Abstract
Regulatory T cells (Treg) play an important role in immune regulation. Their development in the thymus requires TCR activation and recognition of peptide-MHC, although the downstream signals controlling commitment to the lineage are unclear. To compare the requirements for positive selection and Treg development, we studied knockout and transgenic mice defective in Raf signalling and the ERK effector SAP-1, a member of the Ternary Complex Factor (TCF) family of Ets domain transcription factors. Although SAP-1 deficient mice display a severe defect in thymocyte positive selection, Treg development was unimpaired as assessed by expression of Foxp3 and the activation markers CD25, GITR, CTLA4 and CD103 in the CD4+ cell population. In contrast, inhibition of Raf signalling by the dominant interfering DN Raf derivative reduced both Foxp3+ and Foxp3- CD4+ populations. In SAP-1 deficient CD4+CD25+ Treg cells, TCR crosslinking efficiently induced ERK activation, but transcriptional induction of the immediate early gene Egr-1 was impaired. Nevertheless, both SAP-1-deficient and DN Raf CD4+CD25+ Treg cells effectively suppressed CD4+CD25- cell proliferation in vitro. Finally the suppressive activity of CD4+CD25+ Treg cells lacking SAP-1 in an in vivo colitis model was not significantly impaired. The signalling requirements for development of Treg cells in the thymus are thus distinct from those required for “conventional” T cell positive selection, and ERK signalling to SAP-1 is not required for the suppressive activity of Treg cells.
Keywords: T Cells, Transcription Factors, Transgenic/Knockout mice, Thymus, Tolerance/Suppression/Anergy
INTRODUCTION
The phenomenon of immune tolerance is a striking feature of the immune system. Avoidance of autoimmunity is achieved both at the level of immune cell development and by direct or indirect suppressive interactions between lymphocyte populations. During thymocyte development, negative selection ensures deletion of cells bearing TCRs with high affinity for self peptide-MHC, while positive selection ensures survival of cells bearing functionally rearranged TCRs. The processes of positive selection and negative selection have been extensively studied using transgenic and gene knockout approaches, which have shown that positive selection is completely dependent on signalling through the calcineurin and Ras-ERK pathways (1-3). Of the nuclear effectors of ERK, SAP-1, a member of the ternary complex factor (TCF) family of Ets-domain transcription factors, is the predominant TCF in the thymus, and is required for positive selection and for TCR-dependent immediate-early gene activation in DP thymocytes (4). Consistent with this, inactivation of Egr-1, a SAP-1 target gene, or its target Id3 also impairs positive selection (5, 6). In contrast, negative selection appears independent of calcineurin and ERK-SAP-1 signalling (2-4), and is more dependent on SAPK / JNK signalling and its upstream regulators (7, 8). A sharp affinity threshold, which is associated with changes in the relative subcellular localisation of active ERK and JNK, separates positive and negative selection outcomes (9).
A second means by which autoimmunity is controlled is through the generation of regulatory T cells, which play an important role in the maintenance of tolerance as well as regulation of immune responses (reviewed in (10, 11). CD4+Foxp3+ T cells represent an important class of Tregs in both mouse and human, and the transcription factor Foxp3 appears necessary and sufficient for their generation in the thymus (12-14); reviewed in (15). CD4+Foxp3+ T cells comprise a large proportion of thymic CD4+CD25+ T cells and also express activation markers such as CTLA-4, GITR and CD103 (12-14). CD4+CD25+ cells act to suppress a variety of autoimmune phenotypes in adoptive transfer models (16, 17) and have the ability to suppress activation of T cells in vitro in a cell contact-dependent manner (18-20). The development of thymic Treg cells is not well understood: it selects cells expressing TCRs with high affinity for MHC-self peptide, although both the level of peptide expression and the nature of the APC affect the efficiency of Treg commitment (21-27), and is dependent on stromal MHC (28). Curiously, Treg development does not always correlate with substantially increased absolute numbers of Treg cells (27), and indeed recent results suggest that some Treg cells may be generated from a non-proliferative DP thymocyte population (29).
In spite of the involvement of TCR signalling in commitment to the Treg lineage, the relation of this process to positive and negative selection remains unclear, as does the mechanism by which Foxp3 expression is controlled. Here we investigated the role of ERK signalling in Treg cells using SAP-1 KO animals and transgenic animals expressing a dominant negative Raf (4, 30). Development of Treg cells occurs normally in animals lacking SAP-1, in contrast to positive selection, but remains dependent on Raf signalling. While SAP-1 is required for Treg cells to exhibit normal levels of Egr-1 induction in response to CD3 crosslinking, this is not associated with impaired Treg function, as assessed by in vitro suppression assays or the ability to suppress colitis in a T cell transfer model. These findings show that signalling to SAP-1 plays different roles in selection of both SP T cells and Tregs, and is not required for the regulatory function of Treg cells.
MATERIALS AND METHODS
Mice
SAP-1 deficient mice and mice expressing a dominant negative Raf transgene have previously been described (4, 30). Mice were maintained in specific pathogen-free conditions in the Cancer Research UK Biological Resources Unit. Reconstitution experiments were performed as previously described (4). Animal experimentation was approved by the Cancer Research UK Research Services Animal Ethics Committee.
Flow cytometry
Cells were prepared from thymi, spleens, lymph nodes and bone marrow of 6-10 week old mice. Antibodies against CD4 (RM4-5), CD25 (7D4), CD90.2 (53-2.1), CD45.1 (A20), CD45.2 (104), CD152 (UC10-4F10-11) and CD103 (M290) for flow cytometry were purchased from BD Pharmingen. Antibodies against GITR were purchased from R&D Systems, CD8a (5H10) was purchased from Caltag and Foxp3 staining kit was purchased from ebioscience. For intracellular staining, cells were fixed in 4% paraformaldehyde and permeabilized in 0.3% Saponin, 5% FCS, 10mM HEPES, pH7.4 in PBS and were stained in 0.1% Saponin. Foxp3 staining was performed to manufacturer’s protocol. Cells were analyzed on a FACSCalibur (Becton Dickinson) with CellQuest software. Cells were sorted by either a MoFlo sorter (DakoCytomation) or a FACSAria (Becton Dickinson) to greater than 95% purity.
Cell stimulation
T cells were resuspended in RPMI medium with 10% FCS and 50μM 2-mercaptoethanol. Cells were stimulated as previously described (4). Briefly, for short timecourses, thymocytes were stimulated with 10μg/ml of α-CD3 (2C11), and crosslinked by the addition of 75μg/ml goat anti-hamster. Stimulation was terminated by fixation with 4% paraformaldehyde in PBS or into lysis buffer for RNA/protein preparation. For longer timecourses, thymocytes were spun directly onto plates coated with 10μg/ml α-CD3. In some cases irradiated T cell depleted splenocytes were used as APCs and added at 1:2 ratio of T cell:APC along with 20ng/ml IL-2 (Chiron). Intracellular staining for p-ERK and Egr-1 was preformed as previously described (4). Immunoblotting was done by standard techniques with detection by monoclonal anti-MAP kinase, activated (Sigma) and anti-MAP kinase (Sigma).
In vitro Suppression Assays
CD4+CD25- and CD4+CD25+ T cell populations were sorted as described above to >95%. For thymidine incorporation assays, CD4+CD25- T cells (2.5×104) were cocultured with irradiated T cell-depleted splenocytes (5×104) as APCs, various ratios of CD4+CD25+ T cells and 2μg/ml soluble α-CD3 for 72 hours at 37°C. Cultures were pulsed with 1μCi/well [3H] thymidine for the last 6 hours. After 72 hours incubation, cells were harvested and analyzed. For CFSE labelled assays, CD4+CD25- from SJL (CD45.1) were stained with 5mM CFSE for 5 min at 37°C and then washed three times in media. These cells were then co-cultured as above with CD4+CD25+ and irradiated T cell-depleted splenocytes from BL6 mice (CD45.2). After 72 hours, cells were analysed by FACS, with the CD45 marker being used to identify the effector cells.
T cell transfer experiments
Splenocytes were first enriched for CD4+ T cells through magnetic separation according to manufacturer’s instructions. Antibodies to CD8, Mac-1 and B220 were purchased from BD Pharmingen. Anti-Rat Ig magnetic beads were purchased from Dynal. Enriched CD4+ T cells were then sorted for CD4+CD45RBhiCD25- and CD4+CD45RBlowCD25+ populations. Rag2-/- mice were injected intraperitoneally with 4×105 CD4+CD45RBhiCD25- T cells alone or were coinjected with 2×105 CD4+CD45RBlowCD25+ T cells. Mice were observed daily and weighed weekly, any mice showing clinical signs of severe disease were sacrificed accordingly.
Histology
Tissue sections were stained with H&E as well as alcian blue (31). Colitis severity was graded semiquantitatively from 0 to 4 as described (16).
Gene Expression Analysis
RNA was prepared using RNeasy Mini kit (Qiagen) according to manufacturer’s instructions. 10-50ng of RNA was reverse transcribed using Superscript III reverse transcriptase (Invitrogen) according to manufacturer’s protocol. cDNA was analysed by real-time PCR using TaqMan probes (Applied biosystems) or sybr green incoporation (Invitrogen). Expression levels normalised to GAPDH or HPRT. PCR for Foxp3 and HPRT was performed using published primers and probes (13). PCR for the TCFs and GAPDH was performed using the following primers and probes: SAP-1 primers 5′-ACAACGCCTGCCAAAAAGC, 5′-GAAAGACTAGGGCTCGTTGC; probe 5′-FAMATCGAGCCTGTCGCTGCTGCCT; Elk-1 primers 5′-TCACGGGATGGTGGTGAGT, 5′-GTTCTTGCGCAGTCCCCAT; probe 5′-FAMCAAGTTGGTGGATGCAGAGGAGGTGG; Net primers 5′-GATGGCGAGTTCAAGCTCCT, 5′TGGTCTTGTTCTTGCGGAGGC; probe 5′-FAMAAGGCAGAAGAAGTGGCCAAGCTGTG; GAPDH primers 5′-ACAACTTTTGGCATTGTGGAAG, ACAGTCTTCTGGGTGGCAG; GAPDH probe 5′-VIC-CTCATGACCACAGTCCATGCCAT. Egr-1 was measured using SYBR green and the following primers were used: 5′-ATTGATGTCTCCGCTGCAGATC and 5′-TCAGCAGCATCATCTCCTCCA.
Electrophoretic mobility-shift analysis
EMSA was performed as described (32). Cells (0.5×106) were activated with 50ng/ml PDBu for 10 min at 37°C following pre-treatment with UO126 (20μM, 10 min) as necessary. Cells were lysed in 20 mM HEPES (pH 7.9), 10% glycerol, 0.4 M NaCl, 0.4% Triton X-100, 10mM EGTA, 5 mM EDTA, 1 mM dithiothreitol, 0.1 μg of okadaic acid/ml, and protease inhibitors. Binding reactions contained 1μl of extract in 20 μl 10 mM Tris-HCl [pH 7.9], 50 mM NaCl, 1 mM EDTA, 3 mM dithiothreitol, 50 ng/ml ovalbumin, 50 ng/ml poly(dI-dC)·poly(dIdC), 10% Ficoll 400, protease inhibitors; recombinant SRF DNA binding domain (SRF[133-265]) and 1 ng c-fos promoter probe and were incubated for 30 min at room temperature. SAP-1 antibody-induced supershifts were performed by adding anti-SAP-1 (SC-13030X, Santa Cruz Biotechnology) and incubating at room temperature for a further 30 min. All reactions were incubated for a total of one hour. Probe was generated by PCR as described previously (33) with primers p10 (5′ CGCACTGCACCCTCGGTGTTGGCTGC 3′) and p11 (5′ ATGGCTCCCCCCAGGGCTACAGGGAAAG 3′). Complexes were resolved in a 5% 37.5:1 acrylamide-bis-acrylamide-0.5 Tris-borate-EDTA gel.
Statistical analysis
Where indicated analysis was performed using unpaired t-Test except for colitis scores, which were analysed using Mann Whitney Test.
RESULTS
SAP-1 deficient mice show normal numbers of thymic Tregs
We previously showed that SAP-1 deficiency results in an approximately two-fold decrease in the proportion of CD4 SP thymocytes with no appreciable change in thymic cellularity (4). To investigate the role of SAP-1 in Treg cell development, we analysed Foxp3+ T cells within the CD4 SP population. There was no significant difference between the WT and SAP-1 deficient animals in the absolute numbers of CD4+Foxp3+ T cells (0.36×106 ± 0.03 vs 0.42×106 ± 0.13), in contrast to the reduction in numbers of CD4+Foxp3- SP T cells (8.54×106 ± 0.67 vs 5.56×106 ± 0.72, p=0.01) (Figure 1B). Thus, the proportion of Foxp3+ T cells increased by approximately two fold in SAP-1 deficient animals (4.0% ± 0.1 vs 8.7% ± 0.5, p<0.0001)(Figure 1A). Furthermore when cells were additionally gated for TCRhi expression, no reduction in absolute numbers of CD4+Foxp3+TCRhi cells was observed in SAP-1 deficient animals, although a substantial reduction in the absolute numbers of CD4+Foxp3-TCRhi cells was detected (8.1×106 ± 0.5 vs 4.0×106 ± 0.5 p=0.002)(Figure 1C). The positive selection defect in SAP-1 deficient animals results in lower numbers of CD4+ and CD8+ T cells in secondary lymphoid organs (4). As in the thymus, the proportion of CD4+Foxp3+ T cells in spleen and lymph node increased almost two-fold (Spleen: WT 12.7% ± 0.8, SAP-1-/- 24.1 ± 1.6 (p<0.0001); LN: WT 13.8% ± 0.5, SAP-1-/- 23.1% ± 1.3 (p<0.0001)). Thus, in contrast to the development of conventional CD4 SP T cells, the development of CD4+Foxp3+ thymocytes appears to be unaffected by the loss of SAP-1.
Figure 1. CD4+Foxp3+ T cells develop in SAP-1 deficient and DN Raf animals.
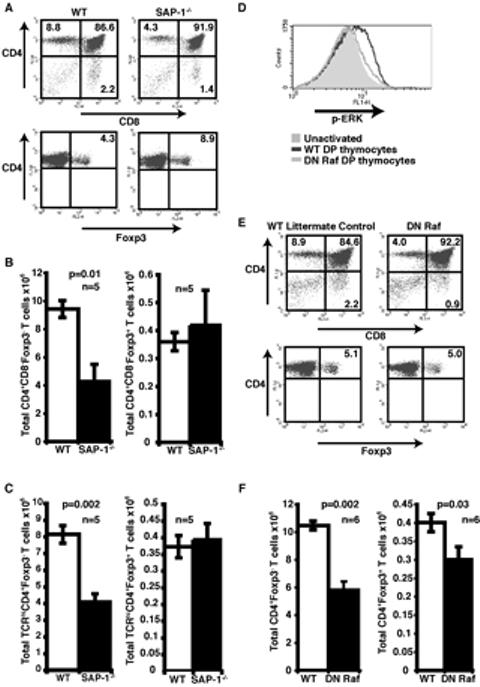
(A) Thymocytes from wild-type and SAP-1-/- mice were stained for CD4, CD8 and Foxp3. Analysis in lower panels was performed on CD4+ SP T cells. Percentage of cells residing within quadrants is shown on the dot plots. (B) Absolute numbers of CD4+CD8-Foxp3- T cells (left panel) and CD4+CD8-Foxp3+ T cells (right panel) were quantified for both WT and SAP-1-/- mice. White bars, wild-type; black bars, SAP-1-/-. (C) Cells were gated on TCRhi and then analysed for expression of CD4 and Foxp3. Absolute numbers of TCRhiCD4+T cells (left panel) and TCRhiCD4+Foxp3+ T cells (right panel) were quantified as in (B). White bars, wild-type; black bars, SAP-1-/-. (D) DP thmocytes were activated by α-CD3 crosslinking for 2 mins and then stained for p-ERK. Grey shaded area, unactivated; dark grey line activated WT; light grey line, activated DN Raf. (E) Thymocytes from wild-type and DN Raf mice were stained for CD4, CD8 and Foxp3 and analysed as in (A). Percentage of cells residing within quadrants is shown on the dot plots. (F) Absolute numbers of CD4+CD8-Foxp3- T cells (left panel) and CD4+CD8-Foxp3+ T cells (right panel) were quantified for both WT and DN Raf mice. White bars, wild-type; black bars, DN Raf.
DN Raf mice display defective development of CD4+Foxp3+ thymocytes
To gain further insight into the role of SAP-1 linked signal pathways in Treg development we analysed thymocytes from transgenic mice expressing a dominant interfering Raf derivative (DN Raf). This protein interferes with Ras-ERK signalling, and consistent with this, these animals were previously shown to exhibit a severe positive selection defect (30); in addition, DN Raf expression is likely to inhibit Mst2- and Ask1-induced apoptosis, since these kinases interact with its regulatory domain (34, 35). We used FACS analysis for activation-loop-phosphorylated ERK to demonstrate that the DN Raf transgene indeed substantially inhibits ERK activation upon TCR crosslinking in DP thymocytes (Figure 1D). DN Raf thymocytes exhibited a slight increase in thymic cellularity (WT 133.8 ± 5.5 ×106, DN Raf 161.4 ± 8.4 × 106; p<0.05) together with an approximately two-fold reduced proportion of CD4+FoxP3- SP cells, comparable to that observed in animals lacking SAP-1 (Figure 1E). In contrast to animals lacking SAP-1, however, the proportion of CD4+Foxp3+ T cells in DN Raf animals was not significantly different from WT littermates (4.5 ± 0.3% vs 5.6 ± 0.5%; n=6). Thus, DN Raf expression reduces the absolute numbers of DN Raf CD4+Foxp3+ thymocytes compared to WT littermates (0.40 ± 0.02 ×106 vs 0.30 ± 0.04 × 106, p=0.03; n=6) (Figure 1F). Taken together with data in the previous section, these results suggest that although Treg development involves Raf signalling it does not require the nuclear ERK effector SAP-1.
Normal CD4+CD25+ T cell markers in SAP-1-/- and DN Raf animals
Previous studies have shown that CD4+Foxp3+ cells account for the majority of thymocytes expressing the activation marker CD25 (12-14). The effects of SAP-1 inactivation or DN Raf expression on the proportion of CD4+CD25+ thymocytes within the CD4+ population mirrored those seen with the CD4+Foxp3+ population, increasing by approximately two fold in animals lacking SAP-1 animals (3.8% ± 0.3 vs 7.2% ± 0.8; p=0.0002) and remaining the same in DN Raf animals (4.2% ± 0.2 vs 4.7% ± 0.4) (Figure 2A). Examination of thymocyte RNA by quantitative RT-PCR showed that Foxp3 mRNA was barely detectable in CD4+CD25- cells but was present at high levels in CD4+CD25+ thymocytes; inactivation of SAP-1 had no effect on this differential mRNA expression (Figure 2B). Approximately 90% of CD4+CD25+ thymocytes expressed Foxp3 protein, as assessed by intracellular staining, consistent with previous reports that the majority of Treg cells are found within the CD4+CD25+ population. Again, deletion of SAP-1 or expression of DN Raf had no effect on Foxp3 protein expression (Figure 2C, top panel). Similar results were observed upon analysis of peripheral lymphoid organs (Spleen: WT 95.1% ± 0.8 Foxp3-positive CD4+ cells, SAP-1-/- 95.1% ± 1.9; DN Raf 86.1% ± 3.3; LN: WT 89.8% ± 3, SAP-1-/- 89.5% ± 2.7, DN Raf 88.6% ± 0.6). Furthermore, bone marrow reconstitution experiments indicated that the apparently normal development of SAP-1 deficient CD4+CD25+ T cells was cell autonomous (data not shown).
Figure 2. SAP-1-/- and DN Raf CD4+CD25+ express high levels of regulatory T cell markers.
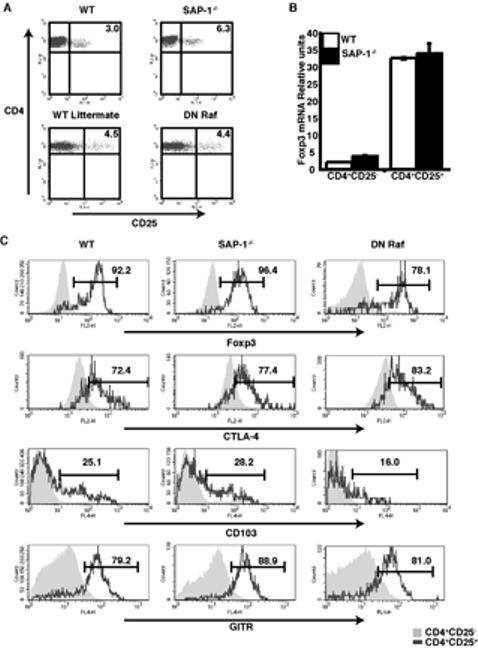
(A) Thymocytes from wild-type, SAP-1-/- and DN Raf mice were stained for CD4, CD8 and CD25. Percentage of cells residing within quadrants is shown on the dot plots. (B) Relative levels of Foxp3 mRNA expression normalised to GAPDH expression in CD4+CD25- and CD25+ thymocytes. White bars, wild-type; black bars, SAP-1-/-. (C) CD4+CD25+ thymocytes from wild-type, SAP-1-/- and DN Raf animals express high levels of Treg markers. Thymocytes stained for Foxp3 (top row) CTLA-4 (second row), CD103 (third row) and GITR (bottom row). Grey shaded area, CD4+CD25-; grey line CD4+CD25+.
Thymic CD4+CD25+ cells also express a number of surface markers associated with the Treg phenotype, including CTLA-4, GITR and CD103. These surface molecules are all more highly expressed on regulatory T cells than on naïve CD4+ T cells, although they can be induced upon activation of naïve T cells. Both thymic and peripheral SAP-1-/- and DN Raf CD4+CD25+ T cells expressed all of these markers at a similar level to WT CD4+CD25+ T cells (Figure 2C; data not shown) and at a higher level than their CD25- counterparts. Taken together these data indicate that the Treg cells from animals deficient for SAP-1 or expressing the DN Raf transgene appear normal, and we therefore used CD4+CD25+ cells for further functional studies.
SAP-1 is the predominant TCF in Regulatory T cells
Previous studies have shown that the suppressive effects of Treg cells in in vitro co-culture assays are dependent on activation of the Treg TCR (18-20). As a prelude to functional studies we therefore examined TCR-mediated activation of immediate-early gene expression in CD4+CD25+ cells. SAP-1 is the major TCF family member expressed in thymus; accounting for approximately 70% of TCF mRNA in total CD4+ thymocytes, with the remainder comprising 20% Net and 10% Elk mRNA, as determined by RNase protection analysis (4). RT-PCR analysis indicated that the relative expression levels of the three TCF family members are similar in CD4+CD25+ and CD4+CD25- thymocytes, with SAP-1 accounting for 80% of the TCF transcripts (Figure 3A). Deletion of SAP-1 did not result in compensatory upregulation of other TCF family members in CD4+CD25+ thymocytes (Figure 3B).
Figure 3. TCFs are expressed in Tregs but responses to TCR stimulus are reduced.
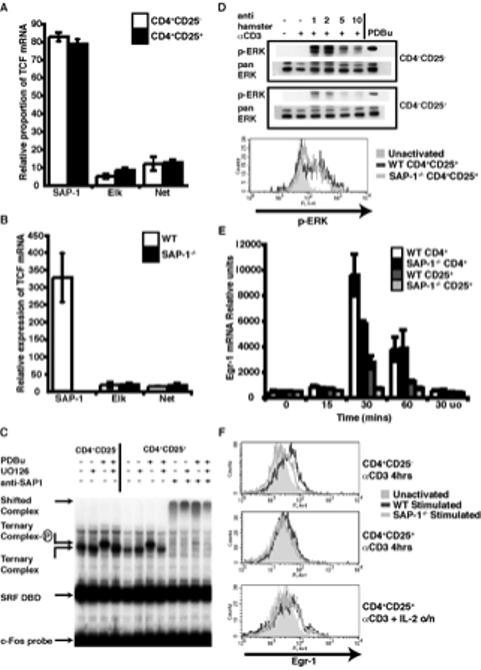
(A) Relative contribution of the three TCF mRNAs in thymic Tregs compared with conventional CD4+ thymocytes. Expression measured by real-time RT-PCR and normalised to GAPDH White bars, CD4+CD25-; black bars, Tregs. (B) Relative expression of TCF expression in wild-type and SAP-1-/- Tregs as measured by real-time RT-PCR shows no compensatory increase in Elk-1 or Net mRNA upon loss of SAP-1 expression. White bars, wild-type; black bars, SAP-1-/-. (C) Gel mobility shift assay performed with c-Fos serum response element probe on peripheral CD4+CD25- or CD4+CD25+ T cell extract with or without PDBu stimulation, in the presence of recombinant SRF DNA binding domain. MEK inhibitor UO126 or anti-SAP-1 antibody were added to the reactions where indicated. Identities of the complexes are indicated. (D) p-ERK levels measured by western blot (top panels) and intracellular staining (bottom panel) upon TCR activation by α-CD3 crosslinking of peripheral and thymic Tregs. Intracellular staining: grey shaded area, unactivated; grey lines, α-CD3 crosslinking for 2mins dark grey - WT CD4+CD25+ thymocytes, light grey SAP-1-/- CD4+CD25+ thymocytes. (E) Egr-1 mRNA induction upon TCR activation measured by real-time RT-PCR and normalised to HPRT. (F) Egr-1 protein levels measured by intracellular staining. Top panel, α-CD3 activation of CD4+CD25- thymocytes (4hrs); middle panel, α-CD3 activation of CD4+CD25+ thymocytes (4hrs); bottom panel, overnight stimulation of CD4+CD25+ thymocytes with α-CD3, APCs and IL-2.
To assess the TCF activity in Treg cells at the biochemical level, peripheral T cell extracts were analysed by gel mobility shift assay, in which formation of ternary complexes between TCFs and the DNA-binding domain of their transcription factor partner SRF can be measured directly (36). In both CD4+CD25- and CD4+CD25+ populations, a well-defined ternary complex was formed on the SRF DNA-binding domain, which antibody supershift analysis confirmed to contain predominantly SAP-1 (Figure 3C). Extracts from PDBu-stimulated cells generated a ternary complex of reduced mobility, characteristic of phosphorylation of the SAP-1 C-terminus (4). No reduction in mobility was observed with extracts of cells stimulated with PDBu in the presence of U0126 indicating that it occurs through activation of MEK-ERK signalling (Figure 3C). Similar results were obtained when complex formation on endogenous SRF was analysed (data not shown). Thus, as in total thymocyte extracts (4), the majority of TCF activity in CD4+CD25+ extracts is accounted for by SAP-1, and activation of ERK in these cells induces its C-terminal phosphorylation.
TCR activation in CD4+CD25+ T cells induces immediate-early gene expression
We next tested whether TCR activation induces ERK activation in CD4+CD25+ T cells. TCR signalling was triggered by antibody cross-linking of surface bound α-CD3 and levels of activation-loop-phosphorylated ERK were then measured by immunoblot. Rapid and transient activation of ERK occurred in both CD4+CD25- and CD4+CD25+ T cell populations, although the degree of activation appeared less in CD4+CD25+ cells than in CD4+CD25- cells (Figure 3D, top). As previously shown for conventional DP thymocytes, the level of ERK phosphorylation in CD4+CD25+ cells was unaffected by the deletion of SAP-1, as assessed by intracellular staining for activated ERK (Figure 3D bottom).
We next examined expression of the TCF target gene Egr-1. CD4+CD25- and CD4+CD25+ populations were activated and levels of Egr-1 mRNA were assessed by quantitative RT-PCR. Maximal induction of Egr-1 transcripts in CD4+CD25- T cells was observed 30 minutes following stimulation. In CD4+CD25+ cells, stimulation activated Egr-1 with similar kinetics, but to a lower maximal level consistent with the lower level of ERK activation in these cells; activation was substantially reduced in cells lacking SAP-1 (Figure 3E). In both CD4+CD25- and CD4+CD25+ cells induction was blocked by the MEK inhibitor UO126, indicating that it was ERK-dependent (Figure 3E). In wildtype CD4+CD25- cells significant up-regulation of Egr-1 protein occurred by 4 hours following stimulation, as assessed by intracellular staining, and this was significantly reduced upon deletion of SAP-1 (Figure 3F top). In contrast no up-regulation of Egr-1 protein was detectable in CD4+CD25+ cells at this time (Figure 3F middle panel). Previous reports have demonstrated that CD4+CD25+ cells become proliferative in vitro following TCR activation in the presence of exogenous IL-2 (37). Indeed a small increase in Egr-1 protein level was observed in CD4+CD25+ following activation and overnight culture with added IL-2 and APCs, although this was not affected by deletion of SAP-1 (Figure 3F, bottom).
SAP-1 deficient and DN Raf Tregs are suppressive in vitro
CD4+CD25+ cells can inhibit proliferation of CD4+CD25- effector cells in a suppressive interaction that requires cell contact and activation of their TCR (18-20). Thymic CD4+CD25+ and CD4+CD25- T cells were mixed at a range of ratios in the presence of anti-CD3 and T-cell depleted irradiated splenocytes and incubated for 72 hours. Proliferation of the effector cells was assessed by either thymidine incorporation or by CFSE dilution. No significant proliferation of either wildtype or SAP-1 deficient CD4+CD25+ T cells alone was detectable in this assay, while CD4+CD25- cells of each genotype proliferated efficiently (Figure 4A). As the CD4+CD25+ / CD4+CD25- ratio was increased, proliferation of the CD4+CD25- cells was inhibited, being effectively blocked at 1:1 ratio. No significant difference could be detected in the abilities of WT and SAP-1 deficient CD4+CD25+ cells to suppress the proliferation of WT CD4+CD25- T cells, whether assessed by thymidine incorporation or CFSE dilution (Figure 4A). Similar results were obtained when SAP-1 deficient CD4+CD25- T cells were used as effector cells, and with CD4+CD25+ and CD4+CD25- T cell populations purified from lymph nodes (data not shown). CD4+CD25+ T cells purified from lymph nodes of DN Raf mice behaved similarly in this assay, suppressing proliferation of both wildtype and DN Raf CD4+CD25- T cells virtually completely at 1:1 ratio (Figure 4B and data not shown).
Figure 4. In vitro suppression assays.
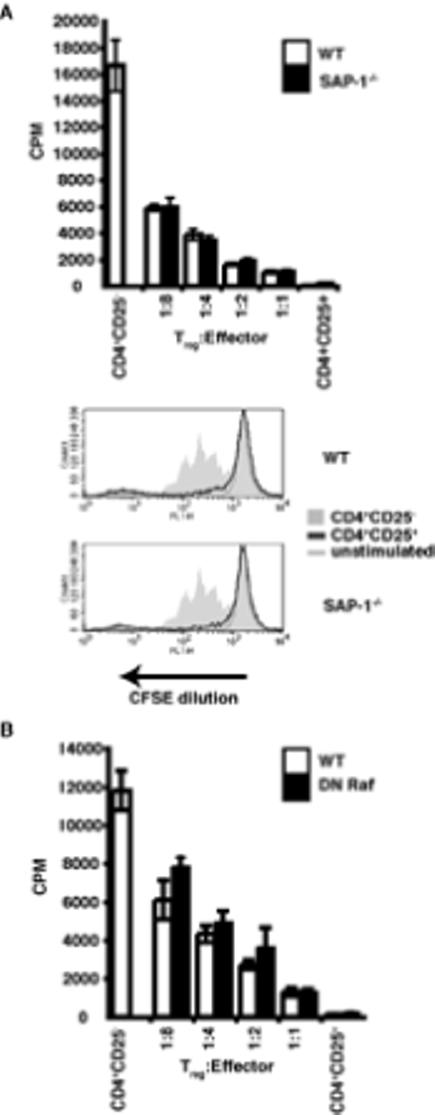
(A) Thymidine incorporation assays were used to assess in vitro suppressive function at a range of effector:Treg ratios. Thymic CD4+CD25- effectors and Tregs were used. +/+, wild-type; -/-, SAP-1 deficient, white bars, wild-type; black bars, SAP-1-/-. CFSE suppression assays performed at a 1:1 ratio of Teffector:Treg. Grey shaded area, activated effectors at 72hrs; dark grey line, 1:1 ratio of Teffectors:Tregs; light grey line, unstimulated effectors. Top panel wild-type thymocytes, Bottom panel SAP-1-/- thymocytes (B) Suppression assay, assessed by thymidine incoportaion after 72hrs. White bars, wild-type; black bars, DN Raf T cells. CD4+CD25- effectors and Tregs were taken from lymph nodes.
SAP-1 deficient CD4+CD25+ cells are functional in vivo
Finally we investigated whether the SAP-1-/- Tregs were capable of suppression in vivo using the adoptive transfer colitis model (38-40). In this system transfer of naïve CD4+RBhiCD25- T cells into immunodeficient hosts such as Scid or Rag-/- mice induces colitis, characterised by weight loss and inflammation of the colon. Induction of colitis in this model is dependent on gut intestinal flora, and can be driven by IL-23 (41). Development of colitis can be prevented by co-injection of CD4+CD25+ regulatory T cells, and apparently reversed by CD4+CD25+ cell transfer 4 weeks subsequent to the initial transfer (38). In contrast to the contact-dependent mechanism of suppression in vitro, suppression of colitis in this model by regulatory T cells is dependent on TGFβ and on IL-10 (42, 43).
Rag2-/- mice were injected with naïve wildtype CD4+RBhiCD25- T cells either alone or in combination with wildtype or SAP-1 deficient CD4+CD25+ T cells. Animals injected with effector cells alone, but not those co-injected with CD4+CD25+ T cells, began losing weight by 4 weeks (Figure 5A). Weight loss observed at 40 days post transfer was significantly prevented by the co-transfer of Tregs at a ratio of 1:2 (WT CD4+CD25+ p=0.02, SAP-1-/- CD4+CD25+ p=0.02). Because wasting does not necessarily correlate with severity of colitis, we performed histological analysis assessing pathology according to the level of T cell infiltrates, loss of colon architecture, and depletion of goblet cells. In mice injected with naïve CD4+CD45RBhiCD25- T cells alone, histological analysis showed clear changes in colon architecture involving elongation of crypt length; substantial lymphocyte infiltration was also apparent (Figure 5B, left). In contrast, animals receiving a co-injection of wildtype CD4+CD25+ T cells showed no such changes (Figure 5B centre). Similarly, staining with Alician Blue revealed loss of goblet cells in those animals injected with WT naïve effectors alone but not those in which wildtype CD4+CD25+ T cells were cotransferred (Figure 5C centre). Pathology was quantified using a standard colitic scoring scheme (0 to 4 with increasing severity). All of the animals injected with CD4+CD45RBhiCD25- T cells alone developed marked colitis (score of 2 or over) whereas transfer of wildtype CD4+CD25+ T cells at 1:2 ratio to effectors prevented development of disease. Similar results were observed with SAP-1 deficient CD4+CD25+ cells (Figure 5C). A two-fold lower dose of CD4+CD25+ cells (1:4 Treg: Teffector ratio), from either wt or SAP-1-/- mice also reduced the incidence of severe disease (Figure 5C). These results indicate that CD4+CD25+ T cells lacking SAP-1 retain suppressor activity both in vitro and in vivo.
Figure 5. SAP-1-/- Tregs are function in vivo.
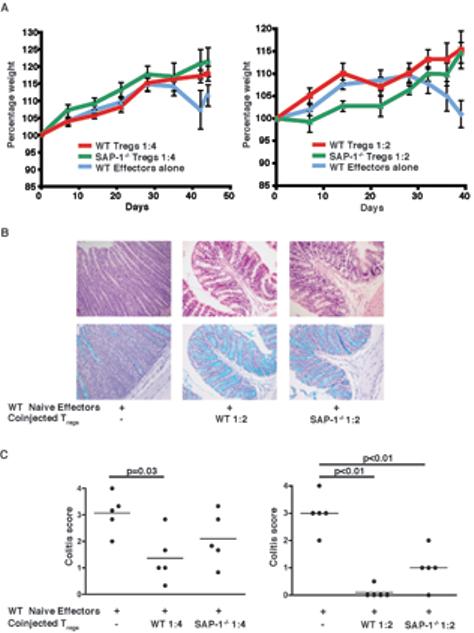
T cell transfer experiments were used to assess the in vivo function of Tregs through prevention of colitis induction in recipient mice. Rag2-/- mice received either WT naïve effectors alone or were coinjected with wild-type or SAP-1-/- Tregs and assessed for presence of disease. Left, Treg:Teffector 1:4; right Treg:Teffector 1:2. (A) Data represent the percentage of initial body weight. Blue line, WT naïve effectors alone; red line, WT Tregs co-transferred with WT effectors; green line, SAP-1-/- Tregs co-transferred with WT effectors. Weight loss was significantly prevented by co-transfer of CD4+CD25+ T cells (p=0.02 for both WT and SAP-1-/-) at 1:2 Treg:Teffector ratio (right panel) (B) Histological sections of distal colon. Left panels, naïve wild-type effectors only; middle panels, co-transfer of wild-type Tregs with effectors (1:2); right panels co-transfer of SAP-1-/- Tregs with effectors (1:2). Upper panels, H & E staining; lower panels, Alcian Blue staining for goblet cells. (C) Colitis scores: left, Treg:Teffector 1:4; right Treg:Teffector 1:2.
DISCUSSION
In this work we used two animal models to study the relationship between thymocyte positive selection and the development of CD4 regulatory T cells. Animals lacking the nuclear ERK effector SAP-1, a member of the TCF family of Ets domain proteins, exhibit a 50% reduction in positive selection (4). Despite this, in SAP-1 deficient animals we found no defect in development of regulatory T cells, which appeared normal according to expression of Foxp3 and of other markers associated with Treg cells including CD25, GITR, CTLA-4 and CD103. In contrast, we found that T cell-restricted expression of a dominant interfering DN Raf transgene (30) inhibited both positive selection and CD4+Foxp3+ cell development to a comparable extent, although it did not affect Treg suppressive function in vitro.
DN Raf, which comprises the N-terminal regulatory domain of Raf, exerts its effects by titrating Ras, its upstream regulator, and we confirmed that its expression inhibits ERK signalling. However, DN Raf also binds two pro-apoptotic MAPKKKs, Mst2 and Ask1 (34, 35). Two considerations lead us to propose that its effects on Treg development are due to its effects on ERK signalling, however. First, studies by the Hedrick group have shown that thymocyte-specific inactivation of both ERK1 and ERK2 reduced CD69hiTCRhi thymocyte numbers by >99% (2). Since Treg cells represent around 4% of the mature CD4+ thymocyte population it is therefore likely that Treg generation is also impaired in these animals. Second, the Raf N-terminus acts to inhibit the pro-apoptotic function of Ask1 and Mst2 (34, 35), and so its expression might if anything be expected to increase rather than decrease cell numbers. We thus favour the interpretation that Treg development requires Ras signalling to ERK but is independent of the nuclear ERK effector SAP-1, or SAP-1 target gene expression.
The two other TCF family members, Elk-1 and Net, are also targets for ERK signalling, and are also expressed in thymus, albeit at lower level than SAP-1. Animals lacking Elk-1 or Net show no obvious signs of autoimmunity (44, 45), and possess normal numbers of CD4+Foxp3+ thymocytes; moreover, simultaneous inactivation of Elk-1 and SAP-1, or Net and SAP-1, does not affect Treg numbers, even though the positive selection defect is even more pronounced in the Elk-1-/- SAP-1-/- animals (JW, RN, PC, A. Nordheim, B. Wasylyk and RT, unpublished observations). It is therefore unlikely that the other TCF family members play specific roles in Treg development, although we cannot rule out the possibility that Treg development requires a low threshold level of TCF activity. In any case, such properties still indicate different signalling requirements for Treg development and positive selection.
A simple interpretation of our findings is that the signalling pathways downstream of the TCR that lead to expression of Foxp3 and commitment to the Treg lineage are different from those involved in positive selection, even though thymic development of CD4+CD25+ Treg cells is dependent on TCR-MHC:self-peptide interactions (21-24, 27). It remains possible, however, that as the strength of signal increases, the dependence of positive selection on ERK-SAP-1 decreases, such that high affinity TCRs, such as those inducing Treg selection, no longer require ERK-SAP-1 signalling to escape death by neglect. According to this view, inactivation of ERK-SAP-1 signalling would differentially affect selection according to the avidity of the TCR-peptide interaction, and it might be interesting to test this idea using transgenic TCR models. Our findings that DN Raf inhibits Treg development raises the possibility that ERK signalling controls activation of Foxp3 expression, but the nature of the link, if any, remains to be determined.
Naturally occurring Tregs have distinct responses to TCR signalling when compared with conventional CD4+ T cells. Initially described as being anergic, recent data has shown that Tregs can proliferate in vitro if exogenous IL-2 is added to the culture (37) and in vivo data has shown that these cells can expand and proliferate. TCR signalling in Tregs thus results in a distinct outcome from that seen in CD4+ T cells. We confirmed that TCR activation in Treg cells results in ERK activation and transcription of the immediate-early gene Egr-1, although to a lesser extent than in CD4+CD25- cells. Indeed at the protein level little Egr-1 induction was observed in the absence of secondary stimulation by IL-2. As in CD4+CD25- cells, SAP-1 is the predominant TCF in Treg cells, and its inactivation results in reduced Egr-1 transcription in response to TCR activation. It is tempting to speculate that the reduced efficiency of IE gene induction in Treg cells underlies their non-proliferation, since reduction of the TCF gene dose in T cells impairs proliferation in response to TCR activation (PC and RT, unpublished observations).
In spite of the reduced activation of immediate-early gene transcription seen in SAP-1 deficient CD4+CD25+ cells, we found that these cells remained fully competent to suppress proliferation of CD4+CD25+ cells in vitro, as did DN Raf CD4+CD25+ cells. CD4+CD25+ cells lacking SAP-1 also retained the ability to inhibit colitis in the T cell transfer model. Together the data suggest that although the Raf/ERK signalling pathway plays a role in the development of Treg thymocytes, it does not appear to be required for their suppressive functions, even though these involve TCR activation.
ACKNOWLEDGMENTS
We thank Cancer Research UK Biological Resources Unit for animal husbandry; Rob Nicolas for genotyping and technical support; Derek Davies and the staff of the LRI FACS facility for cell sorting and technical support; Emma Nye of the LRI Experimental Pathology laboratory for histology; and Caetano Reis e Sousa, Facundo Batista, and members of the Transcriptional Laboratory for helpful discussions and comments on the manuscript.
This research was supported by Cancer Research UK. J. Willoughby was additionally supported by a grant from the MRC. FP and NR are supported by the Wellcome Trust .
Abbreviations used in this paper
- Treg
Regulatory T cell
- SP
single positive
- DN
dominant negative
- p-ERK
phospho-ERK
- WT
wild-type
- TCF
ternary complex factor
REFERENCES
- 1.Alberola-Ila J, Hernandez-Hoyos G. The Ras/MAPK cascade and the control of positive selection. Immunol Rev. 2003;191:79–96. doi: 10.1034/j.1600-065x.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 2.Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Neilson JR, Winslow MM, Hur EM, Crabtree GR. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity. 2004;20:255–266. doi: 10.1016/s1074-7613(04)00052-4. [DOI] [PubMed] [Google Scholar]
- 4.Costello PS, Nicolas RH, Watanabe Y, Rosewell I, Treisman R. Ternary complex factor SAP-1 is required for Erk-mediated thymocyte positive selection. Nat Immunol. 2004;5:289–298. doi: 10.1038/ni1038. [DOI] [PubMed] [Google Scholar]
- 5.Bettini M, Xi H, Milbrandt J, Kersh GJ. Thymocyte development in early growth response gene 1-deficient mice. J Immunol. 2002;169:1713–1720. doi: 10.4049/jimmunol.169.4.1713. [DOI] [PubMed] [Google Scholar]
- 6.Rivera RR, Johns CP, Quan J, Johnson RS, Murre C. Thymocyte selection is regulated by the helix-loop-helix inhibitor protein, Id3. Immunity. 2000;12:17–26. doi: 10.1016/s1074-7613(00)80155-7. [DOI] [PubMed] [Google Scholar]
- 7.Gong Q, Cheng AM, Akk AM, Alberola-Ila J, Gong G, Pawson T, Chan AC. Disruption of T cell signaling networks and development by Grb2 haploid insufficiency. Nat Immunol. 2001;2:29–36. doi: 10.1038/83134. [DOI] [PubMed] [Google Scholar]
- 8.McCarty N, Paust S, Ikizawa K, Dan I, Li X, Cantor H. Signaling by the kinase MINK is essential in the negative selection of autoreactive thymocytes. Nat Immunol. 2005;6:65–72. doi: 10.1038/ni1145. [DOI] [PubMed] [Google Scholar]
- 9.Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NR, Palmer E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 11.Shevach EM. Regulatory T cells in autoimmmunity*. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 12.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 13.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 14.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 15.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 18.Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, Shevach EM. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 20.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 22.Jordan MS, Riley MP, von Boehmer H, Caton AJ. Anergy and suppression regulate CD4(+) T cell responses to a self peptide. Eur J Immunol. 2000;30:136–144. doi: 10.1002/1521-4141(200001)30:1<136::AID-IMMU136>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 24.Kawahata K, Misaki Y, Yamauchi M, Tsunekawa S, Setoguchi K, Miyazaki J, Yamamoto K. Generation of CD4(+)CD25(+) regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J Immunol. 2002;168:4399–4405. doi: 10.4049/jimmunol.168.9.4399. [DOI] [PubMed] [Google Scholar]
- 25.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lerman MA, Larkin J, 3rd, Cozzo C, Jordan MS, Caton AJ. CD4+ CD25+ regulatory T cell repertoire formation in response to varying expression of a neo-self-antigen. J Immunol. 2004;173:236–244. doi: 10.4049/jimmunol.173.1.236. [DOI] [PubMed] [Google Scholar]
- 27.van Santen HM, Benoist C, Mathis D. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. J Exp Med. 2004;200:1221–1230. doi: 10.1084/jem.20041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, Laufer TM. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells. J Exp Med. 2001;194:427–438. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pennington DJ, Silva-Santos B, Silberzahn T, Escorcio-Correia M, Woodward MJ, Roberts SJ, Smith AL, Dyson PJ, Hayday AC. Early events in the thymus affect the balance of effector and regulatory T cells. Nature. 2006;444:1073–1077. doi: 10.1038/nature06051. [DOI] [PubMed] [Google Scholar]
- 30.O’Shea CC, Crompton T, Rosewell IR, Hayday AC, Owen MJ. Raf regulates positive selection. Eur J Immunol. 1996;26:2350–2355. doi: 10.1002/eji.1830261012. [DOI] [PubMed] [Google Scholar]
- 31.Bancroft J. a. M. G. Theory and Practice of Histological Techniques. New York: Churchill Livingstone; 2002. [Google Scholar]
- 32.Murai K, Treisman R. Interaction of serum response factor (SRF) with the Elk-1 B box inhibits RhoA-actin signaling to SRF and potentiates transcriptional activation by Elk-1. Mol Cell Biol. 2002;22:7083–7092. doi: 10.1128/MCB.22.20.7083-7092.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treisman R, Marais R, Wynne J. Spatial flexibility in ternary complexes between SRF and its accessory proteins. Embo J. 1992;11:4631–4640. doi: 10.1002/j.1460-2075.1992.tb05565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Fujii K, Zhang L, Roberts T, Fu H. Raf-1 promotes cell survival by antagonizing apoptosis signal-regulating kinase 1 through a MEK-ERK independent mechanism. Proc Natl Acad Sci U S A. 2001;98:7783–7788. doi: 10.1073/pnas.141224398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Neill E, Rushworth L, Baccarini M, Kolch W. Role of the kinase MST2 in suppression of apoptosis by the proto-oncogene product Raf-1. Science. 2004;306:2267–2270. doi: 10.1126/science.1103233. [DOI] [PubMed] [Google Scholar]
- 36.Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 37.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 38.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 39.Powrie F, Correa-Oliveira R, Mauze S, Coffman RL. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J Exp Med. 1994;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 41.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J Exp Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ayadi A, Zheng H, Sobieszczuk P, Buchwalter G, Moerman P, Alitalo K, Wasylyk B. Net-targeted mutant mice develop a vascular phenotype and up-regulate egr-1. Embo J. 2001;20:5139–5152. doi: 10.1093/emboj/20.18.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cesari F, Brecht S, Vintersten K, Vuong LG, Hofmann M, Klingel K, Schnorr JJ, Arsenian S, Schild H, Herdegen T, Wiebel FF, Nordheim A. Mice deficient for the ets transcription factor elk-1 show normal immune responses and mildly impaired neuronal gene activation. Mol Cell Biol. 2004;24:294–305. doi: 10.1128/MCB.24.1.294-305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


