Abstract
In fungal hyphae, apical dominance refers to the suppression of secondary polarity axes in the general vicinity of a growing hyphal tip. The mechanisms underlying apical dominance remain largely undefined, although calcium signaling may play a role. Here, we describe the localized accumulation of reactive oxygen species (ROS) in the apical region of Aspergillus nidulans hyphae. Our analysis of atmA (ATM) and prpA (PARP) mutants reveals a correlation between localized production of ROS and enforcement of apical dominance. We also provide evidence that NADPH oxidase (Nox) or related flavoproteins are responsible for the generation of ROS at hyphal tips and characterize the roles of the potential Nox regulators NoxR, Rac1, and Cdc42 in this process. Notably, our genetic analyses suggest that Rac1 activates Nox, whereas NoxR and Cdc42 may function together in a parallel pathway that regulates Nox localization. Moreover, the latter pathway may also include Bem1, which we propose represents a p40phox analog in fungi. Collectively, our results support a model whereby localized Nox activity generates a pool of ROS that defines a dominant polarity axis at hyphal tips.
THE fungal mycelium consists of individual hyphae that radiate outward from the colony center. Each hypha grows solely by tip extension and generates new hyphae via the formation of lateral branches. It is generally thought that nutrients are assimilated at hyphal tips and then transported into the interior of the mycelium for subsequent utilization in support of colony growth and development (Carlile 1995). To achieve this characteristic pattern of mycelial organization, individual hyphae must exhibit apical dominance, whereby the growing tip is dominant and appears to suppress the formation of other tips (i.e., lateral branches) in its general vicinity (Rayner 1991). At a physiological level, apical dominance presumably reflects the exclusive targeting of cellular resources (i.e., vesicles laden with precursors required for cell-surface expansion and cell-wall deposition) to the hyphal tip at the expense of potential branching sites. Accordingly, branch sites would become active only when they are a sufficient distance from the growing tip. Although it has been recognized as a key factor underlying mycelial organization (Rayner 1991; Carlile 1995), the molecular basis of apical dominance in fungi remains poorly understood.
Two general approaches have provided insight into the mechanisms that maintain a single axis of polarized hyphal extension in filamentous fungi. First, genetic screens have identified several mutants that simultaneously form multiple polarity axes due to an apparent loss of apical dominance (Harris et al. 1994; Reynaga-Pena and Bartnicki-Garcia 1997). Characterization of these mutants has revealed that the enforcement of apical dominance requires both the Spitzenkorper and the polarizome (Sharpless and Harris 2002; Harris et al. 2005; Virag and Harris 2006; Steinberg 2007). Additional genetic screens in Aspergillus nidulans and Neurospora crassa have identified numerous mutants that alter the frequency of branching and affect diverse cellular processes, including vesicle trafficking, membrane organization, and cell cycle control (Seiler and Plamann 2003; Gatherar et al. 2004; Lin and Momany 2004). Second, detailed physiological studies emphasize the importance of calcium in the enforcement of apical dominance (Jackson and Heath 1993). Most fungal hyphae exhibit a tip-high calcium gradient and, at least in N. crassa, there is evidence that dissipation of this gradient enables branch formation from the hyphal tip (Schmid and Harold 1988). Furthermore, mutations that perturb calcium signaling in N. crassa and A. fumigatus typically cause hyphal tips to become hyperbranched (Prokisch et al. 1997; Gavric and Griffiths 2003; Steinbach et al. 2006; da Silva Ferreira et al. 2007). Although these genetic and physiological approaches have implicated specific functions in the control of apical dominance, the nature and specificity of their roles are not clear.
Studies in other highly polarized cell types strongly suggest that reactive oxygen species (ROS) play a key role in the spatial regulation of polar growth. In Arabidopsis, the formation of root hairs from epidermal cells is preceded by the localized accumulation of ROS at the polarization site (Foreman et al. 2003). Moreover, genetic or chemical disruption of Nox activity leads to altered root-hair morphology because of defects in calcium uptake (Takeda et al. 2008). Additional studies have identified Rho-related GTPases (i.e., Rop GTPases) as positional regulators of Nox function (Carol et al. 2005; Jones et al. 2007). In animals, the formation of a single axon during neuronal differentiation is associated with the suppression of excessive neurite outgrowths (Arimura and Kaibuchi 2005), an effect that resembles apical dominance and appears to be mediated by localized Nox-mediated generation of ROS (Ibi et al. 2006).
Nox was first characterized in phagocytes, where it is responsible for the respiratory burst triggered by microorganisms or inflammatory mediators (Lambeth 2004). Subsequent studies have identified Nox homologs in diverse cell types and shown that they generate localized ROS used for signaling, with potential effects including modification of MAP kinase activity (Lambeth 2004). Although not conserved in yeast, Nox homologs have been found in filamentous fungi and implicated in the regulation of fungal development (Lalucque and Silar 2003; Aguirre et al. 2005; Takemoto et al. 2007). For example, deletion of A. nidulans NoxA causes defects in the formation of sexual reproductive structures known as cleistothecia (Lara-Ortiz et al. 2003). More recently, roles for Nox homologs in fungal–plant interactions have been described (Tanaka et al. 2006; Egan et al. 2007). In phagocytes, Nox is found in association with a regulatory complex that consists of four subunits: p22phox, p40phox, p47phox, and p67phox (Lambeth 2004). Of these, only p67phox (i.e., NoxR) appears to be conserved in filamentous fungi (Takemoto et al. 2007). In the endophytic fungus Epichloe festucae, NoxR acts in conjunction with the Rho-related GTPase RacA to spatially regulate ROS production and control hyphal branching (Takemoto et al. 2006). These observations suggest that, by analogy to root hairs and neurons, localized generation of ROS by Nox may regulate apical dominance in fungal hyphae.
As part of our effort to characterize features of the DNA damage response in A. nidulans (Goldman et al. 2002), we recently described homologs of two key signaling components of the animal DNA damage response: AtmA (ataxia telangiectasia mutated, or ATM) and PrpA [poly(ADP)-ribose polymerase, or PARP] (Malavazi et al. 2006; Semighini et al. 2006). Notably, in addition to the expected defects in the DNA damage response, mutations in atmA and prpA also affected morphogenesis and development. Of particular interest, disruption of atmA caused defects in hyphal morphogenesis that include delayed establishment of hyphal polarity and the failure to maintain a stable polarity axis (Malavazi et al. 2006). Here, we show that these defects result from the Nox-mediated accumulation of ROS. In addition, through the characterization of NoxR and other candidate Nox regulators, we provide evidence that ROS generation at the hyphal tips plays a key role in enforcing apical dominance.
MATERIALS AND METHODS
Strains and media:
A. nidulans strains used are listed on Table 1. Media used include MAG (2% dextrose, 2% malt extract, 0.2% peptone, trace elements, vitamins, pH 6.5), CM (1% dextrose, 0.2% peptone, 0.1% yeast extract, 0.1% casamino acids, nitrate salts, trace elements, vitamins, pH 6.5), MN (1% dextrose, nitrate salts, trace elements, pH 6.5), MN–ethanol (MN with dextrose replaced by 1.2% ethanol). Nitrate salts, trace elements, and vitamins were as described previously (Kafer 1977). Uridine and uracil were added to a final concentration of 5 and 10 mm, respectively, as necessary to complement the pyrimidine auxotrophy caused by the pyrG89 marker.
TABLE 1.
A. nidulans strains used in this work
| Strains | Genotypes | References |
|---|---|---|
| A28 | pabaA6 biA1 | FGSCa |
| AAV128 | pyroA4 pyrG89; nkuA∷argB Δcdc42∷pyroA | Virag et al. (2007) |
| AAV129 | pyroA4 pyrG89; nkuA∷argB ΔracA∷pyorA | Virag et al. (2007) |
| AAV160 | pyroA4 pyrG89; nkuA∷argBΔnoxR∷pyroA ΔmodA∷pyrG | This work |
| AAV161 | pyroA4 pyrG89; nkuA∷argB ΔnoxR∷pyroA Δcdc42∷pyrG | This work |
| ACS14 | pyrG89/pyrG89; ΔprpA∷pyr4/+ | Semighini et al. (2006) |
| ACS28 | pyroA4 pyrG89; nkuA∷argB ΔnoxR∷pyroA | This work |
| ACS29 | pyroA4 pyrG89; nkuA∷argB ΔnoxR∷pyroA | This work |
| ACS32 | pyroA4 pyrG89; nkuA∷argB ΔnoxR∷pyroA ΔatmA∷pyrG | This work |
| ACS33 | pyroA4 pyrG89; nkuA∷argB ΔnoxR∷pyroA ΔatmA∷pyrG | This work |
| ACS37 | pyroA4 pyrG89; nkuA∷argB Δcdc42∷pyroA ΔatmA∷pyrG | This work |
| ACS38 | pyroA4 pyrG89; nkuA∷argB Δcdc42∷pyroA ΔatmA∷pyrG | This work |
| ACS39 | pyroA4 pyrG89; nkuA∷argB ΔnoxR∷pyroA ΔracA∷pyrG | This work |
| ACS40 | pyroA4 pyrG89; nkuA∷argB ΔnoxR∷pyroA ΔracA∷pyrG | This work |
| ACS42 | pyroA4 pyrG89; nkuA∷argB ΔracA∷pyroA ΔatmA∷pyrG | This work |
| ACS43 | pyroA4 pyrG89; nkuA∷argB ΔracA∷pyroA ΔatmA∷pyrG | This work |
| AKS75 | yA2; sepD5; pyrG89 | Sharpless and Harris (2002) |
| BE 3-15 | argB2; methH2; wA3; yA2; ΔbemA∷argB | Geoff Turnerb |
| IM69 | pyroA4 pyrG89, wA1; ΔatmA∷pyrG | Malavazi et al. (2006) |
| GR5 | pyrG89; wA3; pyroA4 | FGSCa |
| TNO2A3 | pyrG89 pyroA4 argB2 nkuA∷argB | Nayak et al. (2006) |
| TTL3 | pabaA1, yA2, ΔargB∷trpCΔB; ΔnoxA∷argB; trpC801 | Lara-Ortiz et al. (2003) |
Fungal Genetics Stock Center, University of Kansas Medical Center (Kansas City, MO).
Department of Molecular Biology and Biotechnology, University of Sheffield (Sheffield, United Kingdom).
Microscopy:
Strains were germinated on coverslips at 28° in indicated medium and time. When specified, germlings were treated with diphenylene iodonium (DPI; Sigma, St. Louis) or ascorbic acid (AAc; Sigma). Hyphae were fixed and nuclei were stained with Hoechst 33258 (Molecular Probes) as previously described (Harris et al. 1994). Conidiophore morphology was analyzed after growth on coverslips for 4 days on supplemented minimal medium at 28° (Lin and Momany 2003). Slides were viewed using an Olympus BX51 fluorescent microscope. Images were captured with a Photometrics CoolSnap HQ CCD camera (Roper Scientific) and processed using IPLab software (Scanalytics) and Adobe PhotoShop 6.0.
Nitro blue tetrazolium staining:
Staining with nitro blue tetrazolium (NBT) was performed according to Malagnac et al. (2004). Coverslips containing germlings grown in 0.5% yeast extract, 2% glucose, and vitamins (YGV) medium at 28° for 16 hr were flooded in 2.5 mm NBT (Sigma) dissolved in 5 mm MOPS, pH 7.6. After 30 min of incubation in the dark at room temperature, hyphae were immediately examined under bright-field illumination with an Olympus AX70 microscope. To measure the size of germlings, individual images from hyphae from each class were captured with a Photometrics CoolSnap HQ CCD camera. The length of 40 hyphae from each NBT staining class was measured using IPLab software (Scanalytics, Rockville, MD) and analyzed with Microsoft Excel.
For the plate assays, strains were point inoculated 1.5 cm from the center of the plate in a circle in MN + supplements. After growth at 30° for 2 days, each plate was flooded with a solution of 2.5 mm NBT in 5 mm MOPS, pH 7.6, and incubated in the dark at 28° for 30 min. The supernatant was drained and the plates were carefully rinsed with distilled water. Plates were reincubated for 2 days in the dark at 28°. Images of whole colonies were made using an Olympus DP25 Microscope Digital Camera attached to a Navitar Macro Zoom optics and diagnosis instrument. A ring light with a light diffuser was used to give homogeneous illumination of the whole plate. The images were analyzed using MetaMorph software, version 6.3r5 (Molecular Devices, Downingtown, PA). The average intensity of hand-selected regions of interest was measured and analyzed with Microsoft Excel.
Images of the colony edges were captured with an Olympus DP25 Microscope Digital Camera attached to a Nikon SMZ 800 stereoscope. Images were processed using Adobe PhotoShop 6.0.
Strain construction:
A fusion PCR-mediated approach was used to construct the noxR gene replacement strain as previously described (Yang et al. 2004). Three initial PCR reactions generated 5′- and 3′-flanking regions of the noxR gene amplified from the wild-type strain A28 [using primer sets GSP1/GSP2 and GSP3/GSP4, respectively, as well as the pyroA selectable nutritional marker amplified from plasmid pTN1 (Nayak et al. 2006) (using primer set PYRO1/PYRO2)]. A final fusion PCR using the primer set GSP1/GSP4 amplified a full-length deletion cassette containing the three previous fragments. PCR amplifications were performed using the Expand Long Template PCR kit (Roche Diagnostics, Indianapolis) and the amplification conditions specified by Yang et al. (2004). The sequences of oligonucleotide primers can be supplied upon request. Transformation of the pyroA4 TNO2A3 strain was performed using a previously described protocol (Osmani et al. 1987) and 5 μg of the deletion cassette. Positive transformants were identified on the basis of their ability to grow on selective media. Diagnostic PCR analysis as described in Yang et al. (2004) was used to select transformants with the wild-type noxR gene replaced by homologous integration by the fusion PCR product. Two independent transformants were backcrossed with strain GR5 to eliminate the possibility of ectopic integrations of the gene replacement construct. Progeny were tested for 1:1 segregation and cosegregation of the gene replacement marker (pyroA) with mutant phenotypes. The verified ΔnoxR strains were named ACS28 and ACS29.
RESULTS
Reduced PARP function leads to loss of apical dominance:
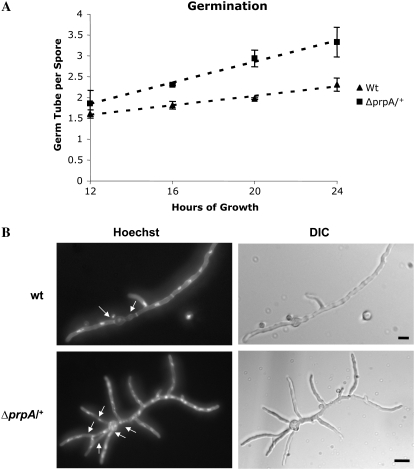
We previously demonstrated that prpA, which encodes the sole PARP homolog in A. nidulans, is an essential gene (Semighini et al. 2006). Accordingly, we constructed a heterozygous deletion strain (i.e., ΔprpA+/−) and showed that it was haplo-insufficient for aspects of the DNA damage response. In addition to these defects, we also noted that the ΔprpA/+ mutant displays subtle defects in hyphal morphology. First, it produces an increased number of germ tubes per spore (Figure 1A). Whereas wild-type diploid control strains typically generate two hyphae per spore on minimal media, the ΔprpA/+ mutant generated three or more. Second, mutant hyphae fail to exhibit a dominant growing tip (Figure 1B). Wild-type strains that have been germinated for 20 hr typically possess two hyphae that grow in opposite directions by apical extension and show no evidence for branch formation (Figure 1B, top). In contrast, ΔprpA/+ hyphae branch repeatedly to yield multiple polarity axes such that there is no single dominant axis of growth (Figure 1B, bottom). Thus, reduced PrpA function leads to the loss of apical dominance. Notably, this phenotype is markedly different from that of ΔatmA mutants, which are significantly impaired in the formation of a single axis of hyphal polarity (Malavazi et al. 2006).
Figure 1.—
The ΔprpA/+ mutant produces an increased number of germ tubes per spore. (A) The number of germ tubes per spore for both wild-type and ΔprpA/+ strains was determined at the indicated times. (B) Wild-type (top) and ΔprpA/+ (bottom) strains were germinated at 28° for 20 hr in YGV medium. The germinated spores were fixed and stained with Hoechst 33258 and analyzed by fluorescent microscopy. Arrows indicate germ tubes emitted from each spore. Bars, 10 μm.
Localization of ROS at hyphal tips is altered in ΔatmA and ΔprpA mutants:
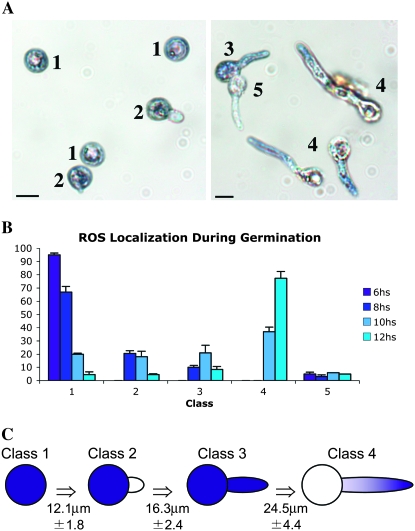
In animals, both ATM and PARP share multiple functions, including roles in the response to oxidative stress (reviewed by Virag and Szabo 2002; Barzilai and Yamamoto 2004). On the basis of this, we surmised that reduced function of these proteins could affect the accumulation of ROS. To monitor the production of ROS, hyphae were stained with NBT, which is reduced by superoxide ions so that it forms a readily detectable blue-purple formazan precipitate (Munkres 1990). We first examined the pattern of ROS localization in wild-type strains during the process of spore germination and formation of polarized hyphae. Five distinct staining patterns were observed (Figure 2A): (i) class 1, in which NBT stained the entire conidium; (ii) class 2, in which NBT stained the conidium but not the germ tube; (iii) class 3, in which NBT stained the entire germling; (iv) class 4, in which NBT stained only the tip of the germ tube; and (v) class 5, in which no NBT staining was observed. To assess the temporal relationship between these patterns, we took samples at hourly intervals during spore germination and counted the number of hyphae in each class. Whereas the majority of the conidia that germinated for 6 hr were present in class 1, the peak had shifted to class 4 by 12 hr of incubation (Figure 2B). Thus, the ROS localization pattern changed in a progressive manner from uniform accumulation in germinating spores to discrete localization at the tips of growing hyphae.
Figure 2.—
ROS localization correlates to germling growth. (A) Light micrographs showing localization of ROS during conidial germination. Germlings of wild-type strain A28 grown on MN medium for 8 hr (left) and 12 hr (right) were stained with NBT. Numbers indicate the five different ROS patterns: class 1, localization throughout the entire conidium; class 2, localization in the conidium but not in the germ tube; class 3, localization throughout the entire germling; class 4, a gradient localized to the hyphal tip; and class 5, no localization. Bars, 10 μm. (B) Distribution of different patterns of ROS localization during germination. Germlings of A28 wild-type strain grown on MN medium for 6, 8, 10, and 12 hr were stained with NBT and classified into the five different ROS patterns. The graph represents the average of three independent experiments where 200 germlings were examined in each replicate. (C) Correlation between ROS localization and germling size. For each of the five patterns of ROS staining, the length of 40 germlings was determined. Average lengths are indicated with the exception of class 5, which showed no relation to hyphal size.
The formation of the first septum represents an important landmark during hyphal growth, as it marks the transition from unicellular to multicellular growth (Harris 1997). Moreover, on the basis of recent observations that suggest the existence of two distinct phases of hyphal growth (Horio and Oakley 2005), septum formation may also be the point at which rapid hyphal tip growth is initiated. To determine if septation is required for ROS localization to hyphal tips (i.e., class 4), we examined the NBT staining pattern in a temperature-sensitive mutant defective in septum formation (i.e., sepD5). At the restrictive temperature, the dynamics of NBT localization were indistinguishable from wild type in this mutant (supplemental Figure 1), suggesting that septation is not required for ROS localization. Furthermore, in wild-type hyphae, we failed to observe any relationship between the dynamics of ROS localization and the presence or absence of septa (data not shown). However, when the sizes of 40 hyphae exhibiting each of the five NBT staining patterns were measured, we did note an apparent correlation between staining pattern and hyphal length (Figure 2C). In particular, the class 4 staining pattern was observed only in hyphae that were > ∼25 μm. This observation suggests that the localization of ROS to hyphal tips correlates with growth and may represent an additional landmark event during spore germination and the formation of a hypha.
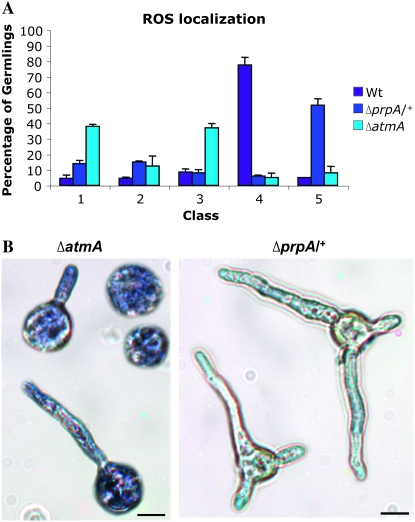
We next used NBT staining to characterize the pattern of ROS localization in the ΔatmA and ΔprpA/+ mutants after 12 hr of germination and compared it to wild type (Figure 3A). Two significant differences were noted. First, whereas the majority of wild-type hyphae exhibited ROS localization at hyphal tips (i.e., 77.5% in class 4), most ΔatmA mutants were present in two classes that are defined by uniform localization of ROS throughout the spore (class 1: 37%, compared to 4% in wild type) or the hypha (class 3: 37%, compared to 8% in wild type) (Figure 3B, left). Second, most ΔprpA/+ hyphae failed to display any ROS accumulation at all (i.e., 51.5% in class 5, compared to only 6% in wild type) (Figure 3B, right). These observations suggest a possible correlation between ROS localization and apical dominance, whereby the failure to discretely localize ROS at hyphal tips can trigger the parallel formation of multiple polarity axes (i.e., ΔprpA/+ mutants) or no axes at all (i.e., ΔatmA mutants). In the former case, the absence of any ROS seemingly abolishes the suppression of branching and enables the formation of multiple branches. In the latter case, the uniform accumulation of ROS appears to repress the timely formation of any polarity axis, whether it is the primary hypha or the lateral branches.
Figure 3.—
Disruption of ROS localization correlates with hyphal morphology defects. (A) Distribution of different patterns of ROS localization in wild-type, ΔatmA, and ΔprpA/+ strains germinated for 12 hr in MN. The graph represents the average of three independent experiments where 200 germlings were examined in each replicate. (B) Light micrographs showing localization of ROS on ΔatmA (left) and ΔprpA/+ (right) germlings grown in MN for 12 hr and stained with NBT. Bars, 10 μm.
ROS production by NADPH oxidase regulates apical dominance:
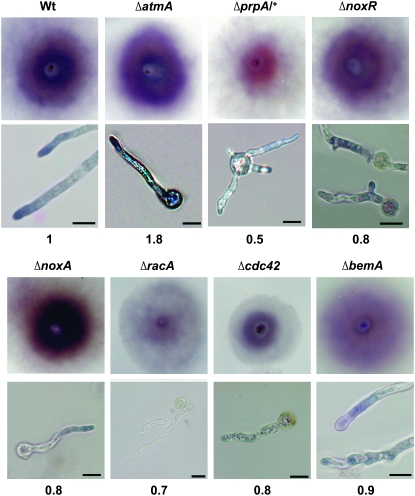
As in animals and plants, increasing evidence shows that Noxs are responsible for the localized generation of ROS signals in filamentous fungi (Takemoto et al. 2006; Egan et al. 2007). Whereas most filamentous fungi possess at least two distinct Nox homologs (Lalucque and Silar 2003; Aguirre et al. 2005; Takemoto et al. 2007), A. nidulans contains only a single recognizable homolog that is required for normal sexual development (NoxA; Lara-Ortiz et al. 2003). Initial examination of a ΔnoxA mutant failed to reveal any defect in hyphal morphogenesis or ROS localization (see Figure 7 and Table 2), suggesting the possible existence of other cytochrome b enzymes that can compensate for the absence of Nox activity (see discussion). Thus, we used two alternate approaches to determine if Nox (or Nox-like) functions regulate apical dominance. First, we treated wild-type hyphae with DPI, which binds flavoproteins and inhibits Nox activity (Cross and Jones 1986). Second, we deleted noxR, which encodes the sole A. nidulans homolog of the Nox regulatory subunit p67phox (Takemoto et al. 2006).
Figure 7.—
ROS accumulation in candidate Nox regulators. NBT staining was used to monitor peroxide production in colonies (top) and hyphae (bottom) of the indicated mutants. Colonies were imaged following 48 hr incubation at 30° on plates. Hyphae were imaged after 16 hr incubation at 28°C on glass coverslips. Bars, 10 μm. Numbers correspond to pixel intensity of colonies stained with NBT compared to wild type (the experiment was repeated three times and similar values were found).
TABLE 2.
ROS staining pattern of different hyphal morphology mutants
| Strain
|
||||||
|---|---|---|---|---|---|---|
| Class | Wild type | ΔnoxA | ΔnoxR | ΔracA | Δcdc42 | ΔbemA |
| 1 | 6.0 (±2.1) | 2.5 (±0.7) | 1.5 (±0.7) | 2.0 (±0.0) | 11.5 (±3.5) | 3.5 (±0.7) |
| 2 | 7.0 (±1.7) | 2.0 (±0.2) | 2.0 (±1.4) | 7.5 (±0.7) | 2.0 (±0.3) | 4.0 (±0.8) |
| 3 | 6.5 (±2.5) | 19.0 (±2.7) | 7.0 (±0.6) | 5.5 (±0.7) | 15 (±0.7) | 25.0 (±4.2) |
| 4 | 74.5 (±7.5) | 72.5 (±7.7) | 23.0 (±4.2) | 21.5 (±3.5) | 19.0 (±1.4) | 62.5 (±3.5) |
| 5 | 6.0 (±1.4) | 4.0 (±1.4) | 25.5 (±4.9) | 44.0 (±2.8) | 7.0 (±1.4) | 5.0 (±1.2) |
| Abnormal | 0.0 (±0.0) | 0.0 (±0.0) | 41.0 (±5.8)a | 19.5 (±4.5)a | 45.5 (±5.5)b | 0.0 (±0.0) |
Hyphae were grown for 16 hr at 28° on coverslips in YGV media before staining with NBT as described in materials and methods. Numbers are percentages.
ROS staining confined to the middle regions of germlings but not at hyphal tip.
Germlings >25 μm with uniform distribution of ROS.
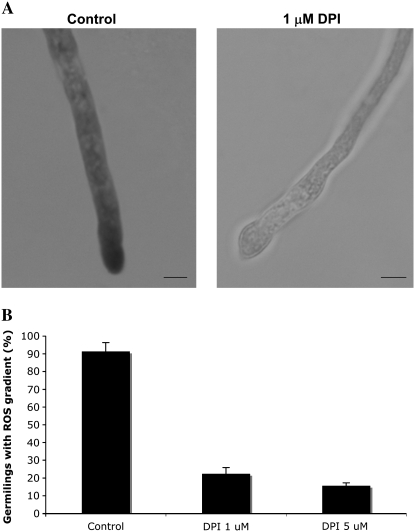
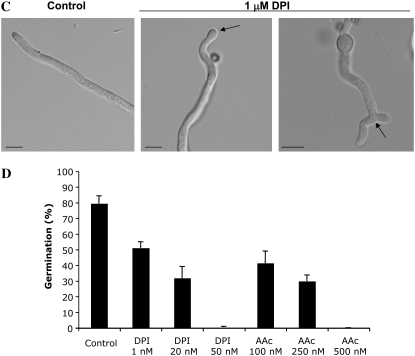
Treatment of growing wild-type hyphae with DPI for 2 hr reduced the localization of ROS at the hyphal tip (Figure 4, A and B). In addition, 71% (±3.3) of DPI-treated hyphae displayed abnormal tips that were either swollen or branched (Figure 4C). In addition, conidia germinated in the presence of DPI or ascorbic acid, an antioxidant molecule that is effective in fungi (i.e., Egan et al. 2007), were unable to form a germ tube (Figure 4D). These observations suggest that the localized generation of ROS is required for both the establishment and the maintenance of an axis of hyphal polarity.
Figure 4.—
DPI disrupts ROS gradient and induces hyphal morphology defects. (A) Light micrographs showing localization of ROS during conidial germination. Germlings of A28 wild-type strain grown on MN medium for 12 hr followed by treatment with 1 μm of DPI for 2 hr and stained with NBT. Bars, 10 μm. (B) The percentage of hyphae >25 μm containing an ROS gradient at the tip in untreated controls or following treatment with 1 or 5 μm of DPI for 2 hr. Bars show the average of three independent experiments where 200 tips were examined in each replicate. (C) Light micrographs of untreated control hyphae or hyphae treated with 1 μm of DPI for 2 hr. Arrows show abnormal hyphal-tip morphologies induced by DPI. (D) The percentage of conidia that germinated in the presence of DPI and ascorbic acid. Germlings of A28 wild-type strain were germinated on YGV medium for 16 hr in the presence of the indicated concentrations of DPI and AAc. Bars show the average of three independent experiments where 200 germlings were examined in each replicate.
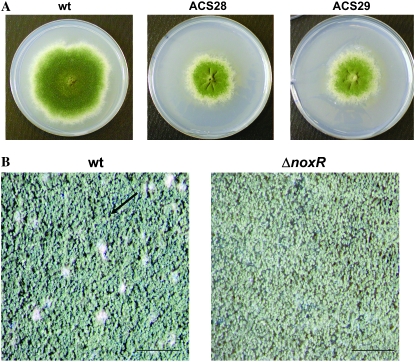
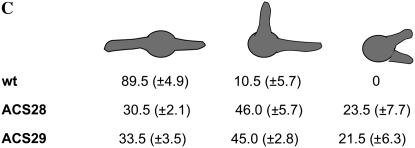
As a more specific means of inhibiting the localized generation of ROS by Nox or Nox-like enzymes, we deleted the p67phox homolog noxR (AN6046.3). Two independent transformants were retained and their genotypes were verified using PCR. Colonies from both transformants exhibited slower growth when compared to the parental wild-type strain, such that they were half the diameter of wild type after 8 days of growth (Figure 5A). Colonies from the mutant also produced fivefold fewer conidia than wild type (Figure 5B), and ∼80% of ΔnoxR conidiophores displayed abnormal morphology, including the absence of vesicles and dichotomously branched stalks, as well as fewer metulae, phialides, and conidia (Figure 5C). These results suggest that NoxR is important for asexual development. In addition, two observations implicate NoxR in sexual development. First, the mutant failed to initiate the formation of cleistothecia at a time (8 days of growth) when sexual development had already begun in wild-type colonies (Figure 5C). Second, ΔnoxR mutants could not form cleistothecia when subjected to a self-cross or when crossed with the ΔnoxA mutant (data not shown).
Figure 5.—
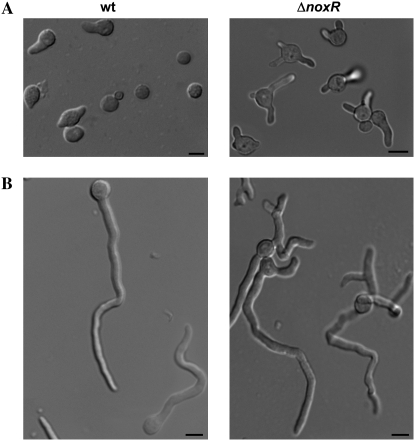
Deletion of NoxR reduces colony growth and results in defective formation of conidia and cleistothecia. (A) Colony morphology of wild type and the two ΔnoxR transformants after 8 days of growth on MN at 28°. (B) Detail of colony morphology of wild-type and ΔnoxR strains after 8 days of growth on MN at 28°. Wild type forms cleistothecia (arrow) while ΔnoxR does not. Bars, 1 mm. (C) Conidiophore morphology of wild-type and ΔnoxR strains after 4 days of growth on MN at 28°. Deletion of noxR induced conidiophore defects such as branched conidiophore stalks (arrow) and the absence of vesicle plus reduced number of metulae, phialides, and conidiospores (arrowhead). Bars, 20 μm.
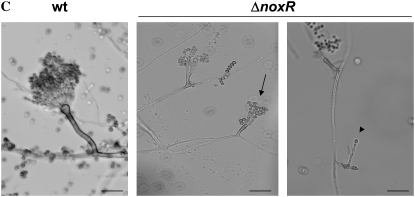
The observation of germinating conidiospores revealed a defective germination pattern in ΔnoxR mutants. Whereas wild-type spores exhibit a strong bias toward bipolar germination that is dependent upon an intact actin cytoskeleton (Harris et al. 1999), ΔnoxR mutants displayed a random pattern of germ-tube emergence (Figure 6, A and C). In addition, almost half (i.e., 45%) of ΔnoxR hyphae exhibited abnormal dichotomous branching at their tips (Figure 6B). NBT staining indicated that ΔnoxR mutants are largely able to generate localized ROS, but do so in abnormal positions such as the middle of hyphae (i.e., the abnormal class in Table 2). Notably, these positions correlate with sites of branch emergence (Figure 7). At the same time, the proportion of hyphae that failed to generate ROS (class 5 in Table 2) was fourfold higher in ΔnoxR mutants compared to wild type. Collectively, these results suggest that NoxR spatially regulates the localized generation of ROS by Nox and, by doing so, enforces apical dominance in growing hyphae.
Figure 6.—
Deletion of NoxR results in altered germination patterns and loss of apical dominance. Germlings from wild-type (left) and ΔnoxR (right) strains grown in MN for 8 hr (A) and 12 hr (B). Bars, 10 μm. (C) Germination pattern of spores possessing two germ tubes. Spores were classified as displaying (from left to right) bipolar, quarterpolar, or random germination patterns.
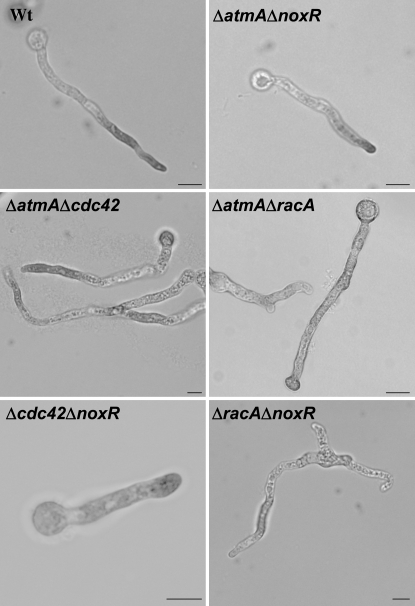
Finally, to determine whether the accumulation of ROS is indeed responsible for repressing the timely formation of polarity axes in the ΔatmA mutant, we generated ΔatmA ΔnoxR double mutants by a standard cross and verified their genotypes using PCR. Strikingly, the ΔnoxR deletion suppressed the accumulation of ROS in the ΔatmA mutant and restored a normal pattern of NBT staining (Figure 8; Table 3). In addition, the deletion of noxR largely suppressed the morphological defects caused by the ΔatmA mutation and restored germ-tube emergence to wild-type levels (Table 4). These observations implicate Nox-dependent accumulation of ROS as a significant factor in impairing the formation of polarity axes in the ΔatmA mutant.
Figure 8.—
ROS localization in double mutants. Light micrographs showing localization of ROS in ΔatmAΔnoxR, ΔatmAΔcdc42, ΔracAΔnoxR, and Δcdc42ΔnoxR germlings grown in MN for 12 hr and stained with NBT. Bars, 10 μm.
TABLE 3.
ROS staining pattern of double mutants
| Strain
|
||||||
|---|---|---|---|---|---|---|
| Class | Wild type | ΔatmA ΔnoxR | ΔatmA Δcdc42 | ΔatmA ΔracA | ΔracA ΔnoxR | Δcdc42 ΔnoxR |
| 1 | 5.0 (±1.6) | 6.0 (±1.4) | 1.5 (±0.7) | 1.5 (±0.7) | 3.5 (±0.7) | 1.5 (±0.7) |
| 2 | 6.0 (±2.0) | 3.0 (±1.4) | 1.5 (±07) | 1.5 (±0.4) | 2.0 (±1.4) | 3 (±1.4) |
| 3 | 8.0 (±2.8) | 12.0 (±0.7) | 12.5 (±3.5) | 14.0 (±2.7) | 7.5 (±3.9) | 19 (±2.8) |
| 4 | 75.0 (±5.6) | 71.0 (±5.6) | 82.5 (±2.1) | 24.5 (±3.5) | 13.5 (±1.7) | 72.5 (±7.7) |
| 5 | 6.0 (±2.8) | 8.0 (±1.4) | 1.5 (±0.7) | 9.5 (±6.3) | 62.5 (±6.3) | 4 (±3.5) |
| Abnormal | 0.0 (±0.0) | 0.0 (±0.0) | 0.0 (±0.0) | 49.0 (±2.8)ab | 11.0 (±2.1)a | 0.0 (±0.0) |
Hyphae were grown for 16 hr at 28° on coverslips in YGV media before staining with NBT as described in materials and methods. Numbers are percentages.
Germlings >25 μm with uniform distribution of ROS.
ROS staining confined to the middle regions of germlings but not at hyphal tip.
TABLE 4.
The ΔatmAΔnoxR double mutant exhibits a wild-type phenotype
| Wild type | ΔatmA | ΔnoxR | ΔatmA ΔnoxR | |
|---|---|---|---|---|
| % pol | 80.6 (±7.5) | 22.6 (4.1) | 48.6 (±6.4) | 80.6 (±2.5) |
| % pol (n ≥ 4) | 100.0 (±0.0) | 67.3 (±3.0) | 99.6 (±1.5) | 100.0 (±0.0) |
| No. of nuclei | ||||
| 1 | 6.6 (±1.1) | 6.0 (±2.0) | 45.0 (±4.2) | 7.6 (±1.4) |
| 2 | 32.3 (±4.0) | 13.0 (±1.0) | 41.0 (±4.4) | 24.3 (±3.2) |
| 4 | 41.0 (±2.6) | 41.3 (±3.1) | 13.0 (±3.7) | 43.0 (±3.6) |
| >4 | 20.0 (±5.2) | 41.6 (±3.7) | 1.0 (±0.4) | 25.3 (±6.1) |
Hyphae were examined following 12 hr growth on YGV at 28°. “% pol” refers to the percentage of conidiospores that possess a recognizable germ tube. “% pol (n ≥ 4)” refers to the percentage of conidiospores with four or more nuclei that possess a recognizable germ tube. The number of nuclei was determined by Hoechst 33258 staining as described in materials and methods. Numbers are percentages.
Regulation of NADPH oxidase function by Rac1 and Cdc42:
In animals and plants, the complex that regulates Nox activity includes a Rho-related GTPase (Rac in animals and Rop in plants: Lambeth 2004; Jones et al. 2007). Initial observations suggest that Rac1 has a similar regulatory role for fungal Noxs (Takemoto et al. 2006; Tanaka et al. 2008). We recently described the functional characterization of the A. nidulans Rac1 (RacA) and Cdc42 homologs and found that, unlike other filamentous fungi that have been tested, RacA has a minimal role in hyphal morphogenesis (Virag et al. 2007). Instead, Cdc42 seems to be the critical GTPase that regulates polarized growth. Accordingly, we surmised that Cdc42 might function as a regulator of Nox activity in A. nidulans, and we tested this notion by examining the effects of both ΔracA and Δcdc42 mutations on the generation of ROS in hyphae.
Analysis of NBT staining patterns revealed that both RacA and Cdc42 have a role in ROS production. In ΔracA mutants, almost sevenfold more hyphae than wild type displayed no detectable ROS accumulation (44% vs. 6%; Table 2; Figure 7). Furthermore, for a significant fraction of those hyphae that did produce ROS, it was abnormally localized in a region distal to the tip (abnormal class; Table 2). By contrast, although Δcdc42 mutants produced ROS at levels that were only slightly lower than wild type, in >60% of hyphae it was uniformly distributed (class 3) or abnormally localized (Table 2; Figure 7). We also determined whether the deletion of racA or cdc42 had an effect on the accumulation of ROS triggered by the loss of AtmA. Strikingly, the Δcdc42 mutation behaved like the ΔnoxR deletion in its ability to restore normal ROS accumulation and permit the proper establishment of hyphal polarity in a ΔatmA mutant background. As shown in Table 3 and Figure 8, >80% of the Δcdc42 ΔatmA double-mutant hyphae exhibited a normal class 4 NBT staining pattern, which coincided with suppression of ΔatmA morphological defects. On the other hand, the ΔracA mutation only partially restored normal ROS accumulation in ΔatmA mutants. In particular, only 24% of ΔracA ΔatmA double-mutant hyphae exhibited a normal class 4 NBT staining pattern, whereas twice as many hyphae displayed abnormal NBT staining in a central region distal to the hyphal tip (Table 3). Moreover, although polarity establishment was restored, abnormal NBT staining appeared to correlate with defects in hyphal morphogenesis such as spurious branch formation and swollen tips (Figure 8). Collectively, these observations implicate both RacA and Cdc42 in the regulation of Nox and further suggest that Rac1 may have a more important role in the activation of Nox whereas Cdc42 regulates its localization.
To further characterize the relative roles of RacA and Cdc42 in the regulation of Nox, we tested for genetic interactions with NoxR. In particular, if NoxR functioned only in partnership with RacA to regulate Nox, we would expect the deletion of noxR to have no effect on the phenotypes displayed by ΔracA mutants. However, in terms of ROS production, the deletion of noxR increased the fraction of ΔracA hyphae that failed to stain with NBT from 44 to 62% (class 5; Tables 2 and 3). Furthermore, deletion of noxR also abolished apical dominance in ΔracA mutants, as the double mutants formed multiple germ tubes and branches (Figure 8). These observations suggest that NoxR has at least one function required for the generation of ROS and/or apical dominance that is independent of RacA. To determine if this function involves Cdc42, we examined ROS production and hyphal morphology in Δcdc42 ΔnoxR mutants. Notably, whereas both parental deletions cause defects in the distribution of ROS (Table 2), the double mutant exhibited a normal pattern of NBT staining (72% class 4; Table 3; Figure 8). This apparent example of reciprocal suppression suggests that Δcdc42 phenotypes in part may reflect unchecked NoxR activity, and vice versa, thereby supporting the possibility that NoxR and Cdc42 function in the same ROS-generating pathway. On the other hand, the deletion of cdc42 completely suppressed the hyperbranching phenotype caused by ΔnoxR (Figure 8), which presumably reflects previously observed Nox-independent roles for Cdc42 in branch formation (Virag et al. 2007). In total, these results are consistent with a model whereby RacA and Cdc42 act via different mechanisms to regulate Nox function.
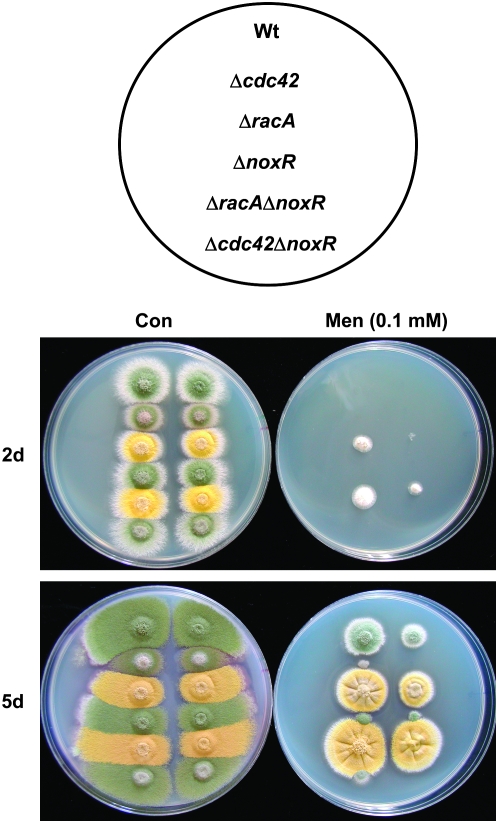
Finally, we used plate tests to determine whether ΔracA or Δcdc42 mutants displayed altered responses to chemical agents that induce oxidative stress. Strikingly, we found that ΔracA mutants were resistant to menadione (Figure 9), which generates increased levels of superoxide (Posci et al. 2005). Moreover, menadione resistance was further enhanced when the ΔnoxR mutation was combined with ΔracA (Figure 9). These phenotypes likely reflect the presence of lower superoxide levels in ΔracA mutants (Figure 7) and suggest that (i) RacA functions as Nox activator in A. nidulans and (ii) RacA and NoxR are capable of exerting independent effects on Nox. By contrast, Δcdc42 deletion mutants displayed pronounced sensitivity to menadione, and, despite its ability to enhance the menadione resistance phenotype of ΔracA mutants, the ΔnoxR mutation alone also caused menadione sensitivity (Figure 9). Notably, Δcdc42 ΔnoxR double mutants exhibited apparent epistasis for menadione sensitivity (Figure 9). The markedly different response of cdc42 mutants to menadione when compared to racA mutants lends further credence to the idea that Cdc42 is not an activator of Nox. Instead, as outlined in the discussion, we propose that Cdc42 and NoxR may function together to regulate Nox localization.
Figure 9.—
Altered response to menadione in racA and cdc42 mutants. Approximately 103 (left column of each plate) or 102 (right column of each plate) conidia were spotted onto plates containing CM or CM plus 0.1 mm menadione. Plates were incubated at 28° and imaged after 2 and 5 days of growth.
Role of Bem1 in the generation of ROS:
To date, comprehensive searches of completed fungal genome sequences have identified only homologs of p91phox (NoxA) and p67phox (NoxR); obvious homologs of the regulatory subunits p47phox, p40phox, or p22phox have not been reported (Takemoto et al. 2007). However, the domain organization of fungal Bem1 homologs suggests that it might be a functional analog of p40phox. In particular, like p40phox, Bem1 possesses a PB1 domain containing the OPCA motif (Kawahara and Lambeth 2008). Moreover, the known role of Bem1 as a scaffold protein required for polarity establishment in yeast (i.e., Park and Bi 2007) supports a potential link between localized production of ROS and polarized growth. To test the possibility that Bem1 might regulate fungal Nox, we used NBT staining to examine ROS localization patterns in a recently characterized A. nidulans mutant possessing a deletion of the Bem1 homolog bemA (Leeder and Turner 2008). This mutant exhibited a modest increase in the number of hyphae with uniform ROS localization (class 3) that was not as dramatic as that observed in ΔnoxR or Δcdc42 mutants (Table 2). In addition, ROS localization in subapical regions of ΔbemA mutants correlated with the appearance of swollen regions that may represent aborted attempts at branching (Figure 7). Taken together, these observations provide initial support for the idea that Bem1 is a functional analog of the Nox organizers p40phox and p47phox in fungi.
DISCUSSION
Apical dominance is a key feature that defines the morphology of fungal hyphae (Rayner 1991). In filamentous fungi such as A. nidulans, the absence of lateral branches in the vicinity of the hyphal tip suggests that their formation may be actively suppressed (Trinci et al. 1994; Dynesen and Nielsen 2003). Evidence from plant pathogenic fungi also implies that apical dominance may be subject to regulation. For example, during infection of rye flowers, Claviceps purpurea hyphae do not branch, although they are capable of doing so under other conditions (see Rolke and Tudzynski 2008). The mechanisms that enforce apical dominance in fungal hyphae remain poorly understood, although recent studies in plants and animals suggest that ROS might play a role (Foreman et al. 2003; Ibi et al. 2006). Here, we present data that support this view. In particular, we show that ROS accumulates at the tips of A. nidulans hyphae and demonstrate that altered patterns of ROS localization correlate with the polarity and branching phenotypes observed in several morphological mutants. Furthermore, we also provide evidence that implicates Nox or related flavoproteins in the generation of ROS at hyphal tips, and we analyze the functional roles of several candidate Nox regulators, including NoxR, RacA, Cdc42, and BemA.
Enforcement of apical dominance at hyphal tips by localized production of ROS:
The asymmetric accumulation of specific molecules in the apical regions of fungal and oomycete hyphae has been known for many years. Tip-high calcium and ion gradients have been reported in several fungi and oomycetes, and the roles of these gradients in polarized hyphal growth have been widely debated (Jackson and Heath 1993). Of particular interest, one study attributed the enforcement of apical dominance in N. crassa hyphae to the presence of calcium gradients (Schmid and Harold 1988). Our results show that ROS accumulates in the apical region of A. nidulans hyphae. This appears to be a common feature of fungal hyphae, since similar localization patterns were recently reported in other fungi such as Magnaporthe grisea and E. festucae (Takemoto et al. 2006; Egan et al. 2007). In addition, older cytochemical studies reported the accumulation of “reducing power” in association with the Spitzenkorper at hyphal tips in multiple fungi (Turian 1976). The pattern of ROS accumulation during conidiospore germination and the formation of hyphae in A. nidulans is dynamic and correlates with growth. Previously, a transition from slow to fast tip extension was noted as “germlings” matured into hyphae (Horio and Oakley 2005). Our results are consistent with the possibility that this transition may coincide with the accumulation of ROS at hyphal tips.
At this time, the nature of the active ROS at hyphal tips and their precise functions remain unknown. Because NBT specifically reacts with superoxide ions (Munkres 1990), our experiments clearly document the accumulation of this form of ROS at hyphal tips. However, superoxide ions are typically converted to hydrogen peroxide by superoxide dismutase, and we cannot rule out the possibility that this occurs at hyphal tips. Regardless of the specific type of ROS that is active at hyphal tips, we envision several possible effects on hyphal morphogenesis that are not mutually exclusive. For example, by analogy to recent observations in plants (Foreman et al. 2003; Takeda et al. 2008), ROS may promote calcium influx by activating plasma-membrane calcium channels. The subsequent accumulation of calcium at hyphal tips would presumably affect the dynamics of the actin cytoskeleton as well as the vesicle trafficking machinery. In addition, ROS may also modulate MAP-kinase-signaling pathways that impinge upon morphogenesis (see Takemoto et al. 2007 for review). Finally, hydrogen peroxide has been implicated in the localized softening of the cell wall during the extension of highly polarized plant cells (e.g., cotton fibers; Hovav et al. 2008) and could conceivably play a similar role at hyphal tips.
Regulation of NADPH oxidase function in fungal hyphae:
Our results provide several important insights into the composition of fungal Nox complexes and the roles of potential Nox regulators such as RacA, Cdc42, and NoxR. Recent phylogenetic analyses demonstrate that most filamentous fungi possess two Nox homologs, NoxA and NoxB (Takemoto et al. 2007; note that obvious Nox homologs are missing from both hemi-ascomycete and archiascomycete yeasts). However, a significant exception to this pattern is found in the Aspergilli, which generally possess only NoxA (A. terreus also harbors a NoxC homolog; Takemoto et al. 2007). In A. nidulans, NoxA is required for sexual development, and as our observations show, has no apparent role in the generation of localized ROS at hyphal tips or in apical dominance. Given the absence of other Nox homologs, it is not clear how ROS is produced in ΔnoxA hyphae. One possible source is homologs of ferric reductase; the Saccharomyces cerevisiae ferric reductase Fre1 is a cytochrome b that shares considerable homology and biochemical features with Nox (Shatwell et al. 1996). A. nidulans possesses at least six potential homologs of Fre1 (AN10893.3, AN0773.3, AN7662.3, AN4906.3, AN8683.3, and AN3207.3) that share domain organization with Nox, and it seems conceivable that one or more of these proteins could in fact be a divergent Nox homolog that generates ROS in the absence of NoxA.
Although results from our genetic analyses are consistent with previous reports that RacA and NoxR regulate Nox function in fungi (Takemoto et al. 2006), they also highlight potential differences in their respective effects (Figure 10). For example, the observation that ΔracA mutants fail to produce detectable ROS and display menadione resistance (presumably due to lowered pools of superoxide) supports the view that RacA activates Nox. On the other hand, two observations imply that NoxR is not needed per se to activate Nox. First, ΔnoxR mutants are able to generate ROS, albeit at abnormal sites. Second, deletion of noxR enhances the menadione sensitivity of ΔracA mutants and also triggers hyperbranching in ΔracA hyphae. Neither of these phenotypes would be expected if NoxR worked solely in conjunction with RacA to regulate Nox. Instead, we propose that NoxR functions in a parallel pathway that regulates Nox localization (Figure 10). Moreover, on the basis of the similar ROS localization and menadione sensitivity phenotypes displayed by ΔnoxR and Δcdc42 mutants, we also propose that Cdc42 functions in the same pathway as NoxR (the absence of hyperbranching in Δcdc42 mutants reflects broader roles for Cdc42 in hyphal morphogenesis; Virag et al. 2007). Finally, we cannot exclude the possibility that NoxR may share functions with Cdc42 that are independent of Nox. In this regard, it is noteworthy that several fungi (i.e., Yarrowia lipolytica, Rhizopus oryzae, Phycomyces blakesleeanus) possess NoxR-like proteins despite the absence of any detectable Nox homolog (Takemoto et al. 2007).
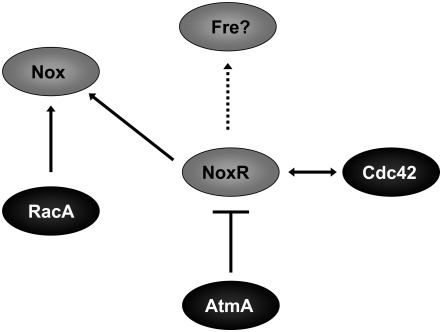
Figure 10.—
Model for the regulatory pathways that affect Nox function. RacA is predicted to function as an activator of Nox, whereas Cdc42 and NoxR may regulate Nox localization. The dashed arrow indicates possible additional functions of NoxR, which may include, for example, the regulation of alternate NADPH oxidases such as the ferric reductase (Fre) homologs. Although the mechanism remains unknown, AtmA is predicted to negatively regulate NoxR.
The role of ROS in the regulation of apical dominance in A. nidulans first became apparent through our analysis of ΔatmA and ΔprpA mutants. The observation that both the ROS accumulation and the hyphal polarity defects caused by the ΔatmA mutation could be suppressed by the deletion of noxR suggests that ATM could act as a negative regulator of NoxR expression. However, transcript levels of noxR, as well as those of noxA, are not altered in ΔatmA mutants (Malavazi et al. 2007). Furthermore, given the known roles of ATM in the response to oxidative stress (Barzilai et al. 2002), the effects on Nox or its regulators caused by loss of ATM could be an indirect consequence of an altered redox state. The mechanism by which prpA haplo-insufficiency leads to a reduction in the levels of localized ROS at hyphal tips also remains unclear. Although this phenotype could conceivably reflect global changes in cellular physiology (i.e., altered NADH/NADPH ratios), there is some evidence that suggests that PARP may act in conjunction with Nox in the production of ROS (i.e., Abdallah et al. 2007).
Bem1 may be an ancestral Nox regulator in fungi:
Both fungal NoxR homologs and their animal counterparts p67phox possess a PB1 domain with conserved amino acids that mediate interactions with the PB1 domain of p40phox (Kawahara and Lambeth 2008). However, no obvious p40phox homolog has yet been found in fungi (Takemoto et al. 2007). Recent phylogenomic analyses have identified three fungal proteins that harbor PB1 domains similar to those found in p40phox: Bem1, Cdc24, and a cystathione β-synthase (Kawahara and Lambeth 2008). Notably, the domain organization of Bem1 resembles that of p40phox (i.e., SH3-SH3-PX-PB1 for Bem1 and PX-SH3-PB1 for p40phox), and Bem1 regulates polarized growth in yeast via interactions with proteins such as Cdc42 (Park and Bi 2007). The A. nidulans Bem1 homolog, BemA, is required for normal hyphal morphology and localizes to a surface cap at hyphal tips (Leeder and Turner 2008). Our observation that ROS accumulates at abnormal subapical locations in ΔbemA mutants suggests that Bem1 homologs may act together with NoxR and Cdc42 to spatially regulate Nox function. Whether Bem1 represents a true analog of p40phox is a question that requires future investigation (i.e., testing for possible interactions between their respective PB1 domains). Nevertheless, our results suggest that an ancestral function of Bem1 in fungi may have been the regulation of Nox.
Acknowledgments
We thank Christian Elowsky and Terri Fangman from the University of Nebraska at Lincoln Microscopy Core Facility for their assistance with the use of the microscope; Aleksandra Virag, Barry Scott, and Aiko Tanaka for their help with NBT staining; and Jesus Aguirre and Geoff Turner for providing noxA and bemA mutant strains, respectively. Awards from the Nebraska Research Foundation and the National Science Foundation (BES-0519080) were used to support this work.
References
- Abdallah, Y., D. Gligorievski, S. A. Kasseckert, L. Dieterich, M. Schafer et al., 2007. The role of poly(ADP-ribose) polymerase (PARP) in the autonomous proliferative response of endothelial cells to hypoxia. Cardiovasc. Res. 73 568–574. [DOI] [PubMed] [Google Scholar]
- Aguirre, J., M. Rios-Momberg, D. Hewitt and W. Hansberg, 2005. Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol. 13 111–118. [DOI] [PubMed] [Google Scholar]
- Arimura, N., and K. Kaibuchi, 2005. Key regulators in neuronal polarity. Neuron 48 881–884. [DOI] [PubMed] [Google Scholar]
- Barzilai, A., and K. Yamamoto, 2004. DNA damage responses to oxidative stress. DNA Repair 3 1109–1115. [DOI] [PubMed] [Google Scholar]
- Barzilai, A., G. Rotman and Y. Shiloh, 2002. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair 1 3–25. [DOI] [PubMed] [Google Scholar]
- Carlile, M. J., 1995. The success of the hypha and mycelium, pp. 3–19 in The Growing Fungus, edited by N. A. R. Gow and G. M. Gadd. Chapman & Hall, London/New York.
- Carol, R. J., S. Takeda, P. Linstead, M. C. Durrant, H. Kakesova et al., 2005. A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature 438 1013–1016. [DOI] [PubMed] [Google Scholar]
- Cross, A. R., and O. T. Jones, 1986. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem. J. 237 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Ferreira, M. E., T. Heinekamp, A. Hartl, A. A. Brakhage, C. P. Semighini et al., 2007. Functional characterization of the Aspergillus fumigatus calcineurin. Fungal Genet. Biol. 44 219–230. [DOI] [PubMed] [Google Scholar]
- Dynesen, J., and J. Nielsen, 2003. Branching is coordinated with mitosis in growing hyphae of Aspergillus nidulans. Fungal Genet. Biol. 40 15–24. [DOI] [PubMed] [Google Scholar]
- Egan, M. J., Z-Y. Wang, M. A. Jones, N. Smirnoff and N. J. Talbot, 2007. Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc. Natl. Acad. Sci. USA 104 11772–11777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman, J., V. Demidchik, J. H. Bothwell, P. Mylona, H. Miedema et al., 2003. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422 442–446. [DOI] [PubMed] [Google Scholar]
- Gatherar, I. M., S. Pollerman, N. Dunn-Coleman and G. Turner, 2004. Identification of a novel gene hbrB required for polarized growth in Aspergillus nidulans. Fungal Genet. Biol. 41 463–471. [DOI] [PubMed] [Google Scholar]
- Gavric, O., and A. J. Griffiths, 2003. Interaction of mutations affecting tip growth and branching in Neurospora. Fungal Genet. Biol. 40 261–270. [DOI] [PubMed] [Google Scholar]
- Goldman, G. H., S. L. McGuire and S. D. Harris, 2002. The DNA damage response in filamentous fungi. Fungal Genet. Biol. 35 183–195. [DOI] [PubMed] [Google Scholar]
- Harris, S. D., 1997. The duplication cycle in Aspergillus nidulans. Fungal Genet. Biol. 22 1–12. [DOI] [PubMed] [Google Scholar]
- Harris, S. D., J. L. Morrell and J. E. Hamer, 1994. Identification and characterization of Aspergillus nidulans mutants defective in cytokinesis. Genetics 136 517–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, S. D., A. F. Hofmann, H. W. Tedford and M. P. Lee, 1999. Identification and characterization of genes required for hyphal morphogenesis in the filamentous fungus Aspergillus nidulans. Genetics 151 1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, S. D., N. D. Read, R. W. Roberson, B. Shaw, S. Seiler et al., 2005. Polarisome meets Spitzenkorper: microscopy, genetics, and genomics converge. Eukaryot. Cell 4 225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio, T., and B. R. Oakley, 2005. The role of microtubules in rapid tip growth of Aspergillus nidulans. Mol. Biol. Cell 16 918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovav, R., J. A. Udall, B. Chaudhary, E. Hovav, L. Flagel et al., 2008. The evolution of spinnable cotton fiber entailed prolonged development and a novel metabolism. PloS Genet. 4 e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi, M., M. Katsuyama, C. Fan, K. Iwata, T. Nishinaka et al., 2006. NOX1/NADPH oxidase negatively regulates nerve growth factor-induced neurite outgrowth. Free Radic. Biol. Med. 40 1785–1795. [DOI] [PubMed] [Google Scholar]
- Jackson, S. L., and I. B. Heath, 1993. Role of calcium ions in hyphal tip growth. Microbiol. Rev. 57 367–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, M. A., M. J. Raymond, Z. Yang and N. Smirnoff, 2007. NADPH oxidase-dependent reactive oxygen species formation required for root hair growth depends on ROP GTPase. J. Exp. Bot. 58 1261–1270. [DOI] [PubMed] [Google Scholar]
- Kafer, E., 1977. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv. Genet. 19 33–131. [DOI] [PubMed] [Google Scholar]
- Kawahara, T., and J. D. Lambeth, 2008. Molecular evolution of Phox-related regulatory subunits for NADPH oxidase enzymes. BMC Evol. Biol. 7 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalucque, H., and P. Silar, 2003. NADPH oxidase: An enzyme for multicellularity? Trends Microbiol. 11 9–12. [DOI] [PubMed] [Google Scholar]
- Lambeth, J. D., 2004. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4 181–189. [DOI] [PubMed] [Google Scholar]
- Lara-Ortiz, T., H. Riveros-Rosas and J. Aguirre, 2003. Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol. Microbiol. 50 1241–1255. [DOI] [PubMed] [Google Scholar]
- Leeder, A. C., and G. Turner, 2008. Characterization of Aspergillus nidulans polarisome component BemA. Fungal Genet. Biol. 45 897–911. [DOI] [PubMed] [Google Scholar]
- Lin, X., and M. Momany, 2003. The Aspergillus nidulans swoC1 mutant shows defects in growth and development. Genetics 165 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, X., and M. Momany, 2004. Identification and complementation of abnormal hyphal branch mutants ahbA1 and ahbB1 in Aspergillus nidulans. Fungal Genet. Biol. 41 998–1006. [DOI] [PubMed] [Google Scholar]
- Malagnac, F., H. Lalucque, G. Lepere and P. Silar, 2004. Two NADPH oxidase isoforms are required for sexual reproduction and ascospore germination in the filamentous fungus Podospora anserina. Fungal Genet. Biol. 41 982–997. [DOI] [PubMed] [Google Scholar]
- Malavazi, I., C. P. Semighini, M. R. Kress, S. D. Harris and G. H. Goldman, 2006. Regulation of hyphal morphogenesis and the DNA damage response by the Aspergillus nidulans ATM homolog AtmA. Genetics 173 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavazi, I., M. Savoldi, M. E. da Silva Ferreira, F. M. Soriani, P. S. Bonato et al., 2007. Transcriptome analysis of the Aspergillus nidulans AtmA (ATM, Ataxia-Telangiectasia Mutated) null mutant. Mol. Microbiol. 66 74–99. [DOI] [PubMed] [Google Scholar]
- Munkres, K. D., 1990. Histochemical detection of superoxide radicals and hydrogen peroxide by Age-1 mutants of Neurospora. Fungal Genet. Newsl. 37 24–25. [Google Scholar]
- Nayak, T., E. Szewczyk, C. E. Oakley, A. Osmani, L. Ukil et al., 2006. A versatile and efficient gene targeting system for Aspergillus nidulans. Genetics 172 1557–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani, S. A., G. R. May and N. R. Morris, 1987. Regulation of the mRNA levels of nimA, a gene required for the G2-M transition in Aspergillus nidulans. J. Cell Biol. 104 1495–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H. O., and E. Bi, 2007. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol. Mol. Biol. Rev. 71 48–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posci, I., M. Miskei, Z. Karanyi, T. Emri, P. Ayoubi et al., 2005. Comparison of gene expression signatures of diamide, H2O2 and menadione exposed Aspergillus nidulans cultures: linking genome-wide transcriptional changes to cellular physiology. BMC Genomics 20 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokisch, H., O. Yarden, M. Dieminger, M. Tropschug and I. B. Barthelmess, 1997. Impairment of calcineurin function in Neurospora crassa reveals its essential role in hyphal growth, morphology and maintenance of the apical Ca2+ gradient. Mol. Gen. Genet. 256 104–114. [DOI] [PubMed] [Google Scholar]
- Rayner, A. D. M., 1991. The challenge of the individualistic mycelium. Mycologia 83 48–71. [Google Scholar]
- Reynaga-Pena, C. G., and S. Bartnicki-Garcia, 1997. Apical branching in a temperature sensitive mutant of Aspergillus niger. Fungal Genet. Biol. 22 153–167. [DOI] [PubMed] [Google Scholar]
- Rolke, Y., and P. Tudzynski, 2008. The small GTPase Rac and the p21-activated kinase Cla4 in Claviceps purpurea: interaction and impact on polarity, development, and pathogenicity. Mol. Microbiol. 68 405–423. [DOI] [PubMed] [Google Scholar]
- Schmid, J., and F. M. Harold, 1988. Dual roles for calcium ions in apical growth of Neurospora crassa. J. Gen. Microbiol. 134 2623–2631. [DOI] [PubMed] [Google Scholar]
- Seiler, S, and M. Plamann, 2003. The genetic basis of cellular morphogenesis in the filamentous fungus Neurospora crassa. Mol. Biol. Cell 14 4352–4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semighini, C. P., M. Savoldi, G. H. Goldman and S. D. Harris, 2006. Functional characterization of the Aspergillus nidulans poly(ADP-ribose) polymerase homologue PrpA. Genetics 173 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless, K. E., and S. D. Harris, 2002. Functional characterization and localization of the Aspergillus nidulans formin SEPA. Mol. Biol. Cell 13 469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatwell, K., A. Dancis, A. R. Cross, R. D. Klausner and A. W. Segal, 1996. The FRE1 ferric reductase of Saccharomyces cerevisiae is a cytochrome b similar to that of NADPH oxidase. J. Biol. Chem. 271 14240–14244. [DOI] [PubMed] [Google Scholar]
- Steinbach, W. J., R. A. Cramer, Jr., B. Z. Perfect, Y. G. Asfaw, T. C. Sauer et al., 2006. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5 1091–1103. [DOI] [PMC free article] [PubMed]
- Steinberg, G., 2007. Hyphal growth: a tale of motors, lipids, and the Spitzenkorper. Eukaryot. Cell 6 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda, S., C. Gapper, H. Kaya, E. Bell, K. Kuchitsu et al., 2008. Local positive feedback regulation determines cell shape in root hair cells. Science 319 1241–1244. [DOI] [PubMed] [Google Scholar]
- Takemoto, D., A. Tanaka and B. Scott, 2006. A p67Phox-like regulator is recruited to control hyphal branching in a fungal-grass mutualistic symbiosis. Plant Cell 18 2807–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto, D., A. Tanaka and B. Scott, 2007. NADPH oxidases in fungi: diverse roles of reactive oxygen species in fungal cellular differentiation. Fungal Genet. Biol. 44 1065–1076. [DOI] [PubMed] [Google Scholar]
- Tanaka, A., M. J. Christensen, D. Takemoto, P. Park and B. Scott, 2006. Reactive oxygen species play a role in regulating a fungus-perennial ryegrass mutualistic association. Plant Cell 18 1052–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, A., D. Takemoto, G.-S. Hyon, P. Park and B. Scott, 2008. NoxA activation by the small GTPase RacA is required to maintain a mutualistic symbiotic association between Epichloe festucae and perennial ryegrass. Mol. Microbiol. 68 1165–1178. [DOI] [PubMed] [Google Scholar]
- Trinci, A. P. J., M. G. Wiebe and G. D. Robson, 1994. The mycelium as an integrated entity, pp. 175–193 in The Mycota, Vol. I: Growth, Differentiation, and Sexuality, edited by J. G. H. Wessels and F. Meinhardt. Springer-Verlag, Berlin.
- Turian, G., 1976. Reducing power of hyphal tips and vegetative apical dominance in fungi. Experientia 32 989–991. [Google Scholar]
- Virag, A., and S. D. Harris, 2006. Functional characterization of Aspergillus nidulans homologues of Saccharomyces cerevisiae Spa2 and Bud6. Eukaryot. Cell 5 881–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virag, A., M. P. Lee, H. Si and S. D. Harris, 2007. Regulation of hyphal morphogenesis by cdc42 and rac1 homologues in Aspergillus nidulans. Mol. Microbiol. 66 1579–1596. [DOI] [PubMed] [Google Scholar]
- Virag, L., and C. Szabo, 2002. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol. Rev. 54 375–429. [DOI] [PubMed] [Google Scholar]
- Yang, L., L. Ukil, A. Osmani, F. Nahm, J. Davies et al., 2004. Rapid production of gene replacement constructs and generation of a green fluorescent protein-tagged centromeric marker in Aspergillus nidulans. Eukaryot. Cell 3 1359–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]