Abstract
The neurotoxicity of β-amyloid protein (AβP) is implicated in the etiology of Alzheimer’s disease. We previously have demonstrated that AβP forms Ca2+-permeable pores on neuronal membranes, causes a marked increase in intracellular calcium level, and leads to neuronal death. Here, we investigated in detail the features of AβP-induced changes in intracellular Ca2+ level in primary cultured rat hippocampal neurons using a multisite Ca2+-imaging system with fura-2 as a fluorescent probe. Only a small fraction of short-term cultured hippocampal neurons (ca 1 week in vitro) exhibited changes in intracellular Ca2+ level after AβP exposure. However, AβP caused an acute increase in intracellular Ca2+ level in long-term cultured neurons (ca 1 month in vitro). The responses to AβP were highly heterogeneous, and immunohistochemical analysis using an antibody to AβP revealed that AβP is deposited on some but not all neurons. Considering that the disruption of Ca2+ homeostasis is the primary event in AβP neurotoxicity, substances that protect neurons from an AβP-induced intracellular Ca2+ level increase may be candidates as therapeutic drugs for Alzheimer’s disease. In line with the search for such protective substances, we found that the preadministration of neurosteroids including dehydroepiandrosterone, dehydroepiandrosterone sulfate, and pregnenolone significantly inhibits the increase in intracellular calcium level induced by AβP. Our results suggest the possible significance of neurosteroids, whose levels are reduced in the elderly, in preventing AβP neurotoxicity.
Keywords: neurotoxicity, pore, calcium homeostasis, channel, aging
Introduction
Alzheimer’s disease (AD) is a senile type of dementia affecting many of the elderly. AD is pathologically characterized by selective neuronal loss, and the presence of numerous extracellular deposits termed senile plaques and intraneuronal neurofibrillary tangles (NFTs) in the patient’s brain (Selkoe 1991). The principal constituent of senile plaques is the β-amyloid protein (AβP), which is a peptide composed of 38–43 amino acids and a proteolytic product of a large precursor protein (amyloid precursor protein; APP). Genetic studies of early-onset cases of familial AD indicated that APP mutations and AβP metabolism are associated with the disease (Goate et al 1991). Yankner and colleagues found that the segment comprising the first 40 amino acid residues of AβP, termed AβP[1–40], has neurotoxic effects on primary cultured neurons of the rat hippocampus (Yankner et al 1990). These lines of evidence support the idea that AβP and its neurotoxicity might be causal agents of neuronal death in AD patients (Small et al 2001).
Although the molecular mechanism underlying AβP neurotoxicity is not yet fully understood, there are a growing number of studies suggesting that the disruption of Ca2+ homeostasis is crucial to AβP neurodegeneration (Mattson et al 1992; Fraser et al 1997). We previously showed that AβP[1–40] is directly incorporated into membranes excised from GT1–7 cells, which are derived from the tumorigenesis of murine hypothalamic neurons, and forms unregulated cation-selective (including Ca2+) ion channels (Kawahara et al 1997). The characteristics of channels formed by AβP, ie, ‘amyloid channels’, on membranes of GT1–7 cells are similar to those of channels formed on artificial planar lipid membranes (Arispe et al 1993a, 1993b, 1996). Other neurotoxic AβP fragments such as AβP[25–35] and AβP[1–42], and the C-terminal fragment peptide of APP termed CT105 were also reported to form ion channels on artificial lipid bilayers or membranes of cultured neurons (Furukawa et al 1994; Mirzabecov et al 1994; Fraser et al 1996; Rhee et al 1998; Hirakura et al 1999). Furthermore, we have demonstrated that AβP[1–40], AβP[25–35] and AβP[1–42] cause the increase in intracellular Ca2+ level ([Ca2+]i) in GT1–7 cells (Kawahara et al 2000; Kawahara and Kuroda 2001; Kawahara 2004). Pretreatment with 2-amino-5-phosphonovalerate (D-APV; an antagonist of the N-methyl-D-aspartate (NMDA)-type glutamate receptor), tetrodotoxin (TTX; a Na+ channel blocker), or nifedipine (an L-type Ca2+ channel blocker) has no effect on the AβP-induced [Ca2+]i increase (Kawahara 2004). On the basis of these lines of evidences, we hypothesized that the ability of AβP to form unregulated ‘amyloid channels’ across neuronal membranes and the resulting disruption of calcium homeostasis explain the molecular mechanism of AβP neurotoxicity (Pollard et al 1995; Kawahara 2004). This mechanism of AβP neurotoxicity is similar to that of the toxicity of toxins, venoms, or the complement system (Tomita et al 1992; Bechinger 1997; Kobayashi et al 2004).
Here, we applied AβP[1–40] and/or AβP[25–35] to cultured hippocampal neurons and observed the spatiotemporal changes in [Ca2+]i induced by AβP using a multisite Ca2+-imaging system with fura-2 as a fluorescent probe. This system enables the simultaneous detection of temporal [Ca2+]i changes in cultured neurons facilitating statistical analysis. In this study, we investigated in detail the features of AβP-induced [Ca2+]i changes and observed heterogeneous cell-to-cell responses to AβP in cultured hippocampal neurons. The localization of AβP to the plasma membrane regions of the neurons was also observed by laser confocal microscopy using immunohistochemistry after AβP exposure. Furthermore, we found that AβP caused an acute increase in [Ca2+]i in many long-term cultured neurons. However, changes in intracellular Ca2+ level after AβP exposure were rarely observed among the short-term cultured hippocampal neurons. Therefore, the difference in susceptibility to AβP among neurons cultured for different periods was also examined.
Thus, the search for substances that protect against AβP neurotoxicity is crucial. Our system for observing Ca2+ influx induced by AβP has contributed to the search for such substances (Kawahara and Kuroda 2001). It requires a relatively short time for carrying out assays. The elevation of [Ca2+]i is considered to be the primary event of AβP neurotoxicity. We previously demonstrated that several lipophilic substances such as phloretin, cholesterol, and 17β-estradiol significantly inhibit the AβP-induced elevation of [Ca2+]i in GT1–7 cells (Kawahara and Kuroda 2001). Phloretin, a plant-derived flavonoid, decreases membrane potential and inhibits the electrostatic interaction between AβP and membrane lipids (Hertel et al 1997). Cholesterol decreases membrane fluidity and inhibits channel formation by peptides (Tomita et al 1992). Cholesterol also blocks the increase in [Ca2+]i induced by AβP in cultured neurons (Hartmann et al 1994). 17β-estradiol, a female hormone, is neuroprotective and affects membrane fluidity (Schwartz et al 1996). All these compounds inhibit AβP neurotoxicity (Zhou and Richardson 1996; Hertel et al 1997; Olivieri et al 2002). Therefore, substances that modulate membrane properties such as membrane potential and fluidity may inhibit AβP neurotoxicity. In line with the search for neuroprotective agents, we focused on neurosteroids including dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEA-S), and pregnenolone. These neurosteroids are steroid hormones synthesized de novo in the central nervous system from cholesterol or peripheral steroid precursors. Several lines of evidence suggest that neurosteroids modulate various functions of the brain and exhibit neuroprotective activities (Tsutsui et al 2000). For example, DHEA-S could protect neurons from NMDA-induced neurotoxicity (Charalampopoulos et al 2006). In addition, there is an age-related decrease in the levels of neurosteroids (Moffat et al 2000), and the levels of neurosteroids in plasma or in the brain are decreased in Alzheimer’s patients (Hillen et al 2000; Marx et al 2006). Thus, neurosteroids have been recognized as anti-aging hormones, and are widely used as supplements for improving the impaired cognitive functions of the elderly (Huppert et al 2000).
Experimental procedures
Chemicals and reagents
AβP[1–40] was obtained from Bachem F.A.G. (Bubendorf, Switzerland). AβP[25–35] was obtained from AnaSpec Inc. (CA, USA). These peptides were dissolved in distilled water at 0.2 mM and stored at −80 °C. DHEA-S, DHEA, and pregnenolone were obtained from Sigma Ltd. (St. Louis, USA), and dissolved in dimethylsulfoxide (DMSO) at 10 mM.
Cell culture
The hippocampus was dissected out from fetal rats (18-day embryonic stage) and neurons were cultured by the method of Banker and Cowan with slight modifications (Banker and Cowan 1977; Muramoto et al 1993). Briefly, after the removal of meninges, tissues were dissociated by mechanical trituration following digestion with 0.15 units/ml papain in phosphate-buffered saline (PBS) containing 0.02% L-cystein, 0.02% BSA, and 0.5% glucose. Dissociated neurons were placed on polyethylenimine-coated coverslips and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% horse serum, 5% newborn calf serum, and 1 mM sodium pyruvate. The culture was maintained in a humidified atmosphere of 93% air and 7% CO2 at 37 ° C. After 3 days in vitro, the medium was replaced with serum-free DMEM supplemented with B27 (Gibco BRL, USA). The medium was changed every 3 days.
Intracellular free-calcium concentration measurements
The culture medium was replaced with a basal salt solution (BSS; in mM: 130 NaCl, 5.5 glucose, 5.4 KCl, 1.8 CaCl2, 20 sodium HEPES, pH 7.4) containing 1.5 mM fura-2 AM (acetoxymethylester cell-permeant form, Molecular Probes, Oregon, USA). The cultured neurons were incubated with fura-2 AM at 37 °C for 60 min, washed and replaced with BSS containing 1 μM TTX to inhibit the endogenous changes in [Ca2+]i related to spontaneous synaptic firings (Robinson et al 1993), and then observed under an inverted fluorescence microscope (Nikon, Japan) equipped with a high-resolution video camera (C-2400, Hamamatsu Photonics Co., Japan). Fluorescence images correspond to [Ca2+]i were recorded at a rate of 30 images/sec on VCR tapes and analyzed using an ad-hoc hardware and software system controlled by a computer (FC-300, Mitsubishi Kasei, Japan). Each image represents the fluorescence intensity from a rectangular optical field (360 × 420 mm2), which enabled the monitoring of approximately 50 neurons simultaneously. The system enabled the recording of the ratio of the intensity emitted at 510 nm from pixels of images obtained by alternating the excitation wavelength between 340 and 360 nm using a rotating filter wheel. Each ratio was converted into [Ca2+]i using a calibration curve (ratio versus [Ca2+]i).
As a rule, prior to AβP addition to the bath solution of the cells, we recorded the resting levels of neuronal [Ca2+]i during a 5-min period. During the experiments, we added aliquots of the peptide stock solution directly to the bath solution (approximately 0.4 ml) of the cells. All recordings were obtained at 37 °C.
Immunohistochemistry
AβP[1–40] (2.5 μM) was applied onto hippocampal neurons cultured for 29 days in vitro (DIV). After 48 h, the cultured neurons were fixed with 4% paraformaldehyde in Ca2+- and Mg2+-free PBS. After fixation, the cultured neurons were incubated in PBS containing 0.5% saponin (Sigma Aldrich, St. Louis, USA) and 5% normal goat serum (Vector, UK) at 37 °C for 30 min to render the cell membranes permeable and to block nonspecific binding sites. The cultured neurons were incubated with primary antibodies diluted with 10% Block Ace (Dai-nihon Seiyaku, Japan) in PBS at 4 °C overnight. For double-immunofluorescence staining, a combination of a mouse monoclonal antibody and a rabbit polyclonal antibody was added to the cell preparation. An anti-microtubule associated protein 2 (MAP2) antibody (1:500, monoclonal, obtained from Boehringer Mannheim, Darmstadt, Germany) and an anti-AβP antibody (1:100, polyclonal, anti-N terminus of human AβP[1-40], obtained from IBL, Tokyo, Japan) were used as primary antibodies. Thereafter, the cells were incubated with anti-mouse Ig, biotinylated species-specific whole antibody (1:500, Amersham Pharmacia Biotech Ltd., Buckinghampshire, England), and then with an FITC-conjugated goat anti-rabbit IgG antibody (1:500, Cappel) and Texas red-conjugated streptavidin (1:500, Bio-Rad Laboratories, California, USA). Stained cells were observed under a confocal laser scanning microscope (LSM 510; Zeiss).
Pretreatment with neurosteroids
Prior to the 20–30 min exposure to AβP, the solutions of DHEA-S, DHEA, and pregnenolone were diluted with BSS and were added to the bath solution of the neurons. Thereafter, we carried out the same protocol designed to test the effects of AβP. As a control, 0.5% DMSO was applied to the cultured neurons, followed by 20 μM AβP[25–35].
Statistical analyses
Our system enabled the accurate measurement of the amplitude of the peak [Ca2+]i increase (Δ[Ca2+]i) in the response. To construct histograms from these data, we grouped the cell responses in bins (bin width, 20 nM) and then plotted the number of cells/bin as a function of peak [Ca2+]i . We obtained both the mean amplitude of the early peak [Ca2+]i for each neuron and the mean percentage of cells responding to AβP. Data are expressed as means ± standard error of mean (SEM) of 25 to 50 neurons in one optical field. All statistical evaluations were carried out using a two-tailed Student’s t-test using Stat View (SAS Institute, NC, USA). A probability level of <0.05 was considered to be significant.
Results
Effects of culture period on AβP-induced [Ca2+]i changes
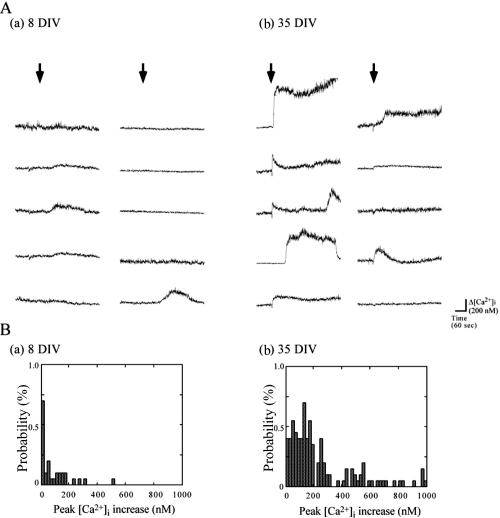
We applied AβP[25–35] (20 μM) solution to rat hippocampal neurons at various culture periods and observed the time course of [Ca2+]i. Little or no changes were observed in [Ca2+]i after AβP exposure in short-term cultured hippocampal neurons. On the other hands, a large proportion of long-term cultured neurons exhibited a marked increase in [Ca2+]i. Figure 1A shows traces of the temporal changes in [Ca2+]i induced by 20 μM AβP[25–35] at 8 DIV and 35 DIV in 10 randomly chosen neurons in one optical field. The extent of increase in [Ca2+]i induced by AβP was different among cultured neurons even in the same optical field. Therefore, we obtained the amplitude of the peak [Ca2+]i increase (Δ[Ca2+]i) in each neuron and the percentage of neurons responding to AβP in an optical field for statistical analysis. Figure 1B shows the histograms constructed from measurements of Δ[Ca2+]i in each neuron at 8 DIV and 35 DIV. In the panels, the vertical axes represent the percentage of neurons responding to AβP, and the horizontal axes represent the amplitude of Δ[Ca2+]i (bin = 20 nM). From these histograms, we obtained the mean amplitude of the increase in [Ca2+]i over basal [Ca2+]i (~100 nM) in hippocampal neurons and the proportion of cultured neurons (in percentage) that responded with a characteristic [Ca2+]i increase. At 8 DIV, the percentage of cells responding to AβP was 8 ± 7 % (mean ± SEM, n = 5), and the mean [Ca2+]i increase was 19 ± 3 nM (mean ± SEM, n = 250). On the other hand, at 35 DIV, the same AβP[25–35] concentration increased [Ca2+]i to 311 ± 37 nM (mean ± SEM, n = 250) in 79 ± 3% (mean ± SEM, n = 5) of neurons in the same optical field.
Figure 1.
Patterns of [Ca2+]i increase in hippocampal neurons cultured at 8 DIV and 35 DIV induced by AβP[25–35]. A. Temporal changes in [Ca2+]i in 10 randomly chosen cultured hippocampal neurons at 8 DIV (a) and 35 DIV (b) in the same optical field were analyzed, and their traces are shown. Arrows indicate the time when 20 μM AβP[25–35] was added to the bath solution. B. Frequency histograms of extent of [Ca2+]i increase at 8 DIV (a) and 35 DIV (b). The vertical axes represent the percentage of neurons responding to AβP, and the horizontal axes represent the amplitude of the peak [Ca2+]i increase (bin = 20 nM).
Heterogeneity of cell-to-cell responses to AβP
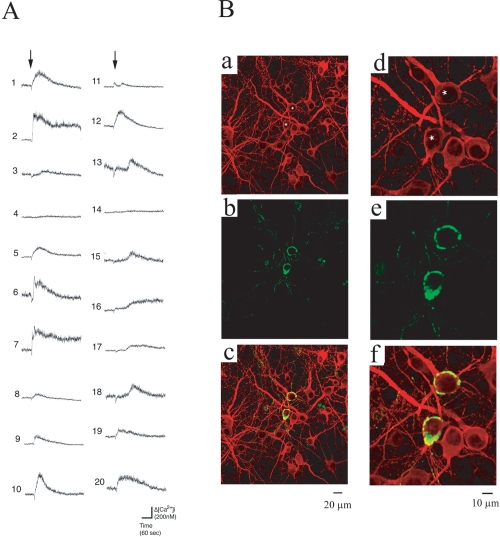
Further detailed analysis of the elevation of [Ca2+]i in many neurons in the same optical field revealed that the type of response of one neuron to AβP was not identical to that of another neuron and that not all neurons exhibited [Ca2+]i increase after the AβP addition. As shown in Figure 1A (b), the cell-to-cell responses to AβP were highly heterogeneous among neighboring neurons. The variability in neuronal responses is similar to our previous work using genetically identical GT1–7 cells, which exhibit heterogeneous responses after their exposure to AβP[25–35] as well as AβP[1–40] (Kawahara et al 2000; Kawahara and Kuroda 2001). The responses of 20 randomly chosen neurons in the same field of view before and after the application of AβP[1–40] were analyzed and their traces are shown in Fig. 2A. The traces were aligned to the time of AβP[1–40] application. The magnitude of the peak [Ca2+]i increase, latency (the time of onset of the first detectable increase in [Ca2+]i from the addition of AβP), and the pattern of [Ca2+]i changes differed among neurons. Some neurons showed an acute and a transient increase in [Ca2+]i within 5 min after the AβP[1–40] exposure (eg, cell nos. 6, 9, 10, 12, and 20 in Figure 2A), whereas [Ca2+]i in other neurons remained high at least 5 min after the exposure (cell nos. 2, 7, and 16). The delayed elevation of [Ca2+]i after the AβP exposure was observed in cell nos. 13, 15, 17, and 18. We observed no detectable elevation of [Ca2+]i in neighboring neurons (eg, cell nos. 4 and 14).
Figure 2.
Heterogenic responses to AβP. A. Patterns of [Ca2+]i increase in 20 hippocampal neurons induced by AβP[1–40]. Traces of temporal changes in [Ca2+]i in 20 randomly chosen cultured hippocampal neurons in the same field of view were analyzed. Numbers represent the number of cells used in the analysis. Arrows indicate the time at which 10 μM AβP[1–40] was added to the bath solution. B. Immunohistochemical identification and localization of AβP[1–40] on cultured hippocampal neurons. Laser confocal microscopy images of cultured hippocampal neurons (29 DIV) after MAP2 binding (Texas Red, red) ((a) and (d)) and AβP binding (FITC, green) ((b) and (e)), and their superimposed images ((c) and (f)). Images (d)-(f) are images of the same areas shown in (a)-(c) at a higher magnification.
Furthermore, we applied AβP[1–40] at a sublethal level (2.5 μM) to cultured hippocampal neurons and observed AβP binding to membrane surfaces. Cultured neurons were fixed and double-immunostained using antibodies to AβP and MAP2, a neuronal marker. Fluorescence images were observed under a laser confocal microscope. Figure 2B shows that the affinities of AβP to neuronal membrane surfaces are also highly heterogeneous. AβP[1–40] was found to deposit on somata and dendrites in some neurons (labeled by asterisks in Figures 2B (a) and (d)); however, we found no detectable AβP[1–40] deposition on surfaces of neighboring neurons. Note that the morphological characteristics of these neurons were similar. Figures 2B (d)-(f) show higher-magnification images of the same area shown in Figures 2B (a)-(c). The AβP[1–40] binding to neurons (asterisks) is not homogeneous. Moreover, AβP localizes to restricted areas of the somata and dendrites (Figure 2B (e)), whereas MAP2 is homogeneously distributed (Figure 2B (d)).
Protection against AβP-induced [Ca2+]i increase by neurosteroids
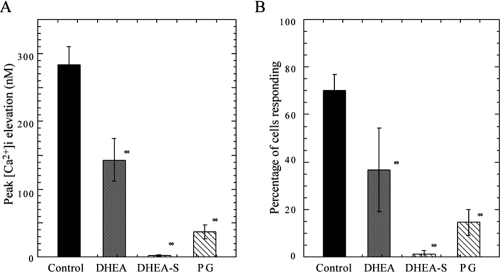
We pretreated hippocampal neurons at 30–36 DIV with solutions of neurosteroids, namely, DHEA, DHEA-S, or pregnenolone to. After 20–30 min, cultured neurons were exposed to AβP[25–35] (20 μM) and the changes in [Ca2+]i were observed using the same protocol. DHEA, DHEA-S, or pregnenolone at 25 μM significantly inhibited the mean increase in [Ca2+]i induced by AβP[25–35] (Figure 3). The mean increases in [Ca2+]i in cultured neurons pretreated with DHEA, DHEA-S, or pregnenolone were 50 ± 9.2%, 0.7 ± 0.5%, 13 ± 4% that of the control (neurons pretreated with 0.5 % DMSO), respectively (mean ± SEM, n = 200) (Figure 3A). The percentages of neurons responding to AβP among those pretreated with DHEA, DHEA-S, and pregnenolone also decreased (Figure 3B).
Figure 3.
Effects of DHEA, DHEA-S, and pregnenolone on [Ca2+]i increase induced by AβP. A. After a 20-min incubation period at 37 °C in 25 μM DHEA-S, DHEA, and pregnenolone (PG), the average increase in [Ca2+]i induced by 20 μM AβP[25–35] was determined. As a control, the same DMSO concentration (0.5%) was added to the cultured neurons by the same protocol. Data are expressed as mean ± SEM, n = 200, ** p < 0.001. B. Percentage of cells responding to AβP under same conditions as those in (A). Data are expressed as mean ± SEM, n = 5.
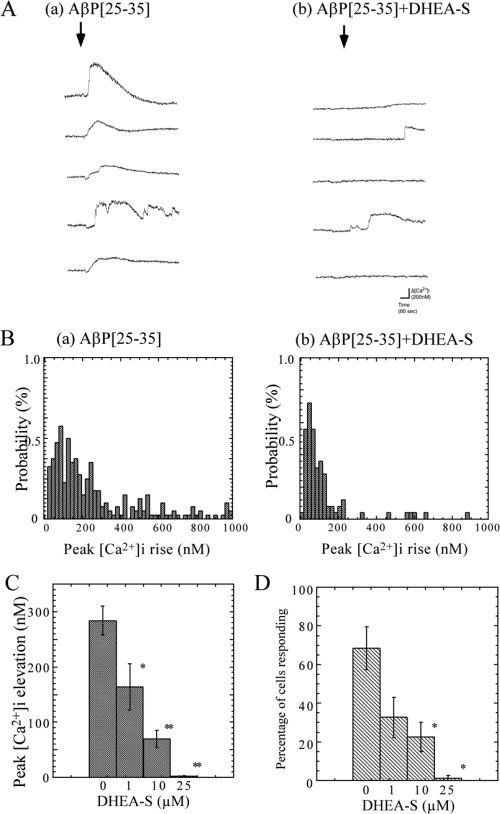
The inhibition by DHEA-S was more marked than those by DHEA or pregnenolone. Figure 4A shows typical responses to AβP[25–35] (20 μM) with (b) or without (a) DHEA-S pretreatment (10 μM) in the same view field. Figure 4B shows the histograms constructed from measurements of the peak [Ca2+]i increase in each neuron. Figures 4C and 4D show the dose-dependent effects of DHEA-S on the mean increase in [Ca2+]i and the percentage of responding neurons induced by AβP[25–35], respectively. DHEA-S at 1 and 10 μM inhibited the mean [Ca2+]i changes to 58 ± 15% and 35 ± 7% that of control (pretreated with 0.5% DMSO) neurons, respectively (mean ± SEM, n = 250). In the case of using DHEA-S at more than 25 μM, most neurons exhibited no significant increase in [Ca2+]i.
Figure 4.
Effects of DHEA-S on AβP-induced [Ca2+]i increase. A. Responses to AβP with or without DHEA-S pretreatment. After a 20-min incubation period at 37 °C with (b) or without (a) DHEA-S (10 μM), cultured hippocampal neurons (35 DIV) were exposed to 20 μM AβP[25–35]. Five typical traces of temporal changes in [Ca2+]i 2 min before and 5 min after the exposure in the same view field are shown. B. Frequency histograms of extent of [Ca2+]i increase with or without DHEA-S pretreatment. The vertical axes represent the percentage of neurons responding to AβP, and the horizontal axes represent the amplitude of the peak [Ca2+]i increase (bin = 20 nM). C. Dose-dependent effects of DHEA-S pretreatment on average [Ca2+]i increase. After a 20-min incubation period at 37 °C with various DHEA-S concentrations, the average increase in [Ca2+]i within 5 min of exposure to 20 μM AβP[25–35] was determined in hippocampal neurons cultured for 35 DIV from histograms in (B). As a control, the same DMSO concentration (0.5%) was added to the cultured neurons by the same protocol. Data are expressed as mean ± SEM, n = 250. * p < 0.05, ** p < 0.001. D. Effects of DHEA-S pretreatment on percentage of cells responding to AβP. The percentage of neurons responding to AβP[25–35] under the same conditions as those in (A) after pretreatment with various DHEA-S concentrations is analyzed from histograms in (B). Data are expressed as mean ± SEM, n = 5, * p < 0.05.
Discussion
We observed the spatiotemporal changes in [Ca2+]i in cultured rat hippocampal neurons induced by AβP[25–35] and AβP[1–40], both exhibiting β-sheet formation and neurotoxicity. Our results indicate that long-term (ca 1 month) cultured neurons are more susceptible to AβP than short-term (ca 1 week) cultured neurons. We have also demonstrated that the responses to AβP[25–35] as well as to AβP[1–40] are highly heterogeneous among neighboring cultured neurons and that AβP[1–40] has an affinity to some type of neuron. Our results are in accordance with the previous results showing that AβP[1–40] induces changes in electrophysiological activities in 3- to 4-week-cultured rat cerebral cortical neurons (Hartley et al 1999). Although factors affecting the susceptibility of neurons to AβP are under investigation, it is widely accepted that the properties of membrane lipids including membrane fluidity and the net charge of membrane surfaces are crucial to peptide binding and the subsequent channel formation (Terzi et al 1994). In particular, the changes in membrane properties such as the level ratio of cholesterol to other phopholipids during the in vitro development of cultured hippocampal neurons may be important. In particular, cholesterol decreases membrane fluidity and thus inhibits the assembly of peptides (Fujii et al 1997). The addition of cholesterol to membrane lipids inhibits the formation of ion channels by prion fragment peptides (Mirzabekov 1994) or Staphylococcus aureus α-toxin (Tomita et al 1992). Numerous epidemiological studies have suggested that a genotype of apolipoprotein E (E4 allele), which plays important roles in the transfer and metabolism of cholesterol, is a risk factor for AD (Corder et al 1993) and that cholesterol is significant in AD pathology (Hartmann 2001). Cholesterol has been reported to block the elevation of [Ca2+]i induced by AβP (Hartmann et al 1994; Kawahara et al 2000), inhibit AβP neurotoxicity (Zhou and Richardson 1996), and affect the secretion of AβP (Frears et al 1999). It is also possible that the percentage of neurons that are resistant to AβP neurotoxicity changes during in vitro development. In particular, gamma-aminobutyric acid (GABA)-immunopositive neurons may be important in the developmental change in susceptibility to AβP because of their resistance to AβP neurotoxicity (Pike and Cotman 1993). We have demonstrated that the developmental changes of GABAergic synapses occur at 7–14 DIV (Kato-Negishi et al 2004).
The search for protective agents against AβP neurotoxicity is of great importance. Our system for observing Ca2+ influx through AβP channels has contributed to the search for such substances (Kawahara and Kuroda 2001). In line with the search for protective agents, we found that neurosteroids including DHEA, DHEA-S, and pregnenolone significantly inhibit the [Ca2+]i elevation induced by AβP. DHEA and DHEA-S have neuroprotective effects against excitotoxicity (Kimonides et al 1998). Considering that plasma DHEA-S level is reduced in healthy individuals in an age-dependent manner and in AD patients (Hillen et al 2000), the implication of DHEA-S in the pathogenesis of AD may be important. Although the mechanism that DHEA-S inhibits AβP-induced elevation of [Ca2+]i is still under investigation, it is possible that DHEA-S modulates membrane fluidity or membrane potential, and influences the affinity of AβP to membranes. Further research about the influences of DHEA-S on the AβP binding to membrane surfaces is necessary. Moreover, it is interesting that DHEA-S can modulate GABA receptors (Meyer et al 1999). Further studies of the mechanism underlying the differences in AβP susceptibility and the mechanism of inhibition by neurosteroids are necessary to confirm the above possibilities. Our results are the first to suggest the role of neurosteroids in AβP neurotoxicity and the development of neurosteroids for AD treatment. In conclusion, our results may aid in improving our understanding of AD and the development of drugs for AD treatment.
Acknowledgments
We thank Ms M Sekiguchi, Ms R Hosoda-Yabe, and Mr M Yanagita for technical assistance. This work was partially supported by a research grant from Sumitomo Marine Welfare Foundation, a research grant from Nissan Science Foundation, and a Grant-in Aid for Scientific Research for the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Abbreviations
- AβP
β-amyloid protein
- AD
Alzheimer’s disease
- DHEA
dehydroepiandrosterone
- DHEA-S
dehydroepiandrosterone sulfate
- DIV
days in vitro
- [Ca2+]i
intracellular calcium level
- NMDA
N-methyl-D-aspartate
References
- Arispe N, Rojas E, Pollard HB. Alzheimer disease amyloid β protein forms calcium channels in bilayer membranes: Blockade by tromethamine and aluminum. Proc Natl Acad Sci USA. 1993a;90:567–71. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe N, Rojas E, Pollard HB. Giant multilevel cation channels formed by Alzheimer disease amyloid β protein [AβP-(1–40)] in bilayer membranes. Proc Natl Acad Sci USA. 1993b;90:10573–7. doi: 10.1073/pnas.90.22.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe N, Pollard HB, Rojas E. Zn2+ interactions with Alzheimer’s amyloid β protein calcium channels. Proc Natl Acad Sci USA. 1996;93:1710–5. doi: 10.1073/pnas.93.4.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–42. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Bechinger B. Structure and functions of channel-forming peptides: Magainins, cecropins, melittin and alamethicin. J Membr Biol. 1997;156:197–211. doi: 10.1007/s002329900201. [DOI] [PubMed] [Google Scholar]
- Charalampopoulos I, Alexaki VI, Tsatsanis C, et al. Neurosteroids as endogenous inhibitors of neuronal cell apoptosis in aging. Ann N Y Acad Sci. 2006;1088:139–52. doi: 10.1196/annals.1366.003. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Fraser SP, Suh YH, Chong YH, et al. Membrane currents induced in Xenopus oocytes by the C-terminal fragment of the β-amyloid precursor protein. J Neurochem. 1996;66:2034–40. doi: 10.1046/j.1471-4159.1996.66052034.x. [DOI] [PubMed] [Google Scholar]
- Fraser SP, Suh YH, Djamgoz MB. Ionic effects of the Alzheimer’s disease β-amyloid precursor protein and its metabolic fragments. Trends Neurosci. 1997;20:67–72. doi: 10.1016/s0166-2236(96)10079-5. [DOI] [PubMed] [Google Scholar]
- Frears ER, Stephens DJ, Walters CE, et al. The role of cholesterol in the biosynthesis of beta-amyloid. Neuroreport. 1999;10:1699–705. doi: 10.1097/00001756-199906030-00014. [DOI] [PubMed] [Google Scholar]
- Fujii G, Chang JE, Coley T, et al. The formation of amphotericin B ion channels in lipid bilayers. Biochemistry. 1997;36:4959–68. doi: 10.1021/bi962894z. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Abe Y, Akaike N. Amyloid β protein-induced irreversible current in rat cortical neurons. Neuroreport. 1994;5:2016–8. doi: 10.1097/00001756-199410270-00006. [DOI] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–6. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Hartley DM, Walsh DM, Ye CP, et al. Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci. 1999;19:8876–84. doi: 10.1523/JNEUROSCI.19-20-08876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann H, Eckert A, Muller WE. Apolipoprotein E and cholesterol affect neuronal calcium signalling: the possible relationship to beta-amyloid neurotoxicity. Biochem Biophys Res Commun. 1994;200:1185–92. doi: 10.1006/bbrc.1994.1576. [DOI] [PubMed] [Google Scholar]
- Hartmann T. Cholesterol, Aβ, and Alzheimer’s disease. Trends Neurosci. 2001;24:S45–S49. doi: 10.1016/s0166-2236(00)01990-1. [DOI] [PubMed] [Google Scholar]
- Hertel C, Terzi E, Hauser N, et al. Inhibition of the electrostatic interaction between beta-amyloid peptide and membranes prevents beta-amyloid-induced toxicity. Proc Natl Acad Sci USA. 1997;94:9412–6. doi: 10.1073/pnas.94.17.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen T, Lun A, Reischies FM, et al. DHEA-S plasma levels and incidence of Alzheimer’s disease. Biol Psychiatry. 2000;47:161–3. doi: 10.1016/s0006-3223(99)00217-6. [DOI] [PubMed] [Google Scholar]
- Hirakura Y, Lin MC, Kagan BL. Alzheimer amyloid Aβ1–42 channels: effects of solvent, pH, and Congo Red. J Neurosci Res. 1999;57:458–66. [PubMed] [Google Scholar]
- Huppert FA, Van Niekerk JK, Herbert J. Dehydroepiandrosterone (DHEA) supplementation for cognition and well-being. Cochrane Database Syst Rev. 2000;2:CD000304. doi: 10.1002/14651858.CD000304. [DOI] [PubMed] [Google Scholar]
- Kato-Negishia M, Muramoto K, Kawahara M, et al. Developmental changes of GABAergic synapses formed between primary cultured cortical neurons. Brain Res Dev Brain Res. 2004;152:99–108. doi: 10.1016/j.devbrainres.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kawahara M, Arispe N, Kuroda Y, et al. Alzheimer’s disease amyloid β-protein forms Zn2+-sensitive, cation-selective channels across excised membrane patches from hypothalamic neurons. Biophys J. 1997;73:67–75. doi: 10.1016/S0006-3495(97)78048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara M, Arispe N, Kuroda Y, et al. Alzheimer’s β-amyloid, human islet amylin and prion protein fragment evoke intracellular free-calcium elevations by a common mechanism in a hypothalamic GnRH neuronal cell-line. J Biol Chem. 2000;275:14077–83. doi: 10.1074/jbc.275.19.14077. [DOI] [PubMed] [Google Scholar]
- Kawahara M, Kuroda Y. Molecular mechanism of neurodegeneration induced by Alzheimer’s β-amyloid protein: channel formation and disruption of calcium homeostasis. Brain Res Bull. 2000;53:389–97. doi: 10.1016/s0361-9230(00)00370-1. [DOI] [PubMed] [Google Scholar]
- Kawahara M, Kuroda Y. Intracellular calcium changes in neuronal cells induced by Alzheimer’s β-amyloid protein are blocked by estradiol and cholesterol. Cell Mol Neurobiol. 2001;21:1–13. doi: 10.1023/A:1007168910582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara M. Disruption of calcium homeostasis in Alzheimer’s disease and other conformational diseases. Current Alzheimer Research. 2004;1:87–95. doi: 10.2174/1567205043332234. [DOI] [PubMed] [Google Scholar]
- Kimonides VG, Khatibi NH, Svendsen CN, et al. Dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc Natl Acad Sci USA. 1998;95:1852–7. doi: 10.1073/pnas.95.4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Chikushi A, Tougu S, et al. Membrane translocation mechanism of the antimicrobial peptide buforin 2. Biochemistry. 2004;43:15610–6. doi: 10.1021/bi048206q. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Ogura A. Glutamate-induced increase in intracellular Ca2+ concentration in isolated hippocampal neurons. Br J Pharmacol. 1986;89:191–8. doi: 10.1111/j.1476-5381.1986.tb11135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx CE, Trost WT, Shampine LJ, et al. The neurosteroid allopregnanolone is reduced in prefrontal cortex in Alzheimer’s disease. Biol Psychiatry. 2006;60:1287–94. doi: 10.1016/j.biopsych.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Davis D, et al. β-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992;12:376–89. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Lee S, Wittenberg GF, et al. Neurosteroid regulation of inhibitory synaptic transmission in the rat hippocampus in vitro. Neuroscience. 1999;90:1177–83. doi: 10.1016/s0306-4522(98)00543-0. [DOI] [PubMed] [Google Scholar]
- Mirzabekov T, Lin MC, Yuan WL, et al. Channel formation in planar lipid bilayers by a neurotoxic fragment of the β-amyloid peptide. Biochem Biophys Res Commun. 1994;202:1142–8. doi: 10.1006/bbrc.1994.2047. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Harman SM, et al. The relationship between longitudinal declines in dehydroepiandrosterone sulfate concentrations and cognitive performance in older men. Arch Intern Med. 2000;160:2193–8. doi: 10.1001/archinte.160.14.2193. [DOI] [PubMed] [Google Scholar]
- Muramoto K, Ichikawa M, Kawahara M, et al. Frequency of synchronous oscillations of neuronal activity increases during development and is correlated to the number of synapses in cultured cortical neuron networks. Neurosci Lett. 1993;163:163–5. doi: 10.1016/0304-3940(93)90372-r. [DOI] [PubMed] [Google Scholar]
- Olivieri G, Novakovic M, Savaskan E, et al. The effects of beta-estradiol on SHSY5Y neuroblastoma cells during heavy metal induced oxidative stress, neurotoxicity and beta-amyloid secretion. Neuroscience. 2002;113:849–55. doi: 10.1016/s0306-4522(02)00211-7. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Cotman CW. Cultured GABA-immunoreactive neurons are resistant to toxicity induced by β-amyloid. Neuroscience. 1993;56:269–74. doi: 10.1016/0306-4522(93)90331-9. [DOI] [PubMed] [Google Scholar]
- Pollard HB, Arispe N, Rojas E. Ion channel hypothesis for Alzheimer amyloid peptide neurotoxicity. Cell Mol Neurobiol. 1995;15:513–26. doi: 10.1007/BF02071314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SK, Quist AP, Lal R. Amyloid β protein-(1–42) forms calcium-permeable, Zn2+-sensitive channel. J Biol Chem. 1998;273:13379–82. doi: 10.1074/jbc.273.22.13379. [DOI] [PubMed] [Google Scholar]
- Robinson HP, Kawahara M, Jimbo Y, et al. Periodic synchronized bursting and intracellular calcium transients elicited by low magnesium in cultured cortical neurons. J Neurophysiol. 1993;70:1606–16. doi: 10.1152/jn.1993.70.4.1606. [DOI] [PubMed] [Google Scholar]
- Schwartz Z, Gates PA, Nasatzky E, et al. Effect of 17 beta-estradiol on chondrocyte membrane fluidity and phospholipid metabolism is membrane-specific, sex-specific, and cell maturation-dependent. Biochim Biophys Acta. 1996;1282:1–10. doi: 10.1016/0005-2736(96)00019-3. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. The molecular pathology of Alzheimer disease. Neuron. 1991;6:487–98. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Small DH, Mok SS, Bornstein JC. Alzheimer’s disease and Aβ toxicity: from top to bottom. Nat Rev Neurosci. 2001;2:595–8. doi: 10.1038/35086072. [DOI] [PubMed] [Google Scholar]
- Terzi E, Holzemann G, Seelig J. Alzheimer β-amyloid peptide 25–35: electrostatic interactions with phospholipid membranes. Biochemistry. 1994;33:7434–41. doi: 10.1021/bi00189a051. [DOI] [PubMed] [Google Scholar]
- Tomita T, Watanabe M, Yasuda T. Influence of membrane fluidity on the assembly of Staphylococcus aureus alpha-toxin, a channel-forming protein, in liposome membrane. J Biol Chem. 1992;267:13391–7. [PubMed] [Google Scholar]
- Tsutsui K, Ukena K, Usui M, et al. Novel brain function: biosynthesis and actions of neurosteroids in neurons. Neurosci Res. 2000;36:261–73. doi: 10.1016/s0168-0102(99)00132-7. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Duffy LK, Kirschner DA. Neurotropic and neurotoxic effects of amyloid β protein: reversal by tachykinin neuropeptides. Nature. 1990;250:279–82. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Richardson JS. Cholesterol protects PC12 cells from β-amyloid induced calcium disordering and cytotoxicity. Neuroreport. 1996;7:2487–90. doi: 10.1097/00001756-199611040-00017. [DOI] [PubMed] [Google Scholar]