Abstract
Anti-apoptotic members of the Bcl-2 family proteins are overexpressed in prostate cancer and are promising molecular targets for modulating chemoresistance of prostate cancer. (-)-Gossypol, a natural BH3-mimetic, is a small molecule inhibitor of Bcl-2/Bcl-xL/Mcl-1 currently in Phase II clinical trials as an adjuvant therapy for human prostate cancer. Our objective is to examine the chemosensitization potential of (-)-gossypol in prostate cancer and its molecular mechanisms of action. (-)-Gossypol inhibited cell growth and induced apoptosis through mitochondria pathway in human prostate cancer PC-3 cells, and synergistically enhanced the anti-tumor activity of docetaxel both in vitro and in vivo in PC-3 xenograft model in nude mouse. (-)-Gossypol blocked the interactions of Bcl-xL with Bax or Bad in cancer cells by fluorescence resonance energy transfer assay, and overcame the Bcl-xL protection of FL5.12 model cells upon IL-3-withdrawal. Western blot and real-time PCR studies showed that a dose-dependent increase of the proapoptotic BH3-only proteins Noxa and Puma contributed to the cell death induced by (-)-gossypol, and to the synergistic effects of (-)-gossypol and docetaxel. The siRNA knock-down studies demonstrated that Noxa and Puma are required in the (-)-gossypol-induced cell death. Taken together, these data suggest that (-)-gossypol exerts its anti-tumor activity through inhibition of the anti-apoptotic protein Bcl-xL, accompanied by an increase of proapoptotic Noxa and Puma. (-)-Gossypol significantly enhances the anti-tumor activity of chemotherapy in vitro and in vivo, representing a promising new regime for the treatment of human hormone-refractory prostate cancer with Bcl-2/Bcl-xL/Mcl-1 overexpression.
Keywords: chemotherapy, (-)-gossypol, Puma, Noxa, prostate cancer
Introduction
Androgen deprivation therapy is the cornerstone treatment for men with de novo or recurrent metastatic prostate cancer (1). Unfortunately, androgen deprivation therapy is primarily palliative, with nearly all patients progressing to an androgen-independent (AI), or hormone-refractory state, for which there is currently no effective therapy (1). Despite several hundred clinical studies of both experimental and approved anti-tumor agents, chemotherapy has limited activity, with an objective response rate of less than 50% and no demonstrated survival benefit (2). Thus, androgen-independent disease is the main obstacle to improving the survival and quality of life in patients with advanced prostate cancer, and has been the focus of extensive studies (3). There is an urgent need for novel therapeutic strategies for the treatment of advanced prostate cancer by specifically targeting the fundamental molecular basis of progression to androgen-independence and the resistance of androgen-independent disease to chemotherapy.
Bcl-2 family proteins are crucial regulators of apoptosis and were first isolated as the products of an oncogene (4). This family of proteins includes both anti-apoptotic molecules such as Bcl-2, Bcl-xL and Mcl-1, and pro-apoptotic molecules such as Bax, Bak, Bid, Bad, Noxa and Puma (5, 6). Bcl-2 and Bcl-xL are closely related proteins, both are highly overexpressed in many types of cancers (7). Overexpression of Bcl-2 is observed in 30-60% of prostate cancer at diagnosis and in nearly 100% of hormone-refractory prostate cancer (8, 9). The expression level of Bcl-2 protein also correlates with resistance to a wide spectrum of chemotherapeutic agents and radiation therapy (9-12). Bcl-xL is also overexpressed in almost 100% of hormone-refractory prostate cancer and is associated with advanced disease, poor prognosis, recurrence, metastasis, and shortened survival (13, 14). The transition of prostate cancer from androgen-dependent to androgen-independent is accompanied by a number of molecular genetic changes, including overexpression of Bcl-2 and Bcl-xL (10, 15). Noxa and Puma are pro-apoptotic BH3 only proteins, work upstream of Bax and Bak to promote mitochondrial depolarization and apoptosis. The BH3 only proteins are classified as activator and sensitizer. Puma is an activator which binds directly to Bax and Bak and promotes their activation, whereas Noxa as a sensitizer binds to the pro-survival proteins and displaces Bim or tBid, allowing them to directly activate Bax and Bak (16). Noxa also engages Mcl-1 and is found to promote Mcl-1 degradation (17, 18).
We have been investigating small molecule inhibitors of the Bcl-2 family proteins as novel therapeutics for cancer. Recently, (-)-gossypol, a natural product from cottonseed with the BH3-mimetic structure, is identified as small molecule inhibitor of Bcl-2/Bcl-xL/Mcl-1, potently induces apoptosis in various cancer cell lines (19, 20). (-)-Gossypol is now in Phase II clinical trials for hormone-refractory prostate cancer and other types of cancer at multiple centers in the United States, as one of the world’s first small molecule Bcl-2 inhibitors entered into clinical trial (http://ClinicalTrials.gov). In the current study, we investigated the therapeutic potential of (-)-gossypol in combination with docetaxel in human hormone-refractory prostate cancer cells in vitro and in vivo. Our hypothesis is that (-)-gossypol may improve the efficacy of chemotherapy by overcoming apoptosis-resistance rendered by Bcl-2/Bcl-xL-overexpression, thereby making the prostate cancer cells more sensitive to chemotherapy. Our results should not only facilitate the rational design of clinical trials, but also refine the selection of patients who will benefit the most from Bcl-2 molecular therapy.
Materials and Methods
Cell culture and reagents
Human prostate cancer cell lines PC-3, DU-145, LNCaP and human lung fibroblast cell line WI-38 were obtained from the American Type Culture Collection. PC-3 cells were routinely maintained in RPMI 1640 (HyClone, Logan, UT), while DU-145, LNCaP and WI-38 were maintained in DMEM (HyClone, Logan, UT), supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT). Murine pro-B lymphoid cell line FL5.12 stably transfected with Bcl-xL or vector were kindly provided by Dr. Gabriel Nunez and were maintained in IMEM (GIBCO, Grand Island, NY) supplemented with 10% FBS and 10% WEHI-3B (D-)-conditional medium as a source of IL-3 (21). The cell cultures were maintained in a humidified incubator at 37°C and with 5% CO2. (-)-Gossypol was purified from natural racemic gossypol. Briefly, racemic gossypol was reacted with L-phenylalanine methyl ester hydrochloride overnight at room temperature and sodium bicarbonate was added to yield gossypol Schiff’s base as a yellow solid. After silica gel column chromatography purification, the solution of the resolved (±)-gossypol-phenylalanine methyl ester Schiff’s base was hydrolyzed by a mixture of tetrahydrofuran, glacial acetic acid and hydrochloric acid at room temperature for 2 hours. The solution was extracted with acetic ether 4 times, then washed and dried. The (-)-gossypol was collected by filtration and evaporation. Some of the (-)-gossypol used for initial in vitro studies was kindly provided by Dr. Shaomeng Wang of the University of Michigan and by Dr. Dajun Yang through the NCI RAID program. For in vitro experiments, (-)-gossypol was dissolved in DMSO at 20mM as a stock solution. For in vivo studies, (-)-gossypol was suspended in carboxy methyl cellulose and then sonicated for 30 min, mixed before each administration. Docetaxel (Taxotere, TXT) was purchased from Sanofi-Aventis (Bridgewater, NJ) and diluted in PBS for in vivo studies.
MTT-based cell viability assay
Cell viability was determined by the MTT-based assay using Cell Proliferation Reagent WST-1 (Roche, Basel, Switzerland) according to the manufacturer’s instruction. Cells (5000 cells/well) were plated in 96-well culture plates, and various concentrations of (-)-gossypol or docetaxel were added to the cells in triplicates. Four days later, WST-1 was added to each well and incubated for 1.5 hours at 37°C. Absorbance was measured with a plate reader at 450 nm with correction at 650 nm. The results are expressed as the % of absorbance of treated wells versus that of vehicle control. IC50, the drug concentration causing 50% growth inhibition, was calculated via sigmoid curve fitting using GraphPad Prism 5.0 (GraphPad, Inc.).
Apoptosis assays
For the detection of apoptotic cells using DAPI staining, PC-3 cells were plated in 6 well plates and treated with various concentrations of (-)-gossypol, then stained with 3mM DAPI (4,6-diamidino-2-phenylindole dihydrochloride) for 10 minutes. The cells with the nuclei showing morphological characteristics of apoptosis, i.e., nuclear karyopyknosis and fragmentation, were counted as positive under a fluorescent microscope as described (22). For mitochondrial transmembrane potential (ΔΨm) assay PC-3 were cultured in a chamber slide, after washed with PBS cells were incubated with the MitoCapture solution at 37°C for 15 min according to the manufacturer’s protocol (BioVision, Mountain View, CA). The fluorescence was detected and recorded using a Zeiss LSM-510 confocal microscope. For apoptosis analysis of tumors in animal studies, tumor tissues were excised and stained for terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) using the ApopTag kit (Chemicon, Temecula, CA), according to the manufacturer’s instructions.
Fluorescence resonance energy transfer (FRET) assay
(-)-Gossypol -mediated disruption of Bcl-xl heterodimerization with Bax and Bad was analyzed with FRET assay as described (23) with modification. Briefly, DU-145 cells were transiently transfected with Bcl-xl-CFP together with either Bax-YFP or Bad-YFP (kindly provided by Dr. Junyin Yuan using FuGene 6 reagent (Roche, Basel, Switzerland). Twenty-four hours later, the cells were treated with increasing doses of (-)-gossypol or DMSO vehicle control for another 15 hours. Cells were then harvested and fixed in 2% paraformaldehyde. The cells were plated in 96-well black plates (0.2 × 106 cells/well) and fluorescence was measured in a Tecan multimode plate reader and calculated based on Dr. Yuan’s method as described (23).
Western blot analysis
Cells were washed with PBS and lysed in an ice-cold RIPA lysis buffer : 1% NP-40, 0.5% Sodium Deoxycholate, 0.1% SDS, and Complete Protease Inhibitor cocktail 100 μg/ml, in PBS. Tumor tissues were incubated with 200 μl lysis buffer in an ice-cold French Douncer for 15 min after homogenization by 40 strokes. The cell lysates and homogenates were cleared by centrifugation at 13,000g for 10 min at 4°C. The supernatants were collected. Protein concentrations were determined with the Bradford method (Bio-Rad, Hercules, CA); 60 μg of protein were electrophoresed by 12% SDS-PAGE. Separated proteins were transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA). After blocking with 5% milk, blots were probed with anti-Mcl-1, anti-Bcl-2, anti-Bim, anti-β-Actin, and anti-GAPDH, which were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), anti-Bcl-xL from BD Biosciences (San Jose, CA), anti-Puma and anti-caspase-3 (Cell Signaling Technology, Danvers, MA), anti-Noxa (CALBIOCHEM, San Diego, CA).
Subcellular fractionation and immunoblotting
PC-3 cells were harvested and washed with PBS. Cell pellets were suspended at 3×107/ml in mitochondrial resuspension buffer (MRB): 250 mM sucrose, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM DTT, 1 mM PMSF, Cocktail 100μg/ml. Digitonin was added to 200 μg/ml. The cells were incubated in digitonin supplimented MRB for 5 min on ice. Cell lysates were then centrifuged for 10 min at 13,000g. Proteins from the supernatant (cytosolic fraction) were re-spun at 13,000g for 10 min to clear any remaining debris, and pellet (membrane fraction) was washed twice with MRB. The pellet was solubilized in RIPA buffer and incubated on ice for 10 min. The cytosolic and mitochondria extracts were analyzed by SDS-PAGE. Antibodies used for immunoblotting were anti-cytochrome c (BD Pharmingen, San Jose, CA), anti-AIF (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-Cox IV (Molecular Probes, Carlsbad, CA).
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from the treated cells using TRIZOL (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Reverse transcription was performed using SuperscriptIII First-stand kits (Invitrogen, Carlsbad, CA). Each qRT-PCR (95°C for 10 min, 40cycles of 95°C for 15 s, 60°C for 1min, 72°C for 10 s) was done using TaqMan Universal PCR Master Mix (Applied Biosystems Foster City, CA). Primers were designed as: Mcl-1, forward: 5’-CTCATTTCTTTTGGTGCCTTT-3’, reverse: 5’- CCAGTCCCGTTTTGTCCTTAC-3’; Noxa, forward: 5’-CTGCAGGACTGTTCGTGTTC -3’, reverse: 5’-TTCTGCCGGAAGTTCAGTTT -3’; Puma, forward: 5’-TCCTCAGCCCTCGCTCTCGC -3’, reverse: 5’-CCGATGCTGAGTCCATCAGC -3’.
Small interfering RNA transfections
Small interfering RNAs (siRNA) oligonucleotides were purchased from Dharmacon Inc. (Chicago, IL). with the sequences for noxa (NM_021127): 5’-CUUCCGGCAGAAACUUCUG-3’ (24); for puma (NM_014417): 5’- UCUCAUCAUGGGACUCCUG-3’ (25); PC-3 cells were transfected 24 hours after being seeded in 6-well plates. siRNA (100 pmol) were dissolved in 200 μl of serum-free, antibiotic-free medium; Lipofectamine 2000 transfection reagent 5μl (Invitrogen, Carlsbad, CA) was dissolved in 200 μl of the same medium; the above two solutions were mixed and allowed to stand at room temperature for 20 min. The resulting 400 μl transfection complexes were then added to each well containing 1.6 ml of medium. Six hours later, the cultures were replaced with fresh medium supplemented with 10% FBS and antibiotics. (-)-Gossypol was added to the cells 24 hours after transfection as indicated. For Western blot, cells were collected after an additional 24 hours; for cell survival assay, after 4 days.
Animal tumor model and in vivo experiments
Double-blinded in vivo experiments were carried out with 5-to-6-week old male athymic NCr-nu/nu nude mice purchased from NCI. After alcohol preparation of the skin, mice were inoculated s.c. with 0.1 ml of PC-3 cell suspension (5 × 106 cells) on both flanks using a sterile 22-gauge needle. When tumors reached 100 mm3, the mice were randomized into 6 groups with 5 to 8 mice per group. Group 1 was given carboxy methyl cellulose as vehicle control; Group 2 was given docetaxel 7.5mg/kg, i.v. 1/week ×3 weeks; Group 3 and Group 4 were respectively given (-)-gossypol 10 mg/kg and 20 mg/kg p.o. q.d. 7 weeks, Group 5 and Group 6 were given a combination of docetaxel 7.5mg/kg, i.v. 1/week ×3 weeks and (-)-gossypol 10 mg/kg or 20 mg/kg p.o. q.d. 7 weeks. The tumor sizes and animal body weights were measured twice weekly. Three weeks after the first treatment, one mouse from each group was sacrificed and the tumors were dissected. Tumor tissues with a size of about 8 mm3 were prepared for Western Blot as described above. All animal experiments were done according to the protocol approved by University of Michigan Guidelines for Use and Care of Animals.
Statistical Analysis
Two-tailed Student’s t-test and two-way ANOVA were employed to analyze the in vitro and in vivo data respectively, using Prism 5.0 software (GraphPad Prism, San Diego, CA).
Results
(-)-Gossypol induces apoptosis through mitochondria pathway in human prostate cancer cells
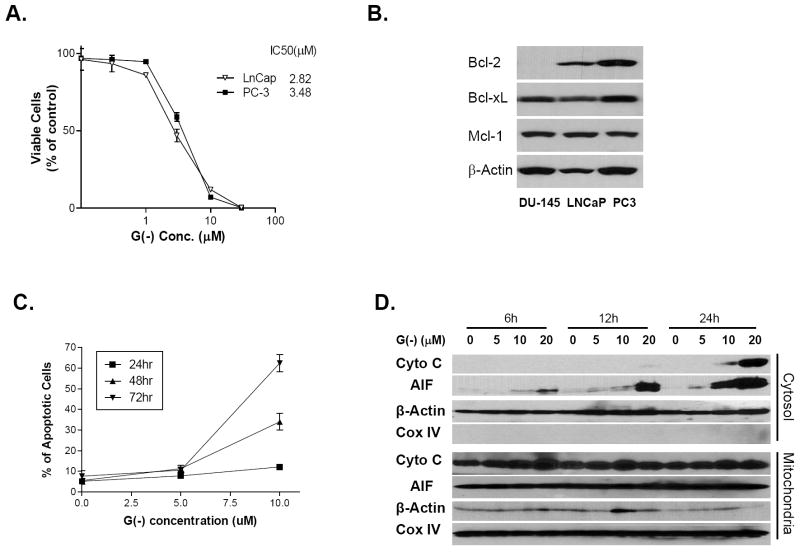
To evaluate the anti-tumor activity of (-)-gossypol in prostate cancer cells, we carried out the MTT-based cell viability assay. Human prostate cancer cell lines PC-3 (p53-null) and LNCaP (p53 wild-type) as well as human lung fibroblast cell line WI-38 were exposed to (-)-gossypol. As shown in Fig. 1A, (-)-gossypol potently inhibited the growth of PC-3 and LNCaP cells but had minimal effect on normal fibroblast WI-38 cells. The IC50 was 3.5 μM for PC-3 cells, 2.8 μM for LNCap cells and 17.4 μM for WI-38 cells (Fig. 1A). It was noted that the protein expression of Bcl-2, Bcl-xL and Mcl-1 was differed among these cell lines. PC-3 cells have higher levels of Bcl-2, Bcl-xL and Mcl-1 (Fig. 1B) than LNCaP cells and form good xenograft tumors in nude mice. Therefore, PC-3 is an appropriate cell line for studying the (-)-gossypol activity and mechanism in prostate cancer.
Figure 1.
(-)-Gossypol causes cell death and induces apoptosis through mitochondria pathway in human prostate cancer cell line PC-3. A. MTT-based cell viability assay of (-)-gossypol in PC-3 and LNCaP cells. Cells were seeded in 96-well plates and treated in triplicates. Results are presented as mean ± SEM and normalized to their respective controls (n=3). B. Western blot analysis showed the difference of Bcl-2, Bcl-xL, and Mcl-1 protein levels in three prostate cell lines (60μg/lane). C. Apoptotic cells were counted with DAPI staining. Cells were counted in five different fields, and plotted as mean ± SEM. D. Cytochrome c and AIF release from mitochondria to Cytosol. PC-3 cells were treated with (-)-gossypol for indicated times, and then subjected to a digitonin-based subcellular fractionation as described in Materials and Methods. Cytosolic fractions (~120 μg/lane) and mitochondria fractions (~60 μg/lane) were subjected to SDS-PAGE, followed by immunoblotting with the indicated antibodies. The COX IV marker was used to determine cross-contamination of cytosolic fractions with mitochondrial proteins.
(-)-Gossypol induced apoptosis was also assessed by DAPI-staining and counting the cells with karyorrhexis, a typical apoptotic change of cells (22). PC-3 cells treated with 10 μM (-)-gossypol for 48 and 72 hours showed dramatically increased apoptosis (Fig. 1C). Cytochrome c and AIF released from the intermembrane space of mitochondria contribute to caspase-dependent and caspase-independent apoptosis (26, 27). We separated the mitochondria from cytosol and examined the cytochrome c and AIF release in (-)-gossypol treated PC-3 (Fig. 1D). Cytochrome c translocated from mitochondria to cytosol 24 hours after (-)-gossypol treatment. Interestingly, AIF was released from mitochondria into cytosol at 6 hours, preceding the cytochrome c release, suggesting that AIF-mediated, caspase-independent apoptosis might also be involved in (-)-gossypol-induced cell death. By using MitoCapture staining, we observed the loss of ΔΨm, one of the early events in apoptosis induction via mitochondrial pathway (28), associated with (-)-gossypol-induced apoptosis. As shown in Figure S1, in control cells, consistent with mitochondrial localization, the bright punctuated yellow/orange fluorescence was found mostly in granular structures distributed throughout the cytoplasm. Exposure of PC-3 cells to 20 μM (-)-gossypol for 6 hours induced a marked change in ΔΨm, as evidenced by the disappearance of bright yellow/orange fluorescence and an increase of green fluorescence in most cells with a predominantly peri-nucleic distribution.
(-)-Gossypol sensitizes PC-3 cells to docetaxel-induced growth inhibition and apoptosis
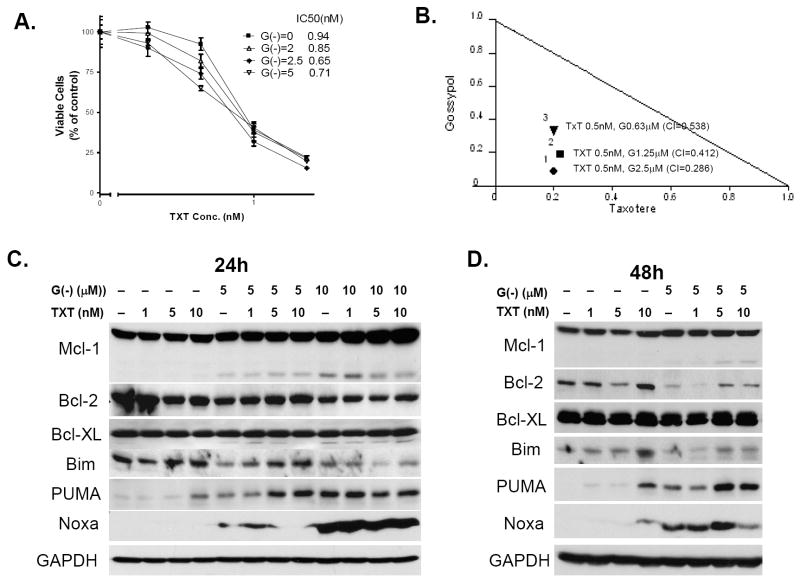
To investigate whether (-)-gossypol sensitizes the prostate cancer cells to chemotherapy, we examined the cytotoxic effect of the combination of (-)-gossypol and docetaxel, a first-line chemotherapy currently used for hormone-refractory prostate cancer. Figure 2A shows that, as the (-)-gossypol dose increased, there was an obvious left shift of the cytotoxicity curves, indicating that the PC-3 cells were sensitized to docetaxel by (-)-gossypol. To assess whether the combined effects were synergistic or additive, the Combination Index (CI) value was calculated and isobologram was plotted (Fig. 2B), as we previously described (20). CI < 1 is considered synergistic of the combination treatment (20). The combination treatment of 0.5 nM docetaxel with 0.63 μM, 1.25 μM or 2.5 μM (-)-gossypol resulted in synergistic effects (CI = 0.286, 0.412, or 0.538, respectively).
Figure 2.
(-)-Gossypol enhances the chemotherapy of docetaxel in human prostate cancer PC-3 cells. A. MTT-based cell viability assay of (-)-gossypol docetaxel in PC-3. B. IC50 isobologram of the combination treatments. In the isobologram, a plot on the diagonal line indicates that the combination is simply additive. A plot to the left under the line indicates that the combination is synergistic, whereas a plot to the right above the line indicates that it is antagonistic. CI (combination index) < 1 is considered synergistic of the combination treatment (20). C, D. Western blot analysis showed the effect of docetaxel and (-)-gossypol on proteins expression in PC-3 cells. Cells were treatment with docetaxel and (-)-gossypol of the indicated doses for 24 hours (C) or 48 hours (D), then collected for Western blot (60μg/lane). Data with (-)-gossypol 10 μM treatment for 48 hours were not shown because of severe cell death.
We next investigated the changes of several Bcl-2 family members at protein level. As shown in Figure 2C-D, no significant change was observed in Bcl-2 and Bcl-xL proteins. This is consistent with an earlier report (29) in large cell lymphoma, and supports that (-)-gossypol is a functional inhibitor of Bcl-2 and Bcl-xL via binding to the BH3 domain of the proteins without affecting protein levels directly. (-)-Gossypol-treated PC-3 cells showed an increase of Noxa and Puma as well as a decrease of Bim, and a dose-dependent increase of Mcl-1 (both full-length and a shortened form) (Figure 2C-D). Combination with docetaxel reversed the Bim decrease and further increased Puma levels in a time- and dose-dependent manner (Figure 2C-D), which might contribute to the synergistic effect of the combination therapy.
Noxa and Puma knockdown by siRNA attenuates (-)-gossypol effects on PC-3 cells
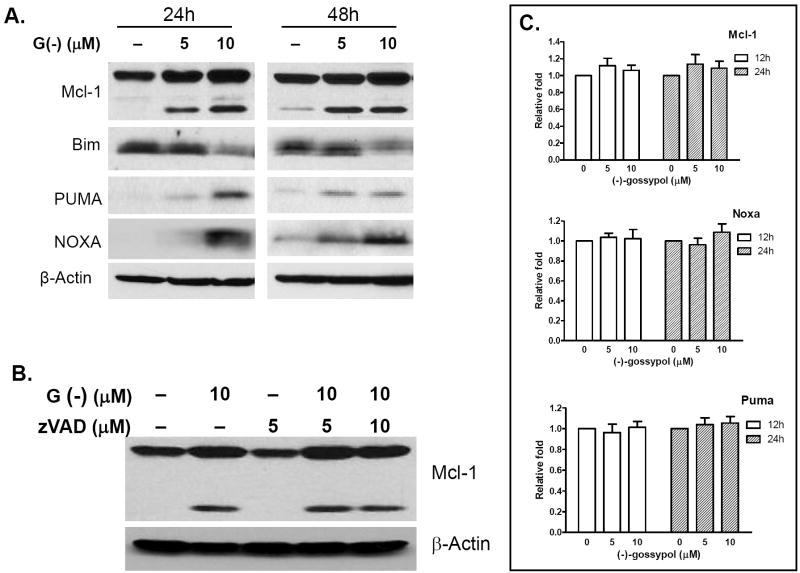
To further investigate the role of Bcl-2 family members in (-)-gossypol-induced apoptosis, we examined the BH3-only proteins Noxa and Puma in (-)-gossypol-treated PC-3 cells by Western Blot. Here again PC-3 cells treated with (-)-gossypol for 24 and 48 hours showed a dose-dependent increase of Noxa and Puma proteins, as well as Mcl-1 (both full-length and a shortened form) (Fig. 3A). This increase was not accompanied by clear and robust increase of their mRNA levels as assessed by qRT-PCR (Fig. 3C), indicating that Mcl-1 increase was post-translational. It is not clear at the present what the shortened form Mcl-1 is. It could be either the alternatively spliced Mcl-1 or caspase-cleaved Mcl-1, both of which have been reported to be pro-apoptotic and associated with apoptosis induction (30, 31). Interestingly, pan-caspase inhibitor zVAD did not block the Mcl-1 increase, nor prevented the increase of the shortened form (Fig. 3B), indicating that the (-)-gossypol-induced Mcl-1 increase was caspase-independent, and the shortened form might not be the caspase-cleaved Mcl-1 but is likely to be the alternatively spliced proapoptotic Mcl-1.
Figure 3.
(-)-Gossypol increases Noxa, Puma, Mcl-1 and decreases Bim at protein levels. A. (-)-gossypol dose-dependently induced Mcl-1, Noxa, Puma increase and Bim decrease. B. (-)-gossypol-induced Mcl-1 increase is caspase independent. PC-3 cells were pretreated with zVAD-fmk for 4 hours, then incubated with or without (-)-gossypol for another 24 hours before collecting for Western blot analysis. C. Changes of mRNA levels of Mcl-1, Noxa and Puma. PC-3 cells were treated with (-)-gossypol at the indicated time-points and doses. qRT-PCR reactions with TaqMan Universal PCR Master Mix (Applied Biosystems) were performed on the Mastercycler realplex 2 system (Eppendorf, Westbury, NY). Target gene mRNA levels were normalized to Actin mRNA according to the following formula: [2ˆ –(CT target – CT Actin)] × 100%, where CT is the threshold cycle. Fold increase was calculated by dividing the normalized target gene expression of the treated sample with that of the untreated control.
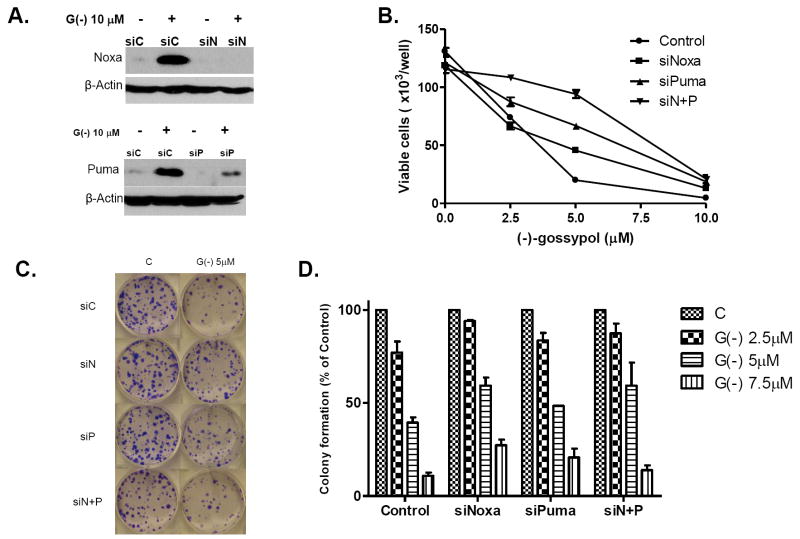
To evaluate the role of Noxa and Puma in the (-)-gossypol–induced cell response, we employed RNA interference to knock down Noxa and Puma in PC-3 cells before treatment with (-)-gossypol. Figure 4A shows that Noxa and Puma were effectively knocked down (>95%) by respective siRNAs in PC-3 cells. Figure 4B shows that knockdown of Noxa or Puma shifted the (-)-gossypol dose-response curve to the right, with the Noxa/Puma double-knock-down cells most resistant to (-)-gossypol (P < 0.001, versus control siRNA, two-way ANOVA, n=3). Similar results were observed with colony formation assay (Fig. 4C-D). These data demonstrate that Noxa and Puma knock-down rendered PC-3 cells resistant to (-)-gossypol-induced cell death and clonogenic growth, whereas Noxa/Puma double knock-down most effectively attenuated the (-)-gossypol activity. Our results support that Noxa and Puma are involved in (-)-gossypol-mediated growth inhibition and cell death in PC-3 cells.
Figure 4.
Noxa and Puma knockdown by siRNA attenuate the effects of (-)-gossypol on PC-3 cells. PC-3 cells transfected with Noxa and Puma siRNAs for 24 hours were used in the following assays. A. Transfected PC-3 cells were treated with 10 μM (-)-gossypol for an additional 24 hours and lysed for Western blot; B. Transfected PC-3 cells were seeded in 24-well plates and treated in triplicates with (-)-gossypol for 4 days, then the viable cells were counted by Trypan blue staining. Data are shown as mean ± SD of cells per well (× 103). C. Transfected PC-3 cells were plated in 6-well plates (300 cells/well) and treated in triplicates with (-)-gossypol for colony formation, the plates were cultured for 14 days and stained. Representative pictures of the colonies in the plates are shown. C. The colonies with over 50 cells were counted and plotted as mean ± SD, n=3. The assays have been repeated in four independent experiments with similar results.
(-)-Gossypol dose-dependently disrupts Bcl-xL heterodimerization with Bax and Bad
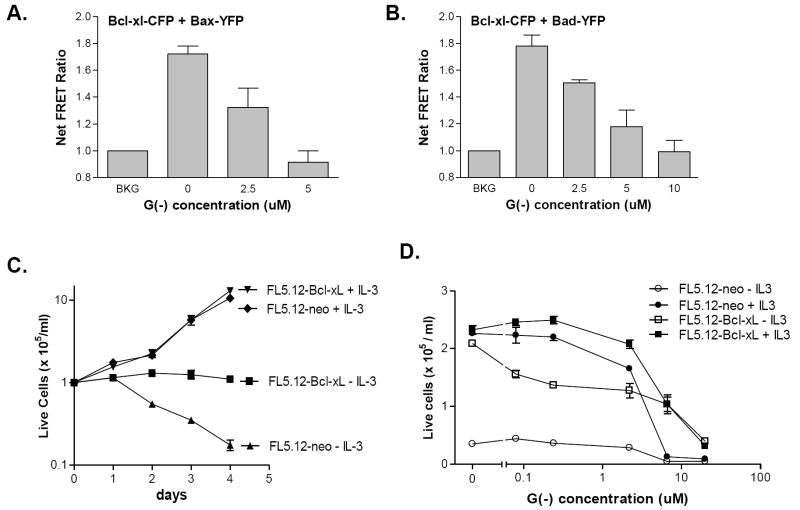
Using the fluorescence resonance energy transfer assay system described by Dr. Yuan’s group (23), we investigated the effect of (-)-gossypol on Bcl-xL heterodimerization with Bax or Bad. (-)-Gossypol dose-dependently inhibited the FRET signal, i.e., it inhibited the interaction between Bcl-xL and Bax (Fig. 5A) or Bad (Fig. 5B). 5 μM (-)-gossypol blocked 98.5% of interaction between Bcl-xL and Bax, and 77% of interaction between Bcl-xL and Bad. The plate-based FRET assay results were also confirmed by FRET analysis under confocal laser scanning microscopy (data not shown). There is a good correlation between (-)-gossypol cellular activity (growth inhibition and apoptosis induction) and the blocking of Bcl-xL-Bax (Pearson’s correlation, r = 0.941) as well as the blocking of Bcl-xL-Bad interaction (Pearson’s correlation, r = 0.994). These data suggest that Bcl-xL might be one of the major molecular targets of (-)-gossypol in cell growth inhibition and apoptosis induction. These data further support that (-)-gossypol is a potent functional inhibitor of the anti-apoptotic protein Bcl-xL.
Figure 5.
(-)-Gossypol disrupts Bcl-xL heterodimerizaion with Bax and Bad and overcomes Bcl-xL protection of FL5.12 cells. A-B, FRET analysis of (-)-gossypol-mediated disruption of Bcl-xL-CFP heterodimerization with Bax-YFP (A) or Bad-YFP (B). DU-145 cells were transiently transfected with Bcl-xL-CFP together with Bax-CFP or Bad-YFP for 24 hours, then treated with (-)-gossypol for 15 hours. The cells were counted and plated in a 96-well black assay plate and the fluorescence was measured in a Tecan multimode plate reader at Ex/Em = 430/485nm (for CFP), Ex/Em = 514/535nm (for YFP), and Ex/Em = 430/535nm (for FRET). Results are Net FRET ratios (n=3) normalized with background (set BKG = 1), which is the fluorescence cross-talk (leak-through) from the cells separately transfected with Bcl-xl-CFP and Bax-YFP or Bad-YFP. C: FL5.12/Neo and FL5.12/Bcl-xL cells were cultured in the presence or absence of IL-3. The cell numbers were counted daily by trypan blue exclusion. Bcl-xL expression protected the cells from cell death induced by IL-3-withdrawal. D: FL5.12/Neo and FL5.12/Bcl-xL cells were treated with the indicated concentrations of (-)-gossypol in the presence or absence of IL-3 for 48 hours, and the viable cells were counted. These experiments were repeated 2-4 times with consistent results and the representative results are shown.
(-)-Gossypol overcomes Bcl-xL protection of FL5.12 model cells from cell death induced by IL-3-withdrawal
FL5.12 cells depend on IL-3 for growth and survival in culture, and rapidly undergo apoptosis upon withdrawal of the growth factor IL-3 (32). However, when anti-apoptotic Bcl-2 or Bcl-xL genes were transfected into FL5.12 cells, they became resistant to apoptosis induction by IL-3-withdrawal (21, 32, 33). Therefore, the FL5.12 is a model system that is frequently used to analyze the anti-apoptotic activity of Bcl-2/Bcl-xL. Parental FL5.12 cells transfected with control vector (FL5.12-neo) undergo apoptosis after IL-3-withdrawal, whereas FL5.12 cells overexpressing Bcl-2 or Bcl-xL are protected (21). As shown in Figure 5C, FL5.12-neo cells rapidly died upon IL-3-withdrawal. FL5.12-Bcl-xL cells remained alive after IL-3-deprivation, but did not grow as well as the cells in the presence of growth factor IL-3. Our data is consistent with the literature (21, 32, 33) in that Bcl-xL is a potent inhibitor of apoptosis, and thus can protect the FL5.12 cells from cell death induced by IL-3-deprivation. On the other hand, because Bcl-xL is not a growth factor on its own, FL5.12-Bcl-xL cells without IL-3 did not grow as fast as those with IL-3 (Fig. 5C). For (-)-gossypol treatment study, the FL5.12 cells were plated in 24-well plates and treated in duplicates with different doses of (-)-gossypol for 2 days, and the live cells were counted by trypan blue exclusion as described (21). As shown in Fig. 5D, the low doses (< 6 μM) of (-)-gossypol did not kill FL5.12-neo and FL5.12-Bcl-xL cells in the presence of IL-3, however, in FL5.12-Bcl-xL cells in the absence of IL-3, whose survival is solely dependent on protection from Bcl-xL, the non-toxic dose of (-)-gossypol potently killed the FL5.12-Bcl-xL cells, demonstrating that (-)-gossypol can overcome the Bcl-xL protection of FL5.12 cells upon IL-3-withdrawal.
(-)-Gossypol enhances prostate cancer response to docetaxel and inhibits tumor growth in vivo
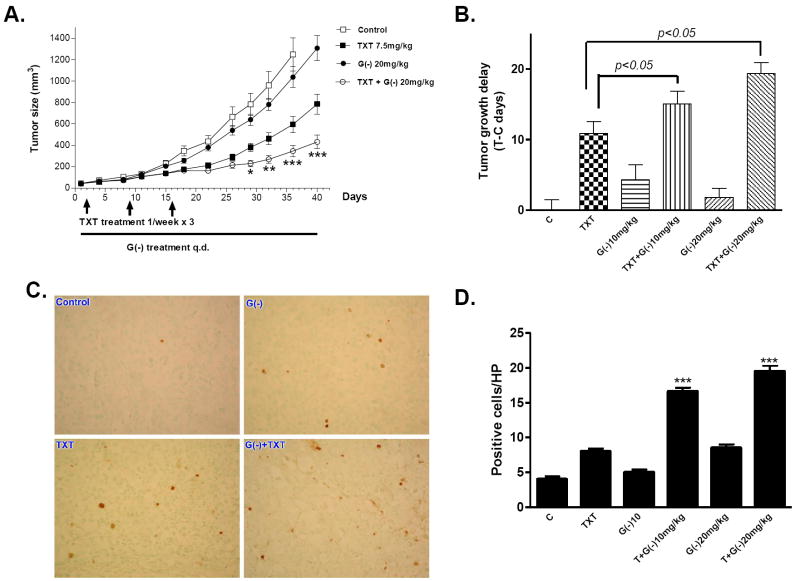
To investigate whether (-)-gossypol can sensitize PC-3 cells to docetaxel chemotherapy in vivo, we employed a PC-3 xenograft model in athymic nude mice. As shown in Figure 6A, oral (-)-gossypol given daily at 20 mg/kg dose significantly improved docetaxel efficacy on PC-3 tumors (p < 0.001 versus docetaxel alone, ANOVA, n = 16). Similar results were shown when 10 mg/kg (-)-gossypol was administrated (Fig. S2A). Tumor growth delay (T-C) analysis (Fig. 6B) showed that docetaxel combined with (-)-gossypol was more effective in inhibiting tumor growth as compared with either treatment alone (p < 0.05 vs docetaxel alone, p < 0.01 vs (-)-gossypol alone). Table S1 summarizes the tumor growth inhibition (T/C) values, calculated from the median tumor size of the treatment group divided by that of the control group on Day 32. The combination of docetaxel with (-)-gossypol 10 mg/kg and 20 mg/kg had T/C values of 37.3% and 29.4%, both of which are less than 42%, a value considered efficacious according to the NCI criteria (34). It is worth noting that (-)-gossypol alone at 10 mg/kg and 20 mg/kg showed similar but limited effects on PC-3 tumor growth (p > 0.05), nether is active as single treatment according to NCI criteria, consistent with our earlier study (20). The animal body weight loss was moderate, transient and tolerable, and recovered to normal soon after docetaxel treatment was complete (Fig. S2 B), indicating that the observed toxicity in the combination therapy was reversible and can be managed.
Figure 6.
(-)-Gossypol potentiates docetaxel in inhibiting tumor growth and inducing apoptosis in xenograft model of human prostate cancer PC-3. A. PC-3 xenograft tumor growth curves. PC-3 cells (5×106) were s.c. injected into the flanks on both sides of each mouse. When the tumors reached 50mm3, the mice were randomized into 5 to 8 mice per group and treated with (-)-gossypol 20mg/kg; docetaxel 7.5 mg/kg; or combination of the both. (-)-Gossypol was administrated p.o. via oral gavage, q.d.; Docetaxel was administrated i.v. once a week for 3 weeks. The average tumor sizes were shown (n =10–16). * P<0.05, **P<0.01, ***P<0.001 compared with docetaxel alone, two-way ANOVA. (n = 14-18). The data shown are the representative results of at least four independent animal experiments. B. Tumor Growth Delay (T-C) analysis. T is the median time (in days) required for the treatment group tumors to reach 750 mg, C is the median time (in days) for the control group tumors to reach 750 mg (T-C value for control group is zero). C-D. The tumor tissues from each treatment group were taken at the end of the 3rd week and fixed in 10% formalin. The tumor sections were stained for TUNEL using the ApopTag kit. The apoptotic cells have DNA fragmentation and are stained positive as brown nuclei. C. Represented pictures were shown: C (control), TXT (docetaxel), G(-) ((-)-gossypol), TXT+G(-) (docetaxel + (-)-gossypol), All photos are ×400 original. D. Quantification of TUNEL staining positive cells. Positive cells were counted under 400× magnification at 8 different fields, and the average numbers were calculated and plotted. ***P < 0.001 compared with docetaxel alone, t-test.
(-)-Gossypol increases docetaxel-induced apoptosis of PC-3 tumors in vivo
Because our in vitro study showed that (-)-gossypol increased the docetaxel-induced apoptosis, we also sought to determine whether this is still the case in vivo. We randomly picked one mouse from each group at the end of docetaxel treatment (Week 3) and took the tumor tissues to perform TUNEL staining for apoptosis. Results are shown in Figure 6C. (-)-Gossypol plus docetaxel induced significantly more apoptosis than either (-)-gossypol or docetaxel alone. Quantification of TUNEL-positive cells clearly showed that (-)-gossypol enhanced the apoptotic effect of docetaxel in a dose-dependent manner (Fig. 6D). The effects of combination therapy were also evaluated on the proteins related to the apoptosis signaling pathway. Western blot analysis was done on PC-3 tumor xenografts treated with 3 weeks of (-)-gossypol and docetaxel. (-)-Gossypol- and docetaxel-treated tumors showed PARP cleavage ((-)-gossypol 10 mg/kg) and caspase-3 cleavage ((-)-gossypol 20mg/kg) (Fig. S2 C).
Discussion
In this study, we have employed (-)-gossypol, a small molecule inhibitor of Bcl-2/Bcl-xL/Mcl-1, to investigate whether (-)-gossypol potentiate prostate cancer’s response to chemotherapy and whether this potentiation is accompanied with an increase of drug-induced apoptosis. Our in vitro and in vivo data show that (-)-gossypol inhibits tumor growth and induces apoptosis in human prostate cancer PC-3 cells with high levels of Bcl-2/Bcl-xL proteins. The FRET assay confirmed that (-)-gossypol potently blocks the interactions of Bcl-xL with Bax or Bad in live cells, in a dose-dependent manner. In Bcl-xL-transfected model cell lines, (-)-gossypol overcomes the Bcl-xL-mediated protection of FL5.12 cells upon IL-3-withdrawal. The data support that (-)-gossypol exerts its anti-tumor activity, at least in part, through functional inhibition of the anti-apoptotic protein Bcl-xL, although other targets might also be involved. More importantly, (-)-gossypol significantly improved the anti-tumor activity of current chemotherapeutic agent docetaxel in PC-3 cells, both in vitro and in vivo in a nude mice xenograft model. This enhanced response to chemotherapy is correlated with increased induction of apoptosis in vivo by the combination therapy.
(-)-Gossypol has recently been reported as a natural BH3-mimetic small molecule inhibitor of both Bcl-2 and Bcl-xL and induces apoptosis in multiple cancer cell lines with high levels of Bcl-2/Bcl-xL (19, 35). These studies show that (-)-gossypol as a potent inducer of apoptosis in these cancer cells is well tolerated, and is clinically safe. Oliver et al (36) demonstrated that (-)-gossypol acts directly on the mitochondria to overcome Bcl-2- and Bcl-xL-mediated apoptosis resistance. Reports have shown that (-)-gossypol has a significant anti-tumor activity as a potential novel therapy for the treatment of lymphoma in vitro and in vivo (29) as well as head and neck cancer cells in vitro (37). We have previously shown that (-)-gossypol potently enhanced radiation-induced apoptosis and growth inhibition of human prostate cancer PC-3 cells, and improved antitumor activity of X-ray irradiation to PC-3 cells in vivo, resulting in tumor regression even in large tumors (20). Interestingly, the (-)-gossypol dose of 1- 5 μM used in the current study is the dose that can block Bcl-xL--Bax and Bcl-xL--Bad protein-protein interactions in our FRET assay, whereas the same doses of (-)-gossypol potently and specifically overcome the Bcl-xL protection of FL5.12-Bcl-xL cells from cell death induced by IL-3-withdrawal. The same doses of (-)-gossypol also inhibited cell growth of PC-3 and LNCaP cells that have high levels of Bcl-xL in the current study, and potently radiosensitized these cells in our earlier publication (20). Taken together, our data support that (-)-gossypol can kill cancer cells, at least in part, through inhibiting the anti-apoptotic function of Bcl-xL. Our results demonstrate that Bcl-xL is one of the major targets of (-)-gossypol in prostate cancer cell growth inhibition.
The Bcl-2 family of proteins, which includes 17 or more members in mammalian cells, functions as a ‘life/death switch’ that integrates diverse inter- and intracellular cues to determine whether or not a cell should undergo apoptosis in response to diverse damage signals (38). The switch operates through the interactions between the proteins within three sub-families of the Bcl-2 protein family. The pro-survival subfamily (Bcl-2, Bcl-xL, Bcl-w, Mcl-1, A1 and also Bcl-B in humans) protects cells exposed to diverse cytotoxic conditions; whereas two other subfamilies, many members of which were identified as Bcl-2-binding proteins, instead promote cell death (38). They are the Bax-like apoptotic sub-family (Bax, Bak and Bok) and the BH3-only proteins sub-family (includes at least eight members: Bik, Bad, Bid, Bim, Bmf, Hrk, Noxa and Puma) (16, 39). Earlier studies with Noxa- and Puma-knockouts demonstrate that Noxa and Puma are critical mediators of the apoptotic responses induced by p53 and cytotoxic drugs. BH3-only proteins Noxa, Puma, and Bim have both overlapping and specialized roles as death initiators in either p53-dependent or p53-independent apoptosis (39). In the current study, (-)-gossypol dose-dependently increased the protein levels of proapoptotic Noxa and Puma independent of p53 (PC-3 is p53 null). This effect on Puma was augmented by combination with docetaxel. Noxa and Puma knock-down by siRNA attenuated the (-)-gossypol-induced cell death and growth inhibition in PC-3 cells, indicating that Noxa and Puma are involved in (-)-gossypol-mediated growth inhibition and cell death in PC-3 cells. Thus, our study provides first proof that (-)-gossypol exerts its anti-tumor activity not only through inhibition of anti-apoptotic protein Bcl-xL (as well as Bcl-2, Mcl-1), but also through a p53-independent induction of proapoptotic BH3-only proteins Noxa and Puma.
The molecular mechanism(s) underlying the (-)-gossypol-induced, p53-independent increase of Noxa and Puma are not yet clear. Our data show that (-)-gossypol-induced Noxa and Puma increase appears to be post-translational, without significant change of mRNA levels. The interaction of Mcl-1 with Noxa and Puma in the apoptosis process draws increasing attention recently (40). Mcl-1 stability is tightly regulated; BH3-only proteins Noxa and Bim preferentially bind to Mcl-1 and also target it for proteasomal degradation (41). A recent study (40) using a novel BH3 ligand that selectively binds to Mcl-1 demonstrated that apoptosis can proceed without Mcl-1 degradation. This BH3 mimetic ligand, derived from Bim BH3 mutation, preferentially binds to and stabilizes Mcl-1, and promotes cell death only when Bcl-xL is absent or neutralized (40). Blockage of BH3 domain-containing E3 ubiquitin ligase, Mule, which regulates the basal level of Mcl-1 by targeting it for proteasomal degradation, might be involved in this apparent Mcl-1 stabilization (40). This might account for our results that (-)-gossypol dose-dependently increased Mcl-l protein level. Based on our data and other’s reports (42, 43), we propose that the BH3 mimetic (-)-gossypol might work in the same way as this unique BH3 ligand: (1) binds to and inactivates the anti-apoptotic function of Bcl-xL (and/or Bcl-2); (2) binds to Mcl-1, blocks and displaces Noxa (and/or Puma), thus blocks Noxa-Mule-mediated Mcl-1 degradation, stabilizes Mcl-1 and increases both full-length and shortened form Mcl-1 (the latter is pro-apoptotic); (3) the displaced Noxa and Puma accumulate and promote apoptosis. This mode of action by (-)-gossypol lies upstream of Bax/Bak in the mitochondrial apoptosis pathway. Indeed, a recent report with melanoma cells (43) lends support to this action by (-)-gossypol, which showed that (-)-gossypol was effective in killing cancer cells dependent on Mcl-1/Bcl-xL for survival. This effect was enhanced when the proteasome was blocked, and accompanied with increase of Noxa (43). We are currently carrying out detailed studies to delineate the mechanism of Mcl-1 increase and Noxa increase induced by (-)-gossypol.
In conclusion, our study demonstrates that (-)-gossypol significantly enhances the anti-tumor activity of docetaxel in human prostate cancer both in vitro and in vivo, and may represent a promising new adjuvant therapy with a novel molecular mechanism for the treatment of hormone-refractory human prostate cancer with Bcl-2/Bcl-xL/Mcl-1 overexpression. (-)-Gossypol is now in Phase II clinical trials for hormone-refractory prostate cancer. Our results provide insight into how (-)-gossypol works with chemotherapy in prostate cancer cells. Our finding contributes to the rational design of coming clinical trials and refines the selection of patients who will benefit the most from Bcl-2 molecular therapy as a promising novel strategy for overcoming chemoresistance of human prostate cancer.
Supplementary Material
Acknowledgments
We thank Susan Harris for help with the manuscript; Dr. Junyin Yuan of Harvard University for kindly providing Bcl-xL-CFP, Bax-YFP and Bad-YFP plasmids for the FRET assay; Dr. Gabriel Nunez of the University of Michigan for kindly providing murine pro-B lymphoid cell line FL5.12 stably transfected with Bcl-xL or control vector; the University of Michigan Comprehensive Cancer Center (UMCCC) Histology Core for immunohistology study; UMCCC Unit of Laboratory Animal Medicine (ULAM) for help with animal experiments, and the UMCCC Flow Cytometry Core for flow cytometry analysis.
Grant support: This study was supported in part by Department of Defense Prostate Cancer Research Program W81XWH-04-1-0215 and W81XWH-06-1-0010 (to L. X.), NIH NCI Prostate Cancer SPORE in University of Michigan Developmental Project 2P50 CA069568-06A1 (to L. X.), NIH R01 CA121830-01 and R21 CA128220-01 (to L. X.), and by NIH through the University of Michigan’s Cancer Center Support Grant (5 P30 CA46592).
References
- 1.Assikis V, Simons JW. Novel therapeutic strategies for androgen-independent prostate cancer: An update. Semin Oncol. 2004;31:26–32. doi: 10.1053/j.seminoncol.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Oh WK, Kantoff PW. Management of hormone refractory prostate cancer: Current standards and future prospects. J Urol. 1998;160:1220–9. [PubMed] [Google Scholar]
- 3.Rago R. Management of hormone-sensitive and hormone-refractory metastatic prostate cancer. Cancer Control. 1998;5:513–21. doi: 10.1177/107327489800500604. [DOI] [PubMed] [Google Scholar]
- 4.Reed JC. Bcl-2 family proteins: Strategies for overcoming chemoresistance in cancer. Adv Pharmacol. 1997;41:501–32. doi: 10.1016/s1054-3589(08)61070-4. [DOI] [PubMed] [Google Scholar]
- 5.Reed JC, Jurgensmeier JM, Matsuyama S. Bcl-2 family proteins and mitochondria. Biochim Biophys Acta. 1998;1366:127–37. doi: 10.1016/s0005-2728(98)00108-x. [DOI] [PubMed] [Google Scholar]
- 6.Korsmeyer SJ. Bcl-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59:1693s–700s. [PubMed] [Google Scholar]
- 7.Makin G, Dive C. Apoptosis and cancer chemotherapy. Trends in Cell Biol. 2001;11:S22–6. doi: 10.1016/s0962-8924(01)02124-9. [DOI] [PubMed] [Google Scholar]
- 8.Konopleva M, Zhao S, Hu W, et al. The anti-apoptotic genes Bcl-x(l) and Bcl-2 are over-expressed and contribute to chemoresistance of non-proliferating leukaemic CD34+ cells. Br J Haematol. 2002;118:521–34. doi: 10.1046/j.1365-2141.2002.03637.x. [DOI] [PubMed] [Google Scholar]
- 9.Furuya Y, Krajewski S, Epstein JI, Reed JC, Isaacs JT. Expression of Bcl-2 and the progression of human and rodent prostatic cancers. Clin Cancer Res. 1996;2:389–98. [PubMed] [Google Scholar]
- 10.Krajewska M, Krajewski S, Epstein JI, et al. Immunohistochemical analysis of Bcl-2, Bax, Bcl-x, and Mcl-1 expression in prostate cancers. Am J Pathol. 1996;148:1567–76. [PMC free article] [PubMed] [Google Scholar]
- 11.Beale PJ, Rogers P, Boxall F, Sharp SY, Kelland LR. Bcl-2 family protein expression and platinum drug resistance in ovarian carcinoma. Br J Cancer. 2000;82:436–40. doi: 10.1054/bjoc.1999.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferlini C, Raspaglio G, Mozzetti S, et al. Bcl-2 down-regulation is a novel mechanism of paclitaxel resistance. Mol Pharmacol. 2003;64:51–8. doi: 10.1124/mol.64.1.51. [DOI] [PubMed] [Google Scholar]
- 13.Grad JM, Zeng XR, Boise LH. Regulation of Bcl-xl: A little bit of this and a little bit of Stat. Curr Opin Oncol. 2000;12:543–9. doi: 10.1097/00001622-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Lebedeva I, Rando R, Ojwang J, Cossum P, Stein CA. Bcl-xl in prostate cancer cells: Effects of overexpression and down-regulation on chemosensitivity. Cancer Res. 2000;60:6052–60. [PubMed] [Google Scholar]
- 15.Gleave M, Nelson C, Chi K. Antisense targets to enhance hormone and cytotoxic therapies in advanced prostate cancer. Curr Drug Targets. 2003;4:209–21. doi: 10.2174/1389450033491190. [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Rafiuddin-Shah M, Tu HC, et al. Hierarchical regulation of mitochondrion-dependent apoptosis by Bcl-2 subfamilies. Nat Cell Biol. 2006;8:1348–58. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- 17.Willis SN, Chen L, Dewson G, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xl, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams KW, Cooper GM. Rapid turnover of Mcl-1 couples translation to cell survival and apoptosis. J Biol Chem. 2007;282:6192–200. doi: 10.1074/jbc.M610643200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem. 2003;46:4259–64. doi: 10.1021/jm030190z. [DOI] [PubMed] [Google Scholar]
- 20.Xu L, Yang D, Wang S, et al. (-)-gossypol enhances response to radiation therapy and results in tumor regression of human prostate cancer. Mol Cancer Ther. 2005;4:197–205. [PubMed] [Google Scholar]
- 21.Simonian PL, Grillot DA, Nunez G. Bcl-2 and Bcl-xl can differentially block chemotherapy-induced cell death. Blood. 1997;90:1208–16. [PubMed] [Google Scholar]
- 22.Biggiogera M, Bottone MG, Pellicciari C. Nuclear ribonucleoprotein-containing structures undergo severe rearrangement during spontaneous thymocyte apoptosis. A morphological study by electron microscopy. Histochem Cell Biol. 1997;107:331–6. doi: 10.1007/s004180050118. [DOI] [PubMed] [Google Scholar]
- 23.Degterev A, Lugovskoy A, Cardone M, et al. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xl. Nat Cell Biol. 2001;3:173–82. doi: 10.1038/35055085. comment. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Galan P, Roue G, Villamor N, Montserrat E, Campo E, Colomer D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of Ros and Noxa activation independent of p53 status. Blood. 2006;107:257–64. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- 25.Castedo M, Coquelle A, Vivet S, et al. Apoptosis regulation in tetraploid cancer cells. Embo J. 2006;25:2584–95. doi: 10.1038/sj.emboj.7601127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Susin SA, Lorenzo HK, Zamzami N, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–6. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 27.Donovan M, Cotter TG. Control of mitochondrial integrity by Bcl-2 family members and caspase-independent cell death. Biochim Biophys Acta. 2004;1644:133–47. doi: 10.1016/j.bbamcr.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Smaili SS, Hsu YT, Sanders KM, Russell JT, Youle RJ. Bax translocation to mitochondria subsequent to a rapid loss of mitochondrial membrane potential. Cell Death Differ. 2001;8:909–20. doi: 10.1038/sj.cdd.4400889. [DOI] [PubMed] [Google Scholar]
- 29.Mohammad RM, Wang S, Aboukameel A, et al. Preclinical studies of a nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-x(l) [(-)-gossypol] against diffuse large cell lymphoma. Mol Cancer Ther. 2005;4:13–21. [PubMed] [Google Scholar]
- 30.Podar K, Gouill SL, Zhang J, et al. A pivotal role for Mcl-1 in bortezomib-induced apoptosis. Oncogene. 2008;27:721–31. doi: 10.1038/sj.onc.1210679. [DOI] [PubMed] [Google Scholar]
- 31.Gomez-Bougie P, Oliver L, Le Gouill S, Bataille R, Amiot M. Melphalan-induced apoptosis in multiple myeloma cells is associated with a cleavage of Mcl-1 and bim and a decrease in the Mcl-1/Bim complex. Oncogene. 2005;24:8076–9. doi: 10.1038/sj.onc.1208949. [DOI] [PubMed] [Google Scholar]
- 32.Nunez G, London L, Hockenbery D, Alexander M, McKearn JP, Korsmeyer SJ. Deregulated bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990;144:3602–10. [PubMed] [Google Scholar]
- 33.Boise LH, Gonzalez-Garcia M, Postema CE et al. Bcl-x, a Bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 34.Corbett TH. Transplantable syngeneic rodent tumors. Totowa: Humana Press; 2002. [Google Scholar]
- 35.Zhang M, Liu H, Guo R, et al. Molecular mechanism of gossypol-induced cell growth inhibition and cell death of HT-29 human colon carcinoma cells. Biochem Pharmacol. 2003;66:93–103. doi: 10.1016/s0006-2952(03)00248-x. [DOI] [PubMed] [Google Scholar]
- 36.Oliver CL, Miranda MB, Shangary S, Land S, Wang S, Johnson DE. (-)-gossypol acts directly on the mitochondria to overcome Bcl-2- and Bcl-x(l)-mediated apoptosis resistance. Mol Cancer Ther. 2005;4:23–31. [PubMed] [Google Scholar]
- 37.Oliver CL, Bauer JA, Wolter KG, et al. In vitro effects of the BH3 mimetic, (-)-gossypol, on head and neck squamous cell carcinoma cells. Clin Cancer Res. 2004;10:7757–63. doi: 10.1158/1078-0432.CCR-04-0551. [DOI] [PubMed] [Google Scholar]
- 38.Adams JM, Cory S. The bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–37. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villunger A, Michalak EM, Coultas L, et al. P53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science. 2003;302:1036–8. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 40.Lee EF, Czabotar PE, van Delft MF, et al. A novel bh3 ligand that selectively targets mcl-1 reveals that apoptosis can proceed without Mcl-1 degradation. J Cell Biol. 2008;180:341–55. doi: 10.1083/jcb.200708096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han J, Goldstein LA, Hou W, Rabinowich H. Functional linkage between noxa and bim in mitochondrial apoptotic events. J Biol Chem. 2007;282:16223–31. doi: 10.1074/jbc.M611186200. [DOI] [PubMed] [Google Scholar]
- 42.Denisov AY, Sprules T, Fraser J, Kozlov G, Gehring K. Heat-induced dimerization of bcl-xl through alpha-helix swapping. Biochemistry. 2007;46:734–40. doi: 10.1021/bi062080a. [DOI] [PubMed] [Google Scholar]
- 43.Wolter KG, Verhaegen M, Fernandez Y, et al. Therapeutic window for melanoma treatment provided by selective effects of the proteasome on Bcl-2 proteins. Cell Death Differ. 2007;14:1605–16. doi: 10.1038/sj.cdd.4402163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.