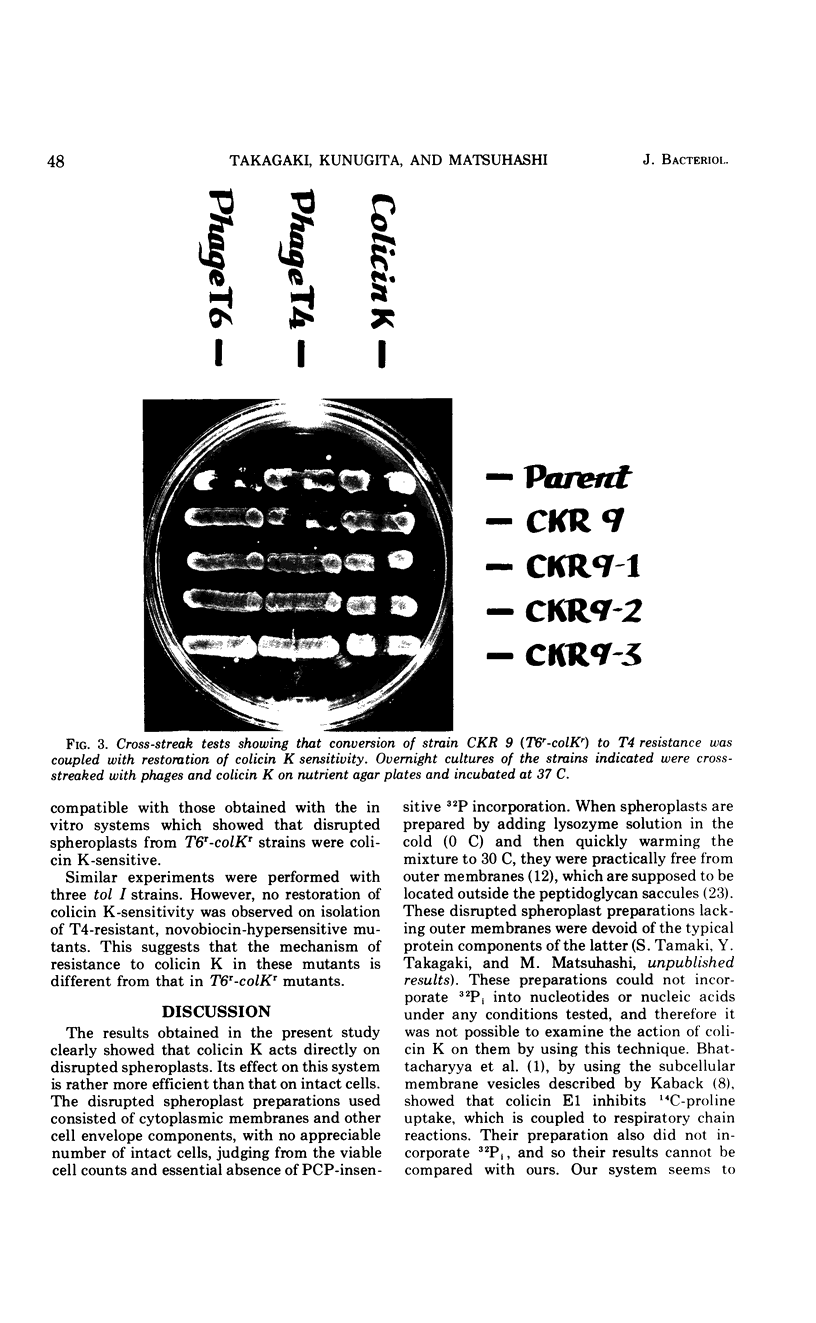
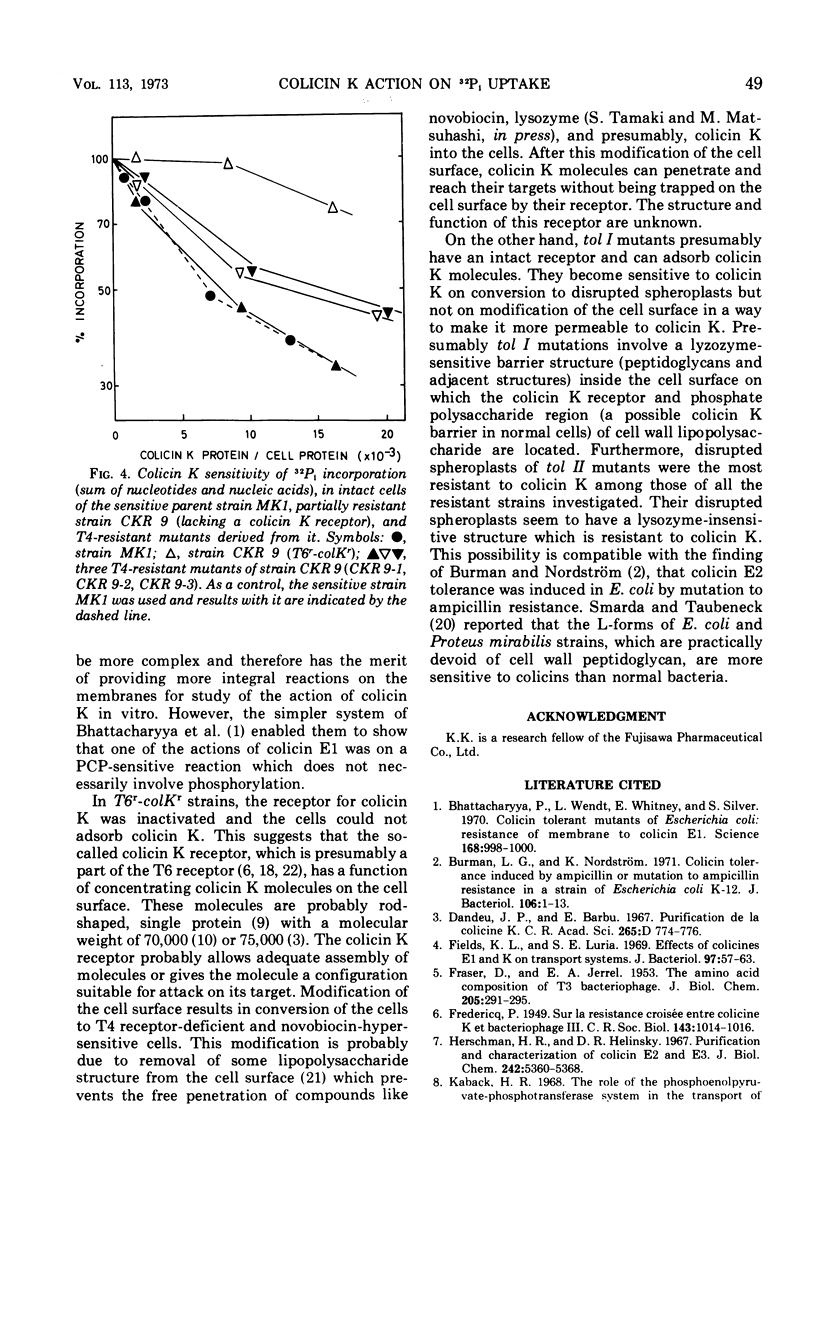
Abstract
Pentachlorophenol (PCP)-sensitive incorporation of 32P-labeled orthophosphate (32Pi) into nucleotides and nucleic acids by disrupted spheroplasts of Escherichia coli was inhibited by addition of colicin K. Incorporation by intact cells was also inhibited by a similar concentration of colicin K. Various colicin K-resistant mutants were isolated, and their ability to incorporate 32Pi was tested. When T6r-colKr mutants (T6 phage-resistant) and tol I mutants (T6-sensitive, colicin E-sensitive) were converted to disrupted spheroplasts, their 32Pi-incorporation became sensitive to colicin K. On the contrary, incorporation by disrupted spheroplasts from tol II mutants (T6-sensitive, colicin E-resistant) was fairly resistant to colicin K like that of intact cells. A modification of the cell surface of T6r-colKr mutants, caused by mutation to novobiocin-permeable, T4 phage-resistant cells, restored the sensitivity of the cells to colicin K. The modified T6r-colKr cells did not adsorb T6 phage or colicin K, indicating that the receptors for T6 phage or colicin K are not reactivated by this modification. Similar treatment of tol I mutants did not have this effect. These observations strongly suggest that colicin K can act on its target on the cell membrane if it can penetrate the cell surface to reach this target. The receptor for colicin K on the cell surface, which may be part of the T6 phage-receptor, may have some unknown function in relation to the action of colicin K in normal cells, but tends to become dispensable if the cells become permeable to colicin K.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharyya P., Wendt L., Whitney E., Silver S. Colicin-tolerant mutants of Escherichia coli: resistance of membranes to colicin E1. Science. 1970 May 22;168(3934):998–1000. doi: 10.1126/science.168.3934.998. [DOI] [PubMed] [Google Scholar]
- Burman L. G., Nordström K. Colicin tolerance induced by ampicillin or mutation to ampicillin resistance in a strain of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):1–13. doi: 10.1128/jb.106.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandeu J. P., Barbu E. Purification de la colicine K. C R Acad Sci Hebd Seances Acad Sci D. 1967 Sep 4;265(10):774–776. [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Fields K. L., Luria S. E. Effects of colicins E1 and K on transport systems. J Bacteriol. 1969 Jan;97(1):57–63. doi: 10.1128/jb.97.1.57-63.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman H. R., Helinski D. R. Purification and characterization of colicin E2 and colicin E3. J Biol Chem. 1967 Nov 25;242(22):5360–5368. [PubMed] [Google Scholar]
- Konisky J., Richards F. M. Characterization of colicin Ia and colicin Ib. Purification and some physical properties. J Biol Chem. 1970 Jun 10;245(11):2972–2978. [PubMed] [Google Scholar]
- Kunugita K., Matsuhashi M. Purification and properties of colicin K. J Bacteriol. 1970 Nov;104(2):1017–1019. doi: 10.1128/jb.104.2.1017-1019.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation and properties of outer and cytoplasmic membranes in Escherichia coli. Biochim Biophys Acta. 1969;193(2):268–276. doi: 10.1016/0005-2736(69)90188-6. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Mizuno S., Maruo B. Preparation and properties of active membrane systems from various species of bacteria. J Biochem. 1966 Apr;59(4):404–410. doi: 10.1093/oxfordjournals.jbchem.a128316. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Shibuya I., Maruo B. Preparation and properties of an active membrane system from Escherichia coli. J Biochem. 1967 May;61(5):623–632. doi: 10.1093/oxfordjournals.jbchem.a128592. [DOI] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M. Colicins and related bacteriocins. Annu Rev Microbiol. 1967;21:257–284. doi: 10.1146/annurev.mi.21.100167.001353. [DOI] [PubMed] [Google Scholar]
- Nomura M., Witten C. Interaction of colicins with bacterial cells. 3. Colicin-tolerant mutations in Escherichia coli. J Bacteriol. 1967 Oct;94(4):1093–1111. doi: 10.1128/jb.94.4.1093-1111.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabet S. F., Schnaitman C. A. Localization and solubilization of colicin receptors. J Bacteriol. 1971 Oct;108(1):422–430. doi: 10.1128/jb.108.1.422-430.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Sato T., Matsuhashi M. Role of lipopolysaccharides in antibiotic resistance and bacteriophage adsorption of Escherichia coli K-12. J Bacteriol. 1971 Mar;105(3):968–975. doi: 10.1128/jb.105.3.968-975.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Weltzien H. U., Jesaitis M. A. The nature of the cilicin K receptor of Escherichia coli Cullen. J Exp Med. 1971 Mar 1;133(3):534–553. doi: 10.1084/jem.133.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]




