit or activate JNK. During overexpression of SHP-2 mutants in COS-7 cells, SHP-2 was shown to be necessary for epidermal growth factor-induced MAPK activation, but SHP-2 was not required for JNK activity in response to myristoylated son of sevenless, activated Ras, or phorbol ester (Deb et al., 1998). However, in a SHP-2 mutant fibroblast cell line, trophic factor induction of ERK activation was diminished or reduced during loss of SHP-2 activity, but heat shock induction in cells that lacked SHP-2 activity significantly increased JNK activity, suggesting that SHP-2 under these conditions was a negative modulator of JNK (Shi et al., 1998). In contrast to these observations, SHP-2 has been shown to enhance insulin induced JNK activation through the Ras signaling pathway (Fukunaga et al., 2000).
SHP and Nuclear Factor κB (NF-κB)
One pathway that may depend upon modulation from SHP-1 and SHP-2 and also is closely tied to Akt involves NF-κB. NF-κB proteins are composed of several homo- and heterodimer proteins that can bind to common DNA elements. It is the phosphorylation of IkB proteins by the IkB kinase (IKK) and their subsequent degradation that lead to the release of NF-κB for its translocation to the nucleus to initiate gene transcription (Hayden and Ghosh, 2004). Dependent upon Akt controlled pathways, the transactivation domain of the p65 subunit of NF-κB is activated by IKK and the IKKa catalytic subunit to lead to the induction of protective anti-apoptotic pathways (Chong et al., 2005b). Increased expression of NF-κB during injury models can occur in inflammatory microglial cells (Chong et al., 2005c, 2007b; Guo and Bhat, 2006) and in neurons (Sanz et al., 2002). Although NF-κB has not consistently been found to enhance cell survival in all cell systems (Esposito et al., 2006; Jacobsen et al., 2006), NF-κB does represent a critical pathway that is responsible for the activation of inhibitors of apoptotic proteins (IAPs), the maintenance of Bcl-xL expression, (Chen et al., 2000; Chong et al., 2005e), and the induction of cytoprotection by trophic factors (Nakata et al., 2004; Chong et al., 2005c; Sae-Ung et al., 2005). In addition, NF-κB p65 is required to maintain the endogenous protection of inflammatory cells, since gene silencing of NF-κB p65 results in significantly increased cell injury during oxidative stress (Chong et al., 2007b).
SHP-1 and SHP-2 can closely regulate the activity of NF-κB. SHP-1 has been shown to limit the activity of NF-κB through Ang II type 2 receptor stimulation in vascular smooth muscle cells (Wu et al., 2004). Increased transcription of NF-κB also has been observed in astrocytes of motheaten mice without functional SHP-1, further supporting a negative modulatory role for SHP-1 over NF-κB (Massa and Wu, 1998) (Fig. 3). Interestingly, the ability of SHP-1 to protect against oxidative stress and prevent the generation of NO also may require the inactivation of Jak2 and ERK1/2 as well as prevention of the nuclear translocation of NF-κB (Forget et al., 2006).
In regards to SHP-2, cytokine generation with interleukin-6 during interleukin-1 or tumor necrosis factor (TNF)-alpha application appears to require both SHP-2 activation and the ability of SHP-2 to promote NF-κB activity (You et al., 2001) (Fig. 4). Another cytokine that depends upon SHP-2 and NF-κB activity is LIGHT, a type II transmembrane protein belonging to the TNF family. It has recently be shown that for LIGHT to activate chemokine CCL27 to foster the migration of mouse embryonic stem cell derived dendritic cells, SHP-2 is required for LIGHT to activate NF-κB for the successful initiation of this pathway (Zou et al., 2006b). In addition, SHP-2 is a positive modulator for some trophic factors to lead to NF-κB activation. For epidermal growth factor to activate NF-κB, SHP-2 must associate with Gab1 to induce a PI3-kinase/Akt signaling axis that subsequently leads to NF-κB activation (Kapoor et al., 2004).
Conclusions
Protein tyrosine phosphorylation oversees a multitude of cellular processes that are relevant during both health and disease. Although more than 100 human PTPs have been identified that form a family of receptor-like and cytosolic enzymes, two particular cytoplasmic PTPs characterized by containing two Src homology 2 (SH2) NH2-terminal domains and a C-terminal protein-tyrosine phosphatase domain and referred to as SHP-1 and SHP-2 are involved in a variety of cellular functions. Both SHP-1 and SHP-2 can modulate progenitor cell development, cellular growth, tissue inflammation, and cellular chemotaxis. More recently, the ability of SHP-1 and SHP-2 to control cell survival and cell protection has come to light. These proteins are integral components for the function of several trophic factor and metabolic pathways that have far reaching implications for disease pathways and disorders such as diabetes, neurodegeneration, neoplastic growth, and oxidative stress. SHP-1 and SHP-2 rely upon parallel, but sometimes opposing downstream cellular mechanisms that can closely regulate early and late apoptotic programs. The activity of SHP-1 and SHP-2 has been associated with the modulation of cellular signals that involve PI 3-K, Akt, Jak2, STAT, MAPKs, ERKs, JNKs, and NF-κB. Our progressive grasp upon the impact of SHP-1 and SHP-2 over critical pathways of cell physiology and survival will continue to enhance our ability to design and development targeted therapeutic strategies for a number of disorders involving multiple organ systems of the body.
Acknowledgements
This research was supported by the following grants (KM): American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, MI Life Sciences Challenge Award, Nelson Foundation Award, NIH NIEHS (P30 ES06639), NIH NINDS, and NIH NIA.
References
- Akagi S, Ichikawa H, Okada T, Sarai A, Sugimoto T, Morimoto H, Kihara T, Yano A, Nakao K, Nagake Y, Wada J, Makino H. The critical role of SRC homology domain 2-containing tyrosine phosphatase-1 in recombinant human erythropoietin hyporesponsive anemia in chronic hemodialysis patients. J. Am. Soc. Nephrol. 2004;15:3215–3224. doi: 10.1097/01.ASN.0000145457.73744.24. [DOI] [PubMed] [Google Scholar]
- Alonso A, Sasin J, Bottini N, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Altomare DA, Guo K, Cheng JQ, Sonoda G, Walsh K, Testa JR. Cloning, chromosomal localization and expression analysis of the mouse Akt2 oncogene. Oncogene. 1995;11:1055–1060. [PubMed] [Google Scholar]
- Andersen JN, Jansen PG, Echwald SM, Mortensen OH, Fukada T, Del Vecchio R, Tonks NK, Moller NP. A genomic perspective on protein tyrosine phosphatases: gene structure, pseudogenes, and genetic disease linkage. FASEB J. 2004;18:8–30. doi: 10.1096/fj.02-1212rev. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Huang Z, Thomas SS, Bhide PG, Huang I, Moskowitz MA, Reeves SA. Increased susceptibility to ischemia-induced brain damage in transgenic mice overexpressing a dominant negative form of SHP2. FASEB J. 2000;14:1965–1973. doi: 10.1096/fj.00-0105com. [DOI] [PubMed] [Google Scholar]
- Araki T, Nawa H, Neel BG. Tyrosyl phosphorylation of Shp2 is required for normal ERK activation in response to some, but not all, growth factors. J. Biol. Chem. 2003;278:41677–41684. doi: 10.1074/jbc.M306461200. [DOI] [PubMed] [Google Scholar]
- Bahlmann FH, Song R, Boehm SM, Mengel M, von Wasielewski R, Lindschau C, Kirsch T, de Groot K, Laudeley R, Niemczyk E, Guler F, Menne J, Haller H, Fliser D. Low-dose therapy with the long-acting erythropoietin analogue darbepoetin alpha persistently activates endothelial Akt and attenuates progressive organ failure. Circulation. 2004;110:1006–1012. doi: 10.1161/01.CIR.0000139335.04152.F3. [DOI] [PubMed] [Google Scholar]
- Bard-Chapeau EA, Yuan J, Droin N, Long S, Zhang EE, Nguyen TV, Feng GS. Concerted functions of Gab1 and Shp2 in liver regeneration and hepatoprotection. Mol. Cell. Biol. 2006;26:4664–4674. doi: 10.1128/MCB.02253-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzing WC, Wujek JR, Ward EK, Shaffer D, Ashe KH, Younkin SG, Brunden KR. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol. Aging. 1999;20:581–589. doi: 10.1016/s0197-4580(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Berchtold S, Volarevic S, Moriggl R, Mercep M, Groner B. Dominant negative variants of the SHP-2 tyrosine phosphatase inhibit prolactin activation of Jak2 (janus kinase 2) and induction of Stat5 (signal transducer and activator of transcription 5)-dependent transcription. Mol. Endocrinol. 1998;12:556–567. doi: 10.1210/mend.12.4.0086. [DOI] [PubMed] [Google Scholar]
- Berg KL, Siminovitch KA, Stanley ER. SHP-1 regulation of p62(DOK) tyrosine phosphorylation in macrophages. J. Biol. Chem. 1999;274:35855–35865. doi: 10.1074/jbc.274.50.35855. [DOI] [PubMed] [Google Scholar]
- Bialy L, Waldmann H. Inhibitors of protein tyrosine phosphatases: next-generation drugs? Angew. Chem. Int. Ed. Engl. 2005;44:3814–3839. doi: 10.1002/anie.200461517. [DOI] [PubMed] [Google Scholar]
- Bittorf T, Buchse T, Sasse T, Jaster R, Brock J. Activation of the transcription factor NF-kappaB by the erythropoietin receptor: structural requirements and biological significance. Cell. Signal. 2001;13:673–681. doi: 10.1016/s0898-6568(01)00189-9. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers MG, Jr, Flier JS. Divergent roles of SHP-2 in ERK activation by leptin receptors. J. Biol. Chem. 2001;276:4747–4755. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- Bousquet C, Susini C, Melmed S. Inhibitory roles for SHP-1 and SOCS-3 following pituitary proopiomelanocortin induction by leukemia inhibitory factor. J. Clin. Invest. 1999;104:1277–1285. doi: 10.1172/JCI7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard AJ, Govewalla P, Yellon DM. Erythropoietin protects the myocardium against reperfusion injury in vitro and in vivo. Basic Res. Cardiol. 2005;100:397–403. doi: 10.1007/s00395-005-0537-4. [DOI] [PubMed] [Google Scholar]
- Burks J, Agazie YM. Modulation of alpha-catenin Tyr phosphorylation by SHP2 positively effects cell transformation induced by the constitutively active FGFR3. Oncogene. 2006;25:7166–7179. doi: 10.1038/sj.onc.1209728. [DOI] [PubMed] [Google Scholar]
- Cai J, Jiang WG, Ahmed A, Boulton M. Vascular endothelial growth factor-induced endothelial cell proliferation is regulated by interaction between VEGFR-2, SH-PTP1 and eNOS. Microvasc. Res. 2006;71:20–31. doi: 10.1016/j.mvr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Chan RJ, Yoder MC. The multiple facets of hematopoietic stem cells. Curr. Neurovasc. Res. 2004;1:197–206. doi: 10.2174/1567202043362324. [DOI] [PubMed] [Google Scholar]
- Chan RJ, Li Y, Hass MN, Walter A, Voorhorst CS, Shelley WC, Yang Z, Orschell CM, Yoder MC. Shp-2 heterozygous hematopoietic stem cells have deficient repopulating ability due to diminished self-renewal. Exp. Hematol. 2006;34:1230–1239. doi: 10.1016/j.exphem.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Pandey P, Hideshima T, Treon S, Raje N, Davies FE, Shima Y, Tai YT, Rosen S, Avraham S, Kharbanda S, Anderson KC. SHP2 mediates the protective effect of interleukin-6 against dexamethasone-induced apoptosis in multiple myeloma cells. J. Biol. Chem. 2000;275:27845–27850. doi: 10.1074/jbc.M003428200. [DOI] [PubMed] [Google Scholar]
- Chen B, Hammonds-Odie L, Perron J, Masters BA, Bixby JL. SHP-2 mediates target-regulated axonal termination and NGF-dependent neurite growth in sympathetic neurons. Dev. Biol. 2002;252:170–187. doi: 10.1006/dbio.2002.0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Edelstein LC, Gelinas C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L) Mol. Cell. Biol. 2000;20:2687–2695. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yu WM, Bunting KD, Qu CK. A negative role of SHP-2 tyrosine phosphatase in growth factor-dependent hematopoietic cell survival. Oncogene. 2004;23:3659–3669. doi: 10.1038/sj.onc.1207471. [DOI] [PubMed] [Google Scholar]
- Chen J, Yu WM, Daino H, Broxmeyer HE, Druker BJ, Qu CK. SHP-2 phosphatase is required for hematopoietic cell transformation by Bcr-Abl. Blood. 2007;109:778–785. doi: 10.1182/blood-2006-04-019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin H, Saito T, Arai A, Yamamoto K, Kamiyama R, Miyasaka N, Miura O. Erythropoietin and IL-3 induce tyrosine phosphorylation of CrkL and its association with Shc, SHP-2, and Cbl in hematopoietic cells. Biochem. Biophys. Res. Commun. 1997;239:412–417. doi: 10.1006/bbrc.1997.7480. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. Erythropoietin employs the phosphatidylinositol 3-kinase pathway, 14-3-3, and FOXO3a nuclear trafficking to foster endothelial cell integrity. Br. J. Pharmacol. 2007;150:839–850. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Angiogenesis and plasticity: role of erythropoietin in vascular systems. J. Hematother. Stem Cell. Res. 2002a;11:863–871. doi: 10.1089/152581602321080529. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002b;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Hematopoietic factor erythropoietin fosters neuroprotection through novel signal transduction cascades. J. Cereb. Blood Flow Metab. 2002c;22:503–514. doi: 10.1097/00004647-200205000-00001. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, Cytochrome c, and Caspase-9 Form the Critical Elements for Cerebral Vascular Protection by Erythropoietin. J. Cereb. Blood Flow Metab. 2003a;23:320–330. doi: 10.1097/01.WCB.0000050061.57184.AE. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br. J. Pharmacol. 2003b;138:1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Metabotropic glutamate receptors promote neuronal and vascular plasticity through novel intracellular pathways. Histol. Histopathol. 2003c;18:173–189. doi: 10.14670/HH-18.173. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Kang JQ, Maiese K. Erythropoietin prevents early and late neuronal demise through modulation of Akt1 and induction of caspase 1, 3, and 8. J. Neurosci. Res. 2003d;71:659–669. doi: 10.1002/jnr.10528. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Kang JQ, Maiese K. The tyrosine phosphatase SHP2 modulates MAP kinase p38 and caspase 1 and 3 to foster neuronal survival. Cell. Mol. Neurobiol. 2003e;23:561–578. doi: 10.1023/A:1025158314016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Akt1 drives endothelial cell membrane asymmetry and microglial activation through Bcl-x(L) and caspase 1, 3, and 9. Exp. Cell. Res. 2004a;296:196–207. doi: 10.1016/j.yexcr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Essential cellular regulatory elements of oxidative stress in early and late phases of apoptosis in the central nervous system. Antioxid. Redox. Signal. 2004b;6:277–287. doi: 10.1089/152308604322899341. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang J, Li F, Maiese K. mGluRI Targets Microglial Activation and Selectively Prevents Neuronal Cell Engulfment Through Akt and Caspase Dependent Pathways. Curr. Neurovasc. Res. 2005a;2:197–211. doi: 10.2174/1567202054368317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Activating Akt and the brain’s resources to drive cellular survival and prevent inflammatory injury. Histol. Histopathol. 2005b;20:299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr. Neurovasc. Res. 2005c;2:387–399. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog. Neurobiol. 2005d;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Stress in the brain: novel cellular mechanisms of injury linked to Alzheimer’s disease. Brain Res. Brain Res. Rev. 2005e;49:1–21. doi: 10.1016/j.brainresrev.2004.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li FQ, Maiese K. Employing new cellular therapeutic targets for Alzheimer’s disease: A change for the better? Curr. Neurovasc. Res. 2005f;2:55–72. doi: 10.2174/1567202052773508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Attempted cell cycle Induction in post-mitotic neurons occurs in early and late apoptotic programs through Rb, E2F1, and caspase 3. Curr. Neurovasc. Res. 2006a;3:25–39. doi: 10.2174/156720206775541741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Group I metabotropic receptor neuroprotection requires Akt and its substrates that govern FOXO3a, Bim, and beta-catenin during oxidative stress. Curr. Neurovasc. Res. 2006b;3:107–117. doi: 10.2174/156720206776875830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell. Signal. 2007a;19:1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int. J. Mol. Med. 2007b;19:263–272. [PMC free article] [PubMed] [Google Scholar]
- Cuevas BD, Lu Y, Mao M, Zhang J, LaPushin R, Siminovitch K, Mills GB. Tyrosine phosphorylation of p85 relieves its inhibitory activity on phosphatidylinositol 3-kinase. J. Biol. Chem. 2001;276:27455–27461. doi: 10.1074/jbc.M100556200. [DOI] [PubMed] [Google Scholar]
- Cui T, Nakagami H, Iwai M, Takeda Y, Shiuchi T, Daviet L, Nahmias C, Horiuchi M. Pivotal role of tyrosine phosphatase SHP-1 in AT2 receptor-mediated apoptosis in rat fetal vascular smooth muscle cell. Cardiovasc. Res. 2001;49:863–871. doi: 10.1016/s0008-6363(00)00299-6. [DOI] [PubMed] [Google Scholar]
- Cui TX, Nakagami H, Nahmias C, Shiuchi T, Takeda-Matsubara Y, Li JM, Wu L, Iwai M, Horiuchi M. Angiotensin II subtype 2 receptor activation inhibits insulin-induced phosphoinositide 3-kinase and Akt and induces apoptosis in PC12W cells. Mol. Endocrinol. 2002;16:2113–2123. doi: 10.1210/me.2001-0284. [DOI] [PubMed] [Google Scholar]
- Cunnick JM, Mei L, Doupnik CA, Wu J. Phosphotyrosines 627 and 659 of Gab1 constitute a bisphosphoryl tyrosine-based activation motif (BTAM) conferring binding and activation of SHP2. J. Biol. Chem. 2001;276:24380–24387. doi: 10.1074/jbc.M010275200. [DOI] [PubMed] [Google Scholar]
- Daigle I, Yousefi S, Colonna M, Green DR, Simon HU. Death receptors bind SHP-1 and block cytokine-induced anti-apoptotic signaling in neutrophils. Nat. Med. 2002;8:61–67. doi: 10.1038/nm0102-61. [DOI] [PubMed] [Google Scholar]
- Deb TB, Wong L, Salomon DS, Zhou G, Dixon JE, Gutkind JS, Thompson SA, Johnson GR. A common requirement for the catalytic activity and both SH2 domains of SHP-2 in mitogen-activated protein (MAP) kinase activation by the ErbB family of receptors. A specific role for SHP-2 in map, but not c-Jun amino-terminal kinase activation. J. Biol. Chem. 1998;273:16643–16646. doi: 10.1074/jbc.273.27.16643. [DOI] [PubMed] [Google Scholar]
- Dombroski D, Balasubramanian K, Schroit AJ. Phosphatidylserine expression on cell surfaces promotes antibody-dependent aggregation and thrombosis in beta2-glycoprotein I-immune mice. J. Autoimmun. 2000;14:221–229. doi: 10.1006/jaut.2000.0365. [DOI] [PubMed] [Google Scholar]
- Doonan F, Cotter TG. Apoptosis: A potential therapeutic target for retinal degenerations. Curr. Neurovasc. Res. 2004;1:41–53. doi: 10.2174/1567202043480215. [DOI] [PubMed] [Google Scholar]
- Du B, Ohmichi M, Takahashi K, Kawagoe J, Ohshima C, Igarashi H, Mori-Abe A, Saitoh M, Ohta T, Ohishi A, Doshida M, Tezuka N, Takahashi T, Kurachi H. Both estrogen and raloxifene protect against beta-amyloid-induced neurotoxicity in estrogen receptor alpha-transfected PC12 cells by activation of telomerase activity via Akt cascade. J. Endocrinol. 2004;183:605–615. doi: 10.1677/joe.1.05775. [DOI] [PubMed] [Google Scholar]
- Dubois MJ, Bergeron S, Kim HJ, Dombrowski L, Perreault M, Fournes B, Faure R, Olivier M, Beauchemin N, Shulman GI, Siminovitch KA, Kim JK, Marette A. The SHP-1 protein tyrosine phosphatase negatively modulates glucose homeostasis. Nat. Med. 2006;12:549–556. doi: 10.1038/nm1397. [DOI] [PubMed] [Google Scholar]
- Dzietko M, Felderhoff-Mueser U, Sifringer M, Krutz B, Bittigau P, Thor F, Heumann R, Buhrer C, Ikonomidou C, Hansen HH. Erythropoietin protects the developing brain against N-methyl-D-aspartate receptor antagonist neurotoxicity. Neurobiol. Dis. 2004;15:177–187. doi: 10.1016/j.nbd.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Easton JB, Royer AR, Middlemas DS. The protein tyrosine phosphatase, Shp2, is required for the complete activation of the RAS/MAPK pathway by brain-derived neurotrophic factor. J. Neurochem. 2006;97:834–845. doi: 10.1111/j.1471-4159.2006.03789.x. [DOI] [PubMed] [Google Scholar]
- Esposito G, De Filippis D, Maiuri MC, De Stefano D, Carnuccio R, Iuvone T. Cannabidiol inhibits inducible nitric oxide synthase protein expression and nitric oxide production in beta-amyloid stimulated PC12 neurons through p38 MAP kinase and NF-kappaB involvement. Neurosci. Lett. 2006;399:91–95. doi: 10.1016/j.neulet.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Feng GS, Shen R, Heng HH, Tsui LC, Kazlauskas A, Pawson T. Receptor-binding, tyrosine phosphorylation and chromosome localization of the mouse SH2-containing phosphotyrosine phosphatase Syp. Oncogene. 1994;9:1545–1550. [PubMed] [Google Scholar]
- Ferrajoli A, Faderl S, Ravandi F, Estrov Z. The JAK-STAT pathway: a therapeutic target in hematological malignancies. Curr. Cancer Drug Targets. 2006;6:671–679. doi: 10.2174/156800906779010227. [DOI] [PubMed] [Google Scholar]
- Ferretti P. Neural stem cell plasticity: Recruitment of endogenous populations for regeneration. Curr. Neurovasc. Res. 2004;1:215–229. doi: 10.2174/1567202043362397. [DOI] [PubMed] [Google Scholar]
- Forget G, Gregory DJ, Whitcombe LA, Olivier M. Role of host protein tyrosine phosphatase SHP-1 in Leishmania donovani-induced inhibition of nitric oxide production. Infect. Immun. 2006;74:6272–6279. doi: 10.1128/IAI.00853-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget G, Matte C, Siminovitch KA, Rivest S, Pouliot P, Olivier M. Regulation of the Leishmania-induced innate inflammatory response by the protein tyrosine phosphatase SHP-1. Eur. J. Immunol. 2005;35:1906–1917. doi: 10.1002/eji.200526037. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Noguchi T, Takeda H, Matozaki T, Hayashi Y, Itoh H, Kasuga M. Requirement for protein-tyrosine phosphatase SHP-2 in insulin-induced activation of c-Jun NH(2)-terminal kinase. J. Biol. Chem. 2000;275:5208–5213. doi: 10.1074/jbc.275.7.5208. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Narita M, Sakaue M, Otsuka T, Kuroha T, Masuko M, Azegami T, Kishi K, Takahashi M, Utsumi J, Koike T, Aizawa Y. Primary familial polycythaemia associated with a novel point mutation in the erythropoietin receptor. Br. J. Haematol. 1997;99:222–227. doi: 10.1046/j.1365-2141.1997.3583172.x. [DOI] [PubMed] [Google Scholar]
- Genc S, Koroglu TF, Genc K. Erythropoietin and the nervous system. Brain Res. 2004;1000:19–31. doi: 10.1016/j.brainres.2003.12.037. [DOI] [PubMed] [Google Scholar]
- Godeny MD, Sayyah J, Vonderlinden D, Johns M, Ostrov DA, Caldwell-Busby J, Sayeski PP. The N-terminal SH2 domain of the tyrosine phosphatase, SHP-2, is essential for Jak2-dependent signaling via the angiotensin II type AT(1) receptor. Cell. Signal. 2006 doi: 10.1016/j.cellsig.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Greco A, Minghetti L. Isoprostanes as biomarkers and mediators of oxidative injury in infant and adult central nervous system diseases. Curr. Neurovasc. Res. 2004;1:341–354. doi: 10.2174/1567202043362036. [DOI] [PubMed] [Google Scholar]
- Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J. Clin. Invest. 2002;109:1625–1633. doi: 10.1172/JCI14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote K, Luchtefeld M, Schieffer B. JANUS under stress—role of JAK/STAT signaling pathway in vascular diseases. Vascul. Pharmacol. 2005;43:357–363. doi: 10.1016/j.vph.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Guo G, Bhat NR. Hypoxia/Reoxygenation Differentially Modulates NF-kappaB Activation and iNOS Expression in Astrocytes and Microglia. Antioxid. Redox Signal. 2006;8:911–918. doi: 10.1089/ars.2006.8.911. [DOI] [PubMed] [Google Scholar]
- Haddad JJ. Mitogen-activated protein kinases and the evolution of Alzheimer’s: a revolutionary neurogenetic axis for therapeutic intervention? Prog. Neurobiol. 2004;73:359–377. doi: 10.1016/j.pneurobio.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Haider UG, Roos TU, Kontaridis MI, Neel BG, Sorescu D, Griendling KK, Vollmar AM, Dirsch VM. Resveratrol inhibits angiotensin II- and epidermal growth factor-mediated Akt activation: role of Gab1 and Shp2. Mol. Pharmacol. 2005;68:41–48. doi: 10.1124/mol.104.005421. [DOI] [PubMed] [Google Scholar]
- Hakak Y, Hsu YS, Martin GS. Shp-2 mediates v-Src-induced morphological changes and activation of the anti-apoptotic protein kinase Akt. Oncogene. 2000;19:3164–3171. doi: 10.1038/sj.onc.1203655. [DOI] [PubMed] [Google Scholar]
- Hardee ME, Rabbani ZN, Arcasoy MO, Kirkpatrick JP, Vujaskovic Z, Dewhirst MW, Blackwell KL. Erythropoietin inhibits apoptosis in breast cancer cells via an Akt-dependent pathway without modulating in vivo chemosensitivity. Mol. Cancer Ther. 2006;5:356–361. doi: 10.1158/1535-7163.MCT-05-0196. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Herrera Abreu MT, Wang Q, Vachon E, Suzuki T, Chow CW, Wang Y, Hong O, Villar J, McCulloch CA, Downey GP. Tyrosine phosphatase SHP-2 regulates IL-1 signaling in fibroblasts through focal adhesions. J. Cell. Physiol. 2006;207:132–143. doi: 10.1002/jcp.20544. [DOI] [PubMed] [Google Scholar]
- Hoffmann PR, deCathelineau AM, Ogden CA, Leverrier Y, Bratton DL, Daleke DL, Ridley AJ, Fadok VA, Henson PM. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J. Cell Biol. 2001;155:649–659. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgado-Madruga M, Wong AJ. Gab1 is an integrator of cell death versus cell survival signals in oxidative stress. Mol. Cell. Biol. 2003;23:4471–4484. doi: 10.1128/MCB.23.13.4471-4484.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JR, Lin GH, Lin CJ, Wang WP, Lee CC, Lin TL, Wu JL. Phosphatidylserine receptor is required for the engulfment of dead apoptotic cells and for normal embryonic development in zebrafish. Development. 2004;131:5417–5427. doi: 10.1242/dev.01409. [DOI] [PubMed] [Google Scholar]
- Horvat A, Schwaiger F, Hager G, Brocker F, Streif R, Knyazev P, Ullrich A, Kreutzberg GW. A novel role for protein tyrosine phosphatase shp1 in controlling glial activation in the normal and injured nervous system. J. Neurosci. 2001;21:865–874. doi: 10.1523/JNEUROSCI.21-03-00865.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivins Zito C, Kontaridis MI, Fornaro M, Feng GS, Bennett AM. SHP-2 regulates the phosphatidylinositide 3’-kinase/Akt pathway and suppresses caspase 3-mediated apoptosis. J. Cell. Physiol. 2004;199:227–236. doi: 10.1002/jcp.10446. [DOI] [PubMed] [Google Scholar]
- Jacobsen EA, Ananieva O, Brown ML, Chang Y. Growth, differentiation, and malignant transformation of pre-B cells mediated by inducible activation of v-Abl oncogene. J. Immunol. 2006;176:6831–6838. doi: 10.4049/jimmunol.176.11.6831. [DOI] [PubMed] [Google Scholar]
- Jessel R, Haertel S, Socaciu C, Tykhonova S, Diehl HA. Kinetics of apoptotic markers in exogeneously induced apoptosis of EL4 cells. J. Cell. Mol. Med. 2002;6:82–92. doi: 10.1111/j.1582-4934.2002.tb00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao H, Berrada K, Yang W, Tabrizi M, Platanias LC, Yi T. Direct association with and dephosphorylation of Jak2 kinase by the SH2- domain-containing protein tyrosine phosphatase SHP-1. Mol. Cell. Biol. 1996;16:6985–6992. doi: 10.1128/mcb.16.12.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JQ, Chong ZZ, Maiese K. Akt1 protects against inflammatory microglial activation through maintenance of membrane asymmetry and modulation of cysteine protease activity. J. Neurosci. Res. 2003a;74:37–51. doi: 10.1002/jnr.10740. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Chong ZZ, Maiese K. Critical role for Akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol. Pharmacol. 2003b;64:557–569. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- Kapoor GS, Zhan Y, Johnson GR, O’Rourke DM. Distinct domains in the SHP-2 phosphatase differentially regulate epidermal growth factor receptor/NF-kappaB activation through Gab1 in glioblastoma cells. Mol. Cell. Biol. 2004;24:823–836. doi: 10.1128/MCB.24.2.823-836.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M, Sekiguchi M, Sato K, Kozaki S, Takahashi M. Erythropoietin receptor-mediated inhibition of exocytotic glutamate release confers neuroprotection during chemical ischemia. J. Biol. Chem. 2001;276:39469–39475. doi: 10.1074/jbc.M105832200. [DOI] [PubMed] [Google Scholar]
- Ke Y, Lesperance J, Zhang EE, Bard-Chapeau EA, Oshima RG, Muller WJ, Feng GS. Conditional deletion of Shp2 in the mammary gland leads to impaired lobulo-alveolar outgrowth and attenuated Stat5 activation. J. Biol. Chem. 2006;281:34374–34380. doi: 10.1074/jbc.M607325200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellerer M, Lammers R, Fritsche A, Strack V, Machicao F, Borboni P, Ullrich A, Haring HU. Insulin inhibits leptin receptor signalling in HEK293 cells at the level of janus kinase-2: a potential mechanism for hyperinsulinaemia-associated leptin resistance. Diabetologia. 2001;44:1125–1132. doi: 10.1007/s001250100614. [DOI] [PubMed] [Google Scholar]
- Kim CH, Qu CK, Hangoc G, Cooper S, Anzai N, Feng GS, Broxmeyer HE. Abnormal chemokine-induced responses of immature and mature hematopoietic cells from motheaten mice implicate the protein tyrosine phosphatase SHP-1 in chemokine responses. J. Exp. Med. 1999;190:681–690. doi: 10.1084/jem.190.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Park SJ, Joe EH, Jou I. Raft-mediated Src homology 2 domain-containing proteintyrosine phosphatase 2 (SHP-2) regulation in microglia. J. Biol. Chem. 2006;281:11872–11878. doi: 10.1074/jbc.M511706200. [DOI] [PubMed] [Google Scholar]
- Klingmuller U, Lorenz U, Cantley LC, Neel BG, Lodish HF. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995;80:729–738. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- Koyama R, Ikegaya Y. Mossy fiber sprouting as a potential herapeutic target for epilepsy. Curr. Neurovasc. Res. 2004;1:3–10. doi: 10.2174/1567202043480242. [DOI] [PubMed] [Google Scholar]
- Krotz F, Engelbrecht B, Buerkle MA, Bassermann F, Bridell H, Gloe T, Duyster J, Pohl U, Sohn HY. The tyrosine phosphatase, SHP-1, is a negative regulator of endothelial superoxide formation. J. Am. Coll. Cardiol. 2005;45:1700–1706. doi: 10.1016/j.jacc.2005.02.039. [DOI] [PubMed] [Google Scholar]
- Kwon M, Ling Y, Maile LA, Badley-Clark J, Clemmons DR. Recruitment of the tyrosine phosphatase Src homology 2 domain tyrosine phosphatase-2 to the p85 subunit of phosphatidylinositol-3 (PI-3) kinase is required for insulin-like growth factor-I-dependent PI-3 kinase activation in smooth muscle cells. Endocrinology. 2006;147:1458–1465. doi: 10.1210/en.2005-1115. [DOI] [PubMed] [Google Scholar]
- Laramee M, Chabot C, Cloutier M, Stenne R, Holgado-Madruga M, Wong AJ, Royal I. The scaffolding adapter Gab1 mediates VEGF signaling and is required for endothelial cell migration and capillary formation. J. Biol. Chem. 2007;282:7758–7769. doi: 10.1074/jbc.M611327200. [DOI] [PubMed] [Google Scholar]
- Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J. Biol. Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Erythropoietin on a tightrope: Balancing neuronal and vascular protection between intrinsic and extrinsic pathways. Neurosignals. 2004;13:265–289. doi: 10.1159/000081963. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Vital elements of the wnt-frizzled signaling pathway in the nervous system. Curr. Neurovasc. Res. 2005;2:331–340. doi: 10.2174/156720205774322557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Cell Life Versus Cell Longevity: The Mysteries Surrounding the NAD(+) Precursor Nicotinamide. Curr. Med. Chem. 2006a;13:883–895. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin, and nuclear factor-kappaB. Curr. Neurovasc. Res. 2006b;3:187–201. doi: 10.2174/156720206778018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol. Histopathol. 2006c;21:103–124. doi: 10.14670/hh-21.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SH, Chong ZZ, Maiese K. Nicotinamide: A nutritional supplement that provides protection against neuronal and vascular injury. J. Med. Food. 2001;4:27–38. doi: 10.1089/10966200152053686. [DOI] [PubMed] [Google Scholar]
- Lin SH, Vincent A, Shaw T, Maynard KI, Maiese K. Prevention of nitric oxide-induced neuronal injury through the modulation of independent pathways of programmed cell death. J. Cereb. Blood Flow Metab. 2000;20:1380–1391. doi: 10.1097/00004647-200009000-00013. [DOI] [PubMed] [Google Scholar]
- Liu D, Martino G, Thangaraju M, Sharma M, Halwani F, Shen SH, Patel YC, Srikant CB. Caspase-8-mediated intracellular acidification precedes mitochondrial dysfunction in somatostatin-induced apoptosis. J. Biol. Chem. 2000;275:9244–9250. doi: 10.1074/jbc.275.13.9244. [DOI] [PubMed] [Google Scholar]
- Lu Y, Yu Q, Liu JH, Zhang J, Wang H, Koul D, McMurray JS, Fang X, Yung WK, Siminovitch KA, Mills GB. Src family protein-tyrosine kinases alter the function of PTEN to regulate phosphatidylinositol 3-kinase/AKT cascades. J. Biol. Chem. 2003;278:40057–40066. doi: 10.1074/jbc.M303621200. [DOI] [PubMed] [Google Scholar]
- Lurie DI, Solca F, Fischer EH, Rubel EW. Tyrosine phosphatase SHP-1 immunoreactivity increases in a subset of astrocytes following deafferentation of the chicken auditory brainstem. J. Comp. Neurol. 2000;421:199–214. [PubMed] [Google Scholar]
- Lyons BL, Smith RS, Hurd RE, Hawes NL, Burzenski LM, Nusinowitz S, Hasham MG, Chang B, Shultz LD. Deficiency of SHP-1 protein-tyrosine phosphatase in “viable motheaten” mice results in retinal degeneration. Invest. Ophthalmol. Vis. Sci. 2006;47:1201–1209. doi: 10.1167/iovs.05-1161. [DOI] [PubMed] [Google Scholar]
- Maas M, Wang R, Paddock C, Kotamraju S, Kalyanaraman B, Newman PJ, Newman DK. Reactive oxygen species induce reversible PECAM-1 tyrosine phosphorylation and SHP-2 binding. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2336–H2344. doi: 10.1152/ajpheart.00509.2003. [DOI] [PubMed] [Google Scholar]
- Maiese K. Organic brain disease. In: Ramachandran VS, editor. Encyclopedia of the human brain. 1st Edition. Elsevier Science; 2002. pp. 509–527. [Google Scholar]
- Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol. Sci. 2003;24:228–232. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ. Insights into oxidative stress and potential novel therapeutic targets for Alzheimer disease. Restor. Neurol. Neurosci. 2004;22:87–104. [PubMed] [Google Scholar]
- Maiese K, Vincent AM. Critical temporal modulation of neuronal programmed cell injury. Cell. Mol. Neurobiol. 2000a;20:383–400. doi: 10.1023/A:1007070311203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Vincent AM. Membrane asymmetry and DNA degradation: functionally distinct determinants of neuronal programmed cell death. J. Neurosci. Res. 2000b;59:568–580. doi: 10.1002/(SICI)1097-4547(20000215)59:4<568::AID-JNR13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Maiese K, Ahmad I, TenBroeke M, Gallant J. Metabotropic glutamate receptor subtypes independently modulate neuronal intracellular calcium. J. Neurosci. Res. 1999;55:472–485. doi: 10.1002/(SICI)1097-4547(19990215)55:4<472::AID-JNR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol. Sci. 2004;25:577–583. doi: 10.1016/j.tips.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Li F. Driving cellular plasticity and survival through the signal transduction pathways of metabotropic glutamate receptors. Curr. Neurovasc. Res. 2005a;2:425–446. doi: 10.2174/156720205774962692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. Erythropoietin and cancer. JAMA. 2005b;293:1858–1859. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005c;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr. Neurovasc. Res. 2007;4:63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat M, Marin-Teva JL, Cheret C. Phagocytosis in the developing CNS: more than clearing the corpses. Curr. Opin. Neurobiol. 2005;15:101–107. doi: 10.1016/j.conb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Mari C, Karabiyikoglu M, Goris ML, Tait JF, Yenari MA, Blankenberg FG. Detection of focal hypoxic-ischemic injury and neuronal stress in a rodent model of unilateral MCA occlusion/reperfusion using radiolabeled annexin V. Eur. J. Nucl. Med. Mol. Imaging. 2004;31:733–739. doi: 10.1007/s00259-004-1473-5. [DOI] [PubMed] [Google Scholar]
- Marrero MB, Venema VJ, Ju H, Eaton DC, Venema RC. Regulation of angiotensin II-induced JAK2 tyrosine phosphorylation: roles of SHP-1 and SHP-2. Am. J. Physiol. 1998;275:C1216–C1223. doi: 10.1152/ajpcell.1998.275.5.C1216. [DOI] [PubMed] [Google Scholar]
- Marsh HN, Dubreuil CI, Quevedo C, Lee A, Majdan M, Walsh GS, Hausdorff S, Said FA, Zoueva O, Kozlowski M, Siminovitch K, Neel BG, Miller FD, Kaplan DR. SHP-1 negatively regulates neuronal survival by functioning as a TrkA phosphatase. J. Cell. Biol. 2003;163:999–1010. doi: 10.1083/jcb.200309036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa PT, Wu C. The role of protein tyrosine phosphatase SHP-1 in the regulation of IFN-gamma signaling in neural cells. J. Immunol. 1996;157:5139–5144. [PubMed] [Google Scholar]
- Massa PT, Wu C. Increased inducible activation of NF-kappaB and responsive genes in astrocytes deficient in the protein tyrosine phosphatase SHP-1. J. Interferon Cytokine Res. 1998;18:499–507. doi: 10.1089/jir.1998.18.499. [DOI] [PubMed] [Google Scholar]
- Massa PT, Saha S, Wu C, Jarosinski KW. Expression and function of the protein tyrosine phosphatase SHP-1 in oligodendrocytes. Glia. 2000;29:376–385. [PubMed] [Google Scholar]
- Massa PT, Wu C, Fecenko-Tacka K. Dysmyelination and reduced myelin basic protein gene expression by oligodendrocytes of SHP-1-deficient mice. J. Neurosci. Res. 2004;77:15–25. doi: 10.1002/jnr.20155. [DOI] [PubMed] [Google Scholar]
- Matsubara H, Shibasaki Y, Okigaki M, Mori Y, Masaki H, Kosaki A, Tsutsumi Y, Uchiyama Y, Fujiyama S, Nose A, Iba O, Tateishi E, Hasegawa T, Horiuchi M, Nahmias C, Iwasaka T. Effect of angiotensin II type 2 receptor on tyrosine kinase Pyk2 and c-Jun NH2-terminal kinase via SHP-1 tyrosine phosphatase activity: evidence from vascular-targeted transgenic mice of AT2 receptor. Biochem. Biophys. Res. Commun. 2001;282:1085–1091. doi: 10.1006/bbrc.2001.4695. [DOI] [PubMed] [Google Scholar]
- Mattoon DR, Lamothe B, Lax I, Schlessinger J. The docking protein Gab1 is the primary mediator of EGF-stimulated activation of the PI-3K/Akt cell survival pathway. BMC Biol. 2004;2:24. doi: 10.1186/1741-7007-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn G, Hollborn M, Schliebs R. Induction of cytokines in glial cells surrounding cortical beta-amyloid plaques in transgenic Tg2576 mice with Alzheimer pathology. Int. J. Dev. Neurosci. 2000;18:423–431. doi: 10.1016/s0736-5748(00)00012-5. [DOI] [PubMed] [Google Scholar]
- Mena-Duran AV, Togo SH, Bazhenova L, Cervera J, Bethel K, Senent ML, Nieva J, Sanz GF, Sanz MA, Saven A, Mustelin T. SHP1 expression in bone marrow biopsies of myelodysplastic syndrome patients: a new prognostic factor. Br. J. Haematol. 2005;129:791–794. doi: 10.1111/j.1365-2141.2005.05516.x. [DOI] [PubMed] [Google Scholar]
- Menon MP, Fang J, Wojchowski DM. Core erythropoietin receptor signals for late erythroblast development. Blood. 2006a;107:2662–2672. doi: 10.1182/blood-2005-02-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon MP, Karur V, Bogacheva O, Bogachev O, Cuetara B, Wojchowski DM. Signals for stress erythropoiesis are integrated via an erythropoietin receptor-phosphotyrosine-343-Stat5 axis. J. Clin. Invest. 2006b;116:683–694. doi: 10.1172/JCI25227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Miura T, Yano T, Takahashi A, Sakamoto J, Tanno M, Kobayashi H, Ikeda Y, Nishihara M, Naitoh K, Ohori K, Shimamoto K. Alteration in erythropoietin-induced cardioprotective signaling by postinfarct ventricular remodeling. J. Pharmacol. Exp. Ther. 2006;317:68–75. doi: 10.1124/jpet.105.095745. [DOI] [PubMed] [Google Scholar]
- Milarski KL, Saltiel AR. Expression of catalytically inactive Syp phosphatase in 3T3 cells blocks stimulation of mitogen-activated protein kinase by insulin. J. Biol. Chem. 1994;269:21239–21243. [PubMed] [Google Scholar]
- Minoo P, Zadeh MM, Rottapel R, Lebrun JJ, Ali S. A novel SHP-1/Grb2-dependent mechanism of negative regulation of cytokine-receptor signaling: contribution of SHP-1 C-terminal tyrosines in cytokine signaling. Blood. 2004;103:1398–1407. doi: 10.1182/blood-2003-07-2617. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Tagawa Y, Watanabe N, Ogimoto M, Yakura H. SLP-76 is recruited to CD22 and dephosphorylated by SHP-1, thereby regulating B cell receptor-induced c-Jun N-terminal kinase activation. Eur. J. Immunol. 2005;35:644–654. doi: 10.1002/eji.200425465. [DOI] [PubMed] [Google Scholar]
- Motohashi T, Nakamura Y, Osawa M, Hiroyama T, Iwama A, Shibuya A, Nakauchi H. Increased cell surface expression of C-terminal truncated erythropoietin receptors in polycythemia. Eur. J. Haematol. 2001;67:88–93. doi: 10.1034/j.1600-0609.2001.t01-1-00446.x. [DOI] [PubMed] [Google Scholar]
- Nakagami Y, Nishimura S, Murasugi T, Kubo T, Kaneko I, Meguro M, Marumoto S, Kogen H, Koyama K, Oda T. A novel compound RS-0466 reverses beta-amyloid-induced cytotoxicity through the Akt signaling pathway in vitro. Eur. J. Pharmacol. 2002;457:11–17. doi: 10.1016/s0014-2999(02)02657-2. [DOI] [PubMed] [Google Scholar]
- Nakata S, Matsumura I, Tanaka H, Ezoe S, Satoh Y, Ishikawa J, Era T, Kanakura Y. NF-kappaB family proteins participate in multiple steps of hematopoiesis through elimination of reactive oxygen species. J. Biol. Chem. 2004;279:55578–55586. doi: 10.1074/jbc.M408238200. [DOI] [PubMed] [Google Scholar]
- Nakatani K, Sakaue H, Thompson DA, Weigel RJ, Roth RA. Identification of a human Akt3 (protein kinase B gamma) which contains the regulatory serine phosphorylation site. Biochem. Biophys. Res. Commun. 1999;257:906–910. doi: 10.1006/bbrc.1999.0559. [DOI] [PubMed] [Google Scholar]
- Nishihara M, Miura T, Miki T, Sakamoto J, Tanno M, Kobayashi H, Ikeda Y, Ohori K, Takahashi A, Shimamoto K. Erythropoietin affords additional cardioprotection to preconditioned hearts by enhanced phosphorylation of glycogen synthase kinase-3 beta. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H748–H755. doi: 10.1152/ajpheart.00837.2005. [DOI] [PubMed] [Google Scholar]
- Owada Y, Utsunomiya A, Yoshimoto T, Kondo H. Expression of mRNA for Akt, serine-threonine protein kinase, in the brain during development and its transient enhancement following axotomy of hypoglossal nerve. J. Mol. Neurosci. 1997;9:27–33. doi: 10.1007/BF02789392. [DOI] [PubMed] [Google Scholar]
- Paling NR, Welham MJ. Role of the protein tyrosine phosphatase SHP-1 (Src homology phosphatase-1) in the regulation of interleukin-3-induced survival, proliferation and signalling. Biochem. J. 2002;368:885–894. doi: 10.1042/BJ20021054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paling NR, Welham MJ. Tyrosine phosphatase SHP-1 acts at different stages of development to regulate hematopoiesis. Blood. 2005;105:4290–4297. doi: 10.1182/blood-2004-08-3271. [DOI] [PubMed] [Google Scholar]
- Pani G, Kozlowski M, Cambier JC, Mills GB, Siminovitch KA. Identification of the tyrosine phosphatase PTP1C as a B cell antigen receptor-associated protein involved in the regulation of B cell signaling. J. Exp. Med. 1995;181:2077–2084. doi: 10.1084/jem.181.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa CJ, Matsumoto A, Kim J, Riel RU, Pascal LS, Walton GB, Thompson RB, Petrofski JA, Annex BH, Stamler JS, Koch WJ. A novel protective effect of erythropoietin in the infarcted heart. J. Clin. Invest. 2003;112:999–1007. doi: 10.1172/JCI18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazdrak K, Adachi T, Alam R. Src homology 2 protein tyrosine phosphatase (SHPTP2)/Src homology 2 phosphatase 2 (SHP2) tyrosine phosphatase is a positive regulator of the interleukin 5 receptor signal transduction pathways leading to the prolongation of eosinophil survival. J. Exp. Med. 1997;186:561–568. doi: 10.1084/jem.186.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluskota E, Chen Y, D’Souza SE. Src homology domain 2-containing tyrosine phosphatase 2 associates with intercellular adhesion molecule 1 to regulate cell survival. J. Biol. Chem. 2000;275:30029–30036. doi: 10.1074/jbc.M000240200. [DOI] [PubMed] [Google Scholar]
- Qu CK, Feng GS. Shp-2 has a positive regulatory role in ES cell differentiation and proliferation. Oncogene. 1998;17:433–439. doi: 10.1038/sj.onc.1201920. [DOI] [PubMed] [Google Scholar]
- Rakesh K, Agrawal DK. Controlling cytokine signaling by constitutive inhibitors. Biochem. Pharmacol. 2005;70:649–657. doi: 10.1016/j.bcp.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Ruhul Amin AR, Oo ML, Senga T, Suzuki N, Feng GS, Hamaguchi M. SH2 domain containing protein tyrosine phosphatase 2 regulates concanavalin A-dependent secretion and activation of matrix metalloproteinase 2 via the extracellular signal-regulated kinase and p38 pathways. Cancer Res. 2003;63:6334–6339. [PubMed] [Google Scholar]
- Rusanescu G, Yang W, Bai A, Neel BG, Feig LA. Tyrosine phosphatase SHP-2 is a mediator of activity-dependent neuronal excitotoxicity. EMBO J. 2005;24:305–314. doi: 10.1038/sj.emboj.7600522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sae-Ung N, Matsushima T, Choi I, Abe Y, Winichagoon P, Fucharoen S, Nawata H, Muta K. Role of NF-kappa B in regulation of apoptosis of erythroid progenitor cells. Eur. J. Haematol. 2005;74:315–323. doi: 10.1111/j.1600-0609.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- Sankarapandi S, Zweier JL, Mukherjee G, Quinn MT, Huso DL. Measurement and characterization of superoxide generation in microglial cells: evidence for an NADPH oxidase-dependent pathway. Arch. Biochem. Biophys. 1998;353:312–321. doi: 10.1006/abbi.1998.0658. [DOI] [PubMed] [Google Scholar]
- Sanz O, Acarin L, Gonzalez B, Castellano B. NF-kappaB and IkappaBalpha expression following traumatic brain injury to the immature rat brain. J. Neurosci. Res. 2002;67:772–780. doi: 10.1002/jnr.10140. [DOI] [PubMed] [Google Scholar]
- Schaapveld R, Wieringa B, Hendriks W. Receptor-like protein tyrosine phosphatases: alike and yet so different. Mol. Biol. Rep. 1997;24:247–262. doi: 10.1023/a:1006870016238. [DOI] [PubMed] [Google Scholar]
- Seki N, Hashimoto N, Suzuki Y, Mori S, Amano K, Saito Y. Role of SRC homology 2-containing tyrosine phosphatase 2 on proliferation of rat smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2002;22:1081–1085. doi: 10.1161/01.atv.0000022878.37277.ec. [DOI] [PubMed] [Google Scholar]
- Servidei T, Bhide PG, Huang Z, Moskowitz MA, Harsh G, Reeves SA. The protein tyrosine phosphatase SHP-2 is expressed in glial and neuronal progenitor cells, postmitotic neurons and reactive astrocytes. Neuroscience. 1998;82:529–543. doi: 10.1016/s0306-4522(97)00292-3. [DOI] [PubMed] [Google Scholar]
- Sharples EJ, Thiemermann C, Yaqoob MM. Mechanisms of disease: Cell death in acute renal failure and emerging evidence for a protective role of erythropoietin. Nat. Clin. Pract. Nephrol. 2005;1:87–97. doi: 10.1038/ncpneph0042. [DOI] [PubMed] [Google Scholar]
- Shi ZQ, Lu W, Feng GS. The Shp-2 tyrosine phosphatase has opposite effects in mediating the activation of extracellular signal-regulated and c-Jun NH2-terminal mitogen-activated protein kinases. J. Biol. Chem. 1998;273:4904–4908. doi: 10.1074/jbc.273.9.4904. [DOI] [PubMed] [Google Scholar]
- Siu AW, To CH. Nitric oxide and hydroxyl radical-induced retinal lipid peroxidation in vitro. Clin. Exp. Optom. 2002;85:378–382. [PubMed] [Google Scholar]
- Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc. Natl. Acad. Sci. USA. 1987;84:5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal SP, Huebner K, Croce CM, Parsa NZ, Testa JR. The AKT1 proto-oncogene maps to human chromosome 14, band q32. Genomics. 1988;2:96–98. doi: 10.1016/0888-7543(88)90114-0. [DOI] [PubMed] [Google Scholar]
- Stoker AW. Protein tyrosine phosphatases and signalling. J. Endocrinol. 2005;185:19–33. doi: 10.1677/joe.1.06069. [DOI] [PubMed] [Google Scholar]
- Sugano M, Tsuchida K, Hata T, Makino N. RNA interference targeting SHP-1 attenuates myocardial infarction in rats. FASEB J. 2005;19:2054–2056. doi: 10.1096/fj.05-4020fje. [DOI] [PubMed] [Google Scholar]
- Sun XM, Cohen GM. Mg(2+)-dependent cleavage of DNA into kilobase pair fragments is responsible for the initial degradation of DNA in apoptosis. J. Biol. Chem. 1994;269:14857–14860. [PubMed] [Google Scholar]
- Suzuki T, Matozaki T, Mizoguchi A, Kasuga M. Localization and subcellular distribution of SH-PTP2, a protein-tyrosine phosphatase with Src homology-2 domains, in rat brain. Biochem. Biophys. Res. Commun. 1995;211:950–959. doi: 10.1006/bbrc.1995.1904. [DOI] [PubMed] [Google Scholar]
- Takai S, Yamada M, Araki T, Koshimizu H, Nawa H, Hatanaka H. Shp-2 positively regulates brain-derived neurotrophic factor-promoted survival of cultured ventral mesencephalic dopaminergic neurons through a brain immunoglobulin-like molecule with tyrosine-based activation motifs/Shp substrate-1. J. Neurochem. 2002;82:353–364. doi: 10.1046/j.1471-4159.2002.00960.x. [DOI] [PubMed] [Google Scholar]
- Torriglia A, Chaudun E, Courtois Y, Counis MF. On the use of Zn2+ to discriminate endonucleases activated during apoptosis. Biochimie. 1997;79:435–438. doi: 10.1016/s0300-9084(97)86153-6. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44:248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- Um M, Lodish HF. Antiapoptotic Effects of Erythropoietin in Differentiated Neuroblastoma SH-SY5Y Cells Require Activation of Both the STAT5 and AKT Signaling Pathways. J. Biol. Chem. 2006;281:5648–5656. doi: 10.1074/jbc.M510943200. [DOI] [PubMed] [Google Scholar]
- Valencia AM, Oliva JL, Bodega G, Chiloeches A, Lopez-Ruiz P, Prieto JC, Susini C, Colas B. Identification of a protein-tyrosine phosphatase (SHP1) different from that associated with acid phosphatase in rat prostate. FEBS Lett. 1997;406:42–48. doi: 10.1016/s0014-5793(97)00235-4. [DOI] [PubMed] [Google Scholar]
- Vantler M, Huntgeburth M, Caglayan E, Ten Freyhaus H, Schnabel P, Rosenkranz S. PI3-kinase/Akt-dependent antiapoptotic signaling by the PDGF alpha receptor is negatively regulated by Src family kinases. FEBS Lett. 2006;580:6769–6776. doi: 10.1016/j.febslet.2006.11.034. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Maiese K. Nitric oxide induction of neuronal endonuclease activity in programmed cell death. Exp. Cell. Res. 1999;246:290–300. doi: 10.1006/excr.1998.4282. [DOI] [PubMed] [Google Scholar]
- Vincent AM, TenBroeke M, Maiese K. Metabotropic glutamate receptors prevent programmed cell death through the modulation of neuronal endonuclease activity and intracellular pH. Exp. Neurol. 1999;155:79–94. doi: 10.1006/exnr.1998.6966. [DOI] [PubMed] [Google Scholar]
- Wadley GD, Konstantopoulos N, Macaulay L, Howlett K, Garnham A, Hargreaves M, Cameron-Smith D. Increased insulin-stimulated Akt pSer473 and cytosolic SHP2 protein abundance in human skeletal muscle following acute exercise and short-term training. J. Appl. Physiol. 2007;102:1624–1631. doi: 10.1152/japplphysiol.00821.2006. [DOI] [PubMed] [Google Scholar]
- Wang H, MacNaughton WK. Overexpressed beta-catenin blocks nitric oxide-induced apoptosis in colonic cancer cells. Cancer Res. 2005;65:8604–8607. doi: 10.1158/0008-5472.CAN-05-1169. [DOI] [PubMed] [Google Scholar]
- Wang JF, Zhang X, Groopman JE. Activation of vascular endothelial growth factor receptor-3 and its downstream signaling promote cell survival under oxidative stress. J. Biol. Chem. 2004;279:27088–27097. doi: 10.1074/jbc.M314015200. [DOI] [PubMed] [Google Scholar]
- Wang JY, Shum AY, Ho YJ. Oxidative neurotoxicity in rat cerebral cortex neurons: synergistic effects of H2O2 and NO on apoptosis involving activation of p38 mitogen-activated protein kinase and caspase-3. J. Neurosci. Res. 2003;72:508–519. doi: 10.1002/jnr.10597. [DOI] [PubMed] [Google Scholar]
- Wang N, Li Z, Ding R, Frank GD, Senbonmatsu T, Landon EJ, Inagami T, Zhao ZJ. Antagonism or synergism. Role of tyrosine phosphatases SHP-1 and SHP-2 in growth factor signaling. J. Biol. Chem. 2006;281:21878–21883. doi: 10.1074/jbc.M605018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JT. Calcium homeostasis following traumatic neuronal injury. Curr. Neurovasc. Res. 2004;1:151–171. doi: 10.2174/1567202043480134. [DOI] [PubMed] [Google Scholar]
- Wheadon H, Edmead C, Welham MJ. Regulation of interleukin-3-induced substrate phosphorylation and cell survival by SHP-2 (Src-homology protein tyrosine phosphatase 2) Biochem. J. 2003;376:147–157. doi: 10.1042/BJ20031160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DW, Tartaglia LA. Leptin and OB-R: body weight regulation by a cytokine receptor. Cytokine Growth Factor Rev. 1996;7:303–309. doi: 10.1016/s1359-6101(96)00040-8. [DOI] [PubMed] [Google Scholar]
- Wickrema A, Chen F, Namin F, Yi T, Ahmad S, Uddin S, Chen YH, Feldman L, Stock W, Hoffman R, Platanias LC. Defective expression of the SHP-1 phosphatase in polycythemia vera. Exp. Hematol. 1999a;27:1124–1132. doi: 10.1016/s0301-472x(99)00043-0. [DOI] [PubMed] [Google Scholar]
- Wickrema A, Uddin S, Sharma A, Chen F, Alsayed Y, Ahmad S, Sawyer ST, Krystal G, Yi T, Nishada K, Hibi M, Hirano T, Platanias LC. Engagement of Gab1 and Gab2 in erythropoietin signaling. J. Biol. Chem. 1999b;274:24469–24474. doi: 10.1074/jbc.274.35.24469. [DOI] [PubMed] [Google Scholar]
- Wilks AF, Harpur AG, Kurban RR, Ralph SJ, Zurcher G, Ziemiecki A. Two novel protein-tyrosine kinases, each with a second phosphotransferase-related catalytic domain, define a new class of protein kinase. Mol. Cell. Biol. 1991;11:2057–2065. doi: 10.1128/mcb.11.4.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DW, Stark KC, Dunnington D, Dillon SB, Yi T, Jones C, Pelus LM. SH2-Containing protein tyrosine phosphatase-1 (SHP-1) association with Jak2 in UT-7/Epo cells. Blood Cells Mol. Dis. 2000;26:15–24. doi: 10.1006/bcmd.2000.0273. [DOI] [PubMed] [Google Scholar]
- Wu L, Iwai M, Li Z, Shiuchi T, Min LJ, Cui TX, Li JM, Okumura M, Nahmias C, Horiuchi M. Regulation of inhibitory protein-kappaB and monocyte chemoattractant protein-1 by angiotensin II type 2 receptor-activated Src homology protein tyrosine phosphatase-1 in fetal vascular smooth muscle cells. Mol. Endocrinol. 2004;18:666–678. doi: 10.1210/me.2003-0053. [DOI] [PubMed] [Google Scholar]
- Xie ZH, Zhang J, Siraganian RP. Positive regulation of c-Jun N-terminal kinase and TNF-alpha production but not histamine release by SHP-1 in RBL-2H3 mast cells. J. Immunol. 2000;164:1521–1528. doi: 10.4049/jimmunol.164.3.1521. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Maruyama W, Kato Y, Yi H, Shamoto-Nagai M, Tanaka M, Sato Y, Naoi M. Selective nitration of mitochondrial complex I by peroxynitrite: involvement in mitochondria dysfunction and cell death of dopaminergic SH-SY5Y cells. J. Neural Transm. 2002;109:1–13. doi: 10.1007/s702-002-8232-1. [DOI] [PubMed] [Google Scholar]
- Yamao T, Noguchi T, Takeuchi O, Nishiyama U, Morita H, Hagiwara T, Akahori H, Kato T, Inagaki K, Okazawa H, Hayashi Y, Matozaki T, Takeda K, Akira S, Kasuga M. Negative regulation of platelet clearance and of the macrophage phagocytic response by the transmembrane glycoprotein SHPS-1. J. Biol. Chem. 2002;277:39833–39839. doi: 10.1074/jbc.M203287200. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Milarski KL, Saltiel AR, Pessin JE. Protein-tyrosine-phosphatase SHPTP2 is a required positive effector for insulin downstream signaling. Proc. Natl. Acad. Sci. USA. 1995;92:664–668. doi: 10.1073/pnas.92.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Klaman LD, Chen B, Araki T, Harada H, Thomas SM, George EL, Neel BG. An Shp2/SFK/Ras/Erk signaling pathway controls trophoblast stem cell survival. Dev. Cell. 2006;10:317–327. doi: 10.1016/j.devcel.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Yi T, Mui AL, Krystal G, Ihle JN. Hematopoietic cell phosphatase associates with the interleukin-3 (IL-3) receptor beta chain and down-regulates IL-3-induced tyrosine phosphorylation and mitogenesis. Mol. Cell. Biol. 1993;13:7577–7586. doi: 10.1128/mcb.13.12.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi T, Zhang J, Miura O, Ihle JN. Hematopoietic cell phosphatase associates with erythropoietin (Epo) receptor after Epo-induced receptor tyrosine phosphorylation: identification of potential binding sites. Blood. 1995;85:87–95. [PubMed] [Google Scholar]
- Yi TL, Cleveland JL, Ihle JN. Protein tyrosine phosphatase containing SH2 domains: characterization, preferential expression in hematopoietic cells, and localization to human chromosome 12p12-p13. Mol. Cell. Biol. 1992;12:836–846. doi: 10.1128/mcb.12.2.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M, Zhao Z. Positive effects of SH2 domain-containing tyrosine phosphatase SHP-1 on epidermal growth factor- and interferon-gamma-stimulated activation of STAT transcription factors in HeLa cells. J. Biol. Chem. 1997;272:23376–23381. doi: 10.1074/jbc.272.37.23376. [DOI] [PubMed] [Google Scholar]
- You M, Flick LM, Yu D, Feng GS. Modulation of the nuclear factor kappa B pathway by Shp-2 tyrosine phosphatase in mediating the induction of interleukin (IL)-6 by IL-1 or tumor necrosis factor. J. Exp. Med. 2001;193:101–110. doi: 10.1084/jem.193.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M, Yu DH, Feng GS. Shp-2 tyrosine phosphatase functions as a negative regulator of the interferon-stimulated Jak/STAT pathway. Mol. Cell. Biol. 1999;19:2416–2424. doi: 10.1128/mcb.19.3.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CL, Jin YJ, Burakoff SJ. Cytosolic tyrosine dephosphorylation of STAT5. Potential role of SHP-2 in STAT5 regulation. J. Biol. Chem. 2000;275:599–604. doi: 10.1074/jbc.275.1.599. [DOI] [PubMed] [Google Scholar]
- Yu WM, Hawley TS, Hawley RG, Qu CK. Catalytic-dependent and -independent roles of SHP-2 tyrosine phosphatase in interleukin-3 signaling. Oncogene. 2003;22:5995–6004. doi: 10.1038/sj.onc.1206846. [DOI] [PubMed] [Google Scholar]
- Yu Z, Su L, Hoglinger O, Jaramillo ML, Banville D, Shen SH. SHP-1 associates with both platelet-derived growth factor receptor and the p85 subunit of phosphatidylinositol 3-kinase. J. Biol. Chem. 1998;273:3687–3694. doi: 10.1074/jbc.273.6.3687. [DOI] [PubMed] [Google Scholar]
- Yuan L, Yu WM, Xu M, Qu CK. SHP-2 phosphatase regulates DNA damage-induced apoptosis and G2/M arrest in catalytically dependent and independent manners, respectively. J. Biol. Chem. 2005;280:42701–42706. doi: 10.1074/jbc.M506768200. [DOI] [PubMed] [Google Scholar]
- Zhan Y, O’Rourke DM. SHP-2-dependent mitogen-activated protein kinase activation regulates EGFRvIII but not wildtype epidermal growth factor receptor phosphorylation and glioblastoma cell survival. Cancer Res. 2004;64:8292–8298. doi: 10.1158/0008-5472.CAN-03-3143. [DOI] [PubMed] [Google Scholar]
- Zhang EE, Chapeau E, Hagihara K, Feng GS. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc. Natl. Acad. Sci. USA. 2004;101:16064–16069. doi: 10.1073/pnas.0405041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Somani AK, Watt S, Mills GB, Siminovitch KA. The Src-homology domain 2-bearing protein tyrosine phosphatase-1 inhibits antigen receptor-induced apoptosis of activated peripheral T cells. J. Immunol. 1999;162:6359–6367. [PubMed] [Google Scholar]
- Zhang SQ, Tsiaras WG, Araki T, Wen G, Minichiello L, Klein R, Neel BG. Receptor-specific regulation of phosphatidylinositol 3’-kinase activation by the protein tyrosine phosphatase Shp2. Mol. Cell. Biol. 2002;22:4062–4072. doi: 10.1128/MCB.22.12.4062-4072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou GM, Chan RJ, Shelley WC, Yoder MC. Reduction of shp-2 expression by small interfering RNA reduces murine embryonic stem cell-derived in vitro hematopoietic differentiation. Stem Cells. 2006;24:587–594. doi: 10.1634/stemcells.2005-0272. [DOI] [PubMed] [Google Scholar]
- Zou GM, Hu WY, Wu W. TNF family molecule LIGHT regulates chemokine CCL27 expression on mouse embryonic stem cell-derived dendritic cells through NF-kappaB activation. Cell. Signal. 2007;19:87–92. doi: 10.1016/j.cellsig.2006.05.025. [DOI] [PubMed] [Google Scholar]