Abstract
Centrosome duplication and separation are of central importance for cell division. Here we provide a detailed account of this dynamic process in Dictyostelium. Centrosome behavior was monitored in living cells using a γ-tubulin–green fluorescent protein construct and correlated with morphological changes at the ultrastructural level. All aspects of the duplication and separation process of this centrosome are unusual when compared with, e.g., vertebrate cells. In interphase the Dictyostelium centrosome is a box-shaped structure comprised of three major layers, surrounded by an amorphous corona from which microtubules emerge. Structural duplication takes place during prophase, as opposed to G1/S in vertebrate cells. The three layers of the box-shaped core structure increase in size. The surrounding corona is lost, an event accompanied by a decrease in signal intensity of γ-tubulin–green fluorescent protein at the centrosome and the breakdown of the interphase microtubule system. At the prophase/prometaphase transition the separation into two mitotic centrosomes takes place via an intriguing lengthwise splitting process where the two outer layers of the prophase centrosome peel away from each other and become the mitotic centrosomes. Spindle microtubules are now nucleated from surfaces that previously were buried inside the interphase centrosome. Finally, at the end of telophase, the mitotic centrosomes fold in such a way that the microtubule-nucleating surface remains on the outside of the organelle. Thus in each cell cycle the centrosome undergoes an apparent inside-out/outside-in reversal of its layered structure.
INTRODUCTION
Centrosomes are cell organelles involved in the nucleation and organization of the microtubule cytoskeleton in interphase and mitosis. They are singular, centrally located, discrete bodies whose duplication must be tightly coupled to the cell cycle to ensure accurate spindle formation and cell division (Bornens, 1992; Balczon, 1996). At the structural level there is substantial variability in centrosome size, shape, and composition among various organisms. In animal cells, they almost always consist of a pair of centrioles surrounded by a cloud of pericentriolar material (Kellog et al., 1994). Whereas centrioles are the most conspicuous centrosomal constituents, it is the pericentriolar material, rather than the centrioles, that is responsible for microtubule nucleation (Gould and Borisy, 1977; Kalnins, 1992; Moritz et al., 1995). In protozoa, algae and fungi, centrosomes show a much greater variability in morphology, ranging from the electron-dense “bodies” of some fungi (Heath, 1981) or the relatively simple layered designs of yeast or Dictyostelium (McCully and Robinow, 1971; Byers and Goetsch, 1975; Omura and Fukui, 1985) to the elaborate structures of some heliozoans (Bardele, 1977). In contrast, higher plant cells do not possess a clearly defined morphological entity that could easily be defined as a centrosome (Gunning and Hardham, 1982; Marc, 1997).
The biochemical composition of centrosomes is still largely unknown although a few components can be classified as potentially universal (Kalt and Schliwa, 1993; Kellog et al., 1994). They include γ-tubulin (Joshi, 1994), pericentrin (5051 antigen; Doxsey et al., 1994), and possibly centrin (Baron and Salisbury, 1992; Schiebel and Bornens, 1995). Of these, γ-tubulin is a highly conserved component of the centrosome that is required for microtubule nucleation (Oakley et al., 1990; Joshi et al. 1992; Sunkel et al., 1995; Spang et al., 1996). γ-Tubulin is concentrated at microtubule-organizing centers but, in addition, a γ-tubulin ring complex is present in the cytoplasm (Zheng et al., 1995; Marschall et al., 1996; Moudjou et al., 1996) from where it can be recruited to the centrosome (Ohta et al., 1993; Felix et al. 1994; Stearns and Kirschner, 1994). These findings suggest that the association of γ-tubulin with the centrosome can be regulated in a way that correlates with functional changes of the centrosome.
Centrosome duplication is still one of the least understood events in cell biology. A description of the major stages of this process exists for centrioles or yeast spindle pole bodies (Robbins et al., 1968; Moens and Rapport, 1971; Byers and Goetsch, 1975; Kuriyama and Borisy, 1981), but the actual mode of separation has remained elusive. In mammalian cells, daughter centrioles appear near the proximal end of the existing centrioles at the end of G1 phase. It remains unresolved whether the initiation of duplication requires a template for the daughter centrioles or whether these can self-assemble from prefabricated components. Before mitosis the centriole pairs with associated pericentriolar material separate. Exactly how this is achieved both mechanistically and biochemically is unknown. In yeast, spindle pole body duplication begins during G1 with the formation of the satellite on the cellular side of the half-bridge. The satellite persists until a new spindle pole body appears at the same site, but no intermediate structures have been identified so far.
We have studied the dynamics of the centrosome in amebae of the slime mold Dictyostelium discoideum. The interphase centrosome of Dictyostelium is a nucleus-associated body consisting of a box-shaped, three-layered core surrounded by an amorphous matrix, the corona, from which microtubules emanate into the cell periphery (Moens, 1976; Kuriyama et al. 1982; Omura and Fukui, 1985). γ-Tubulin is concentrated in the corona, indicating that the corona is a functional equivalent of the pericentriolar matrix of higher eukaryotic centrosomes (Euteneuer et al., 1998). We have generated a fusion protein of green fluorescent protein (GFP) and γ-tubulin (termed γ-tub–GFP; Ueda et al., 1997) that allows us to follow the centrosome cycle in living cells. We show here that the deployment of centrosomal γ-tubulin during mitosis is modulated in a manner consistent with intriguing structural and functional changes of the centrosome. All morphological events associated with centrosome duplication take place in mitosis. In prophase the core structure increases in size and undergoes a splitting process at the start of prometaphase in which the outer layers peel away from each other. These two layers become the mitotic centrosomes and organize the spindle, only to refold at the end of telophase to generate a new three-layered interphase centrosome in each daughter cell. Thus, in each cell cycle the centrosome undergoes an inside-out/outside-in reversal of its major layers.
MATERIALS AND METHODS
Growth and Synchronization of Cells
Dictyostelium discoideum strain AX2 was cultivated axenically in liquid nutrient medium (Claviez et al., 1982) under constant shaking. To partially synchronize the cells, the temperature-shift method of Maeda (1986) was used with slight modifications. Log-phase cells (∼106 cells/ml) were placed at 4°C for 20–24 h and then brought back to 23°C. An increase in mitotic cells by a factor of 10–30 was observed 2.5–3.5 h after removal from the cold. Amebae expressing the γ-tub–GFP fusion protein were grown with Klebsiella aerogenes on nutrient agar for 24 h at 22°C.
Immunofluorescence Microscopy
Cells were allowed to settle onto clean coverslips for 15–30 min in growth medium and then exposed to 5 mM MgCl2 for 2 min to induce further flattening. Cells were fixed in PHEM buffer (12 mM piperazine-N,N′-bis(2-ethanesulfonic acid), 5 mM HEPES, 2 mM EGTA, 0.5 mM MgCl2) containing 0.5% glutaraldehyde and 0.5% Triton X-100 for 15 min. The specimens were then processed according to a standard immunofluorescence protocol (Schliwa et al., 1981). Briefly, after treatment with sodium borohydride, cells were incubated in primary antibody (YL1/2, Kilmartin et al., 1982; MPM2, Davis et al.., 1983) for 45 min at room temperature. After several rinses in PBS, appropriate secondary antibodies (Dianova, Hamburg, Germany) were applied for the same length of time. For visualization of nuclei or chromosomes, cells were treated with 1 μg/ml DAPI in PBS for 5 min. Coverslips were mounted on glass slides and viewed in a Axiophot microscope (Carl Zeiss, Jena, Germany) equipped with either a 40× Achroplan water immersion lens or a 100× Plan-Neofluar oil immersion lens.
Microscopy of γ-tub-GFP–transfected Cells
Cells expressing the γ-tub–GFP fusion protein were harvested and resuspended in Sörensen’s phosphate buffer, pH 6.0. An aliquot was placed on a glass coverslip and covered with a thin agarose sheet (Fukui and Inoué, 1991). In Dictyostelium, mitosis takes up only 3% of the total cell cycle time; accordingly, dividing cells are rare in a population of logarithmically growing cells. However, mitotic cells observed by light microscopy are distinguished from interphase cells by their round contour, quiescent cytoplasm, and disintegrating nucleoli, resulting in a more uniform appearance of the nucleus (Fukui and Inoué, 1991). These morphological characteristics allow the identification of mitotic cells, even at a very early stage in mitosis, in a population of nonsynchronized cells.
Cells were observed using a Zeiss Axiovert inverted microscope equipped with standard filter sets for fluorescein. In cells imaged by fluorescence microscopy, the length of the stages of mitosis is comparable to that of cells imaged by phase contrast microscopy, indicating no apparent deleterious effects of this method of observation during this time period. Images were recorded in real time by using a Panasonic AG 6720 video recorder through a silicon intensified tube (SIT) camera (Hamamatsu, Herrsching, Germany). For image analysis, frames were captured from the recorded tapes at 5-s intervals with a personal computer (Macintosh IIfx) equipped with an analog-digital converter (PixelPipeline; Perceptions, Knoxville, TN). Fluorescence intensities were quantitated using the NIH Public Domain image software.
Confocal Microscopy
Images were acquired using the Leica TCS NT confocal imaging system (Leica Mikroskopie, Wetzlar, Germany). For image analysis, images were transferred to a personal computer (Power Macintosh 8500/180) and were analyzed using the NIH image software.
Electron Microscopy
To select defined stages of mitosis for electron microscopy, DAPI staining was used to identify stages of chromatin condensation and chromatid segregation, allowing the distinction of metaphase, anaphase, and telophase. To distinguish prophase from prometaphase cells, a combination of MPM-2 antibody labeling (Davis et al., 1983; Vandre et al., 1986) and DAPI staining, or a combination of tubulin antibody labeling and DAPI staining, was employed. In agreement with observations by others (Roos and Camenzind, 1981; Roos et al., 1984), prometaphase in Dictyostelium is defined as the first appearance of spindle microtubules, which is accompanied by the movement of mitotic centrosomes into fenestrae of the nuclear envelope. Cells preselected by light and immunofluorescent microscopic inspection were circled with a diamond marker. Coverslips were rinsed with 0.05 M cacodylate buffer, pH 7.0, and postfixed in 1% osmium tetroxide in cacodylate buffer for 1 h. After dehydration in ethanol and propylene oxide, they were embedded in an Epon-Araldite mixture. After polymerization, coverslips were removed by brief cooling to liquid nitrogen temperatures, and serial sections were cut on a Reichert Ultracut E ultramicrotome (Leica, Bensheim, Germany). Sections were viewed on a JEOL 1200 EXII electron microscope (Kontron, Neufahrn, Germany) and photographed on Ilford EM film at a magnification of 20,000×.
RESULTS
Centrosome Behavior during Mitosis Visualized by a γ-tub–GFP Fusion Protein
In vertebrate and yeast cells, centrosome duplication starts in G1 (Rattner and Phillips, 1973; Byers and Goetsch, 1975). Dictyostelium (strain AX2), however, lacks a G1 phase: mitosis is followed immediately by a short S phase (30 min) and a long G2 phase (8 h) (Weijer et al., 1984). Previous work (Moens, 1976; Roos and Guhl, 1990) and our own light and electron microscopic studies have revealed that all interphase cells possess only one centrosome per nucleus. Since mitotic cells have two, duplication/separation must occur at the G2/M border or in mitosis. This reasoning is fully supported by the observation of γ-tub–GFP dynamics in living cells (Figure 1 and 2).
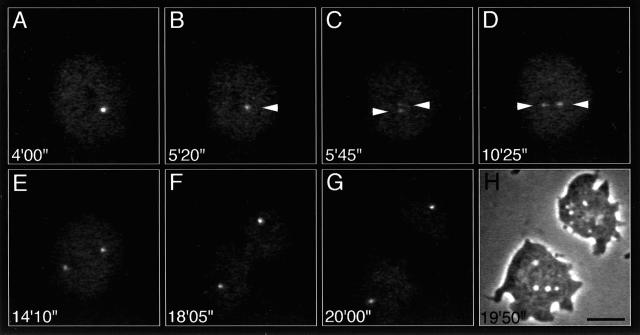
Figure 1.
Centrosome behavior during mitosis visualized by γ-tub–GFP in a D. discoideum ameba. (A and B) Prophase; (C and D) prometaphase; (E) metaphase; (F) anaphase; (G) telophase. Note that the brightness of the centrosome decreases before its separation and reincreases after separation. The times are in minutes and seconds. (H) Phase contrast image corresponding to panel G. Scale bar, 10 μm.
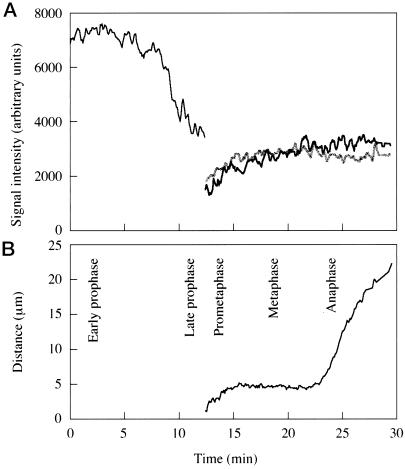
Figure 2.
Changes in the signal intensity of γ-tub–GFP in a dividing Dictyostelium ameba between prophase and anaphase (A) and corresponding changes in the distance between the two spindle poles (B).
In all interphase and prophase cells, γ-tub–GFP is localized in a single fluorescent spot, consistent with the presence of only one centrosome (Figure 1, A and B). While interphase centrosomes move continuously in the cytoplasm in coordination with the nucleus (Ueda et al., 1997), these movements subside during prophase. In early prophase (Figure 1A) the signal intensity of the centrosome is approximately twice that of interphase centrosomes but declines sharply in late prophase (compare Figure 1, A and B, with Figure 2A). At the transition to prometaphase the centrosome splits into two daughter centrosomes (spindle poles), each of which initially exhibits about half the brightness of the mother centrosome at the end of prophase (Figure 2A). The spindle poles move away from each other as the prometaphase spindle elongates (Figures 1, C and D, and 2B) until a constant distance is maintained in metaphase (Figures 1E and 2B). Throughout prometaphase, the signal intensity of γ-tub–GFP gradually increases, indicating a reassociation of γ-tub–GFP with the spindle poles (Figure 2A). Anaphase spindle elongation increases the distance between the spindle poles (Figures 1F and 2B) until the daughter centrosomes suddenly move independently from each other as if an interconnection between them had been severed. Finally, cytokinesis results in the generation of two daughter cells (Figure 1, G and H).
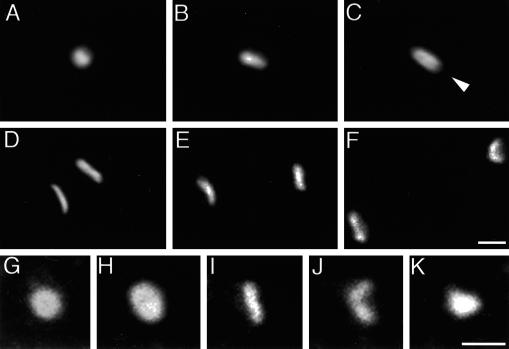
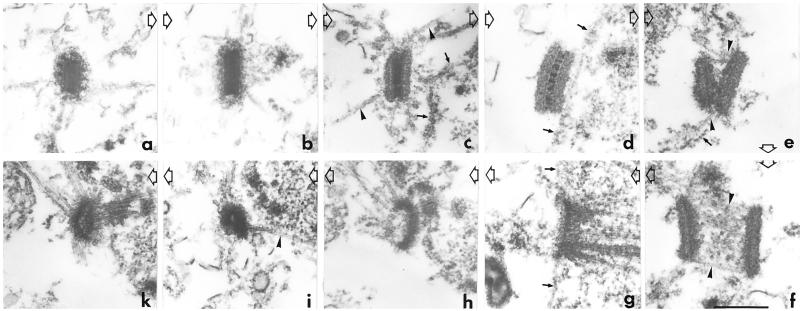
The dynamic changes of γ-tub–GFP in mitotic centrosomes were analyzed in more detail by high-resolution conventional and confocal fluorescence microscopy. The changes in the brightness of γ-tub–GFP labeling in living cells described above are accompanied by significant shape changes of the centrosome. In prophase, the centrosome has a more elongated shape than in interphase, consistent with its increased brightness (compare Figure 3, A and G, with B and H). In late prophase when the intensity of γ-tub–GFP labeling is reduced, centrosomes exhibit a dark zone in the middle of their long axis (Figure 3C, arrowhead). High-resolution image reconstructions of prometaphase and metaphase cells demonstrate a distribution of γ-tub–GFP at the newly formed spindle poles in the form of a rectangular plate with a shallow curvature (Figure 3, D and I). In anaphase (Figure 3, E, F, and J), the curvature of the mitotic centrosome increases until in late telophase (Figure 3K) the γ-tub–GFP fluorescence pattern can hardly be distinguished any more from that of an interphase centrosome (compare Figure 3G with Figure 3K).
Figure 3.
Conventional (A–C) and confocal (D–K) microscopy images of fixed cells illustrating the shape changes of mitotic centrosomes in cells expressing γ-tub–GFP. (A) Interphase; (B) prophase; (C) prophase/prometaphase; arrowhead in panel C indicates a dark zone along the long axis of the centrosome. (D–F) Stacks of 40 optical sections of 40 nm each: (D) metaphase; (E) anaphase; (F) telophase. (G–K) Single optical sections through centrosomes at higher magnification: (G) interphase; (H) early prophase; (I) metaphase; (J) anaphase; (K) late telophase. Note the increase in size from interphase to prophase (G and H) and the curvature in the anaphase centrosome (J). The cells shown here were also stained with DAPI (not shown) to assess the phases of mitosis. Scale bar, 1 μm.
Centrosomal Shape Changes at the Fine Structural Level
Appropriate mitotic stages were preselected in the light microscope as described in MATERIALS AND METHODS. Eighty five mitotic nuclei were serially sectioned; 64 of these were in prophase or prometaphase because these were the stages suspected to be critical for centrosome duplication and separation. The procedures used to select cells in defined stages of mitosis for serial sectioning allow the determination of mitotic stages with considerable accuracy but require extraction with a nonionic detergent. This results in the loss of most membrane systems in the cytoplasm and the disappearance of most of the nuclear envelope. On the other hand, the centrosome now stands out clearly against the lighter background of extracted cytoplasm, facilitating the visualization of structural changes in the corona.
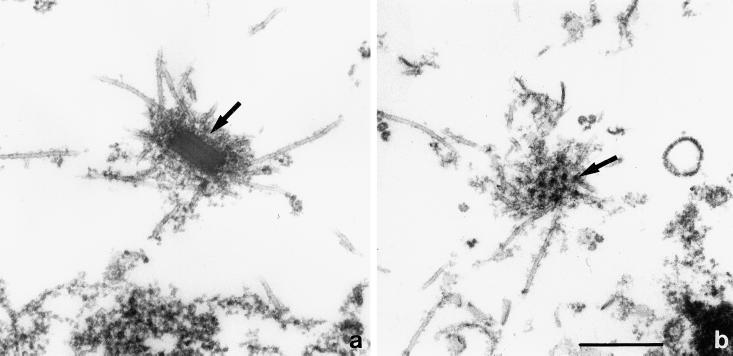
At the electron microscopic level, interphase centrosomes have the shape of a matchbox with rounded edges (Omura and Fukui, 1985). They are approximately 280×220×130 nm in size and show a layered composition (Figure 4a). Microtubules are embedded in a zone of structured fuzz, the corona (Figure 4b), which adds another 70–80 nm on all sides. While centrosome dimensions may vary somewhat depending on the strain used or the fixation protocol employed (Omura and Fukui, 1985; Roos and Guhl, 1990), centrosomes in our preparations rarely diverge from these average dimensions by more than 20 nm.
Figure 4.
Interphase centrosome. (a) Microtubules are originating from the corona (arrow) surrounding the rectangular core of the centrosome. Note the layered composition of the core. (b) Grazing section of the corona, showing the more or less regular arrangement of electron-dense nodules (arrow). Scale bar, 0.5 μm. The electron micrographs of this figure as well as Figure 5 are from cells lysed during fixation according to the protocol described in MATERIALS AND METHODS.
Centrosome size and structure change dramatically during prophase, but, as already demonstrated by the analysis of γ-tub–GFP distribution, the actual separation into two centrosomes takes place at the transition from prophase to prometaphase. Figure 5 illustrates the major structural transformations associated with centrosome duplication inferred from our analysis of serially sectioned mitotic cells. The centrosome increases in size during prophase along all three axes (see Figure 5, a–d). The dimensions at the end of prophase reach 500×350×250 nm. Another major structural change at the end of prophase is the disappearance of the corona (compare Figure 5, panel b, with panels c and d). This stage likely is reflected by, and corresponds to, the decrease in the signal intensity of γ-tub–GFP fluorescence in late prophase (Figures 1B and 2A). Concomitantly, the number of microtubules emanating from the centrosome diminishes (see also Kitanishi-Yumura and Fukui, 1987). In addition to an enlargement of the outer layers that now appear less electron dense, the central layer develops vertical striations. This stage probably corresponds to that seen in Figure 3C at the light microscopical level. Toward the end of prophase the centrosome closely apposes the nuclear envelope (Figure 5d).
Figure 5.
Summary composite of the inferred sequence of morphological changes the centrosome undergoes during duplication, separation, and folding. Progression of time is indicated by large open arrows. For all stages only one section of a section series is shown. (a) Interphase; (b) early prophase; (c) midprophase; (d) late prophase; (e) early prometaphase; (f) prometaphase; (g) metaphase; (h, i, and k) various stages of telophase. Arrowheads indicate microtubules; small arrows indicate remnants of nuclear envelope. Scale bar, 0.5 μm
Centrosomes serially sectioned at the transition from prophase to prometaphase show a gap between the two outer layers as if the centrosome has split open. The central layer is no longer visible except in places where the two outer layers are still closely apposed (Figure 5e). This process, which superficially resembles the separation of sister chromatids in early anaphase, presumably takes place within a few seconds. Although difficult to demonstrate in electron micrographs, at this stage the centrosomes come to lie in openings of the nuclear envelope. Even at very early stages of this separation process (such as that shown in Figure 5e), spindle microtubules are seen to be associated with the inner surfaces of the emerging two mitotic centrosomes. In prometaphase and metaphase all spindle and kinetochore microtubules are assembled inside the nucleus. The two mitotic centrosomes separate from each other as the spindle elongates (Figure 5f), each now occupying its own fenestra in the nuclear envelope. Connections between the poles and kinetochores are also observed at early stages of separation of the newly formed spindle poles. Early attachment of kinetochores is facilitated by the clustering of kinetochores near the centrosome on the inside of the nuclear envelope in prophase (our unpublished results). The attachment is at first monopolar, as in mammalian cells, and becomes bipolar in the process of chromosome alignment.
Since the mitotic centrosome corresponds neither in shape nor in structure to the interphase centrosome, the events leading to its reformation in telophase were studied as well. The high resolution analysis of γ-tub–GFP distribution had demonstrated an increase in the curvature of the mitotic centrosomes from late prometaphase through telophase. This is confirmed by electron microscopy. In anaphase the edges of the mitotic centrosomes are curving away from the nuclear envelope toward the cytoplasm. Microtubules emanating from the edges now frequently extend into the cytoplasm rather than into the nucleoplasm. During telophase, the curling of the edges is even more pronounced (Figure 5h), and more microtubules extend into the cytoplasm. The mitotic centrosome appears sharply curved. One gets the impression that it folds together in a process reminiscent of the folding of a pocket knife (Figure 5i). The centrosome surface that used to face the cytoplasm now becomes buried inside the new interphase centrosome (Figure 5, i and k). At that stage the dimensions of the centrosome, i.e., length, width, and diameter, approach that of the interphase state again (compare Figure 5a and 5k). None of the serial section series of cells in anaphase or telophase has produced any evidence for a direct association of microtubules with the exterior, cytoplasmic face of the spindle pole. There is no indication for a reorganization of the microtubule system in late telophase to restore the interphase network other than the gradual redirection of “mitotic” microtubules into the cytoplasm through the process of centrosome folding. In this transition phase, microtubule distribution already resembles that of an interphase array (our unpublished results).
DISCUSSION
Mitosis and cell division critically depend on the precise doubling of the centrosome. The cell must ensure that there will be two, and only two, identical products of this duplication event; otherwise, ensuing mitoses will be abnormal. Given the central importance of centrosome reproduction for cell survival, surprisingly little is known about the structural changes associated with the generation of two daughter centrosomes. Here we provide a detailed account of the dynamics of centrosome duplication and separation in Dictyostelium amebae. Whereas the structure of both the interphase and the mitotic centrosome (whose appearances are remarkably different) have been known for some time (Moens, 1976; Roos and Camenzind, 1981; Kuriyama et al., 1982; McIntosh et al. 1985; Omura and Fukui, 1985; Roos and Guhl, 1990), the transition between the two has remained elusive. The rapidity of the process requires the use of procedures that allow the preselection of defined mitotic stages for electron microscopic analysis, such as partial synchronization and selection on the basis of DAPI and MPM2 antibody labeling. Otherwise, the events preceding the development of a bipolar mitotic spindle, in particular the fast separation phase of the two outer layers of the prophase centrosome, would have been difficult to uncover.
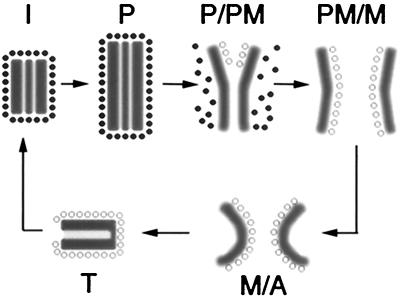
The sequence of events inferred from the correlative light and electron microscopic analysis presented here and summarized diagrammatically in Figure 6 demonstrates that the Dictyostelium centrosome undergoes a set of striking structural changes. A key step is the separation of the two outer layers of the prophase centrosome (Figure 5e): while the middle layer disappears, the two outer layers peel away from each other to form the mitotic centrosomes. The result of this process is the generation of two essentially identical structures at the spindle poles. Thus, the problem of generating two equivalent bodies from one, which initiates the transition from “cellular oneness to twoness” (Mazia, 1978), has been solved by Dictyostelium in a rather elegant way. The structural fidelity of this step is of utmost importance for cell cycle progression. At the end of mitosis, the plate-like mitotic centrosomes fold and convert the telophase centrosome into the trilaminar interphase centrosome. This process requires a certain degree of reorganization, the details of which are unknown. The structural transformations of the centrosome core described here are accompanied by dynamic changes in the distribution of centrosomal γ-tub–GFP, microtubules, and the corona.
Figure 6.
Model of γ-tubulin dynamics and centrosomal shape changes during mitosis in Dictyostelium. The interphase centrosome (I) of Dictyostelium comprises a multilayered core (gray box) surrounded by an amorphous matrix, the corona (•), from which microtubules emanate to the cell periphery. In early prophase (P) the centrosome increases in size. At the transition from prophase to prometaphase (P/PM), the corona, in which γ-tubulin is present, dissociates from the core, resulting in loss of cytoplasmic microtubules. The two outer layers splay apart, and γ-tubulin reassociates with these layers at their inner surfaces (○) to form new nucleation sites for spindle microtubules (P/PM). Beginning in metaphase and throughout anaphase (M/A), the centrosome starts to curl and finally folds in telophase (T), resulting in the reformation of the interphase centrosome.
The centrosome cycle of Dictyostelium is remarkable in several respects. First, structural duplication and separation take place during prophase and the transition to prometaphase, respectively, and thus immediately precede the development of a bipolar spindle. This contrasts markedly with mammalian cells or budding yeast where duplication is initiated in G1 (Winey and Byers, 1992). Second, both duplication and separation are very fast. Within the 8-h cell cycle, mitosis occupies approximately 15 min (Roos et al., 1984; Weijer et al., 1984) and centrosome duplication takes only a few minutes. Third, if our interpretation of the course of structural changes is correct, the centrosome undergoes a reversal of its organization in each cell cycle. The surface from which spindle microtubules emerge after “splitting” was previously buried inside the interphase centrosome, and it remains involved in microtubule organization during the following interphase due to the folding process in telophase. At the end of the next mitosis, however, this centrosomal surface becomes buried, again as a consequence of folding in telophase, in the interphase centrosome of the second generation of daughter cells. This course of events has no precedent in other cellular processes.
Although the analysis of live cells labeled with γ-tub–GFP has proved invaluable for the characterization of the centrosome cycle within the mitotic cycle, it should be interpreted with some caution. No major changes in the intensity of labeling have been observed by conventional immunofluorescence microscopy using γ-tubulin antibodies. However, this may be due to a penetration problem of the antibodies, which only label the outer surface of the corona, as demonstrated by preembedding immunoelectron microscopy (our unpublished results). Although the functional fidelity of γ-tub–GFP has not been demonstrated rigorously, the dynamic changes of γ-tub–GFP labeling observed here correlate well with the structural changes of the centrosome and the dynamics of microtubule organization. Thus the increased labeling intensity and size of the γ-tub-GFP–labeled prophase centrosome is matched by the increased dimensions of this organelle as seen by electron microscopy (compare Figure 3, A–C with Figure 5, a–d). The decrease in γ-tub–GFP association with the centrosome in late prophase, on the other hand, may well correspond to the gradual loss of the corona (Figure 5, c and d) and the release of microtubules from the centrosome, which has previously been observed at this stage by Kitanishi-Yumura and Fukui (1987). Moreover, the tripartite labeling pattern in late prophase (Figure 3C) and the thin plate-like appearance in early prometaphase (Figure 3D) are consistent with the lengthwise splitting/peeling process of the outer layers suggested by the fine structural analysis. Finally, whereas the surfaces of the mitotic centrosomes facing the spindle have γ-tubulin associated with them, no γ-tubulin could be demonstrated on the equivalent surfaces buried in the interphase centrosomal core, suggesting a redistribution during late prophase/early prometaphase (Euteneuer et al., 1998). The slight increase in γ-tub–GFP intensity in prometaphase may therefore indicate additional association of γ-tub–GFP with the nuclear side of the spindle poles.
The process of centrosome separation has not been revealed in detail for other types of centrosomes. In budding yeast, duplication is initiated by the formation of the so-called satellite in association with the half-bridge. Recent genetic and biochemical studies have discovered several novel spindle pole body components and their interactions (e.g., Bullitt et al., 1997; Knop et al., 1997; Schutz et al., 1997), but the series of events that eventually leads to the presence of two fully developed spindle pole bodies lying side-by-side at the start of S-phase (Winey and Byers, 1992) could not be demonstrated. The structural changes that occur in spindle pole bodies of fission yeast have been documented in considerable detail (Ding et al., 1997), although here, too, the transition from a single to a duplicated structure is not fully understood. Likewise, it is unknown how, in animal cells, the rather amorphous cloud of pericentriolar material in which the two pairs of centrioles are embedded separates into two entities of roughly equal size at the beginning of prophase. On the other hand, a process similar to the separation of the two nucleating layers of the Dictyostelium centrosome may occur in diatoms (Pickett-Heaps, 1991) where spindle microtubules form between two plate-like polar complexes that are believed to be derived from a multilayered organelle. However, neither the origin of the multilayered structure nor the early events in polar complex separation are known. Moreover, unlike diatoms where a prominent, bipolar spindle forms outside the intact nuclear envelope and settles into the nucleoplasm at a later stage (Tippit and Pickett-Heaps, 1977; McDonald et al., 1986), in Dictyostelium the separating centrosomes enter an opening in the nuclear envelope before a spindle has developed (see Figure 5e). In this respect the Dictyostelium centrosome resembles the spindle pole body of the fission yeast S. pombe where the interphase centrosome comprises a finely granular ellipsoid with a dark-staining central line that resides next to the nuclear envelope in the cytoplasm (Ding et al., 1997). As in Dictyostelium, duplication takes place late in the cell cycle, in this case in late G2. The duplicated spindle pole body enters an opening in the nuclear envelope first and then forms a spindle, and it leaves the nuclear envelope again at the end of telophase. Thus, the events recorded here for Dictyostelium could be paradigmatic for processes that may occur in a similar manner in many fungal and plant cells.
The morphological events of the centrosome cycle in Dictyostelium as revealed in this study provide a framework for further biochemical, molecular, and immunolocalization studies. We are now in a position to ask specific questions about the role of known centrosomal components in this process and the regulatory mechanisms that trigger these events.
ACKNOWLEDGMENTS
We thank P. Rao for the MPM-2 and J. Kilmartin for the YL 1/2 antibody. We are grateful to T. Zimmermann and D. Menzel for generous assistance with the confocal microscopy and N. Brusis for expert technical assistance. We also thank R. Gräf, U.-P. Roos, M. Bornens, E. Schiebel, and their groups for stimulating discussions. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 184) and the Friedrich Baur Stiftung. M.U. is supported by a Japan Society for the Promotion of Science postdoctoral fellowship for research abroad.
REFERENCES
- Balczon R. The centrosome in animal cells and its functional homologs in plant and yeast cells. Int Rev Cytol. 1996;169:25–82. doi: 10.1016/s0074-7696(08)61984-1. [DOI] [PubMed] [Google Scholar]
- Bardele C. Comparative study of axopodial microtubule patterns and possible mechanisms of pattern control in the centrohelidian Heliozoa Acanthocystis, Raphidiophrys, and Heterophrys. J Cell Sci. 1977;25:205–232. doi: 10.1242/jcs.25.1.205. [DOI] [PubMed] [Google Scholar]
- Baron AT, Salisbury JL. Role of centrin in spindle pole dynamics. In: Kalnins VI, editor. The Centrosome. San Diego, CA: Academic Press; 1992. pp. 167–195. [Google Scholar]
- Bornens M. Structure and function of isolated centrosomes. In: Kalnins VI, editor. The Centrosome. San Diego, CA: Academic Press; 1992. pp. 1–43. [Google Scholar]
- Bullitt E, Rout MP, Kilmartin JV, Akey CW. The yeast spindle pole body is assembled around a central crystal of Spc42p. Cell. 1997;89:1077–1086. doi: 10.1016/s0092-8674(00)80295-0. [DOI] [PubMed] [Google Scholar]
- Byers B, Goetsch L. Behavior of spindles and spindle plaques in the cell cycle and conjugation of Saccharomyces cerevisiae. J Bacteriol. 1975;124:511–523. doi: 10.1128/jb.124.1.511-523.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claviez M, Pagh K, Maruta H, Baltes W, Fisher P, Gerisch G. Electron microscopic mapping of monoclonal antibodies on the tail region of Dictyostelium myosin. EMBO J. 1982;1:1017–1022. doi: 10.1002/j.1460-2075.1982.tb01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FM, Tsao TH, Fowler SK, Rao PN. Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci USA. 1983;80:2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding R, West RR, Morphew M, Oakley BR, McIntosh JR. The spindle pole body of Schizosaccharomyces pombe enters and leaves the nuclear envelope as the cell cycle proceeds. Mol Biol Cell. 1997;8:1461–1479. doi: 10.1091/mbc.8.8.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey SJ, Stein P, Evans L, Calarco PD, Kirschner M. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell. 1994;76:639–650. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- Euteneuer U, Gräf R, Kube-Granderath E, Schliwa M. Dictyostelium γ-tubulin: molecular characterization and ultrastructural localization. J Cell Sci. 1998;111:405–412. doi: 10.1242/jcs.111.3.405. [DOI] [PubMed] [Google Scholar]
- Felix M-A, Antony C, Wright M, Maro B. Centrosome assembly in vitro: role of γ-tubulin recruitment in Xenopus sperm aster formation. J Cell Biol. 1994;124:19–31. doi: 10.1083/jcb.124.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y, Inoué S. Cell division in Dictyostelium with special emphasis on actomyosin organization in cytokinesis. Cell Motil Cytoskeleton. 1991;18:41–54. doi: 10.1002/cm.970180105. [DOI] [PubMed] [Google Scholar]
- Gould RR, Borisy GG. The pericentriolar material in Chinese hamster ovary cells nucleates microtubule formation. J Cell Biol. 1977;73:601–615. doi: 10.1083/jcb.73.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning BES, Hardham AR. Microtubules. Annu Rev Plant Physiol. 1982;33:651–698. [Google Scholar]
- Heath IB. Nucleus-associated organelles in fungi. Int Rev Cytol. 1981;69:191–221. [Google Scholar]
- Joshi HC. Microtubule-organizing centers and gamma-tubulin. Curr Opin Cell Biol. 1994;127:1965–1971. doi: 10.1016/0955-0674(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Joshi HC, Palacios MJ, McNamara L, Cleveland DW. Gamma-tubulin is a centrosomal protein required for cell cycle-dependent microtubule nucleation. Nature. 1992;356:80–83. doi: 10.1038/356080a0. [DOI] [PubMed] [Google Scholar]
- Kalnins VI, editor. The Centrosome. San Diego, CA: Academic Press; 1992. [Google Scholar]
- Kalt A, Schliwa M. Molecular components of the centrosome. Trends Cell Biol. 1993;3:118–128. doi: 10.1016/0962-8924(93)90174-y. [DOI] [PubMed] [Google Scholar]
- Kellog DR, Moritz M, Alberts BM. The centrosome and cellular organization. Annu Rev Biochem. 1994;63:639–674. doi: 10.1146/annurev.bi.63.070194.003231. [DOI] [PubMed] [Google Scholar]
- Kilmartin JV, Wright B, Milstein C. Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line. J Cell Biol. 1982;93:576–582. doi: 10.1083/jcb.93.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitanishi-Yumura T, Fukui Y. Reorganization of microtubules during mitosis in Dictyostelium: dissociation from MTOC and selective assembly/disassembly in situ. Cell Motil Cytoskel. 1987;8:106–117. [Google Scholar]
- Knop M, Pereira G, Geissler S, Grein K, Schiebel E. The spindle pole body component Spc97p interacts with the gamma-tubulin of Saccharomyces cerevisiae and functions in microtubule organization and spindle pole body duplication. EMBO J. 1997;16:1550–1564. doi: 10.1093/emboj/16.7.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R, Borisy GG. Centriole cycle in Chinese hamster ovary cells as determined by whole-mount electron microscopy. J Cell Biol. 1981;91:814–821. doi: 10.1083/jcb.91.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R, Sato C, Fukui Y, Nishibayashi S. In vitro nucleation of microtubules from microtubule-organizing center prepared from cellular slime mold. Cell Motil. 1982;2:257–272. doi: 10.1002/cm.970020306. [DOI] [PubMed] [Google Scholar]
- Maeda Y. A new method for inducing synchronous growth of Dictyostelium discoideum cells using temperature shifts. J Gen Microbiol. 1986;132:1189–1196. [Google Scholar]
- Marc J. Microtubule-organizing centres in plants. Trends Plant Sci. 1997;2:223–230. [Google Scholar]
- Marschall LG, Jeng RL, Mulholland J, Stearns T. Analysis of Tub4p, a yeast γ-tubulin-like protein: implications for microtubule organizing center function. J Cell Biol. 1996;134:443–454. doi: 10.1083/jcb.134.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazia D. Origin of twoness in cell reproduction, In: Dirksen ER, Prescott DM, editors. Cell Reproduction: In Honor of Daniel Mazia. C.F. Fox, New York: Academic Press; 1978. pp. 1–14. [Google Scholar]
- McCully EK, Robinow CF. Mitosis in the fission yeast Schizosaccharomyces pombe: a comparative study with light and electron microscopy. J Cell Sci. 1971;9:475–507. doi: 10.1242/jcs.9.2.475. [DOI] [PubMed] [Google Scholar]
- McDonald KL, Pfister K, Masuda H, Wordeman L, Staiger C, Cande WZ. Comparison of spindle elongation in vivo and in vitro in Stephanopyxis turris. J Cell Sci Suppl. 1986;6:205–227. doi: 10.1242/jcs.1986.supplement_5.14. [DOI] [PubMed] [Google Scholar]
- McIntosh JR, Roos U-P, Neighbors B, McDonald K. Architecture of the microtubule component of mitotic spindles from Dictyostelium discoideum. J Cell Sci. 1985;75:93–129. doi: 10.1242/jcs.75.1.93. [DOI] [PubMed] [Google Scholar]
- Moens PB. Spindle and kinetochore morphology of Dictyostelium discoideum. J Cell Biol. 1976;68:113–122. doi: 10.1083/jcb.68.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens PB, Rapport E. Spindles, spindle plaques, and meiosis in yeast Saccharomyces cerevisiae (Hansen) J Cell Biol. 1971;50:344–361. doi: 10.1083/jcb.50.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M, Braunfield MB, Fung JC, Sedat JW, Alberts BA. Three dimensional characterization of the centrosome from early Drosophila embryos. J Cell Biol. 1995;130:1149–1159. doi: 10.1083/jcb.130.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudjou M, Bordes N, Paintrand M, Bornens M. γ-Tubulin in mammalian cells: the centrosomal and the cytosolic forms. J Cell Sci. 1996;109:875–887. doi: 10.1242/jcs.109.4.875. [DOI] [PubMed] [Google Scholar]
- Oakley BR, Oakley CE, Yoon YS, Jung MK. γ-Tubulin is a component of the spindle pole body that is essential for microtubule function in Aspergillus nidulans. Cell. 1990;61:1289–1301. doi: 10.1016/0092-8674(90)90693-9. [DOI] [PubMed] [Google Scholar]
- Ohta K, Shiina N, Okumura E, Hisanaga S-I, Kishimoto T, Endo S, Gotoh Y, Nishida E, Sakai H. Microtubule nucleating activity of centrosomes in cell-free extracts from Xenopus eggs: involvement of phosphorylation and accumulation of pericentriolar material. J Cell Sci. 1993;104:125–137. doi: 10.1242/jcs.104.1.125. [DOI] [PubMed] [Google Scholar]
- Omura F, Fukui Y. Dictyostelium MTOC: structure and linkage to the nucleus. Protoplasma. 1985;127:212–221. [Google Scholar]
- Pickett-Heaps J. Cell division in diatoms. Int Rev Cytol. 1991;128:63–108. [Google Scholar]
- Rattner JB, Phillips SG. Independence of centriole formation and DNA synthesis. J Cell Biol. 1973;57:359–372. doi: 10.1083/jcb.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins E, Jentsch G, Micali A. The centriole cycle in synchronized HeLa cells. J Cell Biol. 1968;36:329–339. doi: 10.1083/jcb.36.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos U-P, Camenzind R. Spindle dynamics during mitosis in Dictyostelium discoideum. Eur J Cell Biol. 1981;25:248–257. [PubMed] [Google Scholar]
- Roos U-P, De Brabander M, De Mey J. Indirect immunofluorescence of microtubules in Dictyostelium discoideum. Exp Cell Res. 1984;151:183–193. doi: 10.1016/0014-4827(84)90367-7. [DOI] [PubMed] [Google Scholar]
- Roos U-P, Guhl B. Microtubules in interphase and mitosis of cellular slime molds. In: Akkas N, editor. Biomechanics of Active Movement and Deformation of Cells. Berlin: Springer Verlag; 1990. pp. 73–107. [Google Scholar]
- Schiebel E, Bornens M. In search of a function for centrins. Trends Cell Biol. 1995;5:197–201. doi: 10.1016/s0962-8924(00)88999-0. [DOI] [PubMed] [Google Scholar]
- Schliwa M, Euteneuer U, Bulinski JC, Izant JG. Calcium lability of cytoplasmic microtubules and its modulation by microtubule-associated proteins. Proc Natl Acad Sci USA. 1981;78:1037–1041. doi: 10.1073/pnas.78.2.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutz AR, Giddings TH, Steiner E, Winey M. The yeast CDC37 gene interacts with MPS1 and is required for proper execution of spindle pole body duplication. J Cell Biol. 1997;136:969–982. doi: 10.1083/jcb.136.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A, Geissler S, Grein K, Schiebel E. γ-Tubulin-like Tub4p of Saccharomyces cerevisiae is associated with the spindle pole body substructures that organize microtubules and is required for mitotic spindle formation. J Cell Biol. 1996;134:429–441. doi: 10.1083/jcb.134.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T, Kirschner M. In vitro reconstitution of centrosome assembly and function: the central role of gamma-tubulin. Cell. 1994;76:623–637. doi: 10.1016/0092-8674(94)90503-7. [DOI] [PubMed] [Google Scholar]
- Sunkel CE, Gomes R, Sampaio P, Perdigao J, Gonzales C. γ-Tubulin is required for the structure and function of the microtubule organizing centre in Drosophila neuroblasts. EMBO J. 1995;14:28–36. doi: 10.1002/j.1460-2075.1995.tb06972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippit DH, Pickett-Heaps JD. Mitosis in the pennate diatom Surirella ovalis. J Cell Biol. 1977;73:705–727. doi: 10.1083/jcb.73.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M, Gräf R, MacWilliams HK, Schliwa M, Euteneuer U. Centrosome positioning and directionality of cell movement. Proc Natl Acad Sci USA. 1997;94:9674–9678. doi: 10.1073/pnas.94.18.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandre DD, Davis FM, Rao PN, Borisy GG. Distribution of cytoskeletal proteins sharing a conserved phosphorylated epitope. Eur J Cell Biol. 1986;41:72–81. [PubMed] [Google Scholar]
- Weijer CJ, Duschl G, David CN. A revision of the Dictyostelium discoideum cell cycle. J Cell Sci. 1984;70:111–131. doi: 10.1242/jcs.70.1.111. [DOI] [PubMed] [Google Scholar]
- Winey M, Byers B. The Centrosome. V.I. Kalnins, New York: Academic Press; 1992. Spindle pole body of Saccharomyces cerevisiae: a model for genetic analysis of the centrosome cycle; pp. 197–218. [Google Scholar]
- Zheng Y, Wong ML, Alberts B, Mitchison T. Nucleation of microtubuleassembly by a γ-tubulin-containing ring complex. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]