Abstract
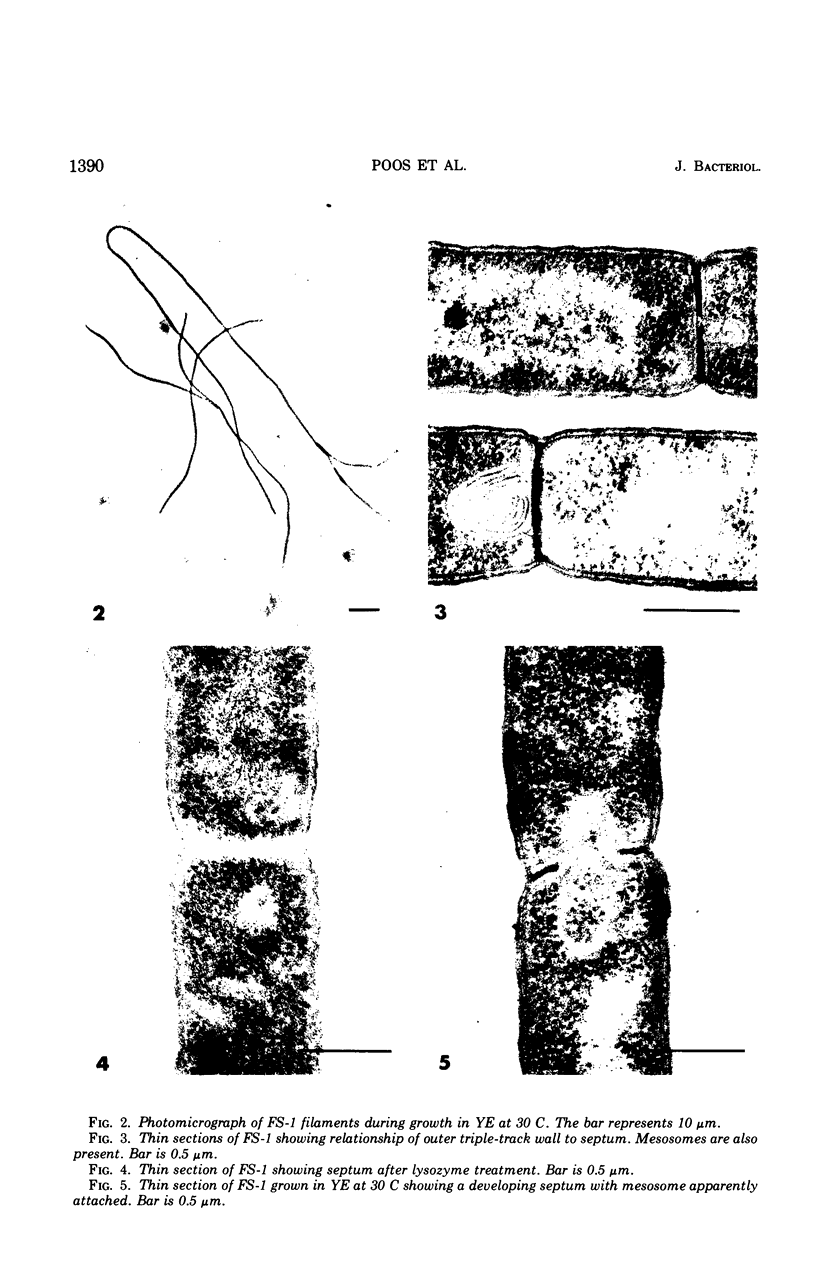
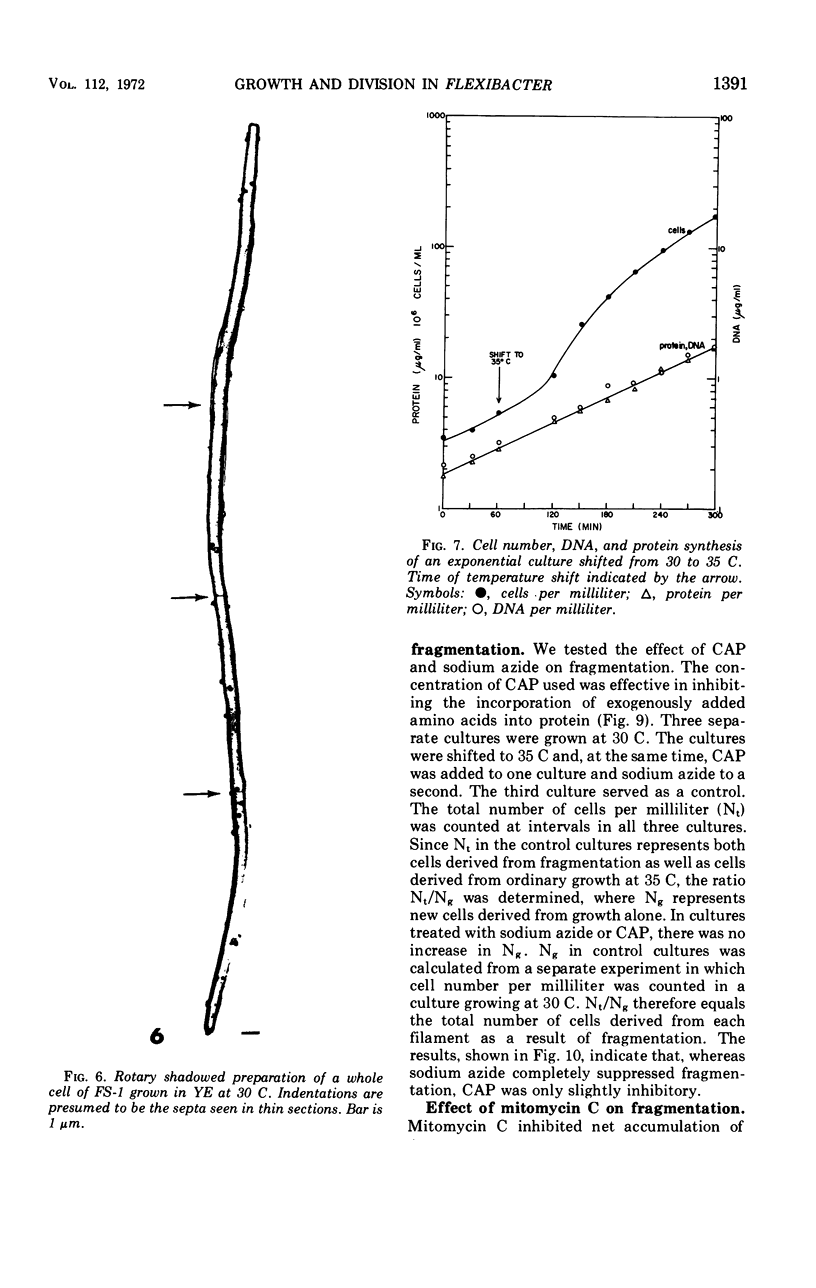
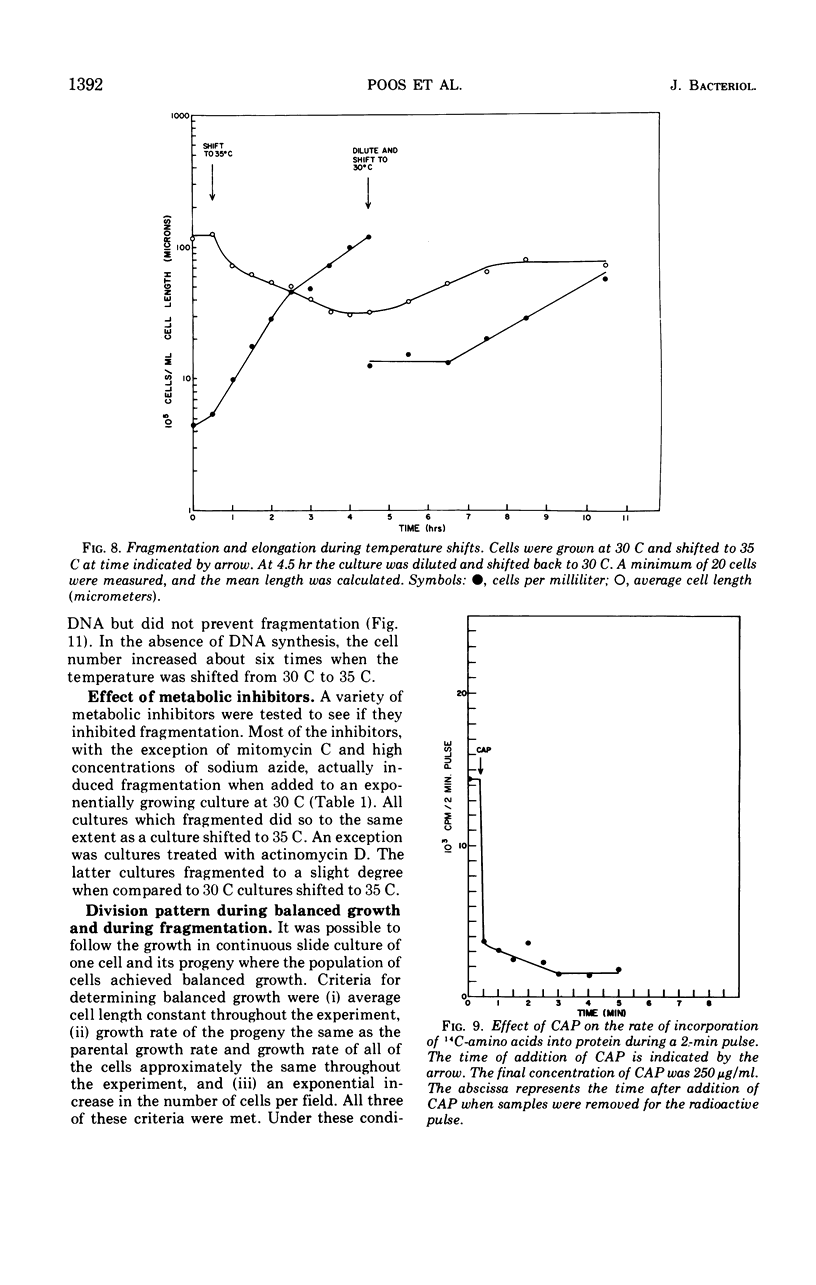
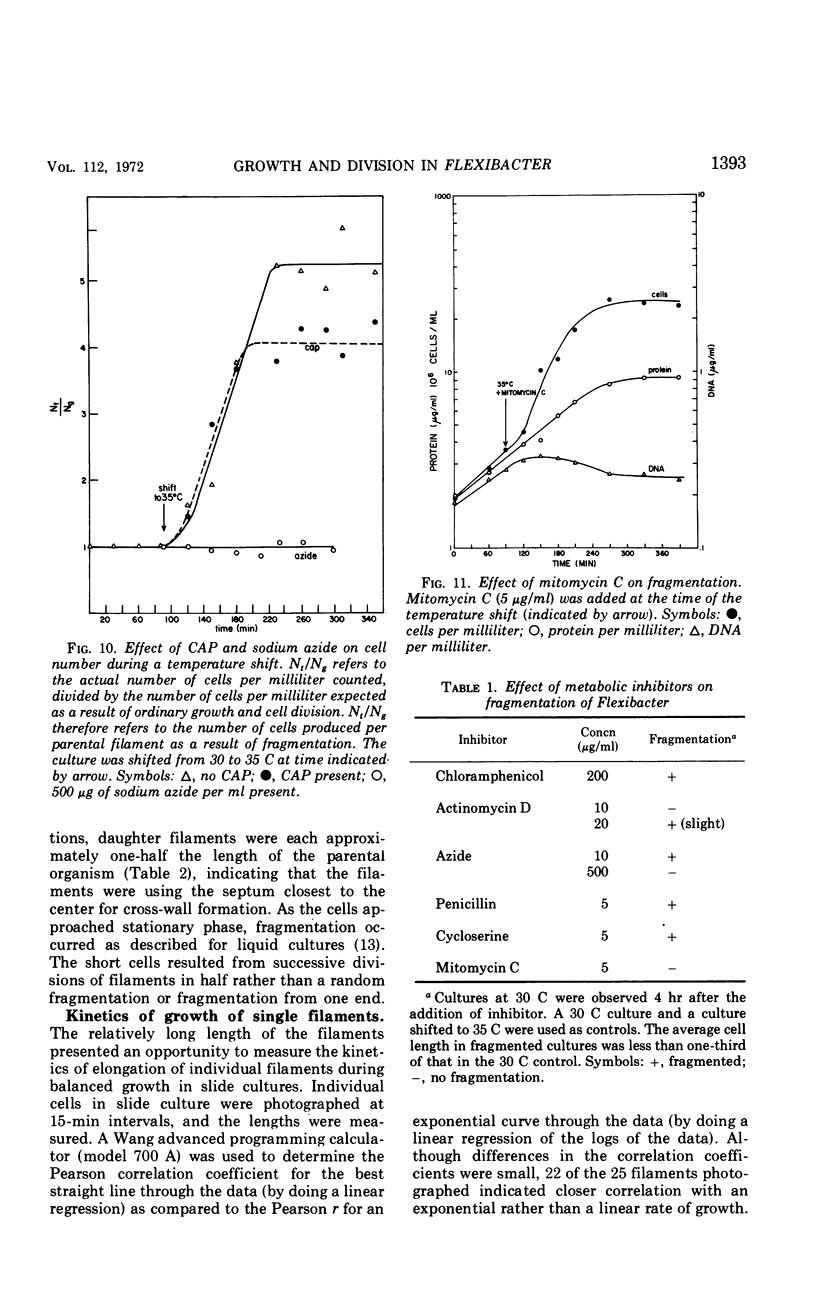
Flexibacter FS-1, a gram-negative gliding bacterium was grown in liquid culture as long (over 100-μm) filaments. The filaments possessed a triple-track wall which resembled that found in other gram-negative bacteria. Although phase-contrast microscopy indicated that the long filaments were nonseptate, electron microscopy revealed three or four septa along the length of each filament. The septa contained lysozyme-sensitive, electron-opaque material, presumed to be peptidoglycan, sandwiched between cell membranes. The outer triple track wall was not part of the septum. Mesosomes were seen in various areas of the cell and frequently were observed attached to septa in different stages of completion. Studies of the organism in slide culture revealed that individual filaments grew in an exponential fashion and divided in the middle despite the long length and multiseptate condition. When the temperature of a liquid culture growing exponentially with a generation time of 90 minutes was shifted from 30 to 35 C, the filaments fragmented into three or four shorter cells within 120 min. The short cells continued to grow exponentially at 35 C at approximately the same rate as at 30 C. When the culture was shifted back to 30 C, the cells immediately stopped dividing and began to elongate. After a period of 2 to 3 hr, cell division resumed. It is suggested that the shift-up in temperature induced the completion of the cross wall (centripetal growth of the triple-track wall) and cell separation at the sites of previously formed septa, whereas the shift-down in temperature caused a transient inhibition of cross-wall formation but not of growth. Fragmentation was inhibited by sodium azide but took place despite the inhibition of protein synthesis by chloramphenicol or the inhibition of deoxyribonucleic acid synthesis by mitomycin C.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., PFENNIG N., KUNISAWA R. THE FINE STRUCTURE OF GREEN BACTERIA. J Cell Biol. 1964 Jul;22:207–225. doi: 10.1083/jcb.22.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Bazire G., Kunisawa R., Poindexter J. S. The internal membranes of Caulobacter crescentus. J Gen Microbiol. 1966 Feb;42(2):301–308. doi: 10.1099/00221287-42-2-301. [DOI] [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G., Slepecky R. A. Fine structure of Bacillus megaterium during synchronous growth. J Bacteriol. 1967 Oct;94(4):1189–1205. doi: 10.1128/jb.94.4.1189-1205.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN H., FRANK M. E. Form and internal structure of cellular aggregations in early Escherichia coli microcultures. J Gen Microbiol. 1961 Jul;25:353–364. doi: 10.1099/00221287-25-3-353. [DOI] [PubMed] [Google Scholar]
- KARUNAIRATNAM M. C., SPIZIZEN J., GEST H. Preparation and properties of protoplasts of Rhodospirillum rubrum. Biochim Biophys Acta. 1958 Sep;29(3):649–650. doi: 10.1016/0006-3002(58)90028-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewin R. A. A classification of flexibacteria. J Gen Microbiol. 1969 Oct;58(2):189–206. doi: 10.1099/00221287-58-2-189. [DOI] [PubMed] [Google Scholar]
- Lewin R. A., Lounsbery D. M. Isolation, cultivation and characterization of flexibacteria. J Gen Microbiol. 1969 Oct;58(2):145–170. doi: 10.1099/00221287-58-2-145. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- SORIANO S., LEWIN R. A. GLIDING MICROBES: SOME TAXONOMIC RECONSIDERATIONS. Antonie Van Leeuwenhoek. 1965;31:66–79. doi: 10.1007/BF02045876. [DOI] [PubMed] [Google Scholar]
- STOVEPOINDEXTER J. L., COHEN-BAZIRE G. THE FINE STRUCTURE OF STALKED BACTERIA BELONGING TO THE FAMILY CAULOBACTERACEAE. J Cell Biol. 1964 Dec;23:587–607. doi: 10.1083/jcb.23.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon G. D., White D. Growth and morphological characteristics of a species of Flexibacter. Arch Mikrobiol. 1971;78(1):1–16. doi: 10.1007/BF00409084. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Steed P., Murray R. G. The cell wall and cell division of gram-negative bacteria. Can J Microbiol. 1966 Apr;12(2):263–270. doi: 10.1139/m66-036. [DOI] [PubMed] [Google Scholar]




