Abstract
The purpose of the present investigation was to examine the association between circadian rhythms of cortisol and physical and relational aggression. Morning arrival, pre-lunch, and afternoon pre-departure salivary cortisol were assessed among 418 maltreated and nonmaltreated children (52% maltreated; 49% female) attending a summer day camp. Counselors and peers rated participants' involvement in physically and relationally aggressive behaviors. Results indicated that physical aggression was associated with heightened cortisol following morning arrival and relatively steep declines in cortisol over the day whereas relational aggression was associated with low cortisol following morning arrival and blunted diurnal change in cortisol. Moreover, maltreatment was a significant moderator of this relationship such that aggression was related to greater cortisol dysregulation among nonmaltreated than maltreated children. The findings suggest that physiological correlates of aggression may differ for physical and relational forms of aggression and among maltreated versus nonmaltreated populations.
Keywords: aggression, gender, cortisol, maltreatment
Involvement in aggressive and antisocial behavior is associated with a host of problems for children and adolescents, including rejection by peers, internalizing symptoms, and academic difficulties (e.g., Buhs, Ladd, & Herald, 2006; Crick, 1996; Farmer et al., 2003; Murray-Close, Ostrov, & Crick, 2007). Mounting evidence suggests that a number of biological processes, including resting heart rate, heart rate variability, blood pressure, and skin conductance are implicated in the development of aggressive and antisocial behavior (e.g., Kibler, Prosser, & Ma, 2004; Murray-Close & Crick, 2007; Scarpa, Fikretoglu, & Luscher, 2000; Scarpa & Raine, 1997; Susman, 2006). Researchers have extended studies assessing biological underpinnings of aggression to examine the role of the stress hormone cortisol (e.g., McBurnett, Lahey, Rathouz, & Loeber, 2000). However, existing research investigating the relation between cortisol and aggression is limited in three important ways. First, most work in this area has examined the association between aggression and resting cortisol at one assessment period rather than diurnal change in cortisol. Second, research has focused on forms of aggression more typical of males (i.e., physical aggression) to the exclusion of aggressive behaviors more common among females (i.e., relational aggression; Crick et al., 1999). Third, many studies have failed to examine potential moderators of the association between cortisol and aggression, including gender and maltreatment. The purpose of the present paper is to address these gaps in the extant literature by examining the association between diurnal change in cortisol and both physical and relational aggression. In addition, the moderating roles of child maltreatment and gender in this relation were assessed.
Cortisol is a major human stress hormone that indexes hypothalamic-pituitary-adrenal (HPA) axis activity. Typical diurnal rhythm manifests a peak in cortisol levels half an hour after waking followed by gradual decrease throughout the day. The neural systems responsible for orchestrating stress responses are plastic and one manifestation of this is the accompanied developmental changes in basal HPA activity and cortisol reactivity throughout childhood (Gunnar & Donzella, 2001). In addition, there is an increase in basal cortisol levels from childhood through adolescence in typically developing children (as reviewed by Klimes-Dougan, Hastings, Granger, Usher, & Zahn-Waxler, 2001).
Some researchers have suggested that low basal cortisol will be associated with heightened involvement in aggression and antisocial behavior (e.g., Oosterlaan, Geurts, Knol, & Sergeant, 2005; Shoal, Giancola, & Kirillova, 2003). These predictions are based on two related theories that both suggest that physiological underarousal contributes to the development of antisocial conduct. From a stimulation-seeking theory perspective, chronic underarousal is experienced as an aversive physiological state that predisposes individuals to involvement in antisocial behavior (Coren, 1999; Quay, 1965; Raine, 2002). In effect, behaviors such as aggression or assault may provide underaroused individuals with stimulation that increases their arousal to more comfortable levels. In contrast, fearlessness theory proposes that underarousal reflects low levels of fear (Raine, 2002). Fearlessness, in turn, is associated with aggressive or antisocial conduct because it lowers one's inhibitions regarding involvement in behaviors such as fighting (Kindlon et al., 1995; Raine, 2002). In addition, fearlessness may indirectly lead to aggressive conduct through interference with conscience development (Frick & Morris, 2004). Although both stimulation-seeking and fearlessness theory emerged from studies assessing autonomic indices of arousal such as heart rate and skin conductance (see Scarpa & Raine, 1997), they have recently been applied to studies exploring the relation between cortisol and aggression (Klimes-Dougan et al., 2001; Loney et al., 2006; Oosterlaan et al., 2005; Shoal et al., 2003; van de Wiel, van Goozen, Matthys, Snoek, & van Engeland, 2004). Moreover, consistent with both perspectives, preliminary work suggests that low cortisol is associated with elevated sensation-seeking (Rosenblitt, Soler, Johnson, & Quadagno, 2002) and impaired fear reactivity (Kagan, Reznick, & Snidman, 1988).
A number of studies have provided support for the hypothesis that low resting cortisol is related to heightened aggression. In animal studies, both low concentrations and low variability of corticosterone have been implicated in aggression (Halász, Liposits, Kruk, & Haller, 2002; Haller, Halasz, Mikics, & Kruk, 2004; Haller, van de Schraaf, & Kruk, 2001). Available human studies have found that low levels of cortisol are related to antisocial and aggressive behavior in adults (Virkkunen, 1985; Woodman et al., 1978), adolescents (Pajer, Gardner, Rubin, Perel, & Neal, 2001; Shoal et al., 2003), and children (McBurnett et al., 2000; van Goozen et al., 1998). Moreover, low basal cortisol appears to be associated with aggressive behaviors in particular rather than problem behaviors such as delinquency (McBurnett et al., 2000; Oosterlaan et al., 2005; Pajer et al., 2001), and longitudinal work suggests that low cortisol predicts future involvement in aggression (Shoal et al., 2003). However, several investigations did not find such associations between cortisol and aggression (Azar et al., 2004; Cashdan, 2003; Klimes-Dougan et al., 2001; Scerbo & Kolko, 1994; Schulz, Halperin, Newcorn, Sharma, & Gabriel, 1997), perhaps reflecting limitations and methodological differences across studies.
An important limitation of research assessing the relation between basal cortisol and aggression is that most studies fail to examine the association between aggression and change in cortisol over the day (Popma et al., 2007; van Goozen, Fairchild, Snoek, & Harold, 2007). In addition to differences in basal cortisol levels at one assessment period, aggressive children and adolescents may exhibit different patterns of change in cortisol when compared to their nonaggressive peers. For example, aggressive children may exhibit low cortisol in the morning, blunted change in cortisol over the day, or both. In other words, cortisol dysregulation among aggressive children may reflect a number of distinct factors, and limited research is currently available to address which aspects of the circadian rhythm are atypical among aggressive children. In addition, cortisol may be more strongly related to aggression during specific periods of the diurnal rhythm, and inconsistent findings across studies might reflect differences in sampling times (van Goozen et al., 2007). Thus, the first goal of the present paper was to assess the association between aggression and trajectories of cortisol over the day.
In fact, research has provided evidence that dysregulation of the diurnal rhythm of cortisol is associated with a number of problems; for example, a flattened cortisol circadian rhythm has been found in individuals with high levels of contextual risk (e.g., child physical abuse, low SES), internalizing symptoms, and stress-related physical disorders (e.g., fibromyalgia; Akil et al., 2003; Gunnar & Vazquez, 2001; Klimes-Dougan et al., 2001). To date, little research has examined the association between aggression and diurnal change in cortisol; however, preliminary work suggests that this is an important avenue for additional research. For example, one study found that children with psychiatric symptoms (including conduct problems) exhibited elevated evening cortisol but similar morning and afternoon levels when compared to their peers (Gustafsson, Gustafsson, & Nelson, 2006). In another study, nonmaltreated boys with externalizing problems exhibited a relatively flat change in cortisol from morning to afternoon (Cicchetti & Rogosch, 2001b). Finally, in a study of preschool and school-aged children in full-day group care settings, aggressive children exhibited greater increases in cortisol over the day than their nonaggressive peers (Dettling, Gunnar, & Donzella, 1999). Based on evidence suggesting that aggression is associated with low cortisol (McBurnett et al., 2000; van Goozen et al., 1998), we expected that aggression would predict relatively low levels of morning cortisol. However, we also hypothesized that aggression would be associated with dysregulation of diurnal change in cortisol over the day, with aggressive children exhibiting a flattened circadian rhythm.
A second limitation of work in this area is that most studies have focused on forms of aggression typical in males (i.e., physical aggression) to the exclusion of types of aggression common among females (i.e., relational aggression). Whereas physical aggression is defined as behaviors that harm others through damage to one's physical well-being, relational aggression includes behaviors that harm others through damage to relationships or feelings of acceptance, friendship, or group inclusion (e.g., rumor-spreading, giving another child the “silent treatment;” Crick & Grotpeter, 1995; Crick et al., 1999; Tomada & Schneider, 1997; for discussion regarding related constructs such as indirect or social aggression, see Bjorkqvist, Lagerspetz, & Kaukianen, 1992; Galen & Underwood, 1997). Although there is substantial overlap between physical and relational aggression (correlations generally range from .5−.75; e.g., Crick, 1996, 1997; Crick & Grotpeter, 1995), factors analyses have provided evidence that physical and relational aggression are distinct constructs (e.g., Crick & Grotpeter, 1995; Crick, 1996). Furthermore, each form of aggression provides unique information regarding children's adjustment (Crick, 1996; Crick & Grotpeter, 1995). Studies assessing relational aggression in addition to physical aggression have provided evidence that girls are more relationally aggressive than boys (Crick & Grotpeter, 1995; Crick et al., 1999; Hawley, 2003; Ostrov & Keating, 2004; Tomada & Schneider, 1997; Xie, Farmer, & Cairns, 2003; although see Henington, Hughes, Cavell, & Thompson, 1998). Thus, an exclusive focus on physical aggression may lead researchers to overlook troubled girls (Crick & Grotpeter, 1995; Crick & Zahn-Waxler, 2003). To gain a gender-balanced understanding of the association between cortisol activity and aggressive behavior, it is important that studies include measures of relational as well as physical aggression.
Given the theoretical explanations regarding why cortisol is associated with aggressive conduct, there is reason to expect that hypocortisol would be related to relational as well as physical aggression. For example, involvement in rumor-spreading or gossip may, like physically aggressive conduct, serve as a relatively stimulating experience. Individuals who experience an aversive state of chronic underarousal may engage in such behaviors as a means of increasing their physiological activity to more comfortable levels. In addition, reduced levels of fear may facilitate children's relationally aggressive conduct because fearless children may be relatively unconcerned by possible retaliation. To date, little research has examined the association between relational aggression and cortisol. However, preliminary work suggests that relational aggression is associated with a flattened diurnal change in cortisol, particularly among girls (Blades & Susman, 2007). In another study, relational aggression was associated with larger increases in cortisol over the day among young children in group child care than was physical aggression (Dettling et al., 1999). In both studies, then, relational aggression was associated with a failure to exhibit the typical diurnal decrease in cortisol over the day. In the present study, we expected that relational aggression would be associated with a blunted diurnal change in cortisol.
Research examining the association between aggression and neuroendocrine functioning is further complicated by scant attention to potential gender differences in this area. Many studies have only included boys as participants (e.g., McBurnett et al., 2000; van de Wiel et al., 2004), although preliminary work suggests that low cortisol is associated with aggression and antisocial behavior among girls as well as boys (Pajer et al., 2001; Pajer et al., 2006). However, studies that have included girls have often failed to examine whether gender moderates the association between cortisol and aggressive conduct (e.g., Oosterlaan et al., 2005). It is possible that cortisol dysregulation promotes aggressive conduct in both boys and girls, but at-risk children employ aggressive behaviors that are relatively typical of their gender (i.e., physical aggression for boys and relational aggression for girls; Crick & Grotpeter, 1995). Although the findings are mixed (Dettling et al., 1999), some work suggests that blunted diurnal cortisol is more strongly related to externalizing symptoms in boys (Cicchetti & Rogosch, 2001b) and relational aggression in girls (Blades & Susman, 2007). Given the limited research in this area, the second goal of the present paper was to investigate whether the association between cortisol dysregulation and physical and relational aggression varied by gender.
A final limitation of most work is a failure to assess the moderating role of contextual risk, such as child maltreatment, in the association between cortisol dysregulation and aggression. In fact, researchers have recently emphasized the importance of examining the interaction between psychosocial and biological processes in the development and maintenance of aggression (Raine, 2002; Raine, Reynolds, Venables, & Mednick, 1997). Two different hypotheses regarding the moderating role of maltreatment in the relation between aggression and cortisol have been offered. On the one hand, the dysregulation of cortisol among aggressive children may be more pronounced among maltreated children (Cicchetti & Rogosch, 2001a,b). Consistent with the perspective that maltreated children will exhibit a stronger relation between cortisol and psychopathology, some researchers have found an association between lower morning cortisol (Hart, Gunnar, & Cicchetti, 1996) and a blunted decrease in cortisol over the day (Cicchetti & Rogosch, 2001b; Hart et al., 1996) and internalizing symptoms among maltreated children only. In other research, the combination of heightened cortisol reactivity to provocation and experiences of victimization (physical abuse and community violence exposure) were associated with particularly high levels of aggression (Scarpa & Ollendick, 2003; Scarpa et al., 1999; as cited in Raine, 2002).
Alternatively, the association between cortisol dysregulation and aggression may be most pronounced among nonmaltreated children. This hypothesis is consistent with the “social push perspective,” in which biological correlates of aggression will be most influential among individuals from relatively benign backgrounds (Raine, 2002). In effect, biological factors may be more important contributors to involvement in aggressive behaviors among children who do not have a strong social push toward such conduct (Raine & Venables, 1981; Raine, 2002). Consistent with this perspective, a number of studies have demonstrated that psychophysiological factors such as low resting heart rate, low skin conductance, and heart rate variability are more strongly related to aggression and antisocial behavior among individuals who have not been victims of violence (Scarpa et al., 1999, as cited in Raine, 2002) and who are from higher social classes (Raine, Reynolds, Venables, & Mednick, 1997; Raine & Venables, 1981, 1984). Thus, it is possible that diurnal change in cortisol will be most strongly related to aggression in nonmaltreated children.
At present, little research is available to address whether diurnal cortisol will be most strongly associated with aggression among maltreated or nonmaltreated children. Preliminary work assessing the moderating role of contextual risk in the relation between cortisol and aggression has focused on cortisol reactivity rather than basal cortisol levels (e.g., Scarpa & Ollendick, 2003), and findings from this research may not be relevant to studies of basal cortisol levels since the relation between aggression and underarousal may reflect different processes than those involved in the association between aggression and physiological reactivity (Scarpa & Raine, 1997). One study, however, provides evidence in support of the social push perspective; specifically, Cicchetti and Rogosch (2001b) found that, for boys, externalizing symptoms were associated with lower morning cortisol and a blunted decrease in cortisol from morning to afternoon among nonmaltreated but not maltreated boys. It is important to note that all children in this study were high risk (e.g., low SES) and thus had a strong social push toward aggressive conduct. Nonetheless, given research suggesting that maltreatment is an important predictor of aggressive behavior (e.g., Cullerton-Sen et al., under review; Manly, Cicchetti, & Barnett, 1994; Shields & Cicchetti, 2001; Tiesl & Cicchetti, in press), it is possible that the social push toward aggression was particularly strong among the maltreated participants. Thus, even among high-risk samples, physiological correlates of aggression may be stronger among participants with fewer environmental and social risk factors. To further explore the possibility, the third goal of the present study was to examine whether maltreatment moderated the relation between cortisol dysregulation and aggression in a high-risk sample. We expected that the relation between aggression and the circadian rhythm of cortisol would be stronger among nonmaltreated than maltreated children.
In sum, there has been a relative lack of systematic research investigating the biological correlates of specific forms of aggressive behaviors (particularly scarce are studies including non-physical forms of aggression). The current study explored the relations among ambulatory salivary cortisol levels, physical aggression, relational aggression, and the moderating role of gender and maltreatment. Based on prior research indicating an association between aggression and neuroendocrine functioning (e.g., Blades & Susman, 2007; Cicchetti & Rogosch, 2001b; Dettling et al., 1999; McBurnett et al., 2000; van Goozen et al., 1998), we predicted that high levels of both physical and relational aggression in childhood would be associated with lower morning cortisol and blunted change in cortisol over the day compared with low levels of aggression. Moreover, based on the social push perspective (Raine, 2002), we expected that this association would be strongest among nonmaltreated children. Finally, we examined whether gender moderated the association between cortisol dysregulation and physical and relational aggression.
Method
Participants
A total of 418 children from a day camp for low-income, inner-city children participated in the present study. None of the participants had attended the camp in previous years. Participants included 219 maltreated children (91 girls and 128 boys) and 199 nonmaltreated children (109 girls and 90 males). Participants ranged in age from 6 to 12 years of age. Fifty-five percent of participants were African-American, 16.5% were Caucasian, 12% were Latino, 13.6% were biracial, and 2.9% were from other ethnic backgrounds.
Prior to enrolling in the study, mothers of both maltreated and nonmaltreated children provided written consent to allow trained project staff to examine any existing Department of Social Services (DSS) records. All maltreated children in the present study had a documented history of family maltreatment with Child Protection and Preventive Services at the DSS resulting in services through the agency, and the sample was representative of children in families receiving services from DSS. Based on the descriptions of maltreatment in the DSS records, the presence of sexual abuse, physical abuse, neglect, and/or emotional maltreatment in each family was assessed by trained doctoral students in clinical psychology using criteria outlined by Barnett, Manly, and Cicchetti (1993). Maltreated children were then assigned a primary maltreatment subtype according to the hierarchy identified by Manly et al. (1994). This hierarchy reflects the extent to which each subtype of abuse violates social norms, with sexual abuse, physical abuse, neglect, and emotional maltreatment, respectively, considered the most severe to least severe violation of social standards. Thus, for example, children who experienced sexual abuse were classified as sexually abused regardless of whether other forms of abuse were present. In the present study, 17 (8%; 3 females and 14 males) of the maltreated children were classified as emotionally maltreated, 77 (36%; 38 females and 39 males) were classified as neglected, 86 were classified as physically abused (41%; 31 females and 55 males), and 31 were classified as sexually abused (15%; 16 females and 15 males). The DSS information was not complete enough to code the subtype of maltreatment for 8 of the maltreated participants.
Demographically comparable nonmaltreated children were recruited from families receiving Temporary Assistance for Needy Families. DSS records were examined to confirm the absence of a history of maltreatment in these families. Mothers of children in the nonmaltreated group were also interviewed to verify a lack of maltreatment and involvement of DSS. Maltreated and nonmaltreated children did not differ in a number of demographic variables, including age, (M = 9.88, SD = 1.75 versus M = 9.99, SD = 1.85, respectively), t(416) = −.62, ns, total family income (in thousands of dollars; M = 23.52, SD = 14.39 versus M = 24.84, SD = 12.92), t(398) = .95, ns, or percentage of families receiving public assistance (82% versus 80% of families, respectively), χ2(1, n=402) = .35, ns. The marital status of the maltreated and nonmaltreated families also did not differ, χ2(2, n=404) = 1.90, ns. For the maltreatment and nonmaltreated groups, respectively, 34% versus 39% of mothers were single, 48% versus 47% were married or living with a partner, and 18% versus 13% were no longer married. Thus, both the maltreated and nonmaltreated participants were characterized by low family income, high need for public assistance, and high levels of single parenting. In addition, race (Caucasian vs. minority) was not associated with maltreatment, χ2(1, n=418) = 2.38, n.s. However, maltreated and nonmaltreated groups did differ in gender. There were more boys than girls in the maltreated sample (58.4% of maltreated participants vs. 45.2% of nonmaltreated participants were male), χ2(1, n=418) = 7.30, p < .01.
Design and Procedure
In 2003 and 2004, parents were approached and asked if they would be willing to allow their child to participate in a weeklong day camp for inner-city children including recreational and research activities (for details of this setting, see Cicchetti & Manly, 1990). Each day at camp lasted 7 hours, providing a total of 35 hours of interaction between children and camp counselors. During the summer camp, children were assigned to groups of same-sex and same-age peers (M number of group members = 7.57 participants, SD = 1.57). During the summer camp, children completed instruments assessing the social behaviors of the peers in their group. In addition, camp counselors completed measures assessing the behavior of children in their group following each camp session. Finally, during each camp day, children provided cortisol samples following their morning arrival (9 am), pre-lunch (12:30 pm), and afternoon pre-departure (4 pm). Cortisol samples were taken at the same time for each child and at the same times each day of camp.
Measures
Aggression
Children's physically and relationally aggressive behaviors were assessed using a multi-informant approach given the limitations of many single measures of aggressive conduct (Xie, Cairns, & Cairns, 2005). A number of researchers have argued that peer reports provide especially valid measures of relational aggression during childhood and adolescence because peers are more likely than others (e.g., observers) to be privy to relationally aggressive behaviors (e.g., episodes of relational aggression that occur in the bathroom; Bjorkqvist et al., 1992; Crick et al., 1999). In addition, although peer nominations are the most commonly used measure of relational aggression in childhood (Crick et al., 1999), some researchers have argued that peer ratings may be more sensitive in the identification of less obvious aggressors (Bjorkqvist, 2001). Thus, both peer nominations and peer ratings were included in the present study. Finally, counselor reports were included to gain an additional perspective regarding participants' aggressive conduct.
The first measure of children's aggression was an instrument developed in previous research for use with teachers (Crick, 1996). In the present study, this instrument was used with camp counselors. Counselors were provided with statements about physically aggressive (4 items; e.g., “This child hits or kicks peers”), relationally aggressive (5 items; e.g., “When mad at a peer, this child ignores the peer or stops talking to the peer”), and prosocial (4 items, positively-toned filler items) behaviors and asked to rate how true each statement was of the target child on a scale from 1 (never true) to 5 (almost always true). Each participating child was rated by two of the three counselors for their group. Subscales were computed by summing across physical and relational aggression items, respectively. These subscales were then averaged across counselors, yielding overall physical and relational aggression scores. Evidence for the validity of this measure has been demonstrated in previous research (Crick, 1996). Cronbach's alpha demonstrated high reliability of the measures for this sample, with α = .97 for physical aggression and α = .90 for relational aggression.
Second, participants completed a peer nomination procedure. The majority of children in each group agreed to participate in the peer nomination procedure (M = 95%, range = 75% −100%). Each child was asked to nominate one child in their group who fit the description of a physically aggressive child (this child “starts fights. He/she says mean things to other kids, or pushes them, or hits them”) and a relationally aggressive child (this child, “when he/she is mad at someone, will refuse to play or talk to the person, will try to get others not to like the person, will spread rumors or talk behind the person's back”). The number of nominations each child received for each item was summed, standardized within group, and then standardized within the summer camp session (2003 or 2004) to yield relational aggression and physical aggression peer nomination scores.
Third, children completed ratings of their peers' physically and relationally aggressive behaviors. Children were provided with one item depicting a physically aggressive child (“starts fights, says mean things, pushes or hits others”) and one item depicting a relationally aggressive child (“when s/he is mad at someone, refuses to play or talk to the person, will try to get others not to like the person, will spread rumors or talk behind the person's back”) and asked to rate how true each item was of each peer in their group on a scale of 0 (“not true”) to 2 (“very true”). The rating scores received from each peer in the group were averaged to yield an overall peer rating of physical aggression and relational aggression for each child
Composite physical and relational aggression scores were computed for each participant based on their counselor report, peer nomination, and peer ratings of aggression. Relational aggression scores and physical aggression scores from each method were z-scored and then averaged to yield a composite relational aggression score and physical aggression score, respectively. The correlations among informants were all significant and tended to be moderate to large in size for physical aggression (r = .51, p < .001 for counselor and peer nominations; r = .71, p < .001 for counselor reports and peer ratings; r = .67, p < 001 for peer nominations and peer ratings) and for relational aggression (r = .37, p < .001 for counselor and peer nominations; r = .42, p < .001 for counselor reports and peer ratings; r = .53, p < 001 for peer nominations and peer ratings). The internal consistency of the composite aggression scales was acceptable, with α = .71 for relational aggression and α = .84 for physical aggression. Composite aggression scores were used in all analyses.
Salivary Cortisol
Each day of the camp week, participants provided saliva samples following their morning arrival (9 am), pre-lunch (12:30 pm), and afternoon pre-departure (4 pm). Participants had not consumed food or drink for 30 minutes prior to cortisol sampling. In addition, cortisol samples were not taken after exercise or sports activities. Given its potential to influence cortisol activity, caffeine was not served at the camp. At each saliva collection, children were asked to rinse their mouth with water and then chew a piece of Trident original flavor sugarless gum to stimulate saliva production. After chewing the gum, children spit through a plastic straw into a 20 ml plastic vial which was immediately frozen and stored at −40° C. Vials were transferred to Salimetrics Laboratories (State College, PA) on dry ice in weekly batches for assay. Saliva samples then underwent high-sensitive enzyme immunoassay (using kit 510k; Salimetrics, PA). Samples were thawed and 4−5 1 ml aliquots were placed into 1.8 ml cryogenic storage vials and then stored at −80° C. When assayed, samples were thawed to room temperature, centrifuged at 3000 RPM for 15 minutes, and the clear top-phase of the sample was used for assay. These assays yielded scores of daily morning arrival, pre-lunch, and afternoon pre-departure ambulatory cortisol, respectively, in mean micrograms per deciliter (μg/dl). The procedure has a lower limit of sensitivity of 0.007 μg/dl and has a range up to 3.0 μg/dl. Composite ambulatory salivary cortisol levels were computed by averaging all available values across the 5 camp days for the morning arrival, pre-lunch, and afternoon pre-departure, respectively. Cronbach's alphas indicated acceptable internal consistency of morning arrival (α = .77), pre-lunch (α = .66), and afternoon pre-departure (α = .58) cortisol.
Results
Generalized Linear Mixed Models
Descriptive information for study variables are presented in Table 1. Inspection of the data indicated that cortisol was positively skewed at each time period. Thus, generalized linear mixed models (GLMMs) with correlated error terms (McCulloch & Searle, 2001) were chosen as the data analytic technique for examining change in cortisol over the day. GLMMs are an extension of regression models and utilize generalized estimating equation procedures to estimate fixed effects (Liang & Zeger, 1986). GLMMs were preferable to more traditional techniques (e.g., Repeated Measures Analysis of Variance) in the present analyses because they allow for non-normal response data. GLMMs permit examination of mean change over time in the response variable as well as the role of covariates (e.g., aggression, maltreatment, gender) in this change. As such, GLMMs allowed us to investigate diurnal changes in cortisol and to assess the role of physical aggression, relational aggression, maltreatment status, and gender in this change.
Table 1.
Descriptive information regarding study
| Mean | Standard Deviation | Range | |
|---|---|---|---|
| Cortisol (μg/dl) | |||
| Morning Arrival | .20 | .12 | .07−1.29 |
| Pre-Lunch | .13 | .08 | .04−.94 |
| Afternoon Pre-Departure | .11 | .07 | .04−1.14 |
| Relational Aggression | |||
| Counselor Reports | 1.81 | .82 | 1−5 |
| Peer Nominations | .02 | 1.02 | −1.47−2.91 |
| Peer Ratings | .49 | .43 | .00−1.83 |
| Physical Aggression | |||
| Counselor Reports | 1.60 | .93 | 1−5 |
| Peer Nominations | .01 | 1.00 | −1.21−3.01 |
| Peer Ratings | .41 | .47 | .00−1.86 |
GLMM analyses with a Gamma distribution with a log-link for the response variable using the GENMOD module in SAS 9.1 for Windows (SAS Institute Inc., 2001) were conducted in the present study. The Gamma distribution with a log-link was chosen because it approximated the cortisol distribution well (i.e., cortisol was a positively skewed, continuous variable with positive values). In the first GLMM, an unconditional model exploring whether cortisol exhibited linear and/or quadratic change over the course of the day was conducted. A second GLMM explored whether physical aggression and relational aggression were related to daily changes in cortisol. A third and fourth GLMM were conducted to explore whether the association between aggression (physical and relational) and diurnal cortisol differed for maltreated versus nonmaltreated children and boys versus girls, respectively.
Describing Change in Cortisol over the Day
The first GLMM examined mean change in cortisol over the day. We expected both linear and quadratic change in cortisol, with the highest levels of cortisol following the morning arrival, a steep decline in the early day, and then a leveling off by the afternoon pre-departure. To address this mean change, a GLMM with a Gamma distribution with a log-link was conducted. Identifying the Gamma distribution with a log-link for the response variable in PROC GENMOD results in the specification of the log for this variable during model estimation (see Davis, 2002). Thus, the equation for the unconditional GLMM was:
| (1) |
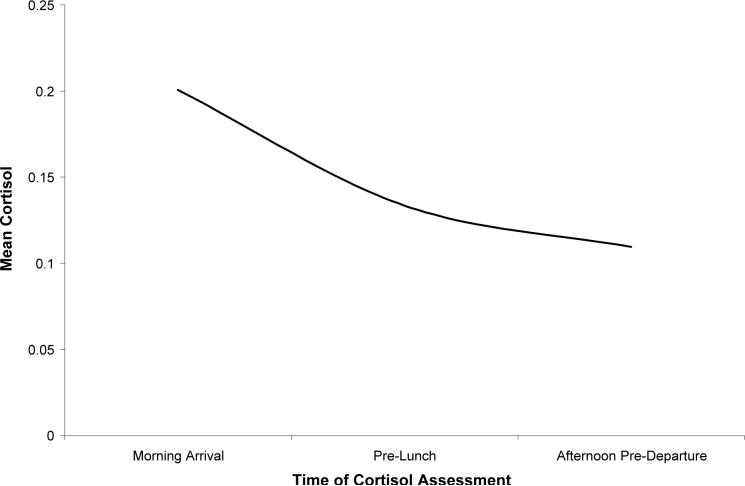
where μj is mean cortisol at the jth time point, γ0 is the intercept (i.e., mean cortisol following the morning arrival), γ1 is the strength of linear change in cortisol over the day, and γ2 is the strength of quadratic change in cortisol over the day. lj represents the linear term (0=morning arrival, 1=pre-lunch, 2=afternoon pre-departure) and qj represents quadratic change (i.e., the linear term squared). The results of this analysis, presented in Table 2, indicated a significant negative linear and positive quadratic trend in mean cortisol over the day (see Figure 1).
Table 2.
Parameter Estimates for Mean Change in Cortisol
| Effect | Parameter | Estimate (SE) | |
|---|---|---|---|
| Intercept | γ0 | Intercept1 | −1.60*** (.03) |
| Linear | γ1 | Linear Change | −.53*** (.05) |
| Quadratic | γ2 | Quadratic Change | .11*** (.02) |
*p < .05 **p < .01
p < .001
Although not pertinent to the hypotheses of the present study, group intercept estimates are reported for completeness.
Figure 1.
Predicted mean cortisol over the day.
Aggression and Cortisol Trajectories
The second GLMM assessed whether aggression was associated with the diurnal rhythm of cortisol and whether this association differed depending on the type of aggression assessed (physical versus relational aggression). Given the overlap between physical and relational aggression (r = .80, p < .001, in the present sample), the analysis was run including both forms of aggression so that the unique association between diurnal cortisol (intercept, linear change, and quadratic change) and each form of aggression could be assessed. In addition, because the potential influence of age on diurnal cortisol, age was included to control for these effects. The following equation was used to estimate the fixed effects:
| (2) |
where μj is mean cortisol at the jth time point, γ0 is the scaling term for the intercept, and γ1, γ2, γ3 indicate intercept differences based on age, physical aggression, and relational aggression, respectively. γ4 is the scaling factor for linear change, with γ5, γ6, and γ7 representing the effect of age, physical aggression, and relational aggression, respectively, on linear change. In addition, γ8 is the scaling factor for quadratic change in cortisol and γ9, γ10, and γ11 indicate the effects of age, physical aggression, and relational aggression, respectively, on quadratic change. Finally, a represents age in years, pagg represents physical aggression, ragg represents relational aggression, lj represents the linear term, and qj represents quadratic change.
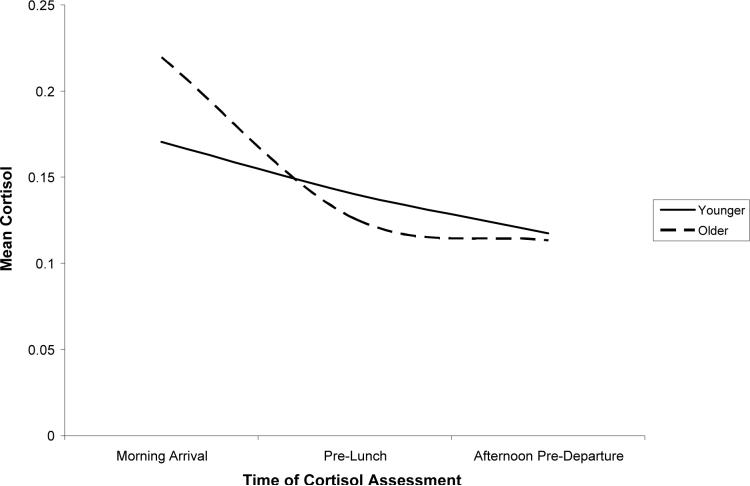
The results of this GLMM are presented in Table 3. Only findings not presented in previous analyses are detailed below. The results indicated that age was associated with cortisol change over the day. Older children exhibited heightened cortisol following the morning arrival, a steeper linear decline in cortisol, and a greater leveling off of cortisol by the afternoon pre-departure relative to their younger peers (see Figure 2 for a comparison of children one SD above and one SD below the mean age).
Table 3.
Association between Aggression and Mean Change in Cortisol
| Effect | Parameter | Estimate (SE) | |
|---|---|---|---|
| Intercept Effects | γ0 | Intercept1 | −2.34*** (.21) |
| γ1 | Age X Intercept | .07*** (.02) | |
| γ2 | Physical Agg X Intercept | .11* (.05) | |
| γ3 | Relational Agg X Intercept | −.15* (.07) | |
| Linear Change | γ4 | Linear Slope Scaling Factor1 | 1.10*** (.26) |
| γ5 | Age X Linear Slope | −.16*** (.03) | |
| γ6 | Physical Agg X Linear Slope | −.19* (.08) | |
| γ7 | Relational Agg X Linear Slope | .18* (.09) | |
| Quadratic Change | γ8 | Quadratic Scaling Factor1 | −.48*** (.12) |
| γ9 | Age X Quadratic Change | .06*** (.01) | |
| γ10 | Physical Agg X Quadratic Change | .07† (.04) | |
| γ11 | Relational Agg X Quadratic Change | −.07† (.04) | |
p < .10
p < .05
**p < .01
p < .001
Although not pertinent to the hypotheses of the present study, scaling estimates for the intercept, linear slope, and quadratic change are reported for completeness.
Figure 2.
Age differences in cortisol over the day.
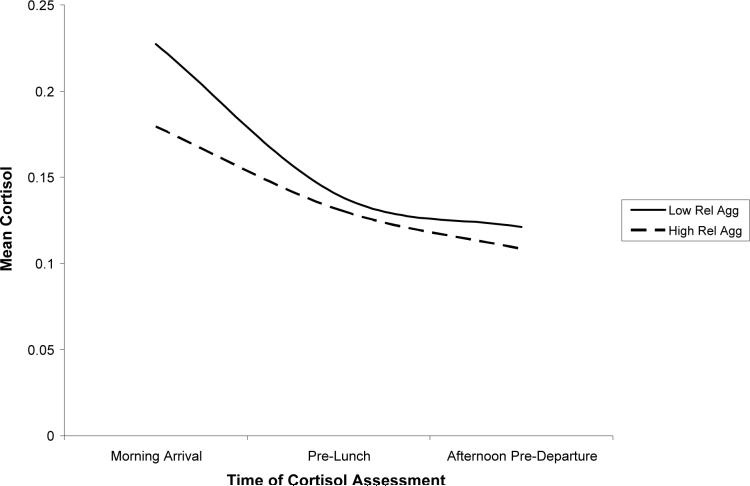
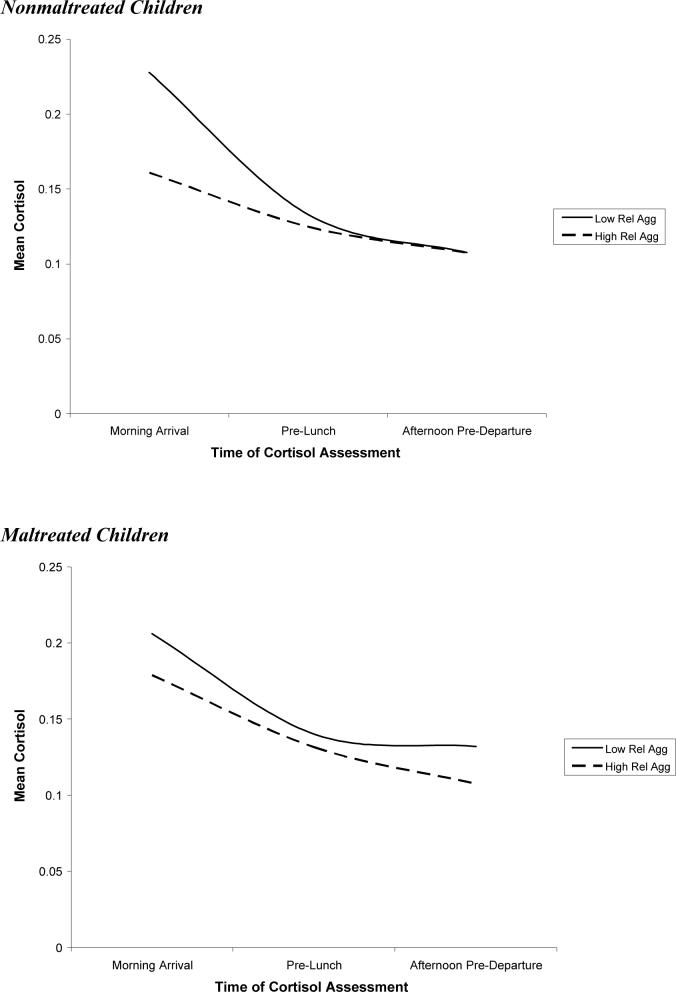
Relational aggression was significantly related to diurnal change in cortisol. The relational aggression X intercept interaction was significant, indicating that high levels of relational aggression predicted lower levels of cortisol following the morning arrival. Relational aggression was also associated with linear change in cortisol over the day. The findings revealed that higher levels of relational aggression predicted a more gradual decline in cortisol over the day. Finally, the association between relational aggression and quadratic change approached significance, p = .08, with higher relational aggression predicting a more gradual leveling off of cortisol over the day. In sum, relational aggression was associated with low cortisol; however, this effect was most pronounced following the morning arrival. High levels of relational aggression also predicted a less steep decline and a more gradual leveling off of cortisol over the day; in other words, children with high levels of relational aggression exhibited blunted diurnal change in cortisol relative to their peers. The cortisol trajectories for children low in relational aggression (1 SD below the mean) and high in relational aggression (1 SD above the mean) are depicted in Figure 3.
Figure 3.
Association between relational aggression and cortisol over the day.
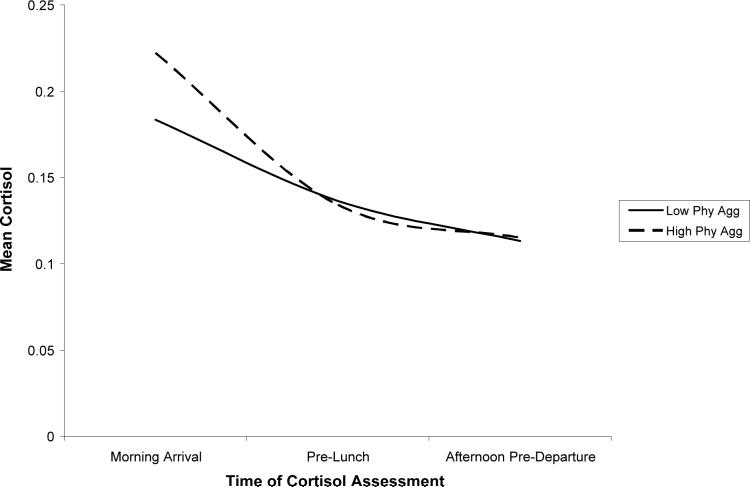
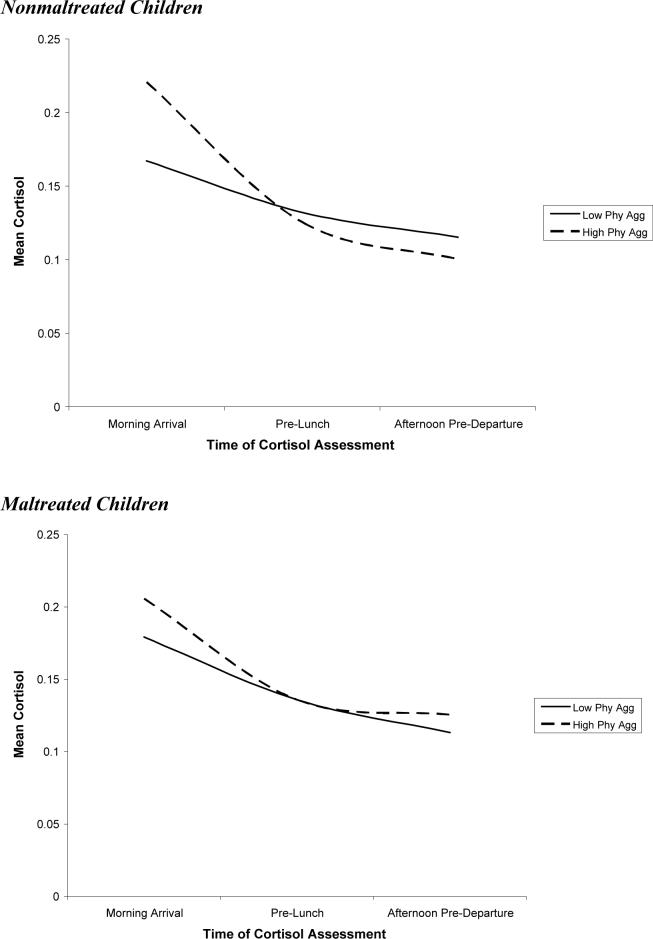
Physical aggression was also related to cortisol trajectories. Physical aggression was associated with heightened cortisol following the morning arrival and a steeper decline in cortisol over the day. In addition, the association between physical aggression and quadratic change approached significance, p = .06, with higher physical aggression predicting a greater leveling off of cortisol. In sum, physical aggression was associated with relatively high cortisol following the morning arrival. In addition, a steeper decline and greater leveling off of cortisol over the day among children with high levels of physical aggression led these children to exhibit more pronounced diurnal change in cortisol than their nonaggressive peers. The cortisol trajectories for children low in physical aggression (1 SD below the mean) and high in physical aggression (1 SD above the mean) are depicted in Figure 4.1,2
Figure 4.
Association between physical aggression and cortisol over the day.
Potential Moderators of the Association between Aggression and Cortisol Trajectories: Maltreatment and Gender
The third goal of the present paper was to examine whether maltreatment status and gender, respectively, moderated the association between aggression and diurnal cortisol. A GLMM was conducted to assess whether the association between aggression and diurnal rhythm of cortisol differed for maltreated and nonmaltreated children. In other words, the role of maltreatment, aggression (physical and relational), and the interaction between maltreatment and aggression on diurnal cortisol was assessed. Preliminary analyses indicated that maltreatment was not associated with quadratic change and did not moderate the association between aggression and quadratic change. As a result, these effects were not included in the final model. The following equation was used to estimate the fixed effects:
| (3) |
where μj is mean cortisol at the jth time point, γ0 is the scaling term for the intercept, and γ1, γ2, γ3, and γ5 indicate the strength of the association between maltreatment, age, physical aggression, and relational aggression, respectively, with the intercept. γ7 is the scaling factor for linear change, and γ8, γ9, γ10, and γ12 represent the effect of maltreatment, age, physical aggression, and relational aggression, respectively, on linear change. γ14 is the scaling term for quadratic change, and γ15, γ16, and γ17 represent the effect of age, physical aggression, and relational aggression, respectively, on quadratic change.
The model assessed whether maltreatment moderated the effects of physical and relational aggression on diurnal cortisol. Specifically, γ4 and γ11 assessed whether the effect of physical aggression on the intercept and linear change, respectively, differed for maltreated and nonmaltreated children. γ6 and γ13 assessed whether the effect of relational aggression on the intercept and linear change, respectively, differed for maltreated and nonmaltreated children. Finally, a represents age in years, mal represents maltreatment status (0=nonmaltreated; 1=maltreated), pagg represents physical aggression, ragg represents relational aggression, lj represents the linear term, and qj represents quadratic change.
The results of this GLMM are presented in Table 4. Maltreatment was not related to morning arrival cortisol and the interaction between maltreatment and relational aggression on the intercept was not significant, indicating that relational aggression predicted lower morning arrival cortisol among both maltreated and nonmaltreated children. However, the association between maltreatment status and linear change in cortisol approached significance, p < .07, with maltreated children exhibiting a less steep decline in cortisol than their nonmaltreated peers. This effect was, however, qualified by a significant interaction of maltreatment status and relational aggression on linear change in cortisol, indicating that the more gradual decline in cortisol over the day among relationally aggressive children was more pronounced among nonmaltreated than maltreated children (see Figure 5). In other words, highly relationally aggressive children who were not maltreated exhibited a more blunted diurnal change in cortisol over the day than their maltreated counterparts.
Table 4.
The Association between Aggression and Mean Change in Cortisol in Maltreated and Nonmaltreated Children
| Effect | Parameter | Estimate (SE) | |
|---|---|---|---|
| Intercept Effects | γ0 | Intercept1 | −2.35*** (.21) |
| γ1 | Maltreatment X Intercept | .00 (.06) | |
| γ2 | Age X Intercept | .07*** (.02) | |
| γ3 | Physical Agg X Intercept | .16* (.08) | |
| γ4 | Physical Agg X Maltreatment X Intercept | −.08 (.10) | |
| γ5 | Relational Agg X Intercept | −.22* (.07) | |
| γ6 | Relational Agg X Maltreatment X Intercept | .13 (.13) | |
| Linear Change | γ7 | Linear Slope Scaling Factor1 | 1.09*** (.26) |
| γ8 | Maltreatment X Linear Slope | .05† (.03) | |
| γ9 | Age X Linear Slope | −.16*** (.03) | |
| γ10 | Physical Agg X Linear Slope | −.26** (.09) | |
| γ11 | Physical Agg X Maltreatment X Linear | .11* (.06) | |
| γ12 | Relational Agg X Linear Slope | .25** (.09) | |
| γ13 | Relational Agg X Maltreatment X Linear | −.13* (.06) | |
| Quadratic Change | γ14 | Quadratic Scaling Factor1 | −.49*** (.12) |
| γ15 | Age X Quadratic Change | .06*** (.01) | |
| γ16 | Physical Agg X Quadratic Change | .07* (.03) | |
| γ17 | Relational Agg X Quadratic Change | −.07† (.04) | |
p < .10
p < .05
p < .01
p < .001
Although not pertinent to the hypotheses of the present study, scaling estimates for the intercept, linear slope, and quadratic change are reported for completeness.
Figure 5.
Association between relational aggression and cortisol over the day among maltreated and nonmaltreated children.
Maltreatment did not moderate the relation between physical aggression and morning arrival cortisol. However, the interaction between maltreatment and physical aggression on linear change was significant, indicating that the steeper decline in cortisol over the day among physically aggressive children was greater among nonmaltreated children (see Figure 6). In other words, highly physically aggressive children who were not maltreated exhibited more pronounced diurnal change in cortisol than their maltreated counterparts.3
Figure 6.
Association between physical aggression and cortisol over the day among maltreated and nonmaltreated Children.
A parallel GLMM was run to assess whether gender moderated the association between diurnal cortisol and aggression. The equation for this analysis was the same as that presented in Equation 3, except that gender rather than maltreatment status served as the moderating variable. The results of this analysis (not shown) indicated that gender did not moderate the association between either physical aggression or relational aggression and cortisol over the course of the day.
Discussion
The first goal of the present paper was to assess the association between diurnal change in cortisol and both physical and relational aggression. We expected that both forms of aggression would be associated with relatively low levels of cortisol following morning arrival and a blunted change in cortisol over the day. Consistent with these predictions, results indicated that high levels of relational aggression predicted lower levels of cortisol following the morning arrival and a more gradual decline in cortisol over the day. This blunted diurnal pattern was largely due to low early morning arrival levels of cortisol which remained low throughout the day. This pattern has been termed “hypocortisolism,” and has recently been proposed to be an indicator of risk in development (for more extensive reviews, see Gunnar & Vazquez, 2001; Heim, Ehlert, & Hellhammer, 2000). However, our findings are some of the first to suggest that hypocortisolism is also related to children's involvement in relationally aggressive behaviors (see Blades & Susman, 2007; Dettling et al., 1999). These findings are consistent with both stimulation-seeking and fearlessness theories of aggression (Raine, 2002). In effect, children with hypocortisolism may engage in relational aggression because it is a stimulating experience that increases their physiological arousal to more comfortable levels or because they are relatively unafraid of potential negative outcomes of their behavior.
In contrast to our hypotheses, physical aggression was actually related to higher levels of cortisol following morning arrival and a steeper decline in cortisol over the day. This finding is surprising, and is not consistent with previous work indicating that physically aggressive children tend to have low basal levels of cortisol (e.g., McBurnett et al., 2000; van Goozen et al., 1998) and blunted change in cortisol over the day (e.g., Gustafsson et al., 2006). One potential explanation for this unexpected finding is that the association between physical aggression and heightened cortisol reflects the cortisol dysregulation in a subset of physically aggressive children: specifically, reactive physical aggressors. In fact, physiological correlates of aggressive conduct may differ for proactive versus reactive aggressors (Scarpa & Raine, 1997). Proactive aggression is defined as planned and goal-directed aggressive behaviors (Crick & Dodge, 1996). In contrast, reactive aggression includes impulsive, emotional aggression in response to perceived negative experiences such as provocation or frustration (Crick & Dodge, 1996). Whereas proactive aggression may be most strongly related to the fearlessness and stimulation-seeking associated with low basal cortisol, reactive aggression is hypothesized to relate to increases in physiological arousal during stress (Hubbard et al., 2002; McBurnett et al., 2005; Scarpa & Raine, 1997). Some research has provided evidence that aggressive individuals exhibit heightened cortisol reactivity following stress or provocation (Gerra et al., 1997; McBurnett et al., 2005). Insofar as attending a summer camp is an even mildly stressful experience for children, the association between physical aggression and elevated levels of cortisol following morning arrival may reflect heightened cortisol reactivity among reactive physical aggressors. Future research should assess proactive and reactive forms of aggression, in addition to basal cortisol and cortisol reactivity, to test this possibility.
A second potential explanation for these surprising findings is that patterns of hypocortisolism may be more strongly associated with relational rather than physical forms of aggression. Because most work in this area has failed to assess relational aggression, previous studies have not examined the association between cortisol and physical aggression while controlling for the effects of relational aggression. Given the relatively high correlation between physical and relational aggression (e.g., Crick & Grotpeter, 1995), it is possible that co-occurring relational aggression accounts for the association between physical aggression and hypocortisolism found in previous research. In fact, in one study, relational aggression was associated with larger increases in cortisol over the day among young children in group child care than physical aggression (Dettling et al., 1999). Thus, it is important to keep in mind that the present study examined the unique associations between each form of aggression and cortisol regulation. Additional research focused on diurnal change in cortisol should include assessments of both relational and physical forms of aggression to address this important point.
The second goal of this paper was to examine whether gender moderates the relationship between aggression and cortisol, with cortisol dysregulation being more strongly related to relational aggression for girls and physical aggression for boys. Contrary to our hypotheses, the results indicated that cortisol dysregulation was associated with involvement in physical and relational aggression for children of both genders. These findings are consistent with a growing number of studies suggesting that low cortisol is associated with physical aggression among girls (e.g., Pajer et al., 2001; Pajer et al., 2006) and is one of the first studies to provide evidence that cortisol dysregulation is implicated in the development of relational aggression among boys (although see Dettling et al., 1999). However, other researchers have found that physiological correlates of aggression are more strongly associated with relational aggression for girls (e.g., Blades & Susman, 2007; Murray-Close & Crick, 2007) and physical aggression for boys (Cicchetti & Rogosch, 2001b; Murray-Close & Crick, 2007). Moreover, patterns of flattened diurnal cortisol may be particularly relevant to the development of co-occurring internalizing disorders (e.g., Rosen & Schulkin, 1998; Yehuda et al., 2000). Thus, hypocortisolism may be a prime candidate mechanism for future exploration of the epigenetic process contributing to gender differences in the development of psychopathology. As such, future research should investigate the conditions under which gender moderates the relation between cortisol dysregulation and physical and relational forms of aggression
Finally, the third goal of this paper was to examine whether maltreatment moderated the association between diurnal cortisol and aggression. Consistent with our hypotheses, the relationship between aggression and diurnal cortisol was stronger among nonmaltreated than maltreated children. Specifically, both the association between relational aggression and a blunted circadian rhythm and the association between physical aggression and a steeper decline in cortisol over day were more pronounced among nonmaltreated than maltreated children.
These findings are consistent with the “social push” perspective, in which biological contributors to involvement in aggression are more influential among children who have a less strong social push toward such conduct (Raine & Venables, 1981; Raine, 2002). Importantly, all participants in the present study were relatively high-risk and thus the social push toward antisocial behaviors was strong in both the maltreated and nonmaltreated samples. Our findings suggest that even among populations experiencing very high levels of contextual risk, biological factors may be most important in predicting aggression among individuals with somewhat lower levels of social risk. The findings are also consistent with the developmental psychopathology tenet of multifinality, in which a particular risk factor does not lead to the same outcome in every individual (Cicchetti & Rogosch, 1996; Sroufe, 1997). It is possible that the processes that underlie the development of aggression differ for maltreated and nonmaltreated children. Evidence suggests that maltreatment is associated with heightened involvement in both physically and relationally aggressive behavior (e.g., Cullerton-Sen et al., under review; Shields & Cicchetti, 2001), and maltreatment is related to cortisol dysregulation (Cicchetti & Rogosch, 2001a,b). Given the overwhelming risk for aggression associated with the maltreatment, it is possible that individual differences in cortisol circadian rhythms may be less influential in the development of aggression in this population. Overall, these results support the recent call among researchers for greater attention to how contextual risk factors, such as childhood neglect and poverty, may moderate the association between aggression and psychophysiological processes (see McBurnett et al., 2005; Raine, 2002).
These results also provide support for the hypothesis that cortisol dysregulation relates to aggression specifically, rather than to life stressors more generally. Gunnar and Vazquez (2001) suggest that the association between cortisol and aggression may be an artifact of other factors that are correlated with aggression. In effect, the association between low basal cortisol and aggression may actually reflect dysregulation of the HPA axis resulting from chronic stressors common in aggressive children's lives (e.g., exposure to violence, poor parent-child relationships, childhood maltreatment). However, our findings are not consistent with this interpretation. Rather, the association between cortisol dysregulation and aggression remained even when controlling for maltreatment and was actually stronger among nonmaltreated compared to maltreated children. Thus, we propose that cortisol functioning is an important correlate of aggressive conduct, and that this relation does not simply reflect an artifact of the relatively high number of stressors in aggressive children's lives. Future research should control for these potential stressors so that the specificity of the relation between cortisol and aggression can be assessed.
Limitations and Future Directions
Although the findings of the present study are both interesting and novel, a number of limitations must be acknowledged. First, in the present paper, we propose that cortisol dysregulation leads to the development of aggressive behavior problems. However, it is also possible that involvement in aggression leads to dysregulated cortisol. Disruptions in peer relations associated with relational aggression during childhood may become a chronic source of stress over time (Blades & Susman, 2007; Dettling et al., 1999) and several investigators have proposed that hypocortisolism results from allostatic adjustments to ongoing stress (Davies, Sturge-Apple, Cicchetti, & Cummings, 2007; Hellhammer & Wade, 1993; McEwen, 1998). Thus, the association between hypocortisolism and relational aggression might reflect down-regulation of the HPA axis in response to chronic stress. We believe that it is likely that cortisol dysregulation leads to involvement in aggressive conduct, which in turn contributes to greater disruption of cortisol activity. However, longitudinal research is necessary to address this possibility.
Longitudinal work would also help clarify the role of low cortisol in the development of aggression over the life course. Although it is not clear how early in development the association between cortisol and aggression emerges, studies have documented this relation in children as young as 3 years of age (Dettling et al., 1999). In fact, some researchers have suggested that cortisol dysregulation places children at risk for severe and persistent involvement in aggression and antisocial behavior (van Goozen et al., 2007). Consistent with this perspective, McBurnett and colleagues (2000) found that low cortisol was associated with early onset and severe aggression. This suggests that biological factors such as low arousal may be an especially strong predictor of life-course persistent antisocial behavior (Lorber, 2004; Moffitt, 1993; Raine et al., 1996). Moreover, personality traits such as low self-control (Shoal et al., 2003) and compromised conscience development (Frick & Morris, 2004) may mediate the relation between low cortisol and aggression over time. Future longitudinal research is necessary to examine the role of dysregulation of diurnal cortisol in the development of persistent aggression and to explicate potential mediators of these relations.
A further limitation in the present investigation is the failure to assess cortisol reactivity and proactive and reactive forms of aggression. Given the surprising findings regarding the association between physical aggression and heightened cortisol following morning arrival, future research should explore the role of cortisol reactivity in the development of aggression (see McBurnett et al., 2005). Studies examining the relation between diurnal cortisol and proactive and reactive aggression should also attend to the unique mechanisms that underlie the development of each form of aggression. Specifically, low physiological arousal may lead to proactive aggression through compromised socialization of conscience among fearless children (Frick & Morris, 2004). In contrast, heightened physiological reactivity may lead to reactive aggression because it places children at risk for deficits in emotion regulation capabilities (Frick & Morris, 2004). Future longitudinal research should consider these proposed pathways from cortisol regulation to proactive and reactive aggression.
In addition to the variable-centered analyses in the present paper, future research may benefit from adopting a person-oriented approach to assessing the relation between cortisol dysregulation and aggression (see Bergman & Magnusson, 1997). Given the overlap between physical and relational aggression, a person-centered approach would allow for the examination of diurnal cortisol among physically aggressive, relationally aggressive, and comorbid children. Such analyses can address a distinct set of questions, including whether co-occurring physical and relational aggression is related to unique patterns of cortisol dysregulation. Moreover, given research suggesting that individual differences in factors such as callous-unemotional traits and anxiety may moderate the association between aggression and cortisol functioning (e.g., Loney, Butler, Lima, Counts, & Eckel, 2006; McBurnett et al., 2000), future research should examine these factors may interact with aggressive conduct in predicting diurnal change in cortisol.
Future research may also benefit from examining the association between diurnal cortisol and aggressive behavior in other contexts (e.g., the classroom). In this study, participants were assigned to same-sex peer groups for activities during the camp. Using these groups for peer reports may have important implications for our assessment of aggression, as the threshold for nominating children as physically and relationally aggressive may vary by the sex composition of the peer group. For instance, a female who exhibits moderate levels of physical aggression may be nominated by peers as physically aggressive when in a group of females (who tend to exhibit relatively low levels of such behavior) but not when in a group including males (who more frequently engage in such behavior). In addition, diurnal cortisol activity may differ when children engage in predominantly same-sex versus opposite-sex peer interactions. Thus, it is important for future research to examine the generalizabiliy of the present findings by replicating this study in contexts involving mixed-sex peer groups.
Future investigations of the association between cortisol and aggression would also benefit from taking account of other relevant hormones. For example, Popma and colleagues (2007) found that testosterone was associated with over aggression among delinquent boys with low cortisol levels. Given that there is evidence of a bidirectional relationship between testosterone and cortisol (e.g., Luine, 2002; Rivier & Rivest, 1991), future studies on the developmental psychobiology of childhood aggression would highly benefit from the simultaneous measurement of both testosterone and cortisol levels (Viau, 2002).
Future work in this area should also explore the role of emotion regulatory processes. Emotion regulation has been thought of as a set of heterogeneous processes by which emotions are modulated, thereby facilitating the function of emotions to coordinate multiple response systems (Levenson, 1999). It has long been posited that emotional processes mediate adrenocortical responses to environmental stressors (for a more extensive review, see Stansbury & Gunnar, 1994). For example, HPA reactivity has been shown to be affected by the perception of control over potentially threatening stimuli (Weiss, 1971). Thus, one potential explanation of previous findings of the link between aggression and low cortisol levels may be that children who employ aggressive strategies believe that such behaviors give them control over events in their social environment. Gunnar and colleagues have conducted studies which suggest that aggression activates the HPA system in normally developing children (as cited in Stansbury & Gunnar, 1994). Thus, future investigations would need to delineate mechanisms that contribute to a reversed pattern of HPA activity for children who exhibit clinical levels of aggressive behaviors.
Finally, the experience of early stress such as childhood maltreatment may produce cascading effects on multiple domains of psychobiological development (e.g., limbic system kindling) which probabilistically constrain ways in which individuals approach new situations (Cicchetti & Tucker, 1994). Thus, an important direction for further exploration is whether HPA-axis dysregulation mediates the association between child maltreatment and the development of aggressive behaviors. Past research suggests that impaired capacities to modulate attention contribute to emotion dysregulation in maltreated children and this mood lability has been linked to reactive aggression (Shields & Cicchetti, 1998). Of note, there is now emerging evidence that both maladaptive social information processing and emotion dysregulation partially mediate the association between maltreatment and aggression (e.g., Teisl & Cicchetti, in press). In sum, future investigations need to deal with the challenge of delineating factors contributing to the development of individual differences in emotion processing, HPA activity, and connections to the emergence of externalizing behaviors.
Conclusions
The results of the present study extend previous research assessing the association between salivary cortisol and aggressive conduct in three important ways. First, by assessing basal cortisol over the course of the day, it was possible to examine the relation between aggression and diurnal change in cortisol. In fact, the results indicated that this is an important avenue for research, as aggression was associated with alterations in cortisol following morning arrival and changes in cortisol over the day. Second, this study provides one of the first assessments of the association between cortisol dysregulation and relational aggression in school-aged children. The findings indicated that both physical and relational forms of aggression were associated with neurobiological dysregulation of the limbic-hypothalamic-pituitary-adrenocortical (LHPA) system. However, our results support the view that the nature of this relation differs for physical (i.e., relatively high cortisol) versus relational (i.e., relatively low cortisol) forms of aggression. In addition, the findings indicated that the association between aggression and cortisol dysregulation was most pronounced among nonmaltreated children. Longitudinal studies in the future are necessary to assess the directionality of association between cortisol activity and aggression. Finally, important avenues for future research include the respective roles of other relevant hormones (e.g., testosterone) and emotion regulation associated with the developmental psychoneuroendocrinology of aggression.
Acknowledgments
This paper was supported by grants awarded to Dante Cicchetti, Ph.D. and Fred A. Rogosch, Ph.D. from the National Institute of Drug Abuse (DA 17741) and from the Spunk Fund, Inc and to Nicki R. Crick, Ph.D. from the National Institute on Child Health and Development. We would like to thank the children, families, counselors and research staff at the Mount Hope Family Center, Rochester, New York who participated in this work. Thanks to Jeff Long, Ph.D. for his statistical consultation.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/dev/
It is important to consider the specificity of the association between physical and relational aggression and cortisol dysregulation. In effect, because both physically and relationally aggressive behaviors are associated with internalizing problems (e.g., Crick, 1997; Murray-Close, Ostrov, & Crick, 2007), and internalizing symptoms are related to cortisol dysregulation (e.g., Klimes-Dougan et al., 2001), it is possible that co-occurring internalizing symptoms may account for the relation between aggression and dysregulated diurnal cortisol. Thus, we ran a follow-up model examining the association between physical and relational aggression and diurnal cortisol controlling for the effects of depression (Child Depression Inventory; CDI; Kovacs, 1985). The results indicated that the vast majority of effects presented in Table 3 remained statistically significant with this additional control. However, two findings dropped just below conventional levels of statistical significance in this follow-up. Specifically, the association between physical aggression and morning arrival cortisol dropped to p = .07 and the association between relational aggression and linear change in cortisol dropped to p = .06. It is important to note that power was reduced in this model due to additional statistical control and a slight reduction in sample size (CDI data was missing for 28 participants). Moreover, these changes in significance levels likely reflect this reduction in power because the estimates for these effects were virtually unchanged in the follow-up model (the effect of physical aggression on morning arrival did not change and the effect of relational aggression on linear change dropped from .18 to .17). Overall, then these findings bolster the case that cortisol dysregulation is uniquely associated with aggression in particular and not psychopathology more generally.
Analyses were run to examine whether child age moderated the association between physical and relational aggression and diurnal cortisol. Results indicated that age was not a significant moderator of these effects.
Given research suggesting that pubertal stages is associated with basal cortisol levels (Halligan, Herbert, Goodyer, & Murray, 2004; Netherton, Goodyer, Tamplin, & Herbert, 2004), all analyses were re-run controlling for the association between pubertal stage and cortisol. The findings indicated that all significant results regarding the association between aggression (both physical and relational) were replicated when controlling for pubertal development.
References
- Akil H, Haskett RF, Young EA, Grunhaus L, Kotun J, Weinberg V, Greden J, Watson SJ. Multiple HPA profiles in endogenous depression: Effect of age and sex on cortisol and beta-endorphin. Biological Psychiatry. 1993;33:73–85. doi: 10.1016/0006-3223(93)90305-w. [DOI] [PubMed] [Google Scholar]
- Azar R, Zoccolillo M, Paquette D, Quiros E, Baltzer F, Tremblay RE. Cortisol levels and conduct disorder in adolescent mothers. Journal of American Academy of Child and Adolescent Psychiatry. 2004;43:461–468. doi: 10.1097/00004583-200404000-00012. [DOI] [PubMed] [Google Scholar]
- Barnett D, Manly JT, Cicchetti D. Defining child maltreatment: The interface between policy and research. In: Cicchetti D, Toth SL, editors. Advances in Applied Developmental Psychology: Vol. 8. Child Abuse, Child Development and Social Policy. Ablex; Norwood, NJ: 1993. pp. 7–73. [Google Scholar]
- Bergman LR, Magnusson D. A person-oriented approach in research on developmental psychopathology. Development and Psychopathology. 1997;9:291–319. doi: 10.1017/s095457949700206x. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist K. Different names, same issue. Social Development. 2001;10:272–275. [Google Scholar]
- Bjorkqvist K, Lagerspetz K, Kaukianen A. Do girls manipulate and boys fight? Developmental trends in regard to direct and indirect aggression. Aggressive Behavior. 1992;18:117–127. [Google Scholar]
- Blades KT, Sussman EJ. The effect of relational aggression on the diurnal rhythm of cortisol in young adolescents. Poster presented at the biennial meeting of the Society of Research on Child Development. 2007 [Google Scholar]
- Buhs ES, Ladd GW, Herald SL. Peer exclusion and victimization: Processes that mediate the relation between peer group rejection and children's classroom engagement and achievement? Journal of Educational Psychology. 2006;98:1–13. [Google Scholar]
- Cashdan E. Hormones and competitive agression in women. Aggressive Behavior. 2003;29:107–115. [Google Scholar]
- Cicchetti D, Manly JT. A personal perspective on conducting research with maltreating families: Problems and solutions. In: Brody E, Sigel I, editors. Family Research: Vol. 2: Families at Risk. Lawrence Erlbaum Associates, Inc.; Hillsdale, NJ: 1990. pp. 87–133. [Google Scholar]
- Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental psychopathology. Development and Psychopathology. 1996;8:597–600. [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001a;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001b;13:783–804. [PubMed] [Google Scholar]
- Cicchetti D, Tucker D. Development and self-regulatory structures of the mind. Development and Psychopathology. 1994;6(4):533–549. [Google Scholar]
- Coren S. Arousal predisposition as a predictor of antisocial and delinquent behavior. Personality and Individual Differences. 1999;27:815–820. [Google Scholar]
- Crick NR. The role of overt aggression, relational aggression, and prosocial behavior in the prediction of children's future social adjustment. Child Development. 1996;67:2317–2327. [PubMed] [Google Scholar]
- Crick NR. Engagement in gender normative versus non-normative forms of aggression: Links to social-psychological adjustment. Developmental Psychology. 1997;33:610–617. doi: 10.1037//0012-1649.33.4.610. [DOI] [PubMed] [Google Scholar]
- Crick NR, Dodge KA. Social information-processing mechanisms in reactive and proactive aggression. Child Development. 1996;67:993–1002. [PubMed] [Google Scholar]
- Crick NR, Grotpeter JK. Relational aggression, gender, and social-psychological adjustment. Child Development. 1995;66:710–722. doi: 10.1111/j.1467-8624.1995.tb00900.x. [DOI] [PubMed] [Google Scholar]
- Crick NR, Werner NE, Casas JF, O'Brien KM, Nelson DA, Grotpeter JK, Markon K. Childhood aggression and gender: A new look at an old problem. In: Bernstein D, editor. Gender and motivation. Nebraska symposium on motivation. Vol. 45. 1999. pp. 75–141. [PubMed] [Google Scholar]
- Crick NR, Zahn-Waxler C. The development of psychopathology in females and males: Current progress and future challenges. Development and Psychopathology. 2003;15:719–742. [PubMed] [Google Scholar]
- Cullerton-Sen C, Cassidy AR, Murray-Close D, Cicchetti D, Crick NR, Rogosch FA. Childhood maltreatment and the development of relational and physical aggression: The importance of a gender-informed approach. Under Review. doi: 10.1111/j.1467-8624.2008.01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PT, Sturge-Apple ML, Cicchetti D, Cummings EM. The role of child adrenocortical functioning in pathways between interparental conflict and child maladjustment. Developmental Psychology. 2007;43:918–930. doi: 10.1037/0012-1649.43.4.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CS. Statistical Methods for the Analysis of Repeated Measurements. Springer; New York: 2002. [Google Scholar]
- Dettling AC, Gunnar MR, Donzella B. Cortisol levels of young children in full-day childcare centers: relations with age and temperament. Psychoneuroendocrinology. 1999;24:519–536. doi: 10.1016/s0306-4530(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Farmer TW, Estell DB, Leung M, Trott H, Bishop J, Cairns BD. Individual characteristics, early adolescent peer affiliations, and school dropout: An examination of aggressive and popular group types. Journal of School Psychology. 2003;41:217–232. [Google Scholar]
- Frick PJ, Morris AS. Temperament and developmental pathways to conduct problems. Journal of Clinical Child and Adolescent Psychology. 33:54–68. doi: 10.1207/S15374424JCCP3301_6. [DOI] [PubMed] [Google Scholar]
- Fung MT, Raine A, Loeber R, Lynam DR, Steinhauer SR, Venables PH, Stouthamer-Loeber M. Reduced electrodermal activity in psychopathy-prone adolescents. Journal of Abnormal Psychology. 2005;114:187–196. doi: 10.1037/0021-843X.114.2.187. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Donzella B. Social regulation of the LHPA axis in early human development. Psychoneuroendocrinology. 2001;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Gustafsson PE, Gustafsson PA, Nelson N. Cortisol levels and psychosocial factors in preadolescent children. Stress and Health. 2006;22:3–9. [Google Scholar]
- Galen BR, Underwood M. A developmental investigation of social aggression among girls. Developmental Psychology. 1997;33:589–599. doi: 10.1037//0012-1649.33.4.589. [DOI] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Avanzini P, Chittolini B, Giucastro G, Caccavari R, et al. Neurotransmitter-neuroendocrine responses to experimentally induced aggression in humans: Influence of personality variable. Psychiatry Research. 1997;66:33–43. doi: 10.1016/s0165-1781(96)02965-4. [DOI] [PubMed] [Google Scholar]
- Halász J, Liposits Z, Kruk MR, Haller J. Neural background of glucocorticoid dysfunction-induced abnormal aggression in rats: Involvement of fear- and stress-related structures. European Journal of Neuroscience. 2002;15:561–569. doi: 10.1046/j.0953-816x.2001.01883.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Halasz J, Mikics E, Kruk MR. Chronic glucocorticoid deficiency-induced abnormal aggression, autonomic hypoarousal, and social deficit in rats. Journal of Neuroendocrinology. 2004;16:550–557. doi: 10.1111/j.1365-2826.2004.01201.x. [DOI] [PubMed] [Google Scholar]
- Haller J, van de Schraaf J, Kruk MR. Deviant forms of aggression in glucocorticoid hyporeactive rats: A model for ‘pathological’ aggression? Neuroendocrinology. 2001;13:102–107. doi: 10.1046/j.1365-2826.2001.00600.x. [DOI] [PubMed] [Google Scholar]
- Halligan S, Herbert J, Goodyer IM, Murray L. Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biological Psychiatry. 2004;55:376–381. doi: 10.1016/j.biopsych.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hart J, Gunnar M, Cicchetti D. Altered neuroendrocrine activity in maltreated children related to depression. Development & Psychopathology. 1996;8:201–214. [Google Scholar]
- Hawley PH. Strategies of control, aggression, and morality in preschoolers: An evolutionary perspective. Journal of Experimental Child Psychology. 2003;85:213–235. doi: 10.1016/s0022-0965(03)00073-0. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hellhammer D, Wade S. Endocrine correlates of stress vulnerability. Psychotherapy and Psychosomatics. 1993;60:8–17. doi: 10.1159/000288675. [DOI] [PubMed] [Google Scholar]
- Henington C, Hughes JN, Cavell TA, Thompson B. The role of relational aggression in identifying aggressive boys and girls. Journal of School Psychology. 1998;36:457–477. [Google Scholar]
- Hubbard JA, Smithmyer CM, Ramsden SR, Parker EH, Flanagan KD, Dearing KF, Relyea N, Simons RF. Observational, physiological, and self-report measures of children's anger: Relations to reactive versus proactive aggression. Child Development. 2002;73:1101–1118. doi: 10.1111/1467-8624.00460. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kibler JL, Prosser VL, Ma M. Cardiovascular correlates of misconduct in children and adolescents. Journal of Psychophysiology. 2004;18:184–189. [Google Scholar]
- Kindlon DJ, Tremblay RE, Mezzacappa E, Earls F, Laurent D, Schaal B. Longitudinal patterns of heart rate and fighting in 9- through 12-year-old boys. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:371–377. doi: 10.1097/00004583-199503000-00023. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Children's Depression Inventory. Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Levenson RW. The intrapersonal functions of emotion. Cognition and Emotion. 1999;13:481–504. [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Loney BR, Butler MA, Lima EN, Counts CA, Eckel LA. The relation between salivary cortisol, callous-unemotional traits, and conduct problems in an adolescent non-referred sample. Journal of Child Psychology and Psychiatry. 2006;47:30–36. doi: 10.1111/j.1469-7610.2005.01444.x. [DOI] [PubMed] [Google Scholar]
- Lorber MF. Psychophysiology of aggression, psychopathy, and conduct problems: A meta-analysis. Psychological Bulletin. 2004;130:531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- Luine V. Sex differences in chronic stress effects on memory in rats. Stress. 2002;5(3):205–216. doi: 10.1080/1025389021000010549. [DOI] [PubMed] [Google Scholar]
- Manly JT, Cicchetti D, Barnett D. The impact of subtype, frequency, chronicity, and severity of child maltreatment on social competence and behavior problems. Development & Psychopathology. 1994;6:121–143. [Google Scholar]
- McBurnett K, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Archives of General Psychiatry. 2000;57:38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- McBurnett K, Raine A, Stouthamer-Loeber M, Loeber R, Kumar AM, Kumar M, Lahey BB. Mood and hormone responses to psychological challenge in adolescent males with conduct problems. Biological Psychiatry. 2005;57:1109–1116. doi: 10.1016/j.biopsych.2005.01.041. [DOI] [PubMed] [Google Scholar]
- McCulloch CE, Searle SR. Generalized, linear, and mixed models. Wiley; New York: 2001. [Google Scholar]
- McEwen B. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Moffitt T, E. Adolescent-limited and life-course persistent antisocial behavior: A developmental taxonomy. Psychological Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Murray-Close D, Crick NR. Gender differences in the association between cardiovascular reactivity and aggressive conduct. International Journal of Psychophysiology. 2007;65:103–113. doi: 10.1016/j.ijpsycho.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Murray-Close D, Ostrov JM, Crick NR. A short-term study of growth of relational aggression during middle childhood: Associations with gender, friendship intimacy, and internalizing problems. Development and Psychopathology. 2007;19:187–203. doi: 10.1017/S0954579407070101. [DOI] [PubMed] [Google Scholar]
- Netherton CM, Goodyer IM, Tamplin A, Herbert J. Salivary cortisol and dehydroepiandrosterone in relation to puberty and gender. Psychoneuoendocrinology. 2004;29:125–140. doi: 10.1016/s0306-4530(02)00150-6. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Geurts HM, Knol DL, Sergeant JA. Low basal salivary cortisol is associated with teacher-reported symptoms of conduct disorder. Psychiatry Research. 2005;134:1–10. doi: 10.1016/j.psychres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ostrov JM, Keating CF. Gender differences in preschool aggression during free play and structured interactions: An observational study. Social Development. 2004;13:255–277. [Google Scholar]
- Pajer K, Gardner W, Rubin RT, Perel J, Neal S. Decreased cortisol levels in adolescent girls with conduct disorder. Archive of General Psychiatry. 2001;58:297–302. doi: 10.1001/archpsyc.58.3.297. [DOI] [PubMed] [Google Scholar]
- Pajer K, Tabbah R, Gardner W, Rubin RT, Czambel RK, Wang Y. Adrenal androgen and gonadal hormone levels in adolescent girls with conduct disorder. Psychoneuroendocrinology. 2006;31:1245–1256. doi: 10.1016/j.psyneuen.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Popma A, Vermeiren R, Geluk CAML, Rinne T, van den Brink W, Knol DL, Jansen LMC, van Engeland H, Doreleijers TAH. Cortisol moderates the relationship between testosterone and aggression in delinquent male adolescents. Biological Psychiatry. 2007;61:405–411. doi: 10.1016/j.biopsych.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Quay HC. Psychopathic personality as pathological stimulation seeking. American Journal of Psychiatry. 1965;122:180–183. doi: 10.1176/ajp.122.2.180. [DOI] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: A. 2002. [DOI] [PubMed]
- Raine A, Reynolds C, Venables PH, Mednick SA. Biosocial bases of aggressive behavior in childhood. In: Raine A, Brennan PA, Farrington DP, Mednick SA, editors. Biosocial Bases of Violence. Plenum; New York: 1997. pp. 107–126. [Google Scholar]
- Raine A, Venables PH. Classical conditioning and socialization—a biosocial interaction. Personality and Individual Differences. 1981;2:273–283. [Google Scholar]
- Raine A, Venables PH. Tonic heart rate level, social class and antisocial behavior in adolescents. Biological Psychology. 1984;18:123–132. doi: 10.1016/0301-0511(84)90015-2. [DOI] [PubMed] [Google Scholar]
- Rivier C, Rivest S. Effect of stress on the activity of the hypothalamicpituitary-gonadal axis: Peripheral and central mechanisms. Biological Reproduction. 1991;45(4):523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychological Review. 1998;105:325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Rosenblitt JC, Soler H, Johnson S, Quadagno DM. Sensation seeking and hormones in men and women: Exploring the link. Hormones and Behavior. 2002;40:396–402. doi: 10.1006/hbeh.2001.1704. [DOI] [PubMed] [Google Scholar]
- Scarpa A, Fikretoglu D, Luscher KA. Community violence exposure in a young adult sample: Psychophysiology and aggressive behavior. Journal of Community Psychology. 2000;28:417–426. [Google Scholar]
- Scarpa A, Ollendick TH. Community violence exposure in a young adult sample: III. Psychophysiology and victimization interact to affect risk for aggression. Journal of Community Psychology. 2003;31:321–338. [Google Scholar]
- Scarpa A, Raine A. Anger, aggression, and violence: Psychophysiology of anger and violent behavior. Psychiatric Clinics of North America. 1997;20:375–394. doi: 10.1016/s0193-953x(05)70318-x. [DOI] [PubMed] [Google Scholar]
- Scerbo AS, Kolko DJ. Salivary testosterone and cortisol in disruptive children: Relationship to aggressive, hyperactive, and internalizing behaviors. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:1174–1184. doi: 10.1097/00004583-199410000-00013. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Halperin JM, Newcorn JH, Sharma V, Gabriel S. Plasma cortisol and aggression in boys with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;1997;36:605–609. doi: 10.1097/00004583-199705000-00010. [DOI] [PubMed] [Google Scholar]
- Shields A, Cicchetti D. Reactive aggression among maltreated children: The contributions of attention and emotion dysregulation. Journal of Clinical Child Psychology. 1998;27(4):381–395. doi: 10.1207/s15374424jccp2704_2. [DOI] [PubMed] [Google Scholar]
- Shields A, Cicchetti D. Parental maltreatment and emotion dysregulation as risk factors for bullying and victimization in middle childhood. Journal of Clinical Child Psychology. 2001;30(3):349–363. doi: 10.1207/S15374424JCCP3003_7. [DOI] [PubMed] [Google Scholar]
- Shoal GD, Giancola PR, Kirillova GP. Salivary cortisol, personality, and aggressive behavior in adolescent boys: A 5-year longitudinal study. Journal of American Academy of Child and Adolescent Psychiatry. 2003;42:1101–1107. doi: 10.1097/01.CHI.0000070246.24125.6D. [DOI] [PubMed] [Google Scholar]
- Sroufe LA. Psychopathology as an outcome of development. Development and Psychopathology. 1997;9:251–268. doi: 10.1017/s0954579497002046. [DOI] [PubMed] [Google Scholar]
- Stansbury K, Gunnar MR. Adrenocortical activity and emotion regulation. Monographs of the Society for Research in Child Development. 1994;59:108–134. [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neuroscience and Biobehavioral Reviews. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Teisl M, Cicchetti D. Physical abuse, cognitive and emotional processes, and aggressive/disruptive behavior problems. Social Development. (in press) [Google Scholar]
- Tomada G, Schneider BH. Relational aggression, gender, and peer acceptance: Invariance across culture, stability over time, and concordance among informants. Developmental Psychology. 1997;33:601–609. doi: 10.1037//0012-1649.33.4.601. [DOI] [PubMed] [Google Scholar]
- Van de Wiel NMH, van Goozen SHM, Matthys W, Snoek H, van Engeland H. Cortisol and treatment effect in children with disruptive behavior disorders: A preliminary study. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:1011–1018. doi: 10.1097/01.chi.0000126976.56955.43. [DOI] [PubMed] [Google Scholar]
- Van Goozen SH, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychological Bulletin. 2007;133:149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- Van Goozen SHM, Matthys W, Cohen-Hettenis PT, Wied CG, Wiegant VM, van Engeland H. Salivary cortisol and cardiovascular activity during stress in oppositional defiant disorder boys and normal controls. Biological Psychiatry. 1998;43:531–539. doi: 10.1016/S0006-3223(97)00253-9. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitarygonadal and -adrenal axes. Journal of Neuroendocrinology. 2002;14(6):506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Virkkunen M. Urinary free cortisol secretion in habitually violent offenders. Acta Psychiatrica Scandinavica. 1985;72:40–44. doi: 10.1111/j.1600-0447.1985.tb02568.x. [DOI] [PubMed] [Google Scholar]
- Weiss JM. Effects of coping behavior with and without a feedback signal on stress pathology in rats. Journal of Comparative and Physiological Psychology. 1971;77:22–30. doi: 10.1037/h0031581. [DOI] [PubMed] [Google Scholar]
- Woodman DD, Hinton JW, O'Neill MT. Cortisol secretion and stress in maximum security hospital patients. Journal of Psychosomatic Research. 1978;22:133–136. doi: 10.1016/0022-3999(78)90040-5. [DOI] [PubMed] [Google Scholar]
- Xie H, Cairns BD, Cairns RB. The development of aggressive behaviors among girls: Measurement issues, social functions, and differential trajectories. In: Pepler DJ, Madsen KC, Webster C, Levene KS, editors. The Development and Treatment of Girlhood Aggression. Lawrence Erlbaum; Mahwah, New Jersey: 2005. pp. 105–136. [Google Scholar]
- Xie H, Farmer TW, Cairns BD. Different forms of aggression among inner-city African-American children: Gender, configurations, and school social networks. Journal of School Psychology. 2003;41:355–375. [Google Scholar]
- Yehuda R, Bierer LM, Schmeidler J, Aferiat DH, Breslau I, Dolan S. Low cortisol and risk for PTSD in adult offspring of holocaust survivors. American Journal of Psychiatry. 2000;157:1252–1259. doi: 10.1176/appi.ajp.157.8.1252. [DOI] [PubMed] [Google Scholar]