Abstract
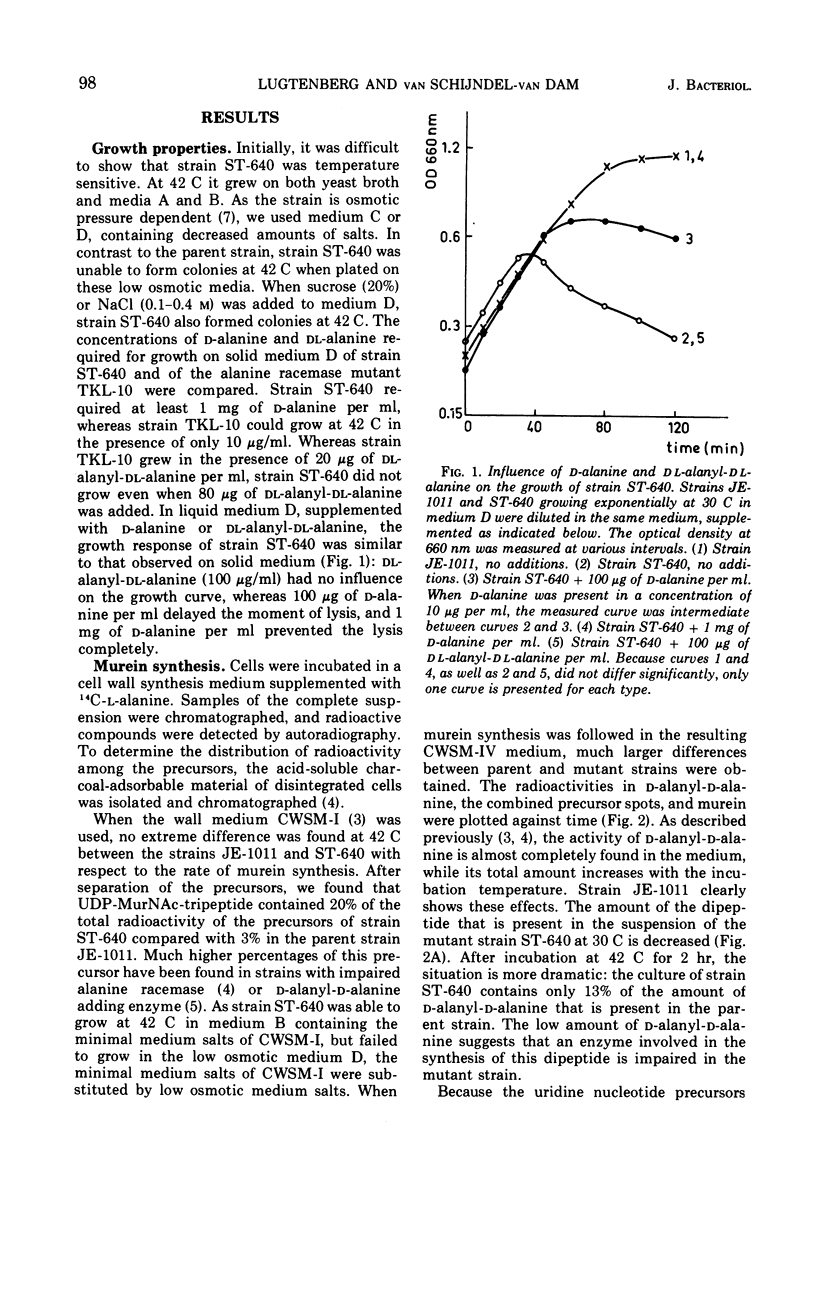
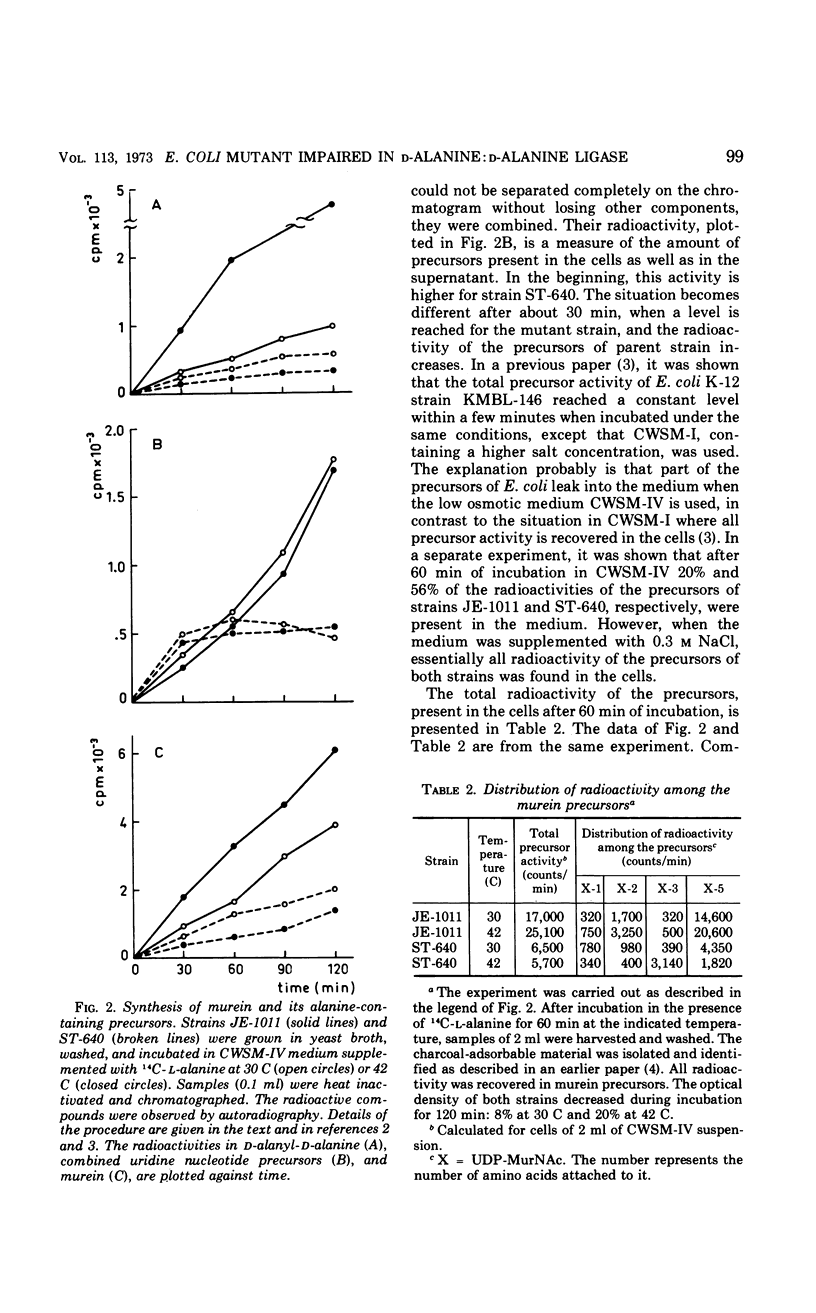
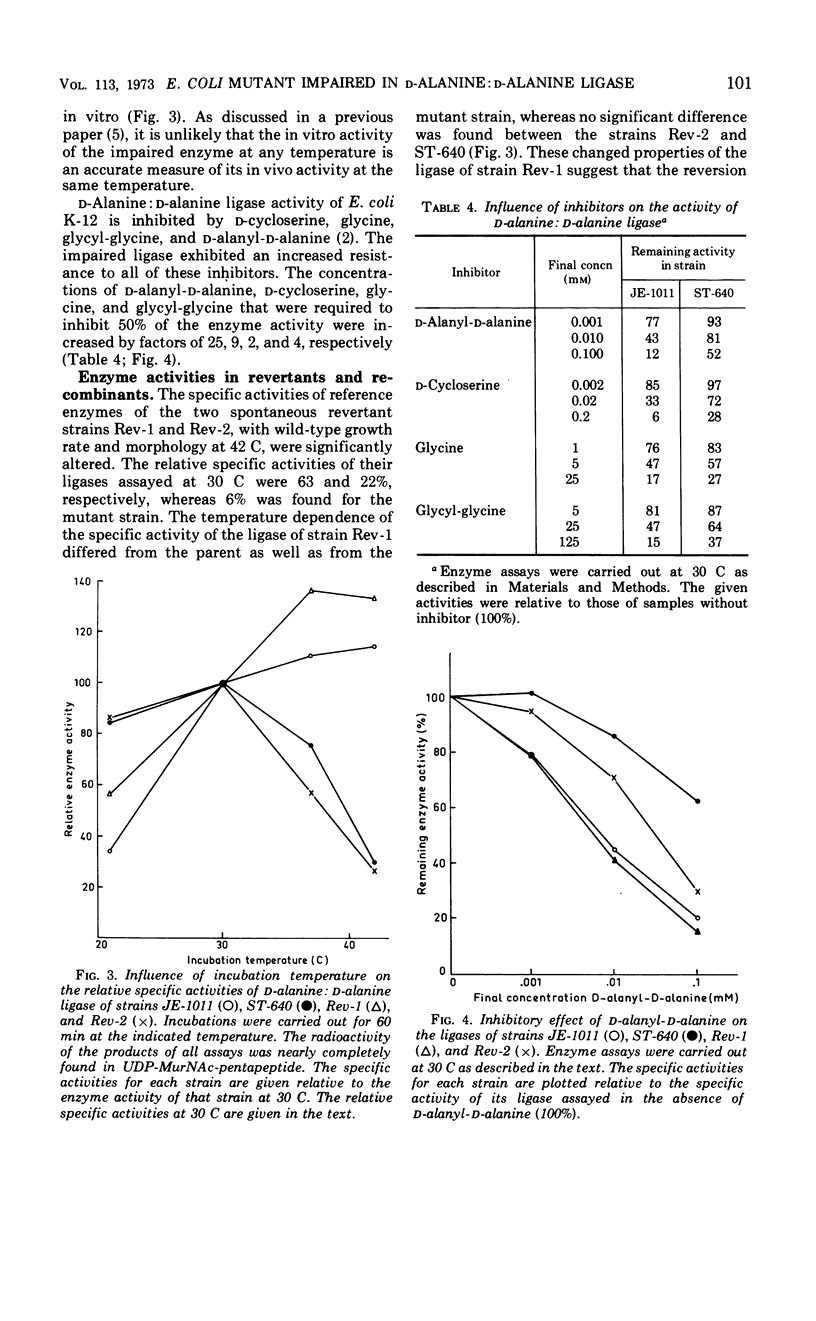
The temperature-sensitive Escherichia coli mutant strain ST-640 lyses at the restrictive temperature except when an osmotic stabilizer or a high concentration of d-alanine is present. The presence of dl-alanyl-dl-alanine does not prevent lysis. The rate of murein synthesis, followed in a wall medium, is decreased at both 30 and 42 C. d-Alanyl-d-alanine and uridine diphosphate-N-acetyl-muramyl (UDP-MurNAc)-pentapeptide are synthesized in decreased amounts, accompanied by accumulation of UDP-MurNAc-tripeptide at 42 C but not at 30 C. Uridine nucleotide precursors leak into the medium, especially out of the mutant cells. This leakage is prevented when NaCl is present. The d-alanine: d-alanine ligase (ADP) (EC 6.3.2.4) of the mutant strain, assayed in crude extracts, is temperature sensitive. The impaired ligase is relatively resistant to d-cycloserine and other inhibitors of the enzyme. Combined genetic and enzymatic results show that the low ligase activity is due to a mutation in the ddl gene, the structural gene for d-alanine: d-alanine ligase.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hendlin D., Stapley E. O., Jackson M., Wallick H., Miller A. K., Wolf F. J., Miller T. W., Chaiet L., Kahan F. M., Foltz E. L. Phosphonomycin, a new antibiotic produced by strains of streptomyces. Science. 1969 Oct 3;166(3901):122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- Lugtenberg E. J., De Haas-Menger L., Ruyters W. H. Murein synthesis and identification of cell wall precursors of temperature-sensitive lysis mutants of Escherichia coli. J Bacteriol. 1972 Jan;109(1):326–335. doi: 10.1128/jb.109.1.326-335.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J. Studies on Escherichia coli enzymes involved in the synthesis of uridine diphosphate-N-acetyl-muramyl-pentapeptide. J Bacteriol. 1972 Apr;110(1):26–34. doi: 10.1128/jb.110.1.26-34.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., de Haan P. G. A simple method for following the fate of alanine-containing components in murein synthesis in Escherichia coli. Antonie Van Leeuwenhoek. 1971;37(4):537–552. doi: 10.1007/BF02218524. [DOI] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A. Temperature-sensitive mutants of Escherichia coli K-12 with low activities of the L-alanine adding enzyme and the D-alanyl-D-alanine adding enzyme. J Bacteriol. 1972 Apr;110(1):35–40. doi: 10.1128/jb.110.1.35-40.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A. Temperature-sensitive mutants of Escherichia coli K-12 with low activity of the diaminopimelic acid adding enzyme. J Bacteriol. 1972 Apr;110(1):41–46. doi: 10.1128/jb.110.1.41-46.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa H., Matsuhashi M., Oka A., Sugino Y. Genetic and biochemical studies on cell wall peptidoglycan synthesis in Escherichia coli K-12. Biochem Biophys Res Commun. 1969 Aug 15;36(4):682–689. doi: 10.1016/0006-291x(69)90360-x. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Izaki K., Matsuhashi M., Tipper D. J. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Fed Proc. 1967 Jan-Feb;26(1):9–22. [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateswaran P. S., Wu H. C. Isolation and characterization of a phosphonomycin-resistant mutant of Escherichia coli K-12. J Bacteriol. 1972 Jun;110(3):935–944. doi: 10.1128/jb.110.3.935-944.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


