Abstract
Break-induced replication (BIR) is an important process of DNA metabolism that has been implicated in the restart of collapsed replication forks, as well as in various chromosomal instabilities, including loss of heterozygosity, translocations, and alternative telomere lengthening. Therefore, knowledge of how BIR is carried out and regulated is important for better understanding the maintenance of genomic stability in eukaryotes. Here we present a new yeast experimental system that enables the genetic control of BIR to be investigated. Analysis of mutations selected on the basis of their sensitivity to various DNA-damaging agents demonstrated that deletion of POL32, which encodes a third, nonessential subunit of polymerase δ, significantly reduced the efficiency of BIR, although some POL32-independent BIR was still observed. Importantly, the BIR defect in pol32Δ cells was associated with the formation of half-crossovers. We propose that these half-crossovers resulted from aberrant processing of BIR intermediates. Furthermore, we suggest that the half-crossovers observed in our system are analogous to nonreciprocal translocations (NRTs) described in mammalian tumor cells and, thus, our system could represent an opportunity to further study the NRT mechanism in yeast.
DOUBLE-strand DNA breaks (DSBs) often cause genetic instability due to the loss of important genetic information and, therefore, DSBs can threaten an organism's homeostasis. DSB-induced changes to the genome are implicated in a variety of human diseases, including birth defects and cancer. Thus, identification and characterization of the molecular mechanisms that repair DSBs are crucial for understanding how the integrity of living cells is maintained. Several different pathways to repair DSBs have been identified. In yeast, gene conversion (GC) is the preferred pathway to repair DSBs generated by endonucleases, ionizing radiation, or mechanical rupture of chromosomes. Several features of GC make it a “safe” pathway for DSB repair. First, GC proceeds via invasion of the two broken DNA ends into a homologous template, which ensures that the donor DNA is homologous to the recipient on both sides of the break. Second, the length of newly synthesized DNA is relatively short because it is limited to a short patch between the sites of invasion. Finally, in vegetative cells, GC is rarely associated with crossing over, which can lead to chromosomal rearrangements (Paques and Haber 1999; Ira et al. 2003).
Another pathway to repair DSBs is break-induced replication (BIR). According to existing models, BIR proceeds by invasion of one broken DNA end into the intact donor molecule, followed by initiation of DNA synthesis that can continue as far as the end of the donor chromosome (McEachern and Haber 2006). BIR can be dangerous for a cell because it can result in the copying of hundreds of kilobases of DNA from the donor molecule, while a large piece of the unrepaired, broken DNA can be lost. In addition, BIR can be initiated through strand invasion at ectopic chromosomal locations, which leads to chromosomal rearrangements, primarily translocations (Bosco and Haber 1998).
BIR was originally studied in bacteria, Escherichia coli, and in bacteriophage T4, where it is called replication-dependent repair (RDR) (Kogoma 1997; Kuzminov 1999; Kreuzer 2000; Marians 2000; Michel et al. 2001). In both of these organisms, BIR was shown to be involved in DSB repair. Several studies demonstrated that BIR operates in yeast, Saccharomyces cerevisiae (Voelkel-Meiman and Roeder 1990; Morrow et al. 1997; Bosco and Haber 1998; Davis and Symington 2004; Malkova et al. 2005). It has been shown that BIR is initiated during transformation of yeast with linearized DNA fragments (Morrow et al. 1997; Davis and Symington 2004). Also, BIR was implicated in the generation of nonreciprocal translocations following DSB induction with HO endonuclease (Bosco and Haber 1998; Lydeard et al. 2007; VanHulle et al. 2007). In addition, it has been suggested that BIR is responsible for telomere maintenance in the absence of telomerase (alternative telomere lengthening (ALT) and for the formation of various types of gross chromosomal rearrangements (GCRs) (Le et al. 1999; Teng and Zakian 1999; Teng et al. 2000; Lemoine et al. 2005; Narayanan et al. 2006; Lydeard et al. 2007; Smith et al. 2007). It is likely that BIR operates in mammalian cells, although a systematic study of this process has yet to be undertaken. Furthermore, it has been suggested that BIR is a probable cause of various genetic instabilities that can lead to cancer, including ALT, loss of heterozygosity (LOH), and chromosomal translocations (Pierce et al. 2001; Neumann and Reddel 2002; Reddel 2003; Stark and Jasin 2003; Liang et al. 2004; Pui et al. 2004; Strauchen 2004).
When both ends of a DSB are capable of invading a homologous sequence, BIR is strongly outcompeted by GC, which accounts for >98% of repair events (Malkova et al. 1996, 2005; Ira et al. 2003). However, BIR is suggested to be the primary repair pathway of one-ended breaks that can be formed as a result of replication fork collapse, which leaves homology on only one side of the DSB and, thus, precludes repair via GC. Therefore, BIR is a unique recombination mechanism capable of repairing collapsed replication forks, suggesting a critically important role for BIR in the life of a cell. Another group of lesions repaired by BIR are DSBs produced in such a way that either only one of the two free DNA ends can find homology for strand invasion or both ends can find homology, but only in different areas of the genome (Malkova et al. 2005; Lydeard et al. 2007).
The key to understanding the mechanism of BIR lies in the knowledge of specific proteins required for the process. So far, three categories of proteins that participate in BIR have been identified: recombination proteins, replication proteins, and proteins mediating recombination and replication (mediator proteins). Recombination proteins, including RecA in E. coli, uvsX in bacteriophage T4, and RAD52, RAD51, RAD54, RAD55, and RAD57 in S. cerevisiae, initiate BIR by promoting strand invasion and D-loop formation (Lark et al. 1978; Formosa and Alberts 1986a,b; Asai et al. 1993; Davis and Symington 2004; Malkova et al. 2005). The role of mediator proteins is to assemble a processive replication fork on the D-loop that is formed during the first step of BIR. So far, these proteins have been identified and studied only in prokaryotes. In E. coli, this function is carried out by PriA with the help of several other proteins, including PriB, PriC, and PriT, while gp59 performs a similar function in bacteriophage T4 (Kogoma and Lark 1975; Kogoma 1976; Marians 2000; Bleuit et al. 2001; George et al. 2001). The last stage of BIR, DNA synthesis, is carried out by processive DNA polymerases working in conjunction with clamp and clamp-loader proteins, including the polymerase III complex in E. coli (Kogoma and Lark 1975), the gp43/gp44/gp45/gp62 complex in T4 (reviewed in Kreuzer 2000), and polα-primase, pol-δ, and pol-ɛ complexes in yeast (Lydeard et al. 2007). However, many details related to DNA synthesis associated with BIR remain unknown. For example, the exact roles of the different polymerases in BIR-related DNA synthesis and the actual mode of synthesis (conservative or semiconservative) remain unclear. In addition, little is known regarding the fidelity of DNA synthesis associated with BIR or regarding the DNA repair systems that might be involved in correcting replication errors resulting from BIR.
Another aspect of BIR that remains largely unknown is its regulation. It is still unclear why BIR is outcompeted by GC and whether different types of BIR (intersister, allelic, and ectopic) are regulated differently. To further understand the regulation of BIR, it is essential to identify the full complement of genes that carry out and regulate the process, as well as to compare the roles of genes in allelic interhomolog vs. ectopic BIR.
Here we report the development of a new experimental system that employs a yeast strain disomic for chromosome III, which provides a convenient way to study the genetic control of allelic interhomolog BIR. Using this system, the effects of various genetic mutations on the efficiency of interhomolog BIR were assessed. We observed that deletion of a gene encoding a nonessential subunit of polymerase δ, POL32, significantly reduced the efficiency of BIR, which is consistent with the reported effect of this mutation in an ectopic BIR system (Lydeard et al. 2007). However, while practically all ectopic BIR events required POL32 (Lydeard et al. 2007), our system allowed for observation of a POL32-independent BIR pathway. In addition, we report that the BIR defect in pol32Δ mutants lead to formation of half-crossovers similar to NRTs reported in mammals, which are implicated in the initiation of cascades of genomic instability characteristic of human cancer cells (Sabatier et al. 2005).
MATERIALS AND METHODS
Yeast strains and plasmids:
The genotypes of all strains used in this study are shown in Table 1. Construction of the primary system, disomic strain AM1003, which contains a haploid chromosome set as well as a second, truncated copy of chromosome III, was accomplished by crossing two newly created haploid strains, AM935 and AM934. AM935 is isogenic to EI515 published in Malkova et al. (1996) and was created by replacing the FS2 region 30 kb proximal to MAT, which consists of two Ty1 elements in inverted orientation (Lemoine et al. 2005; VanHulle et al. 2007), with the NAT (noursothricin-resistance) gene using methods similar to those described in VanHulle et al. (2007). AM934 was derived from AM811, which was obtained through tetrad dissection of the diploid strain MY006 (Malkova et al. 2005) to retrieve a strain that was MATα-inc HML ade1 ura3 leu2 thr4 trp1 met13 hmrΔ∷NAT ade3∷GAL∷HO. Next, the HML locus in AM811 was replaced by ADE3 using the delitto perfetto approach (Storici et al. 2001), which involved transformation with a DNA fragment generated by PCR amplification of pGSKU (Storici et al. 2001) with primers that contained short terminal DNA sequences homologous to the sequences flanking HML, and subsequent transformation with a PCR-amplified fragment of ADE3 with short terminal sequences homologous to HML-flanking sequences to replace pGSKU. Finally, the NAT marker at the HMR locus was replaced by HPH (HYG) using a PCR-derived HPH-MX module (Goldstein and McCusker 1999) with terminal sequences homologous to the NAT–MX module. AM935 and AM934 were crossed to create diploid strain AM1001, which was then transformed with a BamHI–MluI fragment of pMN1 (Malkova et al. 2005) to create a terminal truncation of the MATa-containing chromosome (AM1002). Sporulation of AM1002 allowed for selection of a meiotic product with the desired genotype hml∷ADE1/hml∷ADE3 met13 lys5 by selecting for the Ade+Met−Lys− phenotype. This selected strain (AM1003) was a disome with two copies of chromosome III, which probably resulted from spontaneous meiotic nondisjunction of two copies of chromosome III.
TABLE 1.
List of strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| EI515 | MATaade1 ura3-52 leu2-3,112 lys5 hmlΔ∷ADE1 hmrΔ∷ADE1 ade3∷GAL∷HO | Malkova et al. (1996) |
| AM935 | EI515, but FS2Δ∷NAT | This study |
| AM811 | MATα-inc ade1 ura3 leu2 thr4 trp1met13hmrΔ∷NAT ade3∷GAL∷HO | This study |
| AM934 | AM811, but hmlΔ∷ADE3 hmrΔ∷HYG | This study |
| AM1001 | AM934 × AM935 | This study |
| AM1002 | AM1001, but MATa-LEU2-tel | This study |
| AM1003 | MATa-LEU2-tel/MATα-inc ade1 met13 ura3 leu2-3,112/leu2 thr4 lys5 hmlΔ∷ADE1/hmlΔ∷ADE3 hmrΔ∷HYG ade3∷GAL-HO FS2Δ∷NAT/FS2 | This study |
| AM1014 | AM1003, but pol32Δ∷KAN | This study |
| AM1153 | AM1003, but pho87Δ∷URA3 | This study |
| AM1152 | AM1014, but pho87Δ∷URA3 | This study |
| AM1079 | AM1003, but rad51Δ∷KAN | This study |
| AM1089 | AM1003, but rtt107Δ∷KAN | This study |
| AM1029 | AM1003, but sgs1Δ∷KAN | This study |
| AM1024 | AM1003, but mms1Δ∷KAN | This study |
| AM1030 | AM1003, but tof1Δ∷KAN | This study |
| AM1028 | AM1003, but rtt101Δ∷KAN | This study |
| AM1021 | AM1003, but elg1Δ∷KAN | This study |
| AM1025 | AM1003, but mms2Δ∷KAN | This study |
| AM1027 | AM1003, but rad5Δ∷KAN | This study |
| AM1099 | AM1003, but mms22Δ∷KAN | This study |
| AM1018 | AM1003, but cac2Δ∷KAN | This study |
| AM1108 | AM1003, but ard1Δ∷KAN | This study |
The presence of two copies of chromosome III in AM1003 was confirmed by pulsed-field gel electrophoresis (PFGE). In addition, several lines of evidence were consistent with an n + 1 chromosomal content for AM1003. First, this strain was a MATa/MATα-inc nonmater, but it did not sporulate after replica plating on potassium-acetate (sporulation) medium. Conversely, a hybrid obtained from a cross between an α-mating derivative of AM1003 (obtained by inducing an HO-created DSB at MATa that was repaired by BIR) and a MATa strain demonstrated highly efficient sporulation and spore viability. Finally, various projects performed in our lab have required deletions of genes in the AM1003 background. At this time, >30 genes have been successfully disrupted on 15 different yeast chromosomes (all except IX, where it was not attempted). These deletions were created by single-step transformation with a PCR-derived fragment and confirmed by PCR. For successful disruptions, PCR confirmation indicated that only the disrupted gene was present with no indication of an additional wild-type copy, which supports that only a single copy of these 14 chromosomes (excluding chromosome III) was present.
All single-gene deletion mutants isogenic to AM1003 were constructed using a PCR-derived KAN-MX module flanked by short terminal sequences homologous to the sequences flanking the open reading frame of each gene (Wach et al. 1994).
AM1153 and AM1152 were created by insertion of URA3 at PHO87 of the MATa-containing chromosomes of AM1003 and AM1014, respectively, using methods similar to those described in Malkova et al. (2005). The nucleotide sequences of the primers used to generate all PCR fragments are available upon request.
Media and growth conditions:
Rich medium yeast extract–peptone–dextrose (YEPD), synthetic complete medium with bases and amino acids omitted as specified, and sporulation medium were made as described (Guthrie and Fink 1991). YEP-lactate (YEP-Lac) and YEP-galactose (YEP-Gal) contained 1% yeast extract and 2% Bacto peptone media supplemented with 3.7% lactic acid (pH 5.5) or 2% (w/v) galactose, respectively. Cultures were grown at 30°.
Analysis of DNA repair:
To monitor repair of HO-induced DSBs, logarithmically growing cells grown in YEP-Lac were harvested and plated on YEP-Gal. The resulting colonies were then replica plated onto omission media to examine the ADE1, ADE3, LEU2, and URA3 markers of these strains. To examine retention of the NAT marker, colonies were replica plated onto YEPD containing 25 mg/ml nourseothricin (NAT). Cell viability following HO induction was derived by dividing the number of colony-forming units (CFUs) on YEP-Gal by the number of CFUs on YEPD. A minimum of three plating experiments was used to calculate the averages and standard deviations for viability.
The kinetics of DSB repair were examined in time-course experiments as described previously (Malkova et al. 2005). For PFGE, chromosomal plugs were prepared using the CHEF genomic DNA plug kit (Bio-Rad). PFGE was performed using genomic DNA embedded in plugs of 1% agarose. The DNA was subsequently examined by Southern analysis, and blots were probed with appropriate DNA fragments labeled with P32. Blots were analyzed using a Molecular Dynamics PhosphorImager.
The kinetics of accumulation of BIR product was measured using an ADE1-specific fragment as a probe. To account for variation in DNA loads, intensities of the bands corresponding to the intact chromosome III, as well as to the repaired chromosome III, were normalized to intensities of the bands corresponding to chromosome I, which also hybridizes to the ADE1-specific probe. The efficiency of BIR repair, presented as the percentage of truncated chromosome III that was converted to BIR product, was calculated by dividing the normalized intensity of a repair band by the normalized intensity of uncut, truncated chromosome III. Results of three time-course experiments were used to calculate the average ± SD BIR efficiency at each time point for each strain. BIR efficiencies between strains were concluded to be statistically significantly different if SDs did not overlap.
During the time-course experiments, samples were taken for DAPI staining and analyzed microscopically to determine the percentage of G2/M-arrested cells (as defined by dumbbell-shaped cells with a single nucleus) in the cultures undergoing repair.
Analysis of repair outcomes:
The structures of repair outcomes were analyzed by PFGE, followed by blotting and hybridization with appropriate probes. The ADE1-specific probe was a SalI fragment from pJH879 (Leung et al. 1997). Other probes were generated by PCR amplification using 20–25 bp primers (sequences available upon request) and genomic DNA of AM919 (VanHulle et al. 2007) as a template. The locations of these probes on chromosome III were as follows: (1) THR4 specific, 216,965–217,264 (Figure 1A, probe 1); (2) FEN2 specific, 172,065–172,372 (Figure 1A, probe 2); (3) FS1 proximal, 148,247–148,547 (Figure 1A, probe 3); and (4) MRPL32 specific, 118,654–119,073 (Figure 1A, probe 4). The location of the ADE3-specific probe on chromosome VII was 907,979–908,735. For all probes mentioned above, the starting and ending coordinates on the corresponding chromosomes are derived from the Saccharomyces Genome Database.
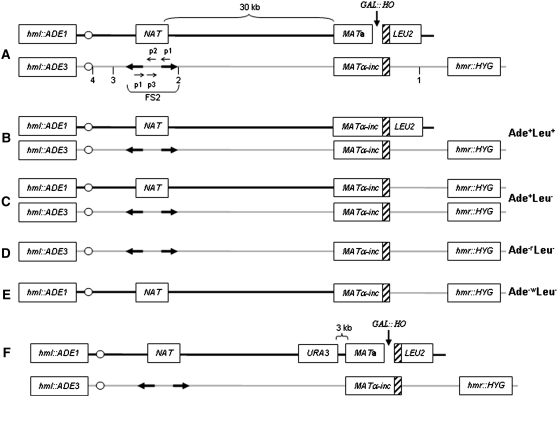
Figure 1.—
Disomic experimental system to study BIR. (A) Arrangement of chromosome (Chr) III markers of disomic strain AM1003 and its derivatives are indicated. A DSB is induced at MATa by a galactose-inducible HO gene. The MATa-containing copy of Chr III is truncated by insertion of a LEU2 gene fused to telomere sequences. The MATα-containing copy is full length and is resistant to cutting by HO. HML sequences are replaced by ADE1 and ADE3 genes in the truncated and full-length chromosomes, respectively, and HMR is replaced by HYG. On the truncated chromosome, FS2 is replaced by NAT. Primers 1, 2, and 3 (p1, p2, and p3, respectively) indicate positions of primers used for PCR analysis of half-crossover repair outcomes. The positions of probes used to analyze the structure of GCR repair outcomes are indicated by numbers (1, 2, 3, and 4; see text for details). (B) GC outcome. (C) BIR repair outcome. (D) Chromosome loss when the HO cut is not repaired. (E) Half-crossovers resulting from fusion of the left portion of the truncated chromosome and the right portion of the full-length chromosome. For outcomes shown in B–E, the observed phenotypes are indicated. (F) Arrangement of Chr III markers of isogenic derivatives of AM1003 (AM1152 and AM1153) containing URA3 inserted 3 kb proximal to MATa.
The site of molecular fusion of half-crossovers was analyzed by PCR amplification using primers 1 and 2 and primers 1 and 3 in combination (Figure 1A). The sequences for these primers were as follows: primer 1 (specific to the Ty1 elements of FS2), 5′-GAGTTAGCCTTAGTGGAAGCCTTC-3′; primer 2 (specific to the inter-Ty1 region of FS2), 5′-GATATGTCGGTATCTAGAATGTAG -3′; and primer 3 (specific to the inter-Ty1 region of FS2), 5′-CTACATTCTAGATACCGACATATC-3′.
Statistical analysis:
All mutants were analyzed for their effect on BIR repair in at least three independent plating experiments. Results from these independent experiments were pooled if it was determined that the distributions of all events were statistically similar to each other using a chi-square test (http://www.psych.ku.edu/preacher/chisq/chisq.htm). The effects of individual mutations on DSB repair were determined by comparing the resulting pooled distributions of repair outcomes obtained for mutants to the distribution obtained for the wild-type strain (AM1003) by chi-square tests. Specifically, to determine the effect of various mutations on the efficiency of BIR, all repair outcomes were divided into two groups: BIR (Ade+Leu− outcomes) and others (combining all other groups). Comparison of the distributions between these two classes in specific mutants vs. wild type was used to determine whether a mutation affected the efficiency of BIR. The effect of mutations on other DSB repair outcomes was determined similarly.
RESULTS
Experimental system to study BIR:
We previously studied the mechanism of BIR using a diploid experimental system wherein a galactose-inducible DSB was initiated at the MATa locus of one copy of chromosome III, while a second copy contained an uncleavable MATα-inc allele and served as the template for DSB repair (Malkova et al. 2005). In this strain, DSBs were predominantly repaired by BIR because the DSB-distal portion of the molecule was truncated via insertion of LEU2 and telomeric sequences, leaving only 46 bp of homology on this side of the DSB and, thus, significantly diminishing the efficiency of GC. This system was used to analyze the kinetics of BIR, as well as the effects of several mutations on the efficiency of BIR. However, the systematic analysis of genetic control of BIR was difficult in this diploid strain due to the necessity of deleting or mutating both copies of the wild-type gene of interest. To facilitate large-scale screening for BIR genes, we created a modified version of our experimental system [see materials and methods for detailed information on construction of this strain (AM1003)].
The experimental strain, AM1003, contains several important features relevant to the study described here (Figure 1A, Table 1): (1) this is a disomic strain that contains a haploid set of all chromosomes and a second copy of chromosome III; (2) as in the original diploid system, the MATa-containing copy of chromosome III is truncated distal to the HO DSB site to increase the efficiency of BIR repair using the uncleavable MATα-inc allele and distal sequences from the full-length copy of chromosome III as the donor; (3) HML on the truncated copy of chromosome III is replaced by ADE1; (4) HML and HMR on the full-length chromosome III are replaced by ADE3 and HPH (HYG), respectively; and (5) the native FS2 region, located on the truncated copy of chromosome III (30 kb proximal to MATa), which consists of two copies of Ty1 transposons in inverted orientation (Lemoine et al. 2005; VanHulle et al. 2007), is replaced by NAT.
The efficiency of BIR in the disomic strain AM1003 was assayed genetically by plating on a galactose-containing medium to induce HO endonuclease, which leads to DSB formation (Table 2). Approximately 78% of the colonies showed the expected BIR phenotype of Ade+Leu−, with only 7.5% of colonies displaying the Ade+Leu+ phenotype indicative of GC. Another 1.5% of colonies were Ade−Leu−, indicating failed repair of the truncated copy of chromosome III. These failed repair events could be easily distinguished by accumulation of red pigment due to the absence of a functional ADE1 gene (this phenotype is hereafter indicated by “Ade−r”). Approximately 7% were sectored Ade+/−r colonies, which were likely to represent cases where one of two sister chromatids completed repair, while the second chromatid was left unrepaired and lost in the next cell division. Alternatively, these sectored Ade+/−r colonies may have resulted from cases in which the broken chromosome was replicated and inherited without repair for one or more divisions, after which some of the broken chromosomes were lost and others were repaired. Approximately 3% of colonies presented a rare and unexpected phenotype: they were Ade−Leu−, but white (hereafter indicated by “Ade−w”). In some of these cases, only a part of the colony was Ade−w, while another part was Ade−r or Ade+. The Ade−w colonies or sectors were determined to represent half-crossover events that resulted from a fusion between the truncated and full-length copies of chromosome III (see below for details on these repair outcomes).
TABLE 2.
Repair of HO-induced DSBs in strain AM1003 and its derivatives
| % phenotype of colonies
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Relevant genotype | Strain | No. of colonies tested | Ade+ Leu+ (GC) | Ade+ Leu+/− (GC/BIR) | Ade+ Leu− (BIR)a | Ade+/−r Leu− (BIR/loss)a | Ade−r Leu− (loss) | Ade−w Leu− (HCO) | Partial Ade−w Leu− (partial HCO)b | Viability YEP-Gal (%) |
| Wt | AM1003 | 671 | 7.5 | 2.1 | 78.1 | 7.4 | 1.5 | 0.3 | 3.1 | 94 ± 11 |
| pol32Δ | AM1014, AM1152c | 1549 | 0.5 | 0.0 | 18.9* | 19.0 | 38.0 | 7.0 | 16.6 | 90 ± 11 |
| ard1Δ | AM1108 | 539 | 6.9 | 7.2 | 72.7 | 7.8 | 2.4 | 1.1 | 1.9 | 96 ± 10 |
| rad51Δ | AM 1079 | 134 | 0.8 | 0.0 | 0.8* | 31.3 | 67.1 | 0.0 | 0.0 | 86 ± 18 |
| rtt107Δ | AM1089 | 351 | 7.4 | 0.0 | 72.6 | 12.3 | 6.0 | 0.0 | 1.7 | 79 ± 15 |
| sgs1Δ | AM1029 | 375 | 19.5 | 1.3 | 66.8* | 5.6 | 2.1 | 1.3 | 3.4 | 95 ± 13 |
| mms1Δ | AM1024 | 356 | 4.5 | 0.0 | 72.8 | 14.0 | 4.2 | 0.6 | 3.9 | 88 ± 11 |
| tof1Δ | AM1030 | 318 | 2.5 | 0.0 | 81.4 | 6.9 | 3.8 | 0.0 | 5.4 | 94 ± 16 |
| rtt101Δ | AM1028 | 393 | 10.4 | 0.8 | 66.2* | 17.0 | 3.8 | 1.0 | 0.8 | 91 ± 5 |
| elg1Δ | AM1021 | 336 | 5.7 | 6.3 | 72.0 | 11.3 | 0.9 | 1.2 | 2.6 | 80 ± 12 |
| mms2Δ | AM1025 | 326 | 8.0 | 1.2 | 69.9 | 14.8 | 1.2 | 0.6 | 4.3 | 98 ± 11 |
| rad5Δ | AM1027 | 372 | 2.2 | 0.0 | 83.9 | 10.8 | 0.0 | 0.0 | 3.1 | 86 ± 9 |
| mms22Δ | AM1099 | 259 | 7.0 | 0.3 | 79.5 | 7.0 | 4.3 | 0.4 | 1.5 | 84 ± 10 |
| cac2Δ | AM1018 | 307 | 6.5 | 0.3 | 77.6 | 12.4 | 0.0 | 0.0 | 3.2 | 99 ± 9 |
Ade−r, Ade−His+ red colonies indicative of the ade1ADE3 genotype; Ade−w, Ade−His− white colonies indicative of the ADE1ade3 genotype; GC, gene conversion; BIR, break-induced replication; HCO, half-crossover; wt, wild type. Sectored colonies are indicated by both phenotypes separated by a slash (/). *A statistically significant difference from the isogenic wild-type strain (AM1003; P < 0.05).
At least some of these events could be half-crossovers (primarily in pol32Δ, see the text for details) or GCRs (primarily in rad51Δ, but also in other strains, see the text for details).
Partial HCOs in which part of the colony was Ade−w while the other part represented chromosome loss or BIR.
The kinetics of repair in AM1003 was tested physically using PFGE (Figure 2A), and Southern blots were probed with an ADE1 probe specific to the truncated chromosome III to follow its repair (see materials and methods for details). We observed that the product of DSB repair was a full-length chromosome III that accumulated between 4 and 10 hr after HO induction (Figure 2, A and C), similar to the kinetics of BIR observed previously in the diploid system (Malkova et al. 2005). At 10 hr after HO induction, the intensity of the BIR repair band was ∼70% of the intensity of the unbroken chromosome III, suggesting that most broken molecules were repaired by BIR. Also consistent with characterization of the diploid system (Malkova et al. 2005), HO induction in the disomic strain led to efficient G2/M arrest, as determined by a high percentage of dumbbell-shaped cells containing a single, undivided nucleus in the interval between 4 and 6 hr after HO induction (Figure 2D). Overall, we conclude that the disomic experimental system (AM1003) is similar to our diploid experimental system (Malkova et al. 2005) with respect to the efficiency, kinetics, and checkpoint response associated with BIR.
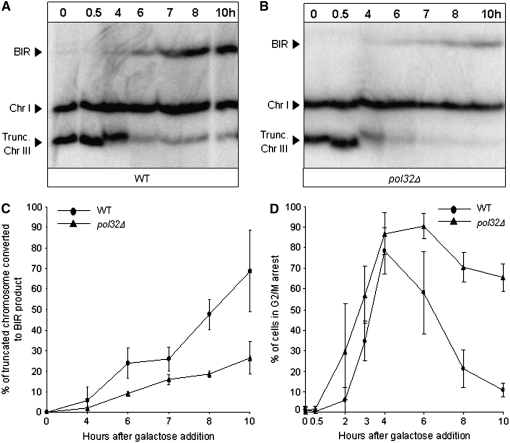
Figure 2.—
Analysis of DSB repair in AM1003 and its pol32Δ derivative. DNA was prepared for pulsed-field gel electrophoresis (PFGE) at intervals after induction of a DSB at MATa. Southern blots were probed with ADE1, which hybridized to the truncated chromosome III (Trunc. Chr III, see Figure 1) and to its native position on Chr I, but not to the full-length Chr III. Analysis of DSB repair in AM1003 (A) confirmed the kinetics of BIR in this strain to be similar to those previously characterized (Malkova et al. 2005). An additional truncated Chr III band observed 4 hr after addition of galactose corresponds to the cut, trunc. Chr III as it is being processed (partially single stranded) (K. VanHulle and A. Malkova, unpublished observation). (B) The BIR-sized repair product was also observed in the pol32Δ derivative of AM1003 (AM1014). (C) Quantification of the BIR repair product performed in AM1003 (WT) and AM1014 (pol32Δ) demonstrated that the kinetics of its accumulation in these two strains was similar, but the amount of BIR product was reduced in pol32Δ compared to WT. Results of three time-course experiments performed for each strain were used to calculate the average ± SD repair efficiency for each time point. (D) G2/M arrest in cells undergoing BIR repair. Cells were removed at intervals during experiments described in A and B. These cells were fixed with ethanol, stained with DAPI, and analyzed by fluorescent microscopy. G2/M-arrested cells were defined as cells with dumbbell morphology, where mother and daughter cells “shared” one nucleus. At least 100 cells were counted for each time point in each experiment. Results from three time-course experiments performed for each strain were used to calculate the results shown.
The effect of various mutations on BIR:
The experimental system described above was used to test various mutations with respect to their effects on BIR. Because yeast homologs for many BIR proteins previously identified in E. coli have been tested for their effects on BIR (excluding “mediator” proteins, for which no yeast homologs have been identified) (Davis and Symington 2004; Malkova et al. 2005), we chose to search for novel BIR proteins based on sensitivity to DNA-damaging agents. Specifically, to identify proteins that might be involved in BIR, repair outcomes were investigated from various mutants in which deletion of a gene conferred sensitivity to methyl methansulfonate (MMS), campothecin, and/or hydroxyurea (Pan et al. 2006). Because all of these drugs induce DNA damage that might be repaired, at least in some cases, by BIR, we hypothesized that the chemical sensitivity of some of these mutants may be the result of impaired BIR. Thus far, we have tested deletions of 12 genes in our disomic system, including RTT107, MMS22, MMS1, MMS2, POL32, ARD1, SGS1, TOF1, RTT101, ELG1, RAD5, and CAC2. In addition, deletion of RAD51 was tested as a control strain. Table 2 shows the distribution of repair outcomes for each of the tested mutants. Comparison of these distributions to the distribution of repair outcomes in AM1003 (wild type, see materials and methods for the details of statistical analysis) allowed us to conclude that deletion of POL32 had a significant effect, as it led to a decrease in BIR repair events and increased chromosome loss. Two other mutants, sgs1Δ and rtt101Δ, showed a mild but significant decrease in the fraction of Ade+Leu− BIR repair outcomes. The decrease observed in sgs1Δ was due to the statistically significant increase in GC, which competes with BIR. Thus, deletion of SGS1 increased GC in our system that provides only 46 bp of homology between donor and recipient molecules on one side of the break. The Sgs1p helicase has been implicated in many processes of DNA metabolism, including regulation of homologous recombination, suppression of crossing over in vegetative cells (Ira et al. 2003), and prevention of aberrant crossing over during meiosis (Oh et al. 2007). Also, Sgs1p suppresses homeologous recombination (Myung et al. 2001; Spell and Jinks-Robertson 2004), and deletion of SGS1 leads to increased translocations between divergent DNA sequences that share only limited homology (Schmidt et al. 2006). The latter function of Sgs1p could explain the increased GC observed in our experimental system.
The difference in distribution of repair events in rtt101Δ was due to an increase in sectored Ade+/−r BIR events. However, the fraction of chromosome loss events (Ade−rLeu−) was not dramatically different from wild type and, therefore, the efficiency of BIR was only mildly affected in this mutant. Deletion of RTT101 leads to several known phenotypes in budding yeast, including an increase in transposition of Ty1 elements (Scholes et al. 2001) and delayed anaphase progression (Michel et al. 2003). Recently, Rtt101p was shown to be required for progression of replication through damaged DNA and through natural replication-impeding loci (Luke et al. 2006; Roberts et al. 2008). It is possible that the partial BIR defect observed in our experiments results from problems associated with passage of BIR through areas on the donor chromosome that impede replication.
As expected, deletion of RAD51 resulted in a strong BIR defect. No significant effect on the efficiency of BIR in any other mutation investigated was observed (Table 2). None of the mutations affected the viability of cells undergoing DSB repair, which was close to 100% in wild type and in all analyzed mutants (Table 2).
BIR efficiency was compromised in a pol32Δ strain:
Analysis of repair in pol32Δ suggested that this mutation significantly reduced the cell's ability to carry out BIR. Thus, while 78.1% of wild-type colonies were fully Ade+Leu−, only 18.9% of colonies from an isogenic pol32Δ strain displayed this BIR phenotype. Moreover, the number of Ade−rLeu− colonies, which indicates loss of the broken chromosome, increased from only 1.5% in wild type to 38% of repair outcomes in isogenic pol32Δ cells (Table 2). Finally, sectored Ade+/−r colonies, indicative of partial repair, increased from 7.4% in wild type to ∼19% in isogenic pol32Δ cells.
The effect of pol32Δ was investigated further by following the kinetics of repair in a time-course experiment. The kinetics of DSB repair was examined by PFGE followed by hybridization with an ADE1-specific probe to detect hml∷ADE1 located on the left arm of the truncated copy of chromosome III. We observed that, in pol32Δ cells, the repair product accumulated with kinetics similar to the kinetics of BIR repair in wild-type cells; i.e., between 4 and 10 hr after galactose induction of the DSB (Figure 2, B and C). However, quantification of Southern blots indicated that the amount of BIR-sized product in pol32Δ mutants was reduced to ∼27% of the intensity of the unbroken chromosome, compared to ∼70% in wild type. Similar to wild-type cells, induction of the DSB in pol32Δ cells led to nearly uniform arrest at the G2/M stage of the cell cycle, which was established ∼4 hr after the DSB (Figure 2D). However, recovery from arrest in pol32Δ was significantly delayed, with >50% of pol32Δ cells maintaining arrest 10 hr after DSB induction, compared to <20% of wild-type cells remaining arrested at this time point. The delayed recovery from arrest is an additional indication that unrepaired DNA persists in pol32Δ cells and also indicates that the defect of pol32Δ resides in its inability to carry out BIR rather than in a defective checkpoint response.
POL32-independent DSB repair:
Despite the fact that BIR was reduced in pol32Δ cells, a substantial number of cells succeeded in DSB repair and formed Ade+Leu− or Ade+/−rLeu− colonies. The majority of these repair outcomes preserved the NAT marker located 30 kb proximal to the DSB site (Figure 1A) and produced the Natr phenotype. However, retention of the NAT marker during break repair was statistically significantly different in pol32Δ cells compared to wild type. In pol32Δ, the NAT marker was preserved in 93% (271 of 293) of Ade+ outcomes compared to 99% (518 of 524) of Ade+ outcomes that were NATr in wild-type cells (P < 0.001). Among Ade+/−r repair outcomes, 79% (230 of 293) of pol32Δ events were NATr, compared to only 62% (31 of 50) of this class of events that retained NAT in wild-type cells (P < 0.05).
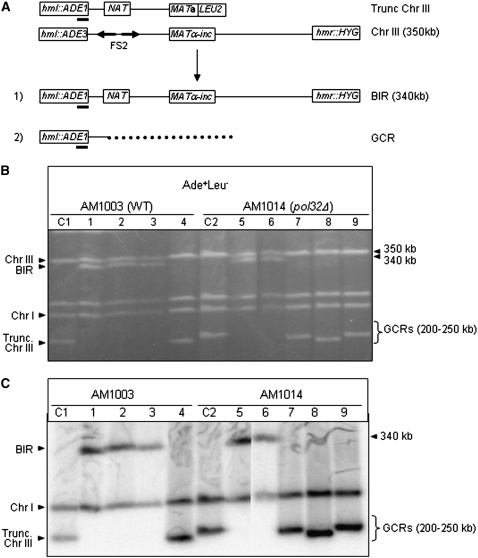
To determine whether the successful repair in pol32Δ cells proceeded through BIR, individual repair outcomes were analyzed by PFGE and Southern blots were probed with an ADE1-specific probe (Figure 3). In total, 33 Ade+NatrLeu− repair outcomes obtained from pol32Δ were analyzed and compared with similar outcomes obtained from wild-type cells. All wild-type events analyzed (six of six) were confirmed to be BIR outcomes (Figure 3, B and C, lanes 1–3) that contained two copies of chromosome III: one copy was an unchanged 350-kb donor chromosome containing ADE3, while the second was the repaired chromosome that contained ADE1 and was ∼340 kb (the difference in length was the result of replacement of the FS2 region by NAT in the truncated chromosome III). In addition, both copies of chromosome III hybridized to a THR4-specific probe (Figure 1A, probe 1; not shown), confirming that the broken molecule had obtained DNA sequences located centromere distal to the MAT break site, as predicted for BIR. Thus, the presence of these two chromosomes was consistent with repair of the DSB via BIR, in which the donor molecule remained unchanged and the broken molecule invaded between NAT and the MAT locus and copied the length of the donor molecule. Most Ade+NatrLeu− repair outcomes obtained from pol32Δ (94%, 31 of 33 analyzed) were similar to the wild-type BIR events (Figure 3, B and C, lanes 5 and 6). The two remaining events contained ∼250-kb repair products (not shown) that were not consistent with the expected BIR products and, thus, were indicative of GCRs, most likely translocations resulting from strand invasion into a nonhomologous chromosome. Thus, we conclude that the chromosome structure of the vast majority (94%) of Ade+NatrLeu− repair outcomes obtained from the progeny of pol32Δ strains was consistent with BIR.
Figure 3.—
Structural analysis of Ade+Leu− repair outcomes. (A) Chromosome (Chr) III in the uncut disomic strain and in Ade+Leu− repair outcomes. The position of the ADE1-specific hybridization probe is indicated (solid bar). (B) Ethidium bromide-stained PFGE gel of repair outcomes obtained from AM1003 (WT) and AM1014 (pol32Δ). (C) Southern blot analysis of the PFGE gel shown in B using an ADE1-specific probe, which hybridized to truncated chromosome III (trunc. Chr III) and to Chr I. Lanes labeled C1 and C2 show DNA from AM1003 and AM1014 cells, respectively, in which the HO site was not cleaved. Other lanes contained DNA obtained from the following Ade+Leu− repair outcomes: 1, 2, and 3, Natr outcomes from AM1003 (WT); 4, Nats outcome from AM1003 (WT); 5 and 6, Natr outcomes from AM1014 (pol32Δ); and 7, 8, and 9, Nats outcomes from AM1014 (pol32Δ). The majority of Natr outcomes contained a 340-kb repair product consistent with BIR, whereas many Nats events contained repair products that were different in size from the Chr III products expected from BIR or GC repair and thus were indicative of GCRs.
Because deletion of POL32 reduced the efficiency of BIR, we hypothesized that the residual BIR repair observed in the absence of POL32 might require more uninterrupted homology between recombining chromosomes. To test this possibility, DSB repair was analyzed in a pol32Δ strain (AM1152) that was isogenic to AM1014 but contained an additional URA3 marker inserted 3 kb proximal to MATa (at PHO87 of the recipient chromosome; Figure 1F). We were able to demonstrate that DSB repair in pol32Δ mutants was associated with loss of URA3 in 47.5% of Ade+NatrLeu− colonies, compared with only 15.8% of wild-type colonies that lost URA3 during BIR repair (Table 3). Among colonies that exhibited partial BIR repair accompanied by chromosome loss (Ade+/−rNatr/sLeu−), 43.4% of pol32Δ colonies lost the URA3 marker compared with only 29.5% in wild type. One possible explanation for this phenomenon could be that successful invasion of the donor molecule takes longer in pol32Δ cells and, therefore, a larger amount of post-DSB resection occurs.
TABLE 3.
Analysis of retention of a URA3 marker among repair outcomes
A second class of repair events analyzed consisted of cells that had lost the NAT marker located 30 kb proximal to the DSB site (Ade+NatsLeu− colonies). These events were much rarer than Natr repair events in both pol32Δ and wild-type cells (see above). PFGE analysis of Ade+NatsLeu− repair outcomes demonstrated that, in pol32Δ cells, over half of them (60%, 9 of 15 analyzed) had a chromosome III of altered size and, therefore, represented GCRs, while the other 40% contained 350-kb repair bands consistent with BIR that proceeded by strand invasion of the broken chromosome into the homolog at positions centromere proximal to NAT. Analysis of five Ade+NatsLeu− repair outcomes obtained from wild type demonstrated that one of them was consistent with a GCR, while the other four were BIR outcomes. Further analysis of six pol32Δ GCR outcomes demonstrated that each of them contained one repaired chromosome that hybridized to an ADE1-specific probe and were between 200 and 250 kb (Figure 3, B and C, lanes 7–9). Hybridization with several other probes demonstrated that these six repair chromosomes did not hybridize to the FEN2 probe (Figure 1A, probe 2) located on chromosome III 30 kb proximal to MAT, or to the FS1-proximal probe (Figure 1A, probe 3) located on chromosome III ∼52 kb proximal to MAT. Because all six repaired chromosomes hybridized to the MRPL32 probe (Figure 1A, probe 4) located on chromosome III 80 kb proximal to MAT, we concluded that repair in these six GCR cases was initiated in the region between positions 118,654 (location of the MRPL32 probe) and 148,247 (location of the FS1-proximal probe) and then likely proceeded via invasion into a nonhomologous chromosome resulting in translocations. These GCRs were similar to GCRs that we previously observed in rad51Δ diploids, where >80% of repair outcomes resulted from invasions into nonhomologous chromosomes (VanHulle et al. 2007). We confirmed this result for the rad51Δ derivative of our disomic strain, where ∼31% of repair outcomes were Ade+/−rNatr/sLeu− (Table 2). PFGE analysis of six of these events demonstrated that each of them contained a repaired chromosome III of altered size consistent with a GCR. The decreased frequency of GCRs in our disomic system (compared to the previously observed frequency in rad51Δ diploids) could be explained by the absence of FS2, which stimulated formation of GCRs in the rad51Δ derivative of our diploid system (VanHulle et al. 2007).
Aberrant processing of BIR leads to formation of half-crossovers:
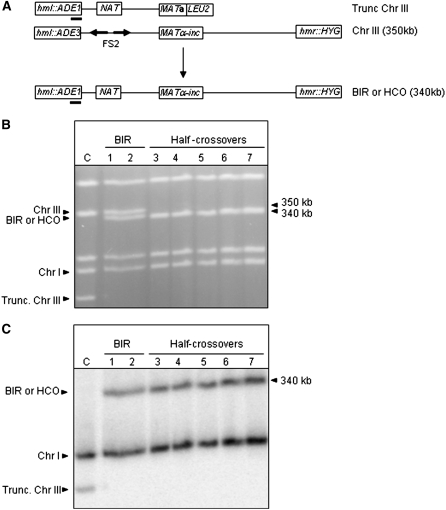
Among repair outcomes obtained in the progeny of pol32Δ mutants, we observed unexpected colonies that were fully or partially Ade−w, as well as His−. This phenotype was very rare in POL32 cells, ∼3% of all repair events, but increased to >23% of repair events in pol32Δ mutants when both full and partial events were combined (Table 2). An allelism test performed by crossing to ade1 and ade3 tester strains demonstrated the Ade−wHis− cells to be ADE1ade3 (mutations in the ADE3 gene affect biosynthesis of both adenine and histidine; thus, ade3 mutants are Ade−wHis−, while ade1 mutants are Ade−rHis+). PFGE analysis confirmed that Ade−wHis− outcomes contained only a single, 340-kb copy of chromosome III (Figure 4B) that hybridized to an ADE1-specific probe (Figure 4C), but not to an ADE3-specific probe (not shown). Thus, these events were determined to be the result of a fusion between the “left” portion of the truncated chromosome III and the “right” portion of the full-length chromosome III, while the remaining two pieces were lost. Such outcomes are similar to previously described half-crossover events in yeast (Haber and Hearn 1985) and also strongly resemble NRT repair events in mammals that are a major pathway of exiting breakage–fusion–bridge cycles (Sabatier et al. 2005).
Figure 4.—
Structural analysis of half-crossover outcomes. (A) Chromosome (Chr) III in the uncut disomic strain and in repair outcomes. The position of the ADE1-specific hybridization probe is indicated (solid bar). (B) Ethidium bromide-stained PFGE gel of Ade−wLeu− (half-crossover) outcomes obtained from AM1014 (pol32Δ). (C) Southern blot analysis of the PFGE gel shown in B using an ADE1-specific probe that hybridizes to truncated chromosome III (trunc. Chr III) and to Chr I. Lane C contained DNA from AM1003 in which the HO site was not cleaved. Other lanes contained DNA from the following repair outcomes: 1, Ade+Leu− from AM1003 (WT BIR control); 2, Ade+Leu− from AM1014 (pol32Δ BIR control); 3–7, Ade−wLeu− half-crossovers from AM1014 (pol32Δ). BIR controls contained both BIR repair product and full-length Chr III. Half-crossovers (HCO) contained only one BIR-sized fusion chromosome while the donor molecule was lost.
The majority (82%) of the half-crossover events recovered from pol32Δ cells (301 of 366 cases) were Natr, while others were Nats. We hypothesized that, in Natr events, chromosomal fusion occurred in the interval between NAT and the MAT locus while, in Nats events, the fusion occurred between NAT and the centromere. This hypothesis was tested by PCR analysis of Natr outcomes. Primers to detect the presence of the FS2 region, which exists only on the full-length copy of chromosome III in the disomic strain, were used in PCR reactions for half-crossover events, as well as for the disomic strains (AM1003 and its pol32Δ derivative, AM1014) in which the HO-created DSB was not made and in their respective BIR repair outcomes as controls (see Figure 1A and materials and methods for the positions and sequences of primers). As predicted, no bands were detected in any of the eight Natr half-crossovers tested, whereas bands of the expected sizes (∼664 bp and 660 bp for the combination of primer 1 and primer 2 and for the combination of primer 1 and primer 3, respectively; Figure 1A) were confirmed both in AM1003 and AM1014, as well as in their respective BIR controls (not shown). This result was consistent with the predicted structure of the fusion chromosome, where the FS2 DNA sequences located centromere proximal to the position of the fusion on the donor chromosome should be lost.
Half-crossover events in wild-type cells were extremely rare, but PFGE analysis of the events we were able to recover demonstrated their structure to be similar to that of half-crossover events obtained from pol32Δ cells (not shown). However, only 35% of half-crossovers in the wild-type strain (8 of 23 cases) were Natr. We believe that formation of half-crossovers in our disomic system resulted from aberrant processing of BIR intermediates, which occurred at a significantly increased frequency in pol32Δ mutants. Specifically, we hypothesize that pol32Δ mutants are proficient at the strand-invasion step of BIR, but defective in initiation and/or progression of DNA synthesis associated with BIR. Supportive of this hypothesis is our finding that half-crossover events were absent in rad51Δ mutants, which are defective at the earlier, strand-invasion step of BIR (Table 2).
An alternative theoretical possibility is that events phenotypically identical to half-crossovers could result from BIR followed by spontaneous loss of the donor (MATα-inc-containing) chromosome. To address this possibility, the frequency of spontaneous Ade−wHis− events was tested in the progeny of Ade+NatrLeu− BIR outcomes. Overall, the frequency of these events was analyzed for three wild-type BIR outcomes and for four pol32Δ BIR outcomes, and the results were combined for each of the strains. After plating on YEPD, 1/1047 (0.09%) AM1003 BIR outcomes and 3/970 (0.3%) AM1014 BIR outcomes were Ade−wHis−Leu−. Thus, the frequencies of these events were not statistically different between wild type and pol32Δ. More importantly, these frequencies were significantly lower than the frequencies of half-crossovers that resulted after repair of the HO-created DSB in wild-type cells (3.4%) or pol32Δ cells (23.6%), making it unlikely that spontaneous chromosome loss is responsible for the Ade−wHis−Leu− events observed in our experiments (Table 2).
Pedigree analysis of pol32Δ cells undergoing DSB repair:
The high occurrence of half-crossovers in pol32Δ required that we consider an additional possibility. Specifically, it was possible that a half-crossover product formed in G2 could segregate into a cell that has an intact copy of the full-length chromosome III. This outcome would be genetically and structurally indistinguishable from BIR (Figure 5). To determine whether pol32Δ Ade+Leu− and Ade+/−rLeu− events were in fact BIR and not half-crossover outcomes, a pedigree analysis of pol32Δ cells undergoing DSB repair was performed (Table 4, AM1014 and AM1152 combined data are shown). Unbudded G1 cells were micromanipulated on YEP-Gal plates, mother and daughter cells were separated, grown into colonies, and scored. In the case of half-crossover molecules cosegregating with a full-length chromosome III, only one cell would be viable, as the second cell would not receive a full-length copy of chromosome III unless the second broken chromosome had actually undergone BIR repair. Conversely, BIR repair events would not preclude survival of both mother and daughter cells (Figure 5). Thus, cell viability among colonies with at least one Ade+ colony was used to determine whether pol32Δ cells were capable of BIR repair.
Figure 5.—
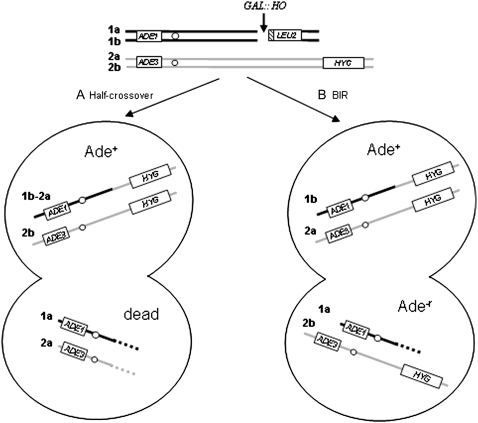
Pedigree analysis rationale. Formation of Ade+ outcomes by two pathways is depicted: (A) half-crossover and (B) BIR. In both scenarios shown, only one broken chromatid was repaired while the other was lost. Labels are provided for sister chromatids of both chromosome III homologs (1a, 1b, 2a, and 2b).
TABLE 4.
Pedigree analysis of DSB repair in pol32Δ strains
| Classes of mother–daughter pairs | No. of mother–daughter pairs | No. (%) (observed phenotypes)
|
|
|---|---|---|---|
| One viable colony | Two viable colonies | ||
| Pairs with at least one Ade+ colony | 44 | 17 (38.6) | 27 (61.4) |
| (17 Ade+:dead) | (1 Ade+:Ade+; 18 Ade+:Ade−r; | ||
| 6 Ade+:Ade+/−r; 1 Ade+:Ade−w; | |||
| 1 Ade+:Ade−w/−r) | |||
| Pairs with only chromosome loss (Ade−r) | 96 | 30 (31.2) | 66 (68.8) |
| (30 Ade−r:dead) | (66 Ade−r:Ade−r) | ||
| Other | 109 | 14 (12.8) | 95 (87.2) |
| (8 Ade+/−r:dead; 3 Ade−w:dead; | (53 Ade−r:Ade+/−r; 11 Ade−r:Ade−w; | ||
| 2 Ade−w/−r:dead; 1 Ade+/−w:dead) | 8 Ade−r:Ade−w/−r; 4 Ade−r:Ade+/−w; | ||
| 13 Ade+/−r:Ade+/−r; 2 Ade−w:Ade−w; | |||
| 2 Ade+/−r:Ade−w; 1 Ade−w/−r:Ade−w/−r; | |||
| 1 Ade+/−r:Ade−w/−r) | |||
| Total | 249 | 61 (24.5) | 188 (75.5) |
Data are pooled from analyses of two isogenic pol32Δ strains, AM1014 and AM1152 (see Figure 1, A and F, and Table 1). Phenotype abbreviations are the same as described in Table 2. All indicated colonies were also Leu−.
A total of 249 mother–daughter cell pairs were dissected and analyzed (Table 4). Of these, 44 pairs gave rise to at least one Ade+ colony. Most of these pairs [27 pairs (61.4%)] resulted in two viable colonies, and it can thus be concluded that these events represent at least one BIR event. This was further confirmed by physical analysis of six of these Ade+NatrLeu− events, in which PFGE demonstrated that they all contained two copies of chromosome III of the expected size for BIR (one 350-kb molecule and one 340-kb molecule; not shown). The remaining 17 pairs of the 44 pairs that produced at least one Ade+ colony (38.6%) resulted in one Ade+Leu− colony and one nonviable colony. Either these events could represent half-crossover events that cosegregated with a full-length copy of chromosome III (as described above) or they could also be explained by a BIR event accompanied by death of the second colony that was unrelated to half-crossover formation. The latter scenario can be estimated by other classes of events. For example, of 96 mother–daughter pairs that produced only colonies that lost the broken chromosome (Ade−r), 30 pairs (31%) produced only a single viable colony. Further, of 109 mother–daughter pairs that constituted the category called “others,” which included various types of repair events, 14 pairs (∼13%) produced only a single viable colony. On the basis of these observations, it is possible that between 13 and 30% of the 17 mother–daughter pairs that produced only a single Ade+ colony could still represent a BIR event accompanied by death of the second colony resulting from an unknown cause.
We acknowledge that our plating experiment (Table 2) and pedigree analysis (Table 4) differed in their estimates of the proportions of full Ade+ vs. sectored Ade+/−r events. While an approximately equal number of Ade+ and Ade+/−r events was observed in pol32Δ cells in plating experiments, only a single Ade+:Ade+ mother–daughter pair was observed during pedigree analysis. At least two possible explanations for this phenomenon exist. In the first scenario, some of the colonies scored as full BIR events in plating experiments could have been half-crossovers that comigrated with a full-length copy of chromosome III; therefore, these events fell into the 1 viable:1 dead category during pedigree analysis. A second possibility is that, while all DSBs were induced in G1 for pedigree analysis, during plating experiments DSBs were induced at different stages of the cell cycle, which may have resulted in different ratios of full vs. sectored repair outcomes. Nevertheless, the pedigree analysis suggests that at least 60% of repair events in pol32Δ that were originally classified as BIR are likely to be formed via BIR, while the remaining 40% could be BIR or half-crossovers.
Pedigree analysis was performed on a limited scale for wild-type cells. Of 17 separated mother–daughter pairs, 16 gave rise to two viable colonies. Among those, 4 pairs produced two Ade+Leu+ colonies indicative of GC, 11 pairs produced two Ade+Leu− colonies indicative of BIR, and 1 pair consisted of one GC and one BIR colony. This result was similar to the results of a large-scale pedigree analysis performed previously in our diploid BIR system (Malkova et al. 2005).
DISCUSSION
BIR is an important process of DNA metabolism, but its mechanism and genetic control remain poorly understood. Therefore, identification of genes that carry out and regulate BIR is a critical step in unraveling the mechanism of BIR. Here we describe our new yeast experimental system to study interhomolog allelic BIR. This system has been optimized for the study of BIR by significantly reducing the amount of homology on one side of a controlled DSB to only 46 bp, resulting in ∼80% efficiency of BIR repair in wild-type cells. Also, our system provides a color-based assay to screen for unsuccessful DSB repair that results in loss of the broken chromosome, which is convenient for large-scale screening of mutants. Finally, our system allows us to recover and analyze aberrant BIR outcomes because cell viability does not depend on successful DSB repair.
Recently, Lydeard et al. (2007) described the effect of several replication mutations on ectopic BIR in a haploid yeast strain. It is possible that cells control allelic and ectopic BIR differently, as these two types of BIR differ from each other in their efficiencies, as well as in their consequences for genomic stability. Therefore, comparison of effects of various mutations on ectopic vs. allelic BIR might shed light on their possible differential regulation.
POL32-dependent BIR:
Here we report that deletion of a gene encoding a third nonessential subunit of polymerase δ, POL32, decreased the efficiency of BIR in our interhomolog allelic system and led to increased chromosome loss. This is consistent with recently published observations of Lydeard et al. (2007), which suggest an essential role of POL32 in ectopic BIR. Although it is still unclear why BIR requires Pol32p while normal DNA replication does not, or what the specific role of Pol32p in BIR is, we suggest that Pol32p might be needed for polymerase δ to be present at DNA regions (the strand invasion sites) other than replication origins, where DNA synthesis is normally initiated. One possible explanation is that the ability of Pol32p to interact with numerous different proteins might be important for the initiation of DNA synthesis associated with BIR (Johansson et al. 2001, 2004). Recently, Pol32p was suggested to play a role in translesion DNA synthesis (TLS). Specifically, it has been suggested that it might serve as an anchor for other proteins required for TLS (Haracska et al. 2001; Huang et al. 2002; Lawrence 2002). Pol32p could play a similar role in the initiation of DNA synthesis associated with BIR. This would be consistent with our data that showed a normal checkpoint response in pol32Δ cells, as well as with data from Lydeard et al. (2007) that suggest the BIR defect in pol32Δ to manifest during initiation of DNA synthesis, possibly suggesting that recruitment of other proteins required for BIR DNA synthesis is defective.
In addition, in our disomic experimental system, deletion of POL32 decreased not only the efficiency of BIR, but also the efficiency of GC. However, on the basis of data from Lydeard et al. (2007), GC does not require POL32 when donor and recipient sequences share a significant amount of homology on both sides of the break. Therefore, the dependency of GC on POL32 that we observed in our disomic system, in which homology between donor and recipient sequences is limited on one side of the DSB, might suggest that the mechanism of GC under these circumstances differs from the mechanism employed to repair DSBs where homology is not limiting. For example, in cases where homology is limited on one side of a DSB, it is possible that only the broken end with a large amount of homology with the donor sequence is capable of strand invasion, while the second end is used only for annealing to the newly synthesized strand. In this scenario, POL32 might be specifically involved in the repair synthesis initiated by one-ended invasion.
POL32-independent BIR:
Despite the fact that repair of DSBs in our experimental system was significantly hindered in pol32Δ cells, some BIR repair was still observed. Specifically, 18.9% of colonies among pol32Δ cells were Ade+Leu−, while an additional 19% were Ade+/−rLeu−, indicative of full or partial BIR, respectively. The results of a pedigree analysis confirmed that BIR repair can be completed in pol32Δ cells. Our data suggested that at least 60% of Ade+ repair outcomes in pol32Δ cells resulted from BIR, while others represented either BIR or cosegregation of half-crossover molecules with an unbroken, full-length copy of chromosome III.
The effect of POL32 on ectopic BIR was recently reported by Lydeard et al. (2007). In their system, deletion of POL32 resulted in very low efficiency of BIR repair (<2%). While this is lower than the frequency of BIR reported in our system, it is difficult to determine whether this reflects a true difference in the role that POL32 plays in ectopic vs. allelic BIR or if it simply reflects a preexisting deficit in the efficiency of ectopic vs. allelic BIR even when POL32 is present. Nevertheless, the possibility of a stronger dependency of ectopic BIR on POL32 is intriguing, as it might be indicative of differential regulation of ectopic vs. allelic BIR. If such differential regulation were confirmed, it might be related to the requirement for long tracts of homology between donor and recipient molecules that we report here.
Failure of BIR leads to half-crossovers:
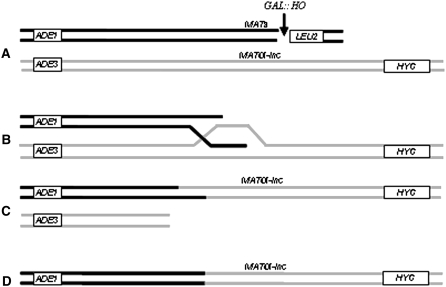
Another important observation reported here is that deletion of POL32 leads to formation of unusual repair outcomes. In ∼24% of pol32Δ cells, initiation of DSBs resulted in fragmentation of both copies of chromosome III such that at least one of the daughter cells inherited a fusion molecule consisting of the left portion of the truncated chromosome III and the right portion of the full-length chromosome III. In fact, the occurrence of these half-crossover outcomes in our system might be even higher than estimated because additional events could be hidden among Ade+ or Ade+/−r events if the half-crossover chromosome cosegregated with an intact, full-length chromosome.
Half-crossovers were documented at a low frequency in vegetative rad52Δ diploid cells (Haber and Hearn 1985; Malkova et al. 1996). Recently, frequent half-crossovers were documented in rad52Δ meiosis (Lao et al. 2008). It has been suggested that rad52Δ prevents capture of the second end, resulting in accumulation of aberrant strand-invasion intermediates that are resolved to produce half-crossovers.
In our experiments, we propose that half-crossovers result from aberrant processing of BIR intermediates (Figure 6). We further hypothesize that pol32Δ mutants stimulate formation of half-crossovers because BIR in these mutants is initiated but not completed. This hypothesis is supported by two pieces of data: (1) the recent finding of Lydeard et al. (2007) that showed deletion of POL32 affected BIR primarily at the step of replication initiation, and (2) the absence of half-crossovers in rad51Δ mutants, which are defective at the earlier step of strand invasion. We predict that mutations in genes that are not involved in strand invasion, but that alter the cell's ability to complete the later stages of BIR, should result in accumulation of unresolved intermediates that are likely acted upon by some version of resolvase to form a half-crossover molecule. This hypothesis is supported by a recent observation made by another laboratory (W.-H. Chung and G. Ira, personal communication) that showed mutation of PIF1 decreased BIR efficiency and stimulated formation of half-crossovers. Importantly, half-crossover formation, while rare, was observed in our wild-type disomic system, and it was also observed at a very low frequency in another study wherein BIR was initiated by transformation of yeast strains with linearized DNA fragments (Smith et al. 2007). Therefore, we propose that initiation of BIR is always associated with a risk of half-crossover formation and that the risk of these deleterious outcomes that threaten genomic stability is further increased in mutants that interfere with BIR progression.
Figure 6.—
Hypothetical mechanism of half-crossover formation. After induction of a DSB at MATa (A) and successful strand invasion (B), BIR progression is interrupted leading to accumulation of a strand-invasion intermediate. Resolution of this intermediate (C) leads to the formation of a fusion (half-crossover) chromosome containing parts of donor and recipient molecules (D), accompanied by the loss of the other chromosome fragments.
Half-crossovers in yeast as a model for NRTs in mammals:
On the basis of our results, we propose that formation of half-crossovers resulting from interruption of BIR may be analogous to NRTs observed in mammalian cells. NRTs were described in mammalian tumor cells as a pathway of telomere acquisition by broken chromosomes that results in the donor molecule losing genetic information—including its telomere—and becoming unstable (Ingvarsson 1999; Gollin 2001; Difilippantonio et al. 2002; Sabatier et al. 2005). This destabilization of the donor makes NRTs especially devastating because the events are self-perpetuating and result in cascades of genomic destabilization events, including chromosome loss and multiple rearrangements (Sabatier et al. 2005). Despite the deleterious consequences of NRTs for genomic stability and their potential relevance for cancer development, our knowledge of this repair pathway remains insufficient because NRT research in mammalian cells has proven to be very difficult. We believe that data from our yeast experimental model raise the possibility that NRTs might result from aberrant processing of BIR intermediates. This hypothesis is consistent with an observation made in mouse tumors where duplications were observed at the sites of NRTs (Difilippantonio et al. 2002), suggesting that NRTs could form as the result of interrupted BIR repair. We anticipate that further study of the half-crossover pathway in yeast will identify which genetic backgrounds predispose cells to the NRT pathway and, therefore, put cells at risk for acquiring genomic instabilities similar to those known to be associated with cancer.
Acknowledgments
We thank Grzegorz Ira, Dmitry Gordenin, Kirill Lobachev, Adam Bailis, Vidhya Narayanan, Youri Pavlov, and Polina Shcherbakova for helpful discussion. This work was supported by National Institutes of Health grant 1R15GM074657-01A1 and Indiana University–Purdue University Indianapolis Research Support Funds grant to A.M.
References
- Asai, T., S. Sommer, A. Bailone and T. Kogoma, 1993. Homologous recombination-dependent initiation of DNA replication from DNA damage-inducible origins in Escherichia coli. EMBO J. 12 3287–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuit, J. S., H. Xu, Y. Ma, T. Wang, J. Liu et al., 2001. Mediator proteins orchestrate enzyme-ssDNA assembly during T4 recombination-dependent DNA replication and repair. Proc. Natl. Acad. Sci. USA 98 8298–8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco, G., and J. E. Haber, 1998. Chromosome break-induced DNA replication leads to nonreciprocal translocations and telomere capture. Genetics 150 1037–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, A. P., and L. S. Symington, 2004. RAD51-dependent break-induced replication in yeast. Mol. Cell. Biol. 24 2344–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difilippantonio, M. J., S. Petersen, H. T. Chen, R. Johnson, M. Jasin et al., 2002. Evidence for replicative repair of DNA double-strand breaks leading to oncogenic translocation and gene amplification. J. Exp. Med. 196 469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formosa, T., and B. M. Alberts, 1986. a DNA synthesis dependent on genetic recombination: characterization of a reaction catalyzed by purified bacteriophage T4 proteins. Cell 47 793–806. [DOI] [PubMed] [Google Scholar]
- Formosa, T., and B. M. Alberts, 1986. b Purification and characterization of the T4 bacteriophage uvsX protein. J. Biol. Chem. 261 6107–6118. [PubMed] [Google Scholar]
- George, J. W., B. A. Stohr, D. J. Tomso and K. N. Kreuzer, 2001. The tight linkage between DNA replication and double-strand break repair in bacteriophage T4. Proc. Natl. Acad. Sci. USA 98 8290–8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, A. L., and J. H. McCusker, 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15 1541–1553. [DOI] [PubMed] [Google Scholar]
- Gollin, S. M., 2001. Chromosomal alterations in squamous cell carcinomas of the head and neck: window to the biology of disease. Head Neck 23 238–253. [DOI] [PubMed] [Google Scholar]
- Guthrie, C., and G. R. Fink, 1991. Guide to Yeast Genetics and Molecular Biology. Academic Press, San Diego.
- Haber, J. E., and M. Hearn, 1985. Rad52-independent mitotic gene conversion in Saccharomyces cerevisiae frequently results in chromosomal loss. Genetics 111 7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haracska, L., I. Unk, R. E. Johnson, E. Johansson, P. M. Burgers et al., 2001. Roles of yeast DNA polymerases delta and zeta and of Rev1 in the bypass of abasic sites. Genes Dev. 15 945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M. E., A. G. Rio, M. D. Galibert and F. Galibert, 2002. Pol32, a subunit of Saccharomyces cerevisiae DNA polymerase delta, suppresses genomic deletions and is involved in the mutagenic bypass pathway. Genetics 160 1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvarsson, S., 1999. Molecular genetics of breast cancer progression. Semin. Cancer Biol. 9 277–288. [DOI] [PubMed] [Google Scholar]
- Ira, G., A. Malkova, G. Liberi, M. Foiani and J. E. Haber, 2003. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell 115 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson, E., J. Majka and P. M. Burgers, 2001. Structure of DNA polymerase delta from Saccharomyces cerevisiae. J. Biol. Chem. 276 43824–43828. [DOI] [PubMed] [Google Scholar]
- Johansson, E., P. Garg and P. M. Burgers, 2004. The Pol32 subunit of DNA polymerase delta contains separable domains for processive replication and proliferating cell nuclear antigen (PCNA) binding. J. Biol. Chem. 279 1907–1915. [DOI] [PubMed] [Google Scholar]
- Kogoma, T., 1976. Two types of temperature sensitivity in DNA replication of an Escherichia coli dnaB mutant. J. Mol. Biol. 103 191–197. [DOI] [PubMed] [Google Scholar]
- Kogoma, T., 1997. Stable DNA replication: interplay between DNA replication, homologous recombination, and transcription. Microbiol. Mol. Biol. Rev. 61 212–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogoma, T., and K. G. Lark, 1975. Characterization of the replication of Escherichia coli DNA in the absence of protein synthesis: stable DNA replication. J. Mol. Biol. 94 243–256. [DOI] [PubMed] [Google Scholar]
- Kreuzer, K. N., 2000. Recombination-dependent DNA replication in phage T4. Trends Biochem. Sci. 25 165–173. [DOI] [PubMed] [Google Scholar]
- Kuzminov, A., 1999. Recombinational repair of DNA damage in Escherichia coli and bacteriophage lambda. Microbiol. Mol. Biol. Rev. 63 751–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark, C. A., J. Riazi and K. G. Lark, 1978. dnaT, dominant conditional-lethal mutation affecting DNA replication in Escherichia coli. J. Bacteriol. 136 1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao, J. P., S. D. Oh, M. Shinohara, A. Shinohara and N. Hunter, 2008. Rad52 promotes post-invasion steps of meiotic double-strand-break repair. Mol. Cell 29 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, C., 2002. Cellular roles of DNA polymerase zeta and Rev1 protein. DNA Repair 1 425–435. [DOI] [PubMed] [Google Scholar]
- Le, S., J. K. Moore, J. E. Haber and C. W. Greider, 1999. RAD50 and RAD51 define two pathways that collaborate to maintain telomeres in the absence of telomerase. Genetics 152 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine, F. J., N. P. Degtyareva, K. Lobachev and T. D. Petes, 2005. Chromosomal translocations in yeast induced by low levels of DNA polymerase a model for chromosome fragile sites. Cell 120 587–598. [DOI] [PubMed] [Google Scholar]
- Leung, W., A. Malkova and J. E. Haber, 1997. Gene targeting by linear duplex DNA frequently occurs by assimilation of a single strand that is subject to preferential mismatch correction. Proc. Natl. Acad. Sci. USA 94 6851–6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, X., S. J. Meech, L. F. Odom, M. A. Bitter, J. W. Ryder et al., 2004. Assessment of t(2;5)(p23;q35) translocation and variants in pediatric ALK+ anaplastic large cell lymphoma. Am. J. Clin. Pathol. 121 496–506. [DOI] [PubMed] [Google Scholar]
- Luke, B., G. Versini, M. Jaquenoud, I. W. Zaidi, T. Kurz et al., 2006. The cullin Rtt101p promotes replication fork progression through damaged DNA and natural pause sites. Curr. Biol. 16 786–792. [DOI] [PubMed] [Google Scholar]
- Lydeard, J. R., S. Jain, M. Yamaguchi and J. E. Haber, 2007. Break-induced replication and telomerase-independent telomere maintenance require Pol32. Nature 448 820–823. [DOI] [PubMed] [Google Scholar]
- Malkova, A., E. L. Ivanov and J. E. Haber, 1996. Double-strand break repair in the absence of RAD51 in yeast: a possible role for break-induced DNA replication. Proc. Natl. Acad. Sci. USA 93 7131–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova, A., M. L. Naylor, M. Yamaguchi, G. Ira and J. E. Haber, 2005. RAD51-dependent break-induced replication differs in kinetics and checkpoint responses from RAD51-mediated gene conversion. Mol. Cell. Biol. 25 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marians, K. J., 2000. PriA-directed replication fork restart in Escherichia coli. Trends Biochem. Sci. 25 185–189. [DOI] [PubMed] [Google Scholar]
- McEachern, M. J., and J. E. Haber, 2006. Break-induced replication and recombinational telomere elongation in yeast. Annu. Rev. Biochem. 75 111–135. [DOI] [PubMed] [Google Scholar]
- Michel, B., M. J. Flores, E. Viguera, G. Grompone, M. Seigneur et al., 2001. Rescue of arrested replication forks by homologous recombination. Proc. Natl. Acad. Sci. USA 98 8181–8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel, J. J., J. F. McCarville and Y. Xiong, 2003. A role for Saccharomyces cerevisiae Cul8 ubiquitin ligase in proper anaphase progression. J. Biol. Chem. 278 22828–22837. [DOI] [PubMed] [Google Scholar]
- Morrow, D. M., C. Connelly and P. Hieter, 1997. “Break copy” duplication: a model for chromosome fragment formation in Saccharomyces cerevisiae. Genetics 147 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung, K., A. Datta, C. Chen and R. D. Kolodner, 2001. SGS1, the Saccharomyces cerevisiae homologue of BLM and WRN, suppresses genome instability and homeologous recombination. Nat. Genet. 27 113–116. [DOI] [PubMed] [Google Scholar]
- Narayanan, V., P. A. Mieczkowski, H. M. Kim, T. D. Petes and K. V. Lobachev, 2006. The pattern of gene amplification is determined by the chromosomal location of hairpin-capped breaks. Cell 125 1283–1296. [DOI] [PubMed] [Google Scholar]
- Neumann, A. A., and R. R. Reddel, 2002. Telomere maintenance and cancer—look, no telomerase. Nat. Rev. Cancer 2 879–884. [DOI] [PubMed] [Google Scholar]
- Oh, S. D., J. P. Lao, P. Y-H. Hwang, A. F. Taylor, G. R. Smith et al., 2007. BLM ortholog, Sgs1, prevents aberrant crossing-over by suppressing formation of multichromatid joint molecules. Cell 130 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, X., P. Ye, D. S. Yuan, X. Wang, J. S. Bader et al., 2006. A DNA integrity network in the yeast Saccharomyces cerevisiae. Cell 124 1069–1081. [DOI] [PubMed] [Google Scholar]
- Paques, F., and J. E. Haber, 1999. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 63 349–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, A. J., J. M. Stark, F. D. Araujo, M. E. Moynahan, M. Berwick et al., 2001. Double-strand breaks and tumorigenesis. Trends Cell Biol. 11 S52–S59. [DOI] [PubMed] [Google Scholar]
- Pui, C. H., M. V. Relling and J. R. Downing, 2004. Acute lymphoblastic leukemia. N. Engl. J. Med. 350 1535–1548. [DOI] [PubMed] [Google Scholar]
- Reddel, R. R., 2003. Alternative lengthening of telomeres, telomerase, and cancer. Cancer Lett. 194 155–162. [DOI] [PubMed] [Google Scholar]
- Roberts, T. M., I. W. Zaidi, J. A. Vaisica, M. Peter and G. W. Brown, 2008. Regulation of Rtt107 recruitment to stalled DNA replication forks by the cullin Rtt101 and the Rtt109 acetyltransferase. Mol. Cell. Biol. 19 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier, L., M. Ricoul, G. Pottier and J. P. Murnane, 2005. The loss of a single telomere can result in instability of multiple chromosomes in a human tumor cell line. Mol. Cancer Res. 3 139–150. [DOI] [PubMed] [Google Scholar]
- Schmidt, K. H., J. Wu and R. D. Kolodner, 2006. Control of translocations between highly diverged genes by Sgs1, the Saccharomyces cerevisiae homolog of the Bloom's syndrome protein. Mol. Cell. Biol. 26 5406–5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes, D. T., M. Banerjee, B. Bowen and M. J. Curicio, 2001. Multiple regulators of Ty1 transposition in Saccharomyces cerevisiae have conserved roles in genome maintenance. Genetics 159 1449–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, C. E., B. Llorente and L. S. Symington, 2007. Template switching during break-induced replication. Nature 447 102–105. [DOI] [PubMed] [Google Scholar]
- Spell, R. M., and S. Jinks-Robertson, 2004. Examination of the roles of Sgs1 and Srs2 helicases in the enforcement of recombination fidelity in Saccharomyces cerevisiae. Genetics 168 1855–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark, J. M., and M. Jasin, 2003. Extensive loss of heterozygosity is suppressed during homologous repair of chromosomal breaks. Mol. Cell. Biol. 23 733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storici, F., L. K. Lewis and M. A. Resnick, 2001. In vivo site-directed mutagenesis using oligonucleotides. Nat. Biotechnol. 19 773–776. [DOI] [PubMed] [Google Scholar]
- Strauchen, J. A., 2004. Immunophenotypic and molecular studies in the diagnosis and classification of malignant lymphoma. Cancer Invest. 22 138–148. [DOI] [PubMed] [Google Scholar]
- Teng, S. C., J. Chang, B. McCowan and V. A. Zakian, 2000. Telomerase-independent lengthening of yeast telomeres occurs by an abrupt Rad50p-dependent, Rif-inhibited recombinational process. Mol. Cell 6 947–952. [DOI] [PubMed] [Google Scholar]
- Teng, S. C., and V. A. Zakian, 1999. Telomere-telomere recombination is an efficient bypass pathway for telomere maintenance in Saccharomyces cerevisiae. Mol. Cell. Biol. 19 8083–8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanHulle, K., F. J. Lemoine, V. Narayanan, B. Downing, K. Hull et al., 2007. Inverted DNA repeats channel repair of distant double-strand breaks into chromatid fusions and chromosomal rearrangements. Mol. Cell. Biol. 27 2601–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkel-Meiman, K., and G. S. Roeder, 1990. Gene conversion tracts stimulated by HOT1-promoted transcription are long and continuous. Genetics 126 851–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wach, A., A. Brachat, R. Pohlmann and P. Philippsen, 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10 1793–1808. [DOI] [PubMed] [Google Scholar]