Abstract
Treatment options for pulmonary arterial hypertension (PAH) have considerably improved in the past few years. Endothelin (ET)-receptor antagonism has been established as a first-line option for the majority of PAH patients. Endothelin-receptor antagonists (ETRAs) comprise sulfonamide and non-sulfonamide agents with different affinities for ET-receptor subtypes (ETA and ETB), and the focus of development has shifted from drugs with less selectivity to those with high selectivity. There is ongoing debate as to whether selective or non-selective ET-receptor antagonism is more beneficial in the treatment of PAH. This paper reviews the current evidence from experimental and clinical studies obtained from a thorough literature search focusing on the three marketed drugs bosentan, sitaxentan, and ambrisentan. A clinically meaningful difference among the three approved ETRAs with respect to their ET-receptor selectivity could not be demonstrated to date. Therefore, in clinical practice, other features are likely to be of greater relevance when considering treatment, such as the potential for serious drug–drug interactions, convenience of dosing schedule, or rates of limiting side effects. These characteristics bear more relation to the chemical or pharmacological properties of the drugs than to receptor selectivity itself.
Keywords: Endothelin, Receptor selectivity, Pulmonary arterial hypertension, ETA/ETB, ETRA, ET-1, Bosentan, Sitaxentan, Ambrisentan
Introduction
Pulmonary arterial hypertension (PAH) is a group of diseases characterized by progressive increases in pulmonary vascular resistance (PVR) and pulmonary arterial pressure (PAP), resulting in right ventricular failure and premature death.1,2 A number of drug classes have been approved for this indication on the basis of randomized, controlled trials, namely prostanoids (epoprostenol, iloprost, and treprostinil), phosphodiesterase-5-inhibitor (sildenafil), and endothelin (ET)-receptor antagonists (ETRAs) (bosentan, sitaxentan, and ambrisentan).3 The ETRA drug class comprises sulfonamide and non-sulfonamide agents with different affinities for endothelin-receptor (ET) subtypes, and there are continuing discussions as to whether selective or non-selective ET-receptor antagonism is more beneficial in the treatment of PAH. This paper reviews the current evidence from experimental and clinical studies obtained from a thorough literature search using the general search terms ‘endothelin receptor antagonist’ and ‘pulmonary (arterial) hypertension’. Particular focus has been placed on clinical articles.
Effects of endothelin-1 mediated via ETA and ETB receptors
The human endothelin (ET) family consists of three 21-amino acid isopeptides: ET-1, ET-2, and ET-3. Of these, only ET-1 plays an important physiological and pathophysiological role, especially in the regulation of vascular tone. ET-1 is released principally from endothelial cells that line blood vessels, but also from other vascular and non-vascular cells. Most of its effects are paracrine, the most striking of which is its extremely potent and long-lasting vasoconstrictor action.4 In addition, ET-1 is profibrotic and involved in the pathogenesis of various diseases, including PAH. Specifically, ET-1 can induce hypertrophy and hyperplasia in various cell types,5 fibroblast proliferation,6 extracellular matrix production,7 inflammation,8 and neuro-humoral stimulation.9 Furthermore, it stimulates the generation of other local mediators of vascular tone, including nitric oxide (NO), prostacyclins, and platelet-activating factors.10 These factors modulate the effects of ET-1 in the cardiovascular system through their vasorelaxant action and anti-proliferative potential.
Circulating plasma ET-1 levels are elevated in atherosclerosis, arterial hypertension, heart failure, and PAH11 when compared with the normal state. Of note, ET-1 plasma levels correlate with parameters of pulmonary haemodynamics,12 and predict survival in patients with untreated PAH.13
ETA- vs. ETB-mediated effects
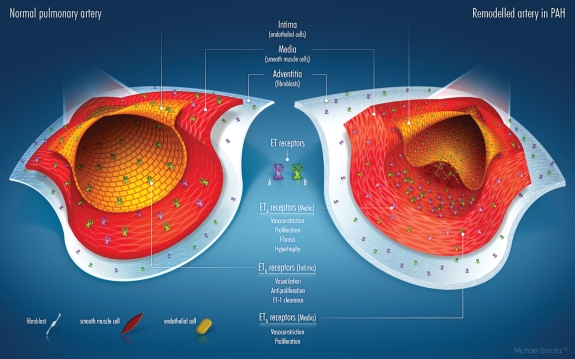
Within the mammalian cardiovascular system, ET-1 acts through two receptor subtypes—ETA and ETB. In the vasculature, ETA receptors are located on smooth muscle cells (SMCs) and fibroblasts, whereas ETB receptors are predominantly localized on endothelial cells and, to a lesser extent, on SMCs, fibroblasts, and macrophages. Recent data using cultured transfected cell lines suggest that ETA and ETB receptors can form constitutive heterodimers (dimerization theory). Functionally, this means that ETB receptors expressed on SMCs couple with ETA receptors, and the former adopt the function of the latter, such that ETB receptors in heterodimers mediate vasoconstriction similar to ETA receptors.14 Furthermore, it has been suggested that selective antagonism of one ET-receptor subtype only may result in compensation by the other receptor. This experimental hypothesis has been called ‘cross-talk’.15,16
Under normal physiological conditions, the receptor types have broadly opposing functions (Figure 1). Activation of ETA receptors mediates vasoconstriction,4 proliferation,17,18 hypertrophy,5,19 cell migration,20 and fibrosis, whereas activation of endothelial ETB receptors stimulates the release of potent vasodilators (NO and prostacyclin), which exhibit anti-proliferative properties, and prevents apoptosis.21 Importantly, ETB receptors on endothelial cells mediate the clearance of circulating ET-1 in the lungs, kidney, and liver, with up to 50% of mature ET-1 in healthy subjects and 40% in patients with PAH cleared via pulmonary ETB receptors.22 Endothelial cell ETB-receptor activation also inhibits ET converting enzyme-1, the enzyme that is required to produce mature ET-1.23
Figure 1.
Schematic of the distribution of ETA and ETB receptors in various layers of the vessel wall of a small pulmonary artery in the healthy state (left) and in PAH (right). In the intima, only ETB receptors are expressed, in the media both ETA and ETB, and in the adventitia only ETA. In the diseased artery, structural changes (intima structure not intact with schematic illustration of plaque; media with smooth muscle cell proliferation, adventitia thickened) are evident. In terms of functional changes, the number/density of ET receptors of both types increases in all vessel layers, however, the ETA receptors to a greater extent.
Alterations in the distribution and number of ETA and ETB receptors in conditions such as PAH suggest that their roles in the disease state may differ from those in normal physiology. For example, there are more ET-1-binding sites in the distal pulmonary vessels of patients with PAH, and ETB receptors are also upregulated.24 ETB receptors may not exclusively mediate pulmonary vasodilatation. Because of the effects of a sub-population of ETB receptors located on SMC and fibroblasts, the spectrum of possible adverse effects of ETB-receptor stimulation in patients with pulmonary hypertension includes the induction of vasoconstriction,15,25 proliferation,24,26 and fibrosis.27
Early suggestions that the endothelium might be dysfunctional, resulting in diminished expression or loss of function of the ETB receptors,28 have recently been challenged by the findings of Langleben et al.,29 who observed intact or only modestly reduced ETB-mediated clearance of ET-1 in patients with pulmonary hypertension of various aetiologies. The authors concluded that the ET-1 levels are increased primarily because of excess synthesis rather than reduced clearance of ET-1.
Receptor selectivity and endothelin plasma levels
Endothelin-receptor antagonists are usually categorized according to their selectivity for ETA or ETB receptors. The ETA pharmacological probe, BQ-123, is considered the benchmark for a selective ETRA, based on an ETA:ETB binding ratio of 2000:1 in a standard in vitro assay.30,31 To some extent, however, the definition of receptor selectivity is arbitrary, given the wide variation in values obtained using different experimental systems. For example, the ETRA ambrisentan has been reported to have an ETA:ETB selectivity ranging from 29:1 for ET-1-mediated contraction in the rat aorta32 to 4000:1 in myocardial membranes.33
An indication of functional selectivity can be gained from observations of the effects of different ETRAs on circulating ET-1 levels in vivo. For example, sitaxentan (in vitro ETA:ETB selectivity >6500:1) acutely decreases ET-1 levels in patients with chronic heart failure,34 indicating that ETB receptors, which play a role in ET-1 clearance, remain functional. In contrast, bosentan and less-selective ETA-receptor antagonists (ETA:ETB ratio <2000:1) increase plasma ET-1 in healthy volunteers and in patients with heart failure or PAH (Table 1). Interestingly, significant increases of ET-1 levels occurring 2 h following ingestion have been reported with ambrisentan (widely reported to be selective for ETA), suggesting that its functional selectivity may differ from that observed in vitro.35 Whether elevated ET-1 levels seen in ETRA-treated PAH patients have pathophysiological or prognostic significance remains unknown.
Table 1.
Effects of endothelin receptor antagonists on ET-1 plasma levels in humans
| Study | Indication | Design | n | Dose | Interval | Effect on ET-1 level |
|---|---|---|---|---|---|---|
| Bosentan | ||||||
| Kiowski et al.101 | CHF | r, pc, db | 24 | 100–200 mg i.v. (single ascending dose) | 1 h | ↑ >2.0× |
| Sütsch et al.102 | CHF | r, pc, db | 36 | 1000 mg b.i.d. oral | 3 h (Day 1) | ↑ >2.0× |
| Chronic 1000 mg b.i.d. | 3 h (Day 14) | ↑ >1.3× | ||||
| Weber et al.103 | Healthy volunteers | r, pc, db | 8 | 3–2400 mg oral | ↑ 2× | |
| 10–750 mg i.v. (single ascending doses) | ↑ 3× | |||||
| Williamson et al.105 | PAH | o | 7 | 50, 150, and 300 mg i.v. | 6 h | ↑ 2× (dose dependent) |
| Hiramoto et al.106 | PAH | o | 7 | 62.5 mg (single oral dose) | 6 h | ↑ 2.0× |
| Sitaxentan | ||||||
| Givertz et al.34 | CHF | o | 47 | 0.5, 3.0, or 6.0 mg/kg | 6 h | ↓ 0.8× |
| Ambrisentan | ||||||
| FDA report35 | Healthy volunteers | o | 7 | 5 mg (single oral dose) | 2 h | ↑ 1.6 pg/mLa |
| 7 | 10 mg (single oral dose) | 2 h | ↑ 1.1 pg/mLa | |||
aPlacebo-subtracted median. CHF, congestive heart failure; db, double-blind; h, hours; i.v., intravenous; o, open; PAH, pulmonary arterial hypertension; pg, picogram; pc, placebo controlled; r, randomized.
Experimental evidence
Endothelin-receptor selectivity and its vasoconstriction and vasodilation effects
Vasodilation is an important goal of therapeutic intervention for PAH. Theoretically, selective ETA-receptor antagonists should be more effective in achieving this than non-selective ETA-/ETB-receptor antagonists, given the role played by ETB receptors in both vasodilation and ET-1 clearance. In animal models of PAH, however, positive dilatory effects have been observed with both selective ETA-receptor blockade and non-selective antagonism36 (see Supplementary material online).
Since direct evaluation of the pulmonary circulation requires invasive procedures, the majority of the available data are extrapolated from human studies performed on blood vessels in the systemic circulation.37,38 Collectively, these studies indicate that: (i) selective ETA-receptor blockade results in a robust vasodilator response and increased blood flow; (ii) selective ETB-receptor blockade results in vasoconstriction and reduced blood flow; and (iii) co-administration of selective ETA- and ETB-receptor antagonists attenuates the vasodilator response relative to selective ETA-receptor blockade (see Supplementary material online). However, although these data provide information regarding the effects of receptor selectivity on blood vessel tone in general, they do not provide precise information on how these drugs work in the pulmonary arterial circulation.
Endothelin-receptor selectivity and its effects on vascular remodelling
Several studies in animal models document that ETRAs, both non-selective39,40 and ETA selective,41–43 prevent, attenuate, or even reverse vascular remodelling and/or hypertrophy. For example, in a rat model, during a 2 week hypoxia exposure, sitaxentan43 and bosentan39 significantly prevented the increase in PAP, and prevented the pulmonary vascular remodelling. During a 6 week hypoxia exposure, both drugs partially reverted pre-established pulmonary vascular remodelling. Interestingly, sitaxentan,43 but not bosentan,39 prevented the increase in ET-1 levels when treatment was initiated early, with hypoxia. In contrast, late treatment, 2 weeks after initiation of hypoxia, did not affect the established elevation of ET-1 level.
Endothelin-receptor selectivity and fibrosis
Extra-vascular anti-mitotic and anti-fibrotic effects of ETRAs may result in greater efficacy in scleroderma than therapies directed exclusively at the vasculature.44–46 Data from animal models using either ETA-selective or non-selective ETRAs47–53 demonstrate an amelioration of ET-1-related effects involving the reduction of growth factor expression, extracellular matrix deposition, and matrix metalloproteinase activity.
In vitro data with skin fibroblasts suggested that targeting both the ETA and the ETB receptors is preferable in order to block collagen type I and III production.54 However, subsequent in vitro data using lung fibroblasts indicate that ET-1 induces collagen matrix contraction through the ETA receptor, but not the ETB receptor.55 Furthermore, while there is evidence that ETB receptors are linked to collagen production in vitro, in vivo animal data with ETA antagonists have shown that they effectively block the accumulation of collagen I, III, and IV,56 normalize pro-collagen I and III mRNA,49 and abolish the effect of ET-1 on pro-collagen metabolism.57 Likewise, although there is evidence that under certain conditions ET-1 can act as a mitogen in vitro through both ETA- and ETB-receptor activation,58 ETB receptors have been shown to inhibit vascular SMC proliferation in vivo.59 It has been suggested that ETB receptors may be up-regulated on SMCs and fibroblasts in certain disease states such as scleroderma lung disease.60 However, the spatial distribution of these receptors among different cell types within the lung microcirculation remains unclear, as does the significance of any increased ETB-receptor expression in PAH.
Clinical trials in pulmonary arterial hypertension patients
ETRAs have been studied in numerous open and several controlled clinical trials in patients with PAH. The differences between the three approved drugs may be because of ET-receptor selectivity, but also linked to other properties, such as pharmacokinetics or drug–drug interactions. Table 2 provides an overview of the pharmacological properties of the three available ETRAs. Patient characteristics and outcomes of the pivotal studies of each agent are shown in Table 3 and discussed below.
Table 2.
Pharmacological and pharmacokinetic characteristics of approved endothelin-receptor antagonists
| Parameter | Bosentan | Sitaxentan | Ambrisentan | |
|---|---|---|---|---|
| Structure | Etherocyclic sulfonamide | Amidothiophene sulfonamide | Diphenyl propionic acid | |
| Selectivity ETA:ETB | 30:1 | 6500:1 | 4000:1 | |
| ET plasma levels after administration | ↑ | ↓ | ↑ | |
| Approved daily dosing | 125–250 mg | 100 mg | 5–10 mg | |
| Titration | Yes | No | Yes | |
| Resorption | ||||
| Absolute bioavailability | ∼50% | 70–100% | High (% not reported) | |
| Food effect on resorption | No | No | No | |
| Time to max. plasma concentration (tmax) (h) | 3–5 | 1–4 | 1.7–3.3 | |
| Distribution | ||||
| Albumin binding (%) | >98 | >99 | 99 | |
| Metabolism and excretion | ||||
| Terminal elimination half-life (h) | 5.4 | 10 | 15 | |
| Steady state (days) | 3–5 | 6 | 3–4 | |
| Metabolism | Hepatic (CYP) | Hepatic (CYP) | Hepatic (CYP and glucuronidation; P-gp) | |
| Cytochromes (CYP) p450 mainly involved | CYP 2C9 ↑, 3A4↑ | CYP 2C9↓ | CYP 3A4↑, 2C19↑ | |
| Excretion in urine (%) | <3 | 50–60 | Low | |
| Significant drug–drug interactions | Sildenafil, glibenclamide, warfain, and cyclosporin A | Warfarin and cyclosporin A | Cyclosporin Aa | |
Table 3.
Characteristics and main outcomes of pivotal studies for approved endothelin receptor antagonists
| Bosentan |
Sitaxentan |
Ambrisentan |
||||
|---|---|---|---|---|---|---|
| Study 351 (Channick et al.61) | BREATHE-1 (Rubin et al.62) | STRIDE-1 (Barst et al.77) | STRIDE-2 (Barst et al.78) | ARIES-1 (Oudiz et al.74 and PI) | ARIES-2 (Olschewski108 and PI) | |
| Selectivity | Oral ETA/ETB antagonist | Oral ETA/ETB antagonist | Oral highly selective ETA antagonist | Oral highly selective ETA antagonist | Oral selective ETA antagonist | Oral selective ETA antagonist |
| Drugs and daily dosages in the study | Placebo/bosentan 125–250 mg | Placebo/bosentan 250 mg/bosentan 500 mg | Placebo/sitaxentan 100 mg/ sitaxentan 300 mg | Placebo/sitaxentan 50 mg/sitaxentan 100 mg/bosentand | Placebo/ambrisentan 5 mg/ambrisentan 10 mg | Placebo/ambrisentan 2.5 mg/ambrisentan 5 mg |
| Setting | US and EUR | US and EUR | US (and 1 centre in Canada) | US and EUR | US and EUR | US and EUR |
| Dosing regimen | 2×/day | 2×/day | 1×/day | 1×/day | 1×/day | 1×/day |
| Study details at inclusion/baseline characteristics | ||||||
| Study duration post-randomization (weeks) | 12 | 16 | 12 | 18 | 12 | 12 |
| Inclusion range for age (years) | ≥18 | ≥12 | ≥16–75 | 12–78 | ≥18 | ≥18 |
| Baseline 6 min walk distance for inclusion (m) | ≥150 and ≤500 | ≥150 and ≤450 | Not defined (only second endpoint) | ≥150 and ≤450 | ≥150 and ≤450 | ≥150 and ≤450 |
| PAH aetiology | IPAH (81%), PAH-SSc (19%) in bosentan group | IPAH (71%), PAH-SSc (23%), PAH-Lupus (6%) | IPAH (53%), PAH-CTD (24%), PAH-CHD (24%) | IPAH (59%), PAH-CTD (30%), PAH-CHD (11%) | IPAH (63%), PAH assoc. with CTD, HIV, anorexigen (37%) | IPAH (65%), PAH assoc. with CTD, HIV, anorexigen (35%) |
| WHO functional class | III (100%) | III (90%), IV (10%) | II (33%), III (66%), IV (1%) | II (37%), III (59%), IV (4%) | I (3%) II (32%), III (58%), IV (7%) | I and II (46%), III and IV (54%) |
| Patient disposition | 36 screened, 32 randomized (2:1). No discontinuations | Screened: n.r., 213 randomized (1:1:1), 14 discontinued | Screened: n.r., 178 randomized (1:1:1), 12 discontinued | Screened: n.r., 247 randomized (1:1:1:1), 31 discontinued | Screened: n.r., 202 randomized (1:1:1). discontinuation n.r. | Screened: n.r., 192 randomized (1:1:1). discontinuation n.r. |
| Males:females (%) | 19:81 (bosentan) | 29:71 (bosentan) | 21:79 | 22:78 | n.r. | n.r. |
| Mean age (years) | 52 (33–73) (bosentan) | 49 (13–80) | 46 (17–74) | 54 (14–78) | n.r. | n.r. |
| Mean 6 min walk distance at baseline (m) | 360 (±86) (bosentan) | 330 (±74) | 398 (±110) | 337 (±80) | 341 (±76) | 348 (±84) |
| mPAP (mmHg) | 54 (±13) (bosentan) | 55 (±16) | 54 (±15) | 48 (±14) | n.r. | n.r. |
| Results at study end in the treatment groups | ||||||
| 6 min walk distance (m)a | −6/70* | −8/27*/47** | −13/22**/20** | −6.5/17.8/24.9/23.0 | −7.8/22.8**/43.6*** | −10.1/22.2*/49.4*** |
| mRAP (mmHg) | 4.9/−1.3** | n.a. | 1/0* | n.a. | n.a. | n.a. |
| mPAP (mmHg) | 5.1/−1.6 * | n.a. | 0 / −3 / −5*** | n.a. | n.a. | n.a. |
| Cardiac output (L/min) | −0.5/0.5*** (CI) | n.a. | 0.0/0.3/0.4*** (CI) | n.a. | n.a. | n.a. |
| PVR (dyn s cm−5) | 191/−223*** | n.a. | 49/−221/−194*** | n.a. | n.a. | n.a. |
| Median change in peak VO2 (mL O2/kg/min)b | n.a. | n.a. | 0.0/0.5/3.1** | n.a. | n.a. | n.a. |
| Improvement in Borg dyspnoea index | 1.4/−0.2 | 0.3/−0.1/−0.6 | n.r. | 0.2/n.r./0.0/n.r. | Ambrisentan yes: details n.r. | Ambrisentan yes: details n.r. |
| Improvement in WHO functional class (%) | 9/43 | 30/43/41 | 15/29/30 | Sitaxentan 100 mg signif. | Ambrisentan yes: details n.r. | Ambrisentan yes: details n.r. |
| Time to clinical worsening | Sign. improved vs. placebo | Sign. improved vs. placebo | n.r. | Sitaxentan 100 mg n.s. (trend to improv.) | PI: significantly delayed (pooled) | PI: significantly delayed (pooled) |
| Incidence of clinical worsening (n) | 27/0* | 14/5/4 | 5/0/2 | 10/6/4/9 | Placebo 7 (10%)/ambrisentan pooled 4 (3%)* | Placebo 13 (22%)/ambrisentan pooled 8 (6%)* |
| LFT elevations >3× ULN (%) | 0/6.3 | 3/4/14 | 3/0/10 | 6/5/3/11 | 3/0/0 | 2/0/0 |
| Peripheral oedema (%) | 5/8c | 5/8c | 17/16/25 | 8/8/11/15 | 10 (placebo), 27 (5+10 mg pooled) | 6/8/13 |
n.a., not applicable (i.e. not done in the study); n.r., not reported; bos, bosentan group only; CI, cardiac index; CHD, congenital heart disease; CTD, connective tissue disease; L, litres; LFT, liver function tests; mPAP, mean pulmonary arterial pressure; PI, Letairis (ambrisentan) prescribing information (in the US); PVR, pulmonary vascular resistance; ULN, upper limit of normal; VO2, oxygen uptake; WHO, World Health Organization.
aPrimary endpoint in all studies with exception of STRIDE-1.
bPrimary endpoint in STRIDE-1.
cData reported for pooled PAH studies in Tracleer® European Public Assessment Report (EPAR).
dOpen-label bosentan arm at the standard dose, i.e., 62.5 mg orally b.i.d. for 4 weeks, then increasing to the maintenance dose of 125 mg b.i.d.
Statistics vs. placebo: *P < 0.05, **P < 0.01, ***P < 0.001.
Bosentan
Bosentan is an orally active, non-peptidic, non-selective, sulphonamide-class ETA/ETB antagonist with twice-daily (b.i.d.) dosing. It was the first ETRA to receive approval for the treatment of patients with PAH in NYHA functional class III (Europe, USA, and Canada) and IV (USA and Canada) at a target dose of 125 mg b.i.d.
In two randomized, controlled trials, bosentan was shown to improve exercise capacity, functional class, haemodynamics, and time to clinical worsening.61,62 Additional open-label, long-term studies in patients with PAH demonstrated persistent efficacy of bosentan over time and potential for improved survival, compared with predicted survival.63,64
Since these first pivotal studies, significant benefits of bosentan treatment have been shown in separate studies ('Bosentan Randomized Trials of Endothelin Antagonist Therapy': BREATHE) in children with PAH65 [BREATHE-3: idiopathic PAH and congenital heart disease (CHD)], in PAH associated with HIV66 (BREATHE-4), in patients with PAH and Eisenmenger syndrome67 (BREATHE-5), and in patients with portopulmonary hypertension.68
In addition, the ‘Endothelin Antagonist tRial in miLdlY symptomatic PAH patients' (EARLY) was the first study specifically designed to evaluate the effects of ETRA treatment in 185 PAH patients in functional class II. Preliminary results from this 6 month trial highlight a significant reduction in PVR while the other primary endpoint, the 6 min walk distance (6MWD), did not reach statistical significance. The secondary endpoint, time to clinical worsening, showed a significant improvement with bosentan, translating into a 70% risk reduction.69
In another group of 157 patients with chronic thrombo-embolic pulmonary hypertension (WHO Group 4), bosentan therapy led to significant reductions in PVR and improved dyspnoea score, while the 6MWD remained unchanged over the 6 month study period (‘BosEntan in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension’: BENEFIT).
Ambrisentan
Ambrisentan is an orally active ETA-receptor antagonist belonging to the propanoic acid class. Although data describing the selectivity of ambrisentan for the ETA receptor vary between 29:132 and >4000:1,33 depending on the assay cited, the drug is considered to be a selective ETA-receptor antagonist.70,71 In the USA, ambrisentan has been approved at a dose of 5–10 mg once daily for PAH patients with WHO functional class II or III symptoms to improve exercise capacity and delay clinical worsening. In Europe, ambrisentan was approved in April 2008 following a positive opinion from the European Committee for Human Medicinal Products for the treatment of PAH patients in functional class II and III.72
Results are based on a 12 week, blinded-to-dose (1, 2.5, 5, or 10 mg daily) Phase II study73 (improvements in 6MWD, functional class, Borg score, quality of life, and pulmonary haemodynamics) and two pivotal studies, ‘AmbRIESentan in patients with moderate to severe PAH’, ARIES-174 and ARIES-2,75 that have not yet been published in full.
The long-term follow-up of patients treated with ambrisentan in the two pivotal studies and the open-label extension (ARIES-E, n = 383) shows that 95% were alive at 1 year and 94% were still receiving ambrisentan monotherapy, with sustained efficacy for 6MWD, dyspnoea score, and functional class.76
Sitaxentan
Sitaxentan sodium, a highly selective ETA-receptor antagonist of the sulphonamide class of ETRA, has received approval for the treatment of PAH patients with WHO functional class III symptoms at an oral dose of 100 mg once daily (European Union, Canada, and Australia). The FDA has not approved sitaxentan to date, and another placebo-controlled study with sitaxentan is currently planned (STRIDE-5) to provide additional data.
The safety and efficacy of sitaxentan in patients with PAH has been clinically tested in the 'sitaxentan to relieve impaired exercise' (STRIDE) programme,70 including three randomized, placebo-controlled pivotal trials (STRIDE-1,77 STRIDE-2,78 and STRIDE-4), two non-controlled studies (Study 211 and STRIDE-6),79 and three long-term studies (STRIDE-1X, STRIDE-2X, and STRIDE-3).
Sitaxentan significantly improved functional class (STRIDE-1, STRIDE-2, STRIDE-4), 6MWD (STRIDE-1, STRIDE-2), dyspnoea score (STRIDE-1), and haemodynamics (Study 211, STRIDE-1). Improvements in time to clinical worsening could only be demonstrated in a post hoc meta-analysis using pooled data from the three pivotal studies.70
Long-term data are available from a small group of patients, suggesting that efficacy and safety are maintained for up to 12 months,80 as well as preliminary data from the extension studies, with mean exposures of 26 (STRIDE-1X70) and 36 weeks (STRIDE-2X81).
Data from subgroup analyses did not exhibit a clinically relevant treatment effect in patients with PAH associated with CHD.70 In contrast, the subgroup of patients with PAH associated with connective tissue disease (CTD) showed an increased 6MWD with sitaxentan treatment.82,83
Endothelin-receptor selectivity and drug efficacy
The most frequent clinical endpoint used to assess drug efficacy in PAH has been exercise capacity, assessed by the 6MWD, although its appropriateness as a measure has been debated.84–86 Moreover, as studies with ETRAs have included different patient populations, it is difficult to judge, using any measure, whether ETA selectivity provides a clinically important benefit for patients with PAH.
6-minute walk distance
Studying the evidence where 6MWD was used as a measure of efficacy, the placebo-corrected improvements from baseline to Week 12 (BREATHE-162 and ARIES-174) or Week 18 (STRIDE-278) were +35, +51, and +31 m for bosentan, ambrisentan, and sitaxentan, respectively (Table 3). A direct comparison is difficult, as BREATHE-1 included only patients in functional class III and IV while ARIES-1 and STRIDE-2 included ≥35% of patients in functional class I and II.
Time to clinical worsening
Significant improvements in the time to clinical worsening have also been reported for all three ETRAs discussed. In BREATHE-1, both the time to and the incidence of clinical worsening were significantly reduced with bosentan compared with placebo.62 In ARIES-1,74 and ARIES-2,75 the differences between ambrisentan and placebo with respect to incidence of and time to clinical worsening reached statistical significance. In a post hoc meta-analysis pooling 512 patients from STRIDE-1, STRIDE-2, and STRIDE-4, significant improvements in time to clinical worsening were seen in patients treated with sitaxentan 100 mg daily compared with placebo;70 this is in contrast to the individual STRIDE-177 and STRIDE-278 studies, where statistical significance was not reached.
Haemodynamics
Since ETA and ETB receptors counter-regulate vascular tone, variations in receptor selectivity could result in different haemodynamic profiles.
The haemodynamic changes from baseline to Week 12 for bosentan and sitaxentan are shown in Table 3. Despite differing study populations, both drugs equally reduced PVR by an average of 220 dynes s cm−5 following a 3 month treatment period; comparable with the decrease of 226 ± 202 dynes s cm−5 reported for ambrisentan.73 Small differences in favour of less-selective ETRAs were observed with respect to right atrial pressure reductions.
Haemodynamic superiority of selective ETA blockade, theoretically mediated by unblocked ETB receptors, cannot be inferred from these data. Likewise, non-selective ETRA blockade does not seem to be associated with clear haemodynamic advantages when indirectly compared with selective blockade.
Survival
There is no definitive study proving a survival benefit for any ETRA, owing to the fact that long-term, placebo-controlled studies are perceived as ethically unjustifiable. Therefore, survival rates for new PAH therapies are generally compared with historical survival rates from patients not receiving PAH-specific drug treatment.87
For patients enrolled in the two placebo-controlled bosentan trials and subsequently followed up for a mean of 2.1 ± 0.5 years, survival estimates were 96 and 89% at 12 and 24 months, compared with a predicted survival of 69 and 57%, respectively.63 At the end of 12 and 24 months, 85 and 70% of patients, respectively, remained on bosentan monotherapy. Another retrospective analysis of 103 consecutive IPAH patients treated with first-line bosentan therapy reported overall survival estimates of 92, 89, and 79% at 1, 2, and 3 years, respectively, compared with a predicted survival of 71, 61, and 51% at these time points64 (85 and 70% on monotherapy at 12 and 24 months, respectively). In this group, 44% of patients received additional intravenous epoprostenol therapy during follow-up.
For ambrisentan, an integrated analysis of 383 PAH patients in ARIES-l, ARIES-2, or ARIES-E reported an l year survival of 95%.76 During long-term, open-label treatment (mean, 1.7 years) of 64 PAH patients treated with ambrisentan, survival in the IPAH subgroup was 89% (67% on monotherapy) compared with a predicted survival of 66%.88,89
Survival data for sitaxentan are available from the STRIDE-2X programme for 145 patients with PAH treated with sitaxentan 100 mg/day.81 At 1 year, survival estimates were 96% for the PAH group and 98% for the subgroup of patients with PAH and CTD. In both groups, additional PAH therapies had been added during this period in 13 and 10% of the patients, respectively.
From these data, differential effects on survival with any of the ETRAs discussed cannot be inferred.
Comparative trials
A unique data set is provided by the STRIDE-2 trial, in which 245 patients were randomized to placebo, sitaxentan (50 or 100 mg q.d.), or bosentan (62.5 mg b.i.d. for 1 month followed by 125 mg b.i.d.).78 The bosentan arm was, however, open label and included only as a comparator arm (events were rater-blinded). At 18 weeks, both sitaxentan 100 mg and bosentan arms showed significant increases in 6MWD, the primary endpoint. Improvements in functional class (secondary endpoint) were observed with sitaxentan 100 mg (P = 0.04). Time to clinical worsening did not improve with either treatment.
After 18 weeks, patients were entered into the extension study STRIDE-2X where patients who received sitaxentan (100 mg) or bosentan during STRIDE-2 continued on their respective therapies, in an open-label fashion. Patients receiving sitaxentan 50 mg daily during STRIDE-2 had their dosages increased to 100 mg daily, and the patients on placebo were assigned to sitaxentan (100 mg daily) or bosentan. Preliminary results of pre-specified analyses for patients treated for up to 1 year (bosentan, n = 84; sitaxentan, n = 145) revealed differences between the treatment arms, with better outcomes for the sitaxentan-treated patients when compared with bosentan therapy in parameters such as risk of discontinuation of monotherapy (25 vs. 45%, P = 0.003) and abnormal liver enzyme levels (4 vs. 14%, P = 0.01). However, no significant differences between both treatment regimens were observed for functional class, 6MWD, or survival.81 Thus, no clinically meaningful differences between these drugs with respect to selectivity-related efficacy were observed.
Selectivity and safety
Clinical side effects
Although ETRAs are generally well tolerated, they are associated with side effects related to their vasodilatory properties including peripheral oedema, headache, and palpitations. Table 4 provides an overview on the incidence of those side effects that have the greatest relevance in everyday clinical care.
Table 4.
Frequent side effects of the three endothelin receptor antagonists in pulmonary arterial hypertension patients according to labelling
| Side effect | Sitaxentan | Bosentan | Ambrisentan |
|---|---|---|---|
| ALT/AST elevations | >3× ULN: 7% for sitaxentan 100 mg/day treated patients (n = 887) vs. 5% of PBO-treated patients (n = 155). | >3× ULN: eight integrated PBO-controlled studies (six other than PAH): 11.2% of the bosentan vs. 1.8% of the PBO-treated patients. | >3× ULN: 0.8% for ambrisentan vs. 0.2% PBO |
| >5× ULN: 4% (36/887) for sitaxentan 100 mg/day vs. 0.6% in the PBO group (1/155). | In PAH: 11.6% for bosentan 125 mg b.i.d., and 14.3% for bosentan 250 mg twice daily. >8× ULN: 2.1% for bosentan 125 mg b.i.d. vs. 7.1% for 250 mg twice daily. | >8× ULN 0.2% for ambrisentan vs. 0% for PBO. | |
| Peripheral oedema | 9% | PBO-controlled studies 4.7 vs. 1.4% PBO BREATHE-5 study: 18.9 vs. 5.9% PBO | 17% (PBO-adjusted 6%) |
| BREATHE-4 study 31% (no PBO comparison) RAPIDS-1, -2: 14 vs. 5% PBO | |||
| Headache | 15% | PBO-controlled studies 15.8 vs. 12.8% PBO BREATHE-5 study: 13.5 vs. 11.8% PBO | 15% (PBO-adjusted 1%) |
| BREATHE-4 study 19% (no PBO comparison) | |||
| Decreased haemoglobin | 7% (PBO-adjusted 4%) | 5.6 (PBO-adjusted 3.0%) | 7% (PBO-adjusted 3%) |
Source: Product information (Summary of Product Characteristics, SmPC) of Tracleer, Thelin, and Letairis. ALT, alanine aminotransferases; AST, aspartate aminotransferases; ULN, upper limit of normal; PBO, placebo.
Abnormal liver function tests
The most clinically relevant side effects reported with ETRA therapy are dose-dependent liver function abnormalities. These present as elevated transaminases and/or bilirubin levels, and are seen more frequently with the sulfonamide-class ETRAs. Because these changes are a marker for potentially serious liver injury, serum aminotransferase levels (and bilirubin if aminotransferase levels are elevated) must be measured prior to initiation of treatment and then monthly thereafter. It has been reported that bosentan inhibits the bile salt export pump, which may lead to cholestatic liver injury as a result of the intracellular accumulation of bile salts, while increasing bile salt-independent bile flow.90,91 While the incidence of hepatotoxicity in the placebo-controlled trials was highest with bosentan (Tables 2 and 4), the inclusion criteria and control group characteristics of the studies should be taken into account. While patients with liver enzyme elevations >1.5× upper limit of normal (ULN) at baseline were excluded from ARIES-1, ARIES-2, and STRIDE-2, patients with elevations up to 3× ULN were included in STRIDE-1 and BREATHE-1. Similarly, the incidence of hepatic aminotransferase elevations >3× ULN in the control groups varied between 0 and 6% (Table 3).
Consequently, drug surveillance programmes (named TRAcleer eXcellence Post-Marketing Surveillance Programme, TRAX, for bosentan, Thelin Outcomes for Patients Surveillance, TOPS, for sitaxsentan, and VOLibris Tracking, VOLT, for ambrisentan) had/have to be conducted in the first years after introduction for all ETRAs. In the USA, the marketed drugs (bosentan and ambrisentan) can only be prescribed in the frameworks of special restricted distribution programmes.
It is a useful finding that in case of elevated transaminases, a switch to another ETRA may be an option. The STRIDE-6 study79 aimed to explore the potential use of sitaxentan in PAH patients who previously discontinued bosentan treatment (13 patients owing to ‘safety issues’, 12 patients with aminotransferase elevations, and one patient with rash). Among the 12 patients with liver enzyme elevations on bosentan treatment, only one individual re-developed this side effect during 12 weeks of sitaxentan therapy. An open-label study of ambrisentan evaluated the hypothesis that patients previously discontinued from bosentan (86%), sitaxentan (6%), or both (8%) because of elevations in hepatic aminotransferases can be successfully treated with ambrisentan without recurrence of hepatotoxicity. None of these 36 patients developed recurrent liver transaminase elevations during the initial 12 week observation period.92 In conclusion, among the various ETRAs currently available for the treatment of patients with PAH, receptor selectivity itself does not appear to be related to the incidence of hepatotoxicity. It is likely that chemical properties of the drugs, the pharmacokinetics or drug–drug interactions, or patient characteristics, may influence the incidence and severity of the side effects rather than differences in ET-receptor selectivity.
Decreased haemoglobin
Owing to an as yet incompletely identified mechanism, potentially related to vasodilatation and subsequent fluid shift producing haemodilution, all ETRAs are associated with a usually modest, dose-dependent, and partially transient reduction in haemoglobin levels. Decreases in haemoglobin were not related to haemolysis, bone marrow depression, or risk of bleeding. They occur in about 5–7% of patients, irrespective of the ETRA used. Haemoglobin (and haematocrit) reductions are likely to be a dose-dependent class effect of the ETRAs that may not be attributable to receptor selectivity.
For all three agents, these symptoms typically do not require discontinuation of therapy or dose adjustment and are usually not dose dependent (up to the approved doses). The occurrence, frequency, and severity of these side effects appear not to be related to the degree of selectivity for the ETA receptor.
Peripheral oedema
There has been speculation as to whether peripheral oedema occurs more frequently as a drug-specific, ETB-mediated side effect. However, the incidences of leg oedema in the pivotal ETRA studies (Table 4) suggest that the incidence is related to patient characteristics (as can be derived from the large variance in the placebo groups), but do not suggest a significant drug-related effect. Notably, a warning label has been issued by the FDA for ambrisentan based on post-marketing reports of fluid retention occurring within weeks after starting ambrisentan.71
Selectivity and PAH associated with connective tissue disease
Of the wide spectrum of diseases encompassed by the term ‘pulmonary hypertension’, PAH with associated CTD (PAH-CTD) is a disease for which patients have a particularly poor prognosis. Importantly, this subgroup has been included in several trials evaluating ETRAs; however, the clinical relevance of ET-receptor selectivity in this patient group has not been specifically explored.
The BREATHE-1 trial included 47 patients with systemic sclerosis (22%).62 In contrast to patients with IPAH, bosentan did not significantly increase 6MWD. However, the decline in walking distance of 40 m at 16 weeks in the systemic sclerosis placebo group (n = 14) was prevented by bosentan (+3 m, n = 33).
For ambrisentan, comparable efficacy with respect to functional capacity, measured as 6MWD, was described for 19 patients (30%) with PAH associated with collagen vascular disease, when compared to patients with idiopathic PAH.73
A post hoc analysis of 42 patients with PAH-CTD enrolled in the STRIDE-1 study83 showed that during the 12 week placebo-controlled phase, sitaxentan (pooled 100 and 300 mg groups) increased the placebo-subtracted 6MWD by 58 m (P = 0.027), improved haemodynamics, as well as certain domains within the quality-of-life assessment. Notably, in contrast to the bosentan data, sitaxentan not only prevented deterioration of exercise capacity but also significantly improved 6MWD by 20 m (P = 0.037), compared with baseline. In another post hoc meta-analysis pooling 512 patients from STRIDE-1, STRIDE-2, and STRIDE-4, a subgroup of 129 patients with PAH-CTD was analysed. Within this subgroup, 39 patients treated with sitaxentan 100 mg daily showed a significantly improved 6MWD by 38 m (P = 0.0419), compared with placebo.70 This effect was not seen with sitaxentan 50 or 300 mg daily in this PAH subgroup.
Long-term outcomes in PAH-CTD
Retrospective analyses have been published examining the long-term effects of ETRAs in patients with PAH-CTD. In two randomized, controlled studies investigating bosentan in PAH,61,62 66 patients with PAH-CTD were randomized to receive either bosentan (n = 44) or placebo (n = 22). Forty-four patients on bosentan were stable in 6MWD at study end, while the placebo patients deteriorated; the placebo-subtracted difference was 22 m (non-significant). Subsequently, in an open-label, long-term extension study (1.6 ± 0.9 years), survival rate in the 64 patients receiving bosentan was 86% after 1 year and 73% after 2 years.93 These outcome data are comparable with the 81 and 71% survival rates at 1 and 3 years, respectively, seen among 45 patients with PAH associated with scleroderma, treated with bosentan (mono- or combination therapy), as detailed in the Royal Free Hospital registry. These findings compare favourably to the 68 and 47% survival rates at 1 and 2 year, respectively, in a historical cohort of 47 patients in the same institution treated with conventional therapy.94
Within the STRIDE-2X study, 52 patients with PAH-CTD (scleroderma, n = 38; overlap syndrome, n = 9; lupus, n = 5) were included. According to a preliminary report,82 for this subgroup, time to discontinuation owing to adverse events or elevated hepatic aminotransferase, time to clinical worsening, and 1 year survival were all improved with sitaxentan therapy compared with placebo. In the STRIDE-1X study, at the end of the blinded extension phase, following mean treatment duration of 26 weeks, significantly more patients were in functional class I or II with sitaxentan, compared with baseline.70
Taken together, these data document short- and long-term clinical efficacy for ETRAs in the subgroup of patients with PAH-CTD. When comparing these post hoc analyses, an advantage of selective ETA blockade appears possible, since some of the efficacy endpoints reached statistical significance only with sitaxentan treatment. Differential effects on survival with these ETRAs cannot be evaluated from these data.
Drug metabolism, drug interactions, and combination therapy
Bosentan, ambrisentan, and sitaxentan have divergent pharmacological and pharmacokinetic characteristics, resulting in clinically important differences with respect to drug metabolism, drug interactions, and their potential for use in combination therapy (Table 2). Of interest are the interactions of bosentan with sildenafil, a frequently used combination therapy, where sildenafil plasma levels are reduced by about 50% while bosentan concentrations rise by approximately 50%.95,96 Theoretically, sub-therapeutic sildenafil levels as well as increased bosentan-related liver toxicity may result. However, in clinical practice, this combination is well tolerated and appears to be effective.97 No such interaction with sildenafil has been described for ambrisentan or sitaxentan.
Other important co-medications in patients with PAH are vitamin-K antagonists. Bosentan and sitaxentan have different effects on the doses of oral anticoagulants (vitamin-K antagonists): bosentan partially induces the cytochrome P450 system, thereby increasing warfarin metabolism and the required dose.98,104 In contrast, sitaxentan inhibits the liver isoenzyme CYP2C9. Thus, combining sitaxentan and warfarin in healthy volunteers can lead to a 2.4-fold increase in exposure to warfarin, therefore, requiring a substantial reduction in dose (∼80%) at initiation of therapy to avoid bleeding complications.99 No such interaction occurs with ambrisentan; however, according to the labelling, the drug interaction potential of ambrisentan ‘has not been well characterized’.71
In summary, most of these characteristics are related to the class of drug and differences in metabolism instead of reflecting differences in ET-receptor selectivity.
Conclusions
Together, these data, derived mainly from a series of randomized, controlled trials and their open-label extensions, confirm that ET antagonism is an effective and generally well-tolerated treatment option for patients with symptomatic PAH. They represent a major advance within the available therapeutic armamentarium for this severely compromised patient population.
Considering the entire group of PAH patients who have been prospectively studied in these trials, a clinically meaningful difference between the three approved ETRAs with respect to their ET-receptor selectivity could not be demonstrated to date. Therefore, in clinical practice, other features are likely to be of greater relevance when considering treatment, such as the potential for serious drug–drug interactions, convenience of dosing schedule, or rates of limiting side effects. These characteristics bear more relation to the chemical or pharmacological properties of the drug than to receptor selectivity itself.
Another important limitation of the data discussed in this paper relates to the design of the studies. As mentioned earlier, ET-1 creates short-term effects, mainly vasoconstriction, as well as medium and long-term sequelae such as proliferation, inflammation, and fibrosis. Most of the studies discussed in this paper investigated the effects of pharmacological interventions over the periods of 12–16 weeks, thereby assessing only the short-term vasodilator potential of the drug under study.100 From this perspective, the agents examined all resulted in a small, though significant change in exercise capacity over 12–16 weeks, irrespective of the drug used.86 Considering these limitations, much longer follow-up periods and probably other endpoints might be necessary to detect clinically important differences related to receptor selectivity when comparing different ETRAs. Until the results from such long-term trials become available, other strategies can be used to acquire data on the effects of different ETRAs in the treatment of patients with PAH. As one of these projects, the ‘Comparison of Endothelin Receptor Antagonist therapy in routine care' (CompERA, http://compera.org/) has recently been initiated in the European Union. This prospective large-scale register documents safety and efficacy parameters in consecutive pulmonary hypertension patients treated with any of the approved ETRAs (as mono- or combination therapy). These data should contribute to the optimization of the current ETRA-based drug therapy and provide further insight into selectivity-related differences among the currently available drugs.
Supplementary material
Supplementary material is available at European Heart Journal online.
Conflict of interest: none declared.
Funding
Funding to pay the Open Access publication charges for this article was provided by Christian Opitz and David Pittrow.
Supplementary Material
References
- 1.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 2.Galie N, Torbicki A, Barst R, Dartevelle P, Haworth S, Higenbottam T, Olschewski H, Peacock A, Pietra G, Rubin LJ, Simonneau G, Priori SG, Garcia MA, Blanc JJ, Budaj A, Cowie M, Dean V, Deckers J, Burgos EF, Lekakis J, Lindahl B, Mazzotta G, McGregor K, Morais J, Oto A, Smiseth OA, Barbera JA, Gibbs S, Hoeper M, Humbert M, Naeije R, Pepke-Zaba J. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur Heart J. 2004;25:2243–2278. doi: 10.1016/j.ehj.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425–1436. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- 4.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 5.Ito H, Hirata Y, Hiroe M, Tsujino M, Adachi S, Takamoto T, Nitta M, Taniguchi K, Marumo F. Endothelin-1 induces hypertrophy with enhanced expression of muscle-specific genes in cultured neonatal rat cardiomyocytes. Circ Res. 1991;69:209–215. doi: 10.1161/01.res.69.1.209. [DOI] [PubMed] [Google Scholar]
- 6.Kahaleh MB. Endothelin, an endothelial-dependent vasoconstrictor in scleroderma. Enhanced production and profibrotic action. Arthritis Rheum. 1991;34:978–983. doi: 10.1002/art.1780340807. [DOI] [PubMed] [Google Scholar]
- 7.Xu SW, Denton CP, Dashwood MR, Abraham DJ, Black CM. Endothelin-1 regulation of intercellular adhesion molecule-1 expression in normal and sclerodermal fibroblasts. J Cardiovasc Pharmacol. 1998;31(Suppl. 1):S545–S547. doi: 10.1097/00005344-199800001-00157. [DOI] [PubMed] [Google Scholar]
- 8.Filep JG, Fournier A, Foldes-Filep E. Acute pro-inflammatory actions of endothelin-1 in the guinea-pig lung: involvement of ETA and ETB receptors. Br J Pharmacol. 1995;115:227–236. doi: 10.1111/j.1476-5381.1995.tb15868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belloni AS, Rossi GP, Andreis PG, Neri G, Albertin G, Pessina AC, Nussdorfer GG. Endothelin adrenocortical secretagogue effect is mediated by the B receptor in rats. Hypertension. 1996;27:1153–1159. doi: 10.1161/01.hyp.27.5.1153. [DOI] [PubMed] [Google Scholar]
- 10.Schiffrin EL, Touyz RM. Vascular biology of endothelin. J Cardiovasc Pharmacol. 1998;32(Suppl. 3):S2–S13. [PubMed] [Google Scholar]
- 11.Nootens M, Kaufmann E, Rector T, Toher C, Judd D, Francis GS, Rich S. Neurohormonal activation in patients with right ventricular failure from pulmonary hypertension: relation to hemodynamic variables and endothelin levels. J Am Coll Cardiol. 1995;26:1581–1585. doi: 10.1016/0735-1097(95)00399-1. [DOI] [PubMed] [Google Scholar]
- 12.Rubens C, Ewert R, Halank M, Wensel R, Orzechowski HD, Schultheiss HP, Hoeffken G. Big endothelin-1 and endothelin-1 plasma levels are correlated with the severity of primary pulmonary hypertension. Chest. 2001;120:1562–1569. doi: 10.1378/chest.120.5.1562. [DOI] [PubMed] [Google Scholar]
- 13.Galie N, Grigioni F, Bacchi-Reggiani L. Relation of endothelin-1 to survival in patients with primary pulmonary hypertension [abstract 273] Eur J Clin Invest. 1996;26:A48. [Google Scholar]
- 14.Gregan B, Schaefer M, Rosenthal W, Oksche A. Fluorescence resonance energy transfer analysis reveals the existence of endothelin-a and endothelin-b receptor homodimers. J Cardiovasc Pharmacol. 2004;44:S30–S33. doi: 10.1097/01.fjc.0000166218.35168.79. [DOI] [PubMed] [Google Scholar]
- 15.Sauvageau S, Thorin E, Caron A, Dupuis J. Endothelin-1-induced pulmonary vasoreactivity is regulated by ET(A) and ET(B) receptor interactions. J Vasc Res. 2007;44:375–381. doi: 10.1159/000102534. [DOI] [PubMed] [Google Scholar]
- 16.Clozel M, Gray GA. Are there different ETB receptors mediating constriction and relaxation? J Cardiovasc Pharmacol. 1995;26(Suppl. 3):S262–S264. [PubMed] [Google Scholar]
- 17.Ohlstein EH, Arleth A, Bryan H, Elliott JD, Sung CP. The selective endothelin ETA receptor antagonist BQ123 antagonizes endothelin-1-mediated mitogenesis. Eur J Pharmacol. 1992;225:347–350. doi: 10.1016/0922-4106(92)90109-9. [DOI] [PubMed] [Google Scholar]
- 18.Alberts GF, Peifley KA, Johns A, Kleha JF, Winkles JA. Constitutive endothelin-1 overexpression promotes smooth muscle cell proliferation via an external autocrine loop. J Biol Chem. 1994;269:10112–10118. [PubMed] [Google Scholar]
- 19.Andrawis NS, Wang E, Abernethy DR. Endothelin-1 induces an increase in total protein synthesis and expression of the smooth muscle alpha-actin gene in vascular smooth muscle cells. Life Sci. 1996;59:523–528. doi: 10.1016/0024-3205(96)00332-3. [DOI] [PubMed] [Google Scholar]
- 20.Kernochan LE, Tran BN, Tangkijvanich P, Melton AC, Tam SP, Yee HF., Jr Endothelin-1 stimulates human colonic myofibroblast contraction and migration. Gut. 2002;50:65–70. doi: 10.1136/gut.50.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shichiri M, Kato H, Marumo F, Hirata Y. Endothelin-1 as an autocrine/paracrine apoptosis survival factor for endothelial cells. Hypertension. 1997;30:1198–1203. doi: 10.1161/01.hyp.30.5.1198. [DOI] [PubMed] [Google Scholar]
- 22.Dupuis J, Cernacek P, Tardif JC, Stewart DJ, Gosselin G, Dyrda I, Bonan R, Crepeau J. Reduced pulmonary clearance of endothelin-1 in pulmonary hypertension. Am Heart J. 1998;135:614–620. doi: 10.1016/s0002-8703(98)70276-5. [DOI] [PubMed] [Google Scholar]
- 23.Naomi S, Iwaoka T, Disashi T, Inoue J, Kanesaka Y, Tokunaga H, Tomita K. Endothelin-1 inhibits endothelin-converting enzyme-1 expression in cultured rat pulmonary endothelial cells. Circulation. 1998;97:234–236. doi: 10.1161/01.cir.97.3.234. [DOI] [PubMed] [Google Scholar]
- 24.Davie N, Haleen SJ, Upton PD, Polak JM, Yacoub MH, Morrell NW, Wharton J. ET(A) and ET(B) receptors modulate the proliferation of human pulmonary artery smooth muscle cells. Am J Respir Crit Care Med. 2002;165:398–405. doi: 10.1164/ajrccm.165.3.2104059. [DOI] [PubMed] [Google Scholar]
- 25.Masaki T. Possible role of endothelin in endothelial regulation of vascular tone. Annu Rev Pharmacol Toxicol. 1995;35:235–255. doi: 10.1146/annurev.pa.35.040195.001315. [DOI] [PubMed] [Google Scholar]
- 26.Dong F, Zhang X, Wold LE, Ren Q, Zhang Z, Ren J. Endothelin-1 enhances oxidative stress, cell proliferation and reduces apoptosis in human umbilical vein endothelial cells: role of ETB receptor, NADPH oxidase and caveolin-1. Br J Pharmacol. 2005;145:323–333. doi: 10.1038/sj.bjp.0706193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagnall A, Webb D. Are selective endothelin A receptor antagonists better than mixed antagonists? J Cardiovasc Pharmacol. 2001;38(Suppl. 2):S43–S46. doi: 10.1097/00005344-200111002-00011. [DOI] [PubMed] [Google Scholar]
- 28.Clozel M. Effects of bosentan on cellular processes involved in pulmonary arterial hypertension: do they explain the long-term benefit? Ann Med. 2003;35:605–613. doi: 10.1080/07853890310017477. [DOI] [PubMed] [Google Scholar]
- 29.Langleben D, Dupuis J, Langleben I, Hirsch AM, Baron M, Senecal JL, Giovinazzo M. Etiology-specific endothelin-1 clearance in human precapillary pulmonary hypertension. Chest. 2006;129:689–695. doi: 10.1378/chest.129.3.689. [DOI] [PubMed] [Google Scholar]
- 30.Goddard J, Johnston NR, Hand MF, Cumming AD, Rabelink TJ, Rankin AJ, Webb DJ. Endothelin-A receptor antagonism reduces blood pressure and increases renal blood flow in hypertensive patients with chronic renal failure: a comparison of selective and combined endothelin receptor blockade. Circulation. 2004;109:1186–1193. doi: 10.1161/01.CIR.0000118499.69469.51. [DOI] [PubMed] [Google Scholar]
- 31.Dupuis J. Endothelin receptor antagonists and their developing role in cardiovascular therapeutics. Can J Cardiol. 2000;16:903–910. [PubMed] [Google Scholar]
- 32.Bolli MH, Marfurt J, Grisostomi C, Boss C, Binkert C, Hess P, Treiber A, Thorin E, Morrison K, Buchmann S, Bur D, Ramuz H, Clozel M, Fischli W, Weller T. Novel benzo[1,4]diazepin-2-one derivatives as endothelin receptor antagonists. J Med Chem. 2004;47:2776–2795. doi: 10.1021/jm031115r. [DOI] [PubMed] [Google Scholar]
- 33.Greene S, Nunley K, Weber S, Minobe W, Bristow MR. ETA vs. ETB receptor selectivity of endothelin-1 receptor antagonists. J Am Coll Cardiol. 2006;47:307A. [Google Scholar]
- 34.Givertz MM, Colucci WS, LeJemtel TH, Gottlieb SS, Hare JM, Slawsky MT, Leier CV, Loh E, Nicklas JM, Lewis BE. Acute endothelin A receptor blockade causes selective pulmonary vasodilation in patients with chronic heart failure. Circulation. 2000;101:2922–2927. doi: 10.1161/01.cir.101.25.2922. [DOI] [PubMed] [Google Scholar]
- 35.Food and Drug Administration/CDER LETAIRIS® (ambrisentan tables) Clinical Pharmacology. Biopharmaceutics Review. 2007;3:98–99. http://www.fda.gov/cder/foi/nda/2007/022081s000_ClinPharmR_P3.pdf. (28 May 2008) [Google Scholar]
- 36.Opitz CF, Ewert R. Dual ET(A)/ET(B) vs. selective ET(A) endothelin receptor antagonism in patients with pulmonary hypertension. Eur J Clin Invest. 2006;36(Suppl. 3):1–9. doi: 10.1111/j.1365-2362.2006.01691.x. [DOI] [PubMed] [Google Scholar]
- 37.Love MP, Ferro CJ, Haynes WG, Plumpton C, Davenport AP, Webb DJ, McMurray JJ. Endothelin receptor antagonism in patients with chronic heart failure. Cardiovasc Res. 2000;47:166–172. doi: 10.1016/s0008-6363(00)00081-x. [DOI] [PubMed] [Google Scholar]
- 38.Verhaar MC, Strachan FE, Newby DE, Cruden NL, Koomans HA, Rabelink TJ, Webb DJ. Endothelin-A receptor antagonist-mediated vasodilatation is attenuated by inhibition of nitric oxide synthesis and by endothelin-B receptor blockade. Circulation. 1998;97:752–756. doi: 10.1161/01.cir.97.8.752. [DOI] [PubMed] [Google Scholar]
- 39.Chen SJ, Chen YF, Meng QC, Durand J, Dicarlo VS, Oparil S. Endothelin-receptor antagonist bosentan prevents and reverses hypoxic pulmonary hypertension in rats. J Appl Physiol. 1995;79:2122–2131. doi: 10.1152/jappl.1995.79.6.2122. [DOI] [PubMed] [Google Scholar]
- 40.Kim H, Yung GL, Marsh JJ, Konopka RG, Pedersen CA, Chiles PG, Morris TA, Channick RN. Endothelin mediates pulmonary vascular remodelling in a canine model of chronic embolic pulmonary hypertension. Eur Respir J. 2000;15:640–648. doi: 10.1034/j.1399-3003.2000.15d04.x. [DOI] [PubMed] [Google Scholar]
- 41.Chen SJ, Chen YF, Opgenorth TJ, Wessale JL, Meng QC, Durand J, DiCarlo VS, Oparil S. The orally active nonpeptide endothelin A-receptor antagonist A-127722 prevents and reverses hypoxia-induced pulmonary hypertension and pulmonary vascular remodeling in Sprague-Dawley rats. J Cardiovasc Pharmacol. 1997;29:713–725. doi: 10.1097/00005344-199706000-00003. [DOI] [PubMed] [Google Scholar]
- 42.DiCarlo VS, Chen SJ, Meng QC, Durand J, Yano M, Chen YF, Oparil S. ETA-receptor antagonist prevents and reverses chronic hypoxia-induced pulmonary hypertension in rat. Am J Physiol. 1995;269:L690–L697. doi: 10.1152/ajplung.1995.269.5.L690. [DOI] [PubMed] [Google Scholar]
- 43.Tilton RG, Munsch CL, Sherwood SJ, Chen SJ, Chen YF, Wu C, Block N, Dixon RA, Brock TA. Attenuation of pulmonary vascular hypertension and cardiac hypertrophy with sitaxsentan sodium, an orally active ET(A) receptor antagonist. Pulm Pharmacol Ther. 2000;13:87–97. doi: 10.1006/pupt.2000.0237. [DOI] [PubMed] [Google Scholar]
- 44.Clozel M, Salloukh H. Role of endothelin in fibrosis and anti-fibrotic potential of bosentan. Ann Med. 2005;37:2–12. doi: 10.1080/07853890410018925. [DOI] [PubMed] [Google Scholar]
- 45.Hocher B, Schwarz A, Fagan KA, Thone-Reineke C, El-Hag K, Kusserow H, Elitok S, Bauer C, Neumayer HH, Rodman DM, Theuring F. Pulmonary fibrosis and chronic lung inflammation in ET-1 transgenic mice. Am J Respir Cell Mol Biol. 2000;23:19–26. doi: 10.1165/ajrcmb.23.1.4030. [DOI] [PubMed] [Google Scholar]
- 46.Coghlan JG, Mukerjee D. The heart and pulmonary vasculature in scleroderma: clinical features and pathobiology. Curr Opin Rheumatol. 2001;13:495–499. doi: 10.1097/00002281-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Forbes JM, Hewitson TD, Becker GJ, Jones CL. Simultaneous blockade of endothelin A and B receptors in ischemic acute renal failure is detrimental to long-term kidney function. Kidney International. 2001;59:1333–1341. doi: 10.1046/j.1523-1755.2001.0590041333.x. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida J, Yamamoto K, Mano T, Sakata Y, Nishikawa N, Miwa T, Hori M, Masuyama T. Angiotensin II type 1 and endothelin type A receptor antagonists modulate the extracellular matrix regulatory system differently in diastolic heart failure. J Hypertens. 2003;21:437–444. doi: 10.1097/00004872-200302000-00037. [DOI] [PubMed] [Google Scholar]
- 49.Ammarguellat F, Larouche I, Schiffrin EL. Myocardial fibrosis in DOCA-salt hypertensive rats: effect of endothelin ET(A) receptor antagonism. Circulation. 2001;103:319–324. doi: 10.1161/01.cir.103.2.319. [DOI] [PubMed] [Google Scholar]
- 50.Ergul A, Portik-Dobos V, Giulumian AD, Molero MM, Fuchs LC. Stress upregulates arterial matrix metalloproteinase expression and activity via endothelin A receptor activation. Am J Physiol. 2003;285:H2225–H2232. doi: 10.1152/ajpheart.00133.2003. [DOI] [PubMed] [Google Scholar]
- 51.Seccia TM, Belloni AS, Kreutz R, Paul M, Nussdorfer GG, Pessina AC, Rossi GP. Cardiac fibrosis occurs early and involves endothelin and AT-1 receptors in hypertension due to endogenous angiotensin II. J Am Coll Cardiol. 2003;41:666–673. doi: 10.1016/s0735-1097(02)02860-7. [DOI] [PubMed] [Google Scholar]
- 52.Boffa JJ, Tharaux PL, Dussaule JC, Chatziantoniou C. Regression of renal vascular fibrosis by endothelin receptor antagonism. Hypertension. 2001;37:490–496. doi: 10.1161/01.hyp.37.2.490. [DOI] [PubMed] [Google Scholar]
- 53.Park SH, Saleh D, Giaid A, Michel RP. Increased endothelin-1 in bleomycin-induced pulmonary fibrosis and the effect of an endothelin receptor antagonist. Am J Respir Crit Care Med. 1997;156:600–608. doi: 10.1164/ajrccm.156.2.9607123. [DOI] [PubMed] [Google Scholar]
- 54.Shi-Wen X, Denton CP, Dashwood MR, Holmes AM, Bou-Gharios G, Pearson JD, Black CM, Abraham DJ. Fibroblast matrix gene expression and connective tissue remodeling: role of endothelin-1. J Invest Dermatol. 2001;116:417–425. doi: 10.1046/j.1523-1747.2001.01256.x. [DOI] [PubMed] [Google Scholar]
- 55.Shi-Wen X, Chen Y, Denton CP, Eastwood M, Renzoni EA, Bou-Gharios G, Pearson JD, Dashwood M, du Bois RM, Black CM, Leask A, Abraham DJ. Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell. 2004;15:2707–2719. doi: 10.1091/mbc.E03-12-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakamura T, Ebihara I, Tomino Y, Koide H. Effect of a specific endothelin A receptor antagonist on murine lupus nephritis. Kidney Int. 1995;47:481–489. doi: 10.1038/ki.1995.61. [DOI] [PubMed] [Google Scholar]
- 57.Dawes KE, Cambrey AD, Campa JS, Bishop JE, McAnulty RJ, Peacock AJ, Laurent GJ. Changes in collagen metabolism in response to endothelin-1: evidence for fibroblast heterogeneity. Int J Biochem Cell Biol. 1996;28:229–238. doi: 10.1016/1357-2725(95)00124-7. [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Douglas SA, Louden C, Vickery-Clark LM, Feuerstein GZ, Ohlstein EH. Expression of endothelin-1, endothelin-3, endothelin-converting enzyme-1, and endothelin-A and endothelin-B receptor mRNA after angioplasty-induced neointimal formation in the rat. Circ Res. 1996;78:322–328. doi: 10.1161/01.res.78.2.322. [DOI] [PubMed] [Google Scholar]
- 59.Mallat A, Fouassier L, Preaux AM, Gal CS, Raufaste D, Rosenbaum J, Dhumeaux D, Jouneaux C, Mavier P, Lotersztajn S. Growth inhibitory properties of endothelin-1 in human hepatic myofibroblastic Ito cells. An endothelin B receptor-mediated pathway. J Clin Invest. 1995;96:42–49. doi: 10.1172/JCI118052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abraham DJ, Vancheeswaran R, Dashwood MR, Rajkumar VS, Pantelides P, Xu SW, du Bois RM, Black CM. Increased levels of endothelin-1 and differential endothelin type A and B receptor expression in scleroderma-associated fibrotic lung disease. Am J Pathol. 1997;151:831–841. [PMC free article] [PubMed] [Google Scholar]
- 61.Channick R, Badesch DB, Tapson VF, Simonneau G, Robbins I, Frost A, Roux S, Rainisio M, Bodin F, Rubin LJ. Effects of the dual endothelin receptor antagonist bosentan in patients with pulmonary hypertension: a placebo-controlled study. J Heart Lung Transplant. 2001;20:262–263. doi: 10.1016/s1053-2498(00)00606-9. [DOI] [PubMed] [Google Scholar]
- 62.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 63.McLaughlin VV, Sitbon O, Badesch DB, Barst RJ, Black C, Galie N, Rainisio M, Simonneau G, Rubin LJ. Survival with first-line bosentan in patients with primary pulmonary hypertension. Eur Respir J. 2005;25:244–249. doi: 10.1183/09031936.05.00054804. [DOI] [PubMed] [Google Scholar]
- 64.Provencher S, Sitbon O, Humbert M, Cabrol S, Jais X, Simonneau G. Long-term outcome with first-line bosentan therapy in idiopathic pulmonary arterial hypertension. Eur Heart J. 2006;27:589–595. doi: 10.1093/eurheartj/ehi728. [DOI] [PubMed] [Google Scholar]
- 65.Barst RJ, Ivy D, Dingemanse J, Widlitz A, Schmitt K, Doran A, Bingaman D, Nguyen N, Gaitonde M, van Giersbergen PL. Pharmacokinetics, safety, and efficacy of bosentan in pediatric patients with pulmonary arterial hypertension. Clin Pharmacol Ther. 2003;73:372–382. doi: 10.1016/s0009-9236(03)00005-5. [DOI] [PubMed] [Google Scholar]
- 66.Sitbon O, Gressin V, Speich R, Macdonald PS, Opravil M, Cooper DA, Fourme T, Humbert M, Delfraissy JF, Simonneau G. Bosentan for the treatment of human immunodeficiency virus-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;170:1212–1217. doi: 10.1164/rccm.200404-445OC. [DOI] [PubMed] [Google Scholar]
- 67.Galie N, Beghetti M, Gatzoulis MA, Granton J, Berger RM, Lauer A, Chiossi E, Landzberg M. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation. 2006;114:48–54. doi: 10.1161/CIRCULATIONAHA.106.630715. [DOI] [PubMed] [Google Scholar]
- 68.Hoeper MM, Seyfarth HJ, Hoeffken G, Wirtz H, Spiekerkoetter E, Pletz MW, Welte T, Halank M. Experience with inhaled iloprost and bosentan in portopulmonary hypertension. Eur Respir J. 2007;30:1096–1102. doi: 10.1183/09031936.00032407. [DOI] [PubMed] [Google Scholar]
- 69.Galie N. The endothelin antagonist trial in mildly symptomatic PAH patients (EARLY) (Abstract 1011) Eur Heart J. 2007;28:140. [Google Scholar]
- 70.Barst RJ. Sitaxsentan: a selective endothelin-A receptor antagonist, for the treatment of pulmonary arterial hypertension. Expert Opin Pharmacother. 2007;8:95–109. doi: 10.1517/14656566.8.1.95. [DOI] [PubMed] [Google Scholar]
- 71.Gilead Pharmaceuticals. Letairis(R) (Ambrisentan) full prescribing information. 2007 http://www.gilead.com/pdf/letairis_pi.pdf. (27 May 2008) [Google Scholar]
- 72.EMEA. Summary of opinion. Volibris (ambrisentan) 2008 http://www.emea.europa.eu/pdfs/human/opinion/Volibris_3142508en%20.pdf. (22 March 2008) [Google Scholar]
- 73.Galie N, Badesch D, Oudiz R, Simonneau G, McGoon MD, Keogh AM, Frost AE, Zwicke D, Naeije R, Shapiro S, Olschewski H, Rubin LJ. Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2005;46:529–535. doi: 10.1016/j.jacc.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 74.Oudiz R, Torres F, Frost A, Badesch D, Olschewski H, Galie N, McGoon MD, McLaughin VV, Rubin L. ARIES-1: a placebo-controlled, efficacy and safety study of ambrisentan in patients with pulmonary arterial hypertension. Chest. 2006;130:121S. [Google Scholar]
- 75.Oudiz R, Olschewski H, Galie N, Frost A, Badesch D, McGoon MD, McLaughin VV, Rubin L. Ambrisentan improves exercise capacity and time to clinical worsening in patients with pulmonary arterial hypertension: results of the ARIES-2 study. International Conference of the American Thoracic Society; 19–24 May 2006; San Diego, CA: American Thoracic Society; 2006. [Google Scholar]
- 76.Oudiz R, Badesch D, Rubin L. ARIES-E: Long-term safety and efficacy of ambrisentan in pulmonary arterial hypertension. Chest. 2007;132:474a. [Google Scholar]
- 77.Barst RJ, Langleben D, Frost A, Horn EM, Oudiz R, Shapiro S, McLaughlin V, Hill N, Tapson VF, Robbins IM, Zwicke D, Duncan B, Dixon RA, Frumkin LR. Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;169:441–447. doi: 10.1164/rccm.200307-957OC. [DOI] [PubMed] [Google Scholar]
- 78.Barst RJ, Langleben D, Badesch D, Frost A, Lawrence EC, Shapiro S, Naeije R, Galie N. Treatment of pulmonary arterial hypertension with the selective endothelin-A receptor antagonist sitaxsentan. J Am Coll Cardiol. 2006;47:2049–2056. doi: 10.1016/j.jacc.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 79.Benza RL, Mehta S, Keogh A, Lawrence EC, Oudiz RJ, Barst RJ. Sitaxsentan treatment for patients with pulmonary arterial hypertension discontinuing bosentan. J Heart Lung Transplant. 2007;26:63–69. doi: 10.1016/j.healun.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 80.Langleben D, Hirsch AM, Shalit E, Lesenko L, Barst RJ. Sustained symptomatic, functional, and hemodynamic benefit with the selective endothelin-A receptor antagonist, sitaxsentan, in patients with pulmonary arterial hypertension: a 1-year follow-up study. Chest. 2004;126:1377–1381. doi: 10.1378/chest.126.4.1377. [DOI] [PubMed] [Google Scholar]
- 81.Benza RL, Frost A, Girgis R, Langleben D, Lawrence EC, Naeije R. Chronic treatment of pulmonary arterial hypertension (PAH) with sitaxentan and bosentan [abstract] Proc Am Thorac Soc. 2006;3:A729. [Google Scholar]
- 82.Highland KB, Strange C, Girgis R, Black C. Comparison of sitaxentan and bosentan in PAH-CTD. Ann Rheum Dis. 2006;65(Suppl. II):393. [Google Scholar]
- 83.Girgis RE, Frost AE, Hill NS, Horn EM, Langleben D, Mc Laughlin VV, Oudiz RJ, Robbins IM, Seibold JR, Shapiro S, Tapson VF, Barst RJ. Selective endothelin A receptor antagonism with sitaxsentan for pulmonary arterial hypertension associated with connective tissue disease. Ann Rheum Dis. 2007;66:1467–1472. doi: 10.1136/ard.2007.069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoeper MM, Oudiz RJ, Peacock A, Tapson VF, Haworth SG, Frost AE, Torbicki A. End points and clinical trial designs in pulmonary arterial hypertension: clinical and regulatory perspectives. J Am Coll Cardiol. 2004;43(Suppl. 12S):48S–55S. doi: 10.1016/j.jacc.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 85.Frost AE, Langleben D, Oudiz R, Hill N, Horn E, McLaughlin V, Robbins IM, Shapiro S, Tapson VF, Zwicke D, DeMarco T, Schilz R, Rubenfire M, Barst RJ. The 6-min walk test (6MW) as an efficacy endpoint in pulmonary arterial hypertension clinical trials: demonstration of a ceiling effect. Vascul Pharmacol. 2005;43:36–39. doi: 10.1016/j.vph.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 86.Rich S. The value of approved therapies for pulmonary arterial hypertension. Am Heart J. 2007;153:889–890. doi: 10.1016/j.ahj.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 87.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 88.Olschewski H. Long-term safety and tolerance of ambrisentan in patients with pulmonary arterial hypertension. (Abstract) Eur Respir J. 2005;26(Suppl. 49):205s. [Google Scholar]
- 89.Barst RJ. A review of pulmonary arterial hypertension: role of ambrisentan. Vasc Health Risk Manag. 2007;3:11–22. [PMC free article] [PubMed] [Google Scholar]
- 90.Fattinger K, Funk C, Pantze M, Weber C, Reichen J, Stieger B, Meier PJ. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther. 2001;69:223–231. doi: 10.1067/mcp.2001.114667. [DOI] [PubMed] [Google Scholar]
- 91.Mano Y, Usui T, Kamimura H. Effects of bosentan, an endothelin receptor antagonist, on bile salt export pump and multidrug resistance-associated protein 2. Biopharm Drug Dispos. 2007;28:13–18. doi: 10.1002/bdd.527. [DOI] [PubMed] [Google Scholar]
- 92.McGoon M, Frost A, Oudiz R, Badesch D, Galie N, Olschewski H, McLaughin VV, Rubin L. Ambrisentan rescue therapy in patients with pulmonary arterial hypertension who discontinued bosentan or sitaxentan due to liver function abnormalities. Chest. 2006:254S. doi: 10.1378/chest.08-1028. [DOI] [PubMed] [Google Scholar]
- 93.Denton CP, Humbert M, Rubin L, Black CM. Bosentan treatment for pulmonary arterial hypertension related to connective tissue disease: a subgroup analysis of the pivotal clinical trials and their open-label extensions. Ann Rheum Dis. 2006;65:1336–1340. doi: 10.1136/ard.2005.048967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Williams MH, Das C, Handler CE, Akram MR, Davar J, Denton CP, Smith CJ, Black CM, Coghlan JG. Systemic sclerosis associated pulmonary hypertension: improved survival in the current era. Heart. 2006;92:926–932. doi: 10.1136/hrt.2005.069484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paul GA, Gibbs JS, Boobis AR, Abbas A, Wilkins MR. Bosentan decreases the plasma concentration of sildenafil when coprescribed in pulmonary hypertension. Br J Clin Pharmacol. 2005;60:107–112. doi: 10.1111/j.1365-2125.2005.02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Burgess G, Hoogkamer H, Collings L, Dingemanse J. Mutual pharmacokinetic interactions between steady-state bosentan and sildenafil. Eur J Clin Pharmacol. 2008;64:43–50. doi: 10.1007/s00228-007-0408-z. [DOI] [PubMed] [Google Scholar]
- 97.Humbert M, Segal ES, Kiely DG, Carlsen J, Schwierin B, Hoeper MM. Results of European post-marketing surveillance of bosentan in pulmonary hypertension. Eur Respir J. 2007;30:338–344. doi: 10.1183/09031936.00138706. [DOI] [PubMed] [Google Scholar]
- 98.Committee for Proprietary Medicinal Products (CPMP) Tracleer(R). Public Assessment Report. Summary of Product Characteristics. Scientific Discussion. http://www.emea.europa.eu/humandocs/Humans/EPAR/tracleer/tracleer.htm. (28 May 2008) [Google Scholar]
- 99.Committee for Proprietary Medicinal Products (CPMP) 2006. Thelin(R). Public Assessment Report. Summary of Product Characteristics. Scientifc Discussion. http://www.emea.europa.eu/humandocs/Humans/EPAR/thelin/thelin.htm. (28 May 2008) [Google Scholar]
- 100.Macchia A, Marchioli R, Marfisi R, Scarano M, Levantesi G, Tavazzi L, Tognoni G. A meta-analysis of trials of pulmonary hypertension: a clinical condition looking for drugs and research methodology. Am Heart J. 2007;153:1037–1047. doi: 10.1016/j.ahj.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 101.Kiowski W, Sutsch G, Hunziker P, Muller P, Kim J, Oechslin E, Schmitt R, Jones R, Bertel O. Evidence for endothelin-1-mediated vasoconstriction in severe chronic heart failure. Lancet. 1995;346:732–736. doi: 10.1016/s0140-6736(95)91504-4. [DOI] [PubMed] [Google Scholar]
- 102.Sütsch G, Kiowski W, Yan XW, Hunziker P, Christen S, Strobel W, Kim JH, Rickenbacher P, Bertel O. Short-term oral endothelin-receptor antagonist therapy in conventionally treated patients with symptomatic severe chronic heart failure. Circulation. 1998;98:2262–2268. doi: 10.1161/01.cir.98.21.2262. [DOI] [PubMed] [Google Scholar]
- 103.Weber C, Schmitt R, Birnboeck H, Hopfgartner G, van Marle SP, Peeters PA, Jonkman JH, Jones CR. Pharmacokinetics and pharmacodynamics of the endothelin-receptor antagonist bosentan in healthy human subjects. Clin Pharmacol Ther. 1996;60:124–137. doi: 10.1016/S0009-9236(96)90127-7. [DOI] [PubMed] [Google Scholar]
- 104.Weber C, Banken L, Birnboeck H, Schulz R. Effect of the endothelin-receptor antagonist bosentan on the pharmacokinetics and pharmacodynamics of warfarin. J Clin Pharmacol. 1999;39:847–854. doi: 10.1177/00912709922008380. [DOI] [PubMed] [Google Scholar]
- 105.Williamson DJ, Wallman LL, Jones R, Keogh AM, Scroope F, Penny R, Weber C, Macdonald PS. Hemodynamic effects of bosentan, an endothelin receptor antagonist, in patients with pulmonary hypertension. Circulation. 2000;102:411–418. doi: 10.1161/01.cir.102.4.411. [DOI] [PubMed] [Google Scholar]
- 106.Hiramoto Y, Shioyama W, Kuroda T, Masaki M, Sugiyama S, Okamoto K, Hirota H, Fujio Y, Hori M, Yamauchi-Takihara K. Effect of bosentan on plasma endothelin-1 concentration in patients with pulmonary arterial hypertension. Circ J. 2007;71:367–369. doi: 10.1253/circj.71.367. [DOI] [PubMed] [Google Scholar]
- 107.Vatter H, Seifert V. Ambrisentan, a non-peptide endothelin receptor antagonist. Cardiovasc Drug Rev. 2006;24:63–76. doi: 10.1111/j.1527-3466.2006.00063.x. [DOI] [PubMed] [Google Scholar]
- 108.Olschewski H, Galie N, Ghofrani H, et al. Ambrisentan improves exercise capacity and time to clinical worsening in patients with pulmonary arterial hypertension: Results of the ARIES-2 study [abstract] Proc Am Thorac Soc. 2006;3:A728. [Google Scholar]
References
The above article uses a new reference style being piloted by the EHJ that shall soon be used for all articles.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.