Abstract
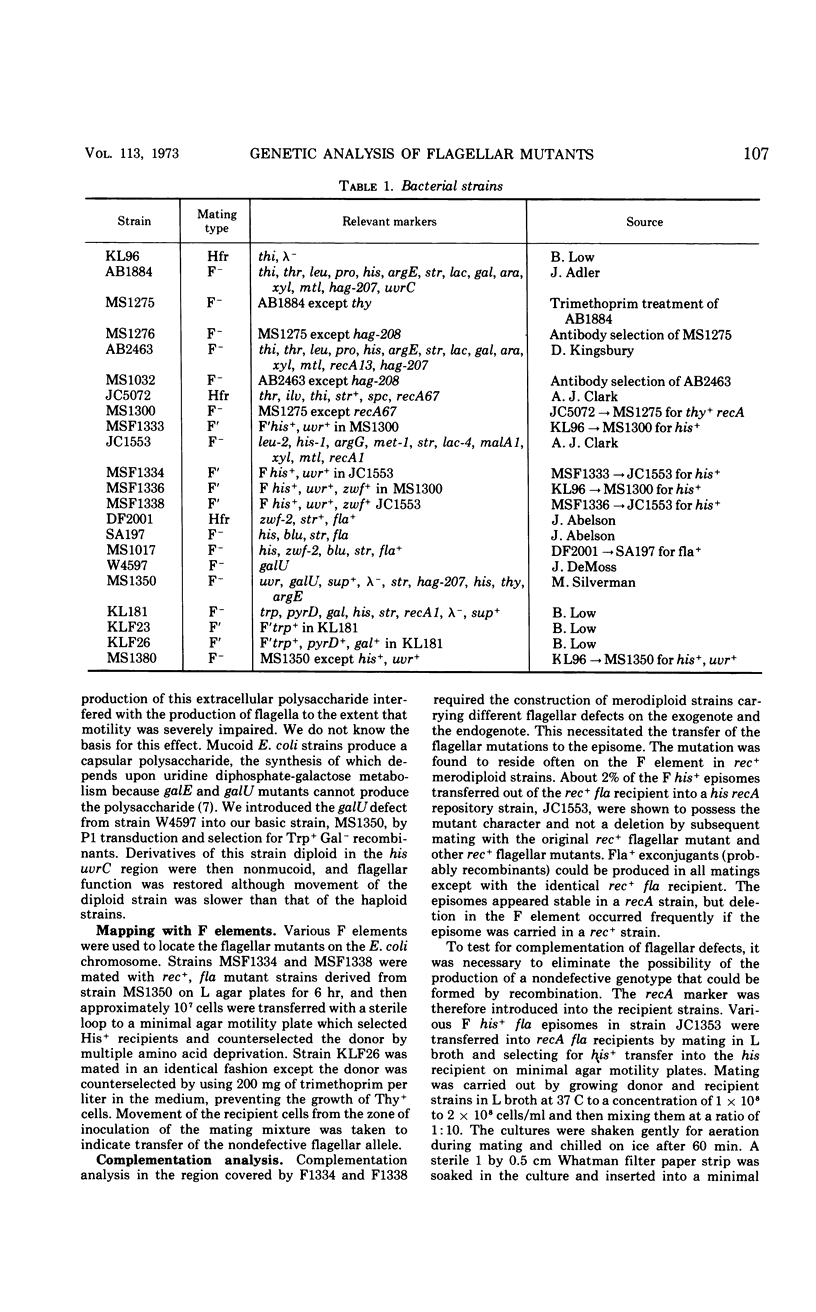
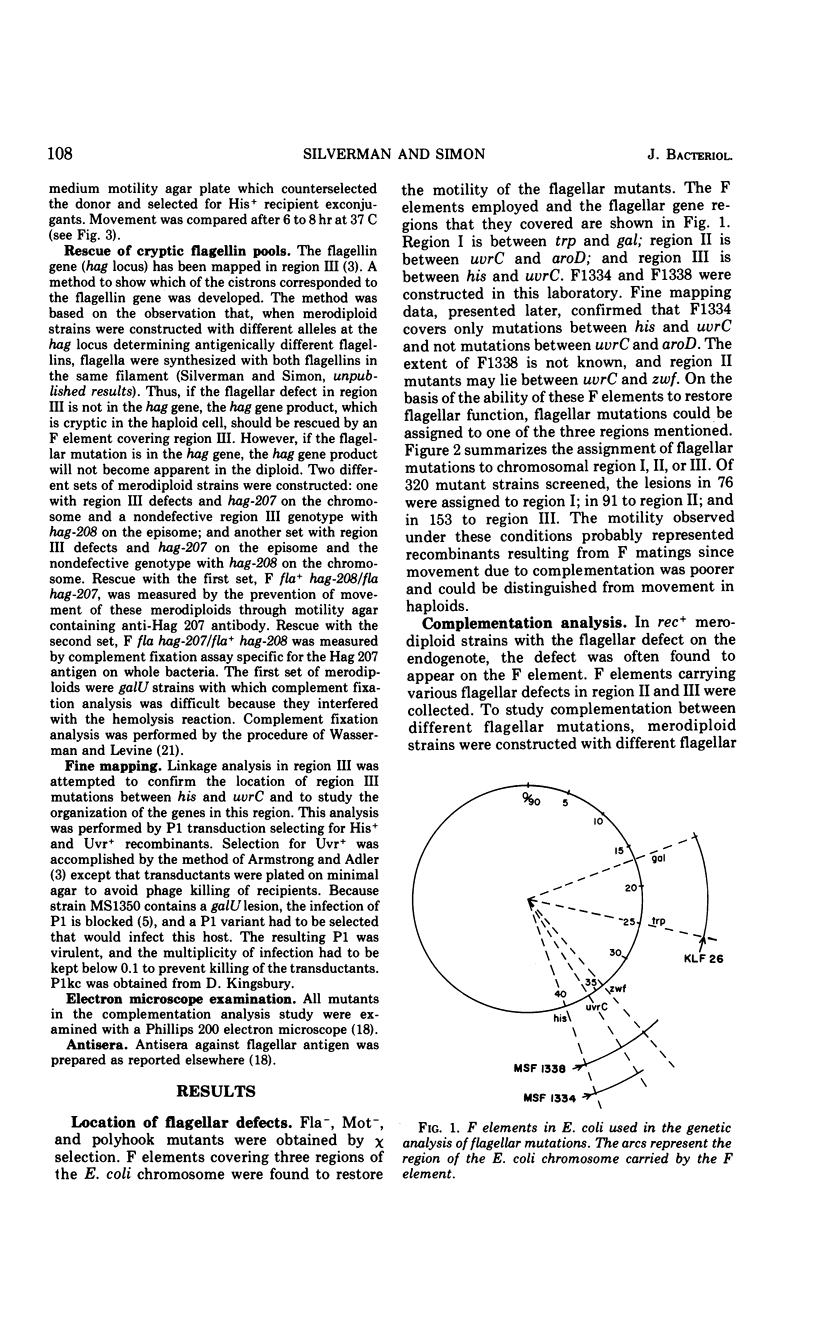
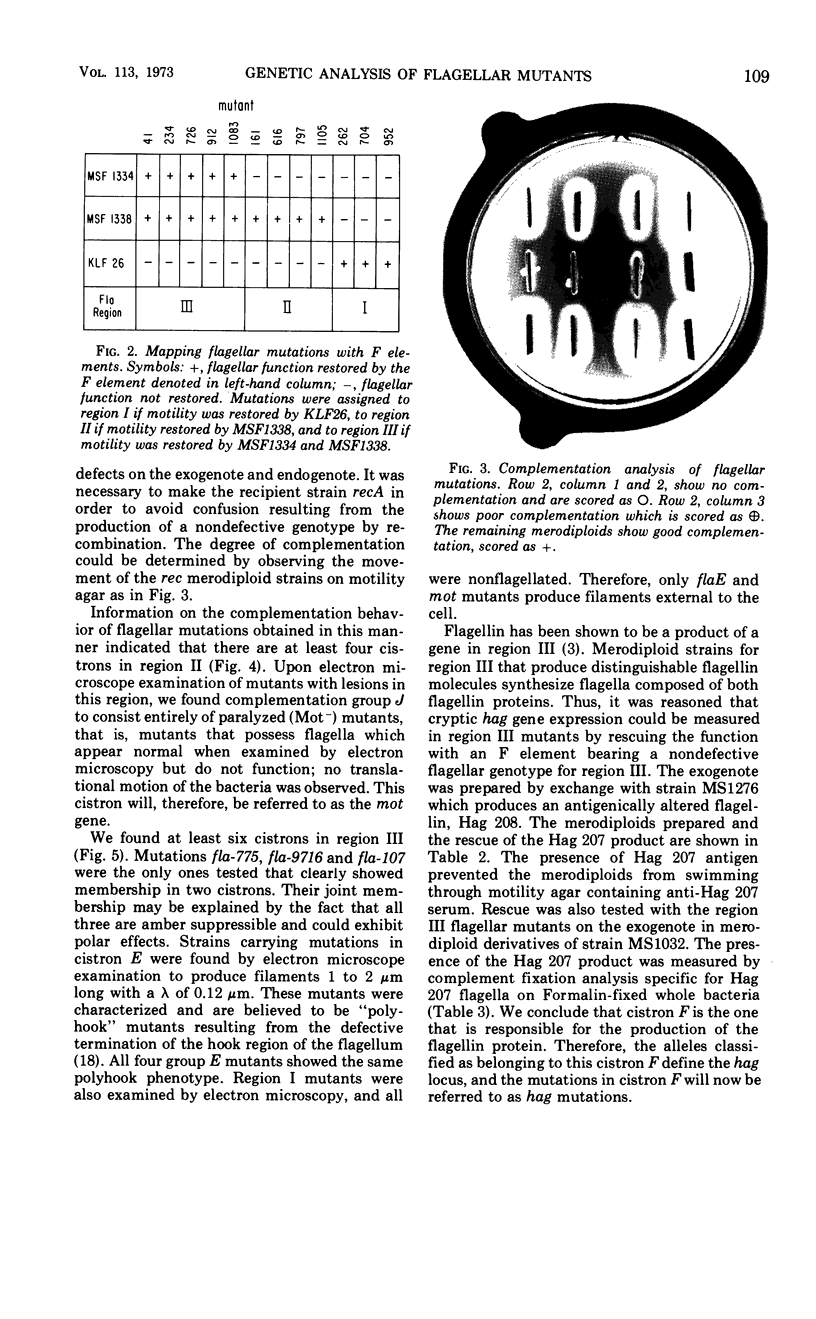
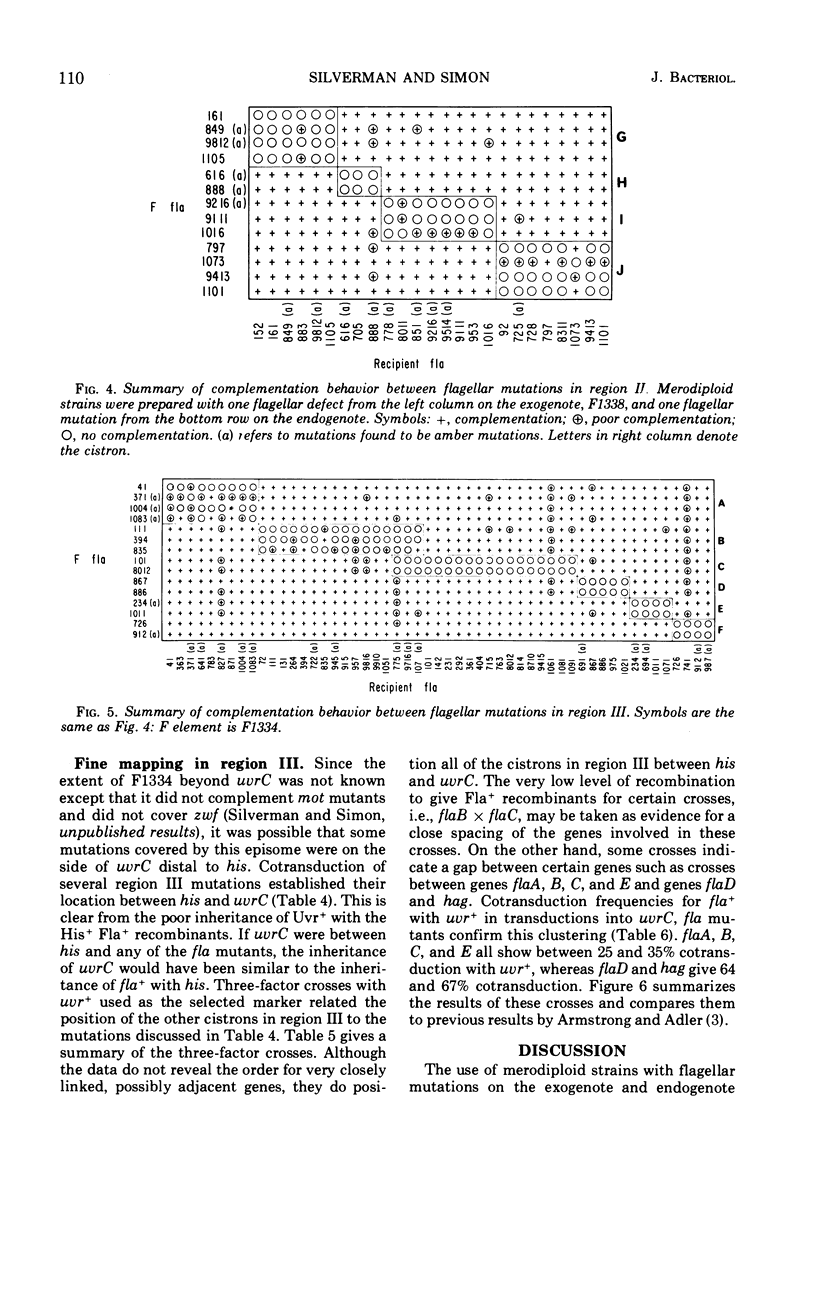
Flagellar mutants in Escherichia coli were obtained by selection for resistance to the flagellotropic phage χ. F elements covering various regions of the E. coli genome were then constructed, and, on the basis of the ability of these elements to restore flagellar function, the mutations were assigned to three regions of the E. coli chromosome. Region I is between trp and gal; region II is between uvrC and aroD; and region III is between his and uvrC. F elements carrying flagellar mutations were constructed. Stable merodiploid strains with a flagellar defect on the exogenote and another on the endogenote were then prepared. These merodiploids yielded information on the complementation behavior of mutations in a given region. Region III was shown to include at least six cistrons, A, B, C, D, E, and F. Region II was shown to include at least four cistrons, G, H, I, and J. Examination of the phenotypes of the mutants revealed that those with lesions in cistron E of region III produce “polyhooks” and lesions in cistron F of region III result in loss of ability to produce flagellin. Mutants with lesions in cistron J of region II were entirely paralyzed (mot) mutants. Genetic analysis of flagellar mutations in region III suggested that the mutations located in cistrons A, B, C, and E are closely linked and mutations in cistrons D and F are closely linked.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
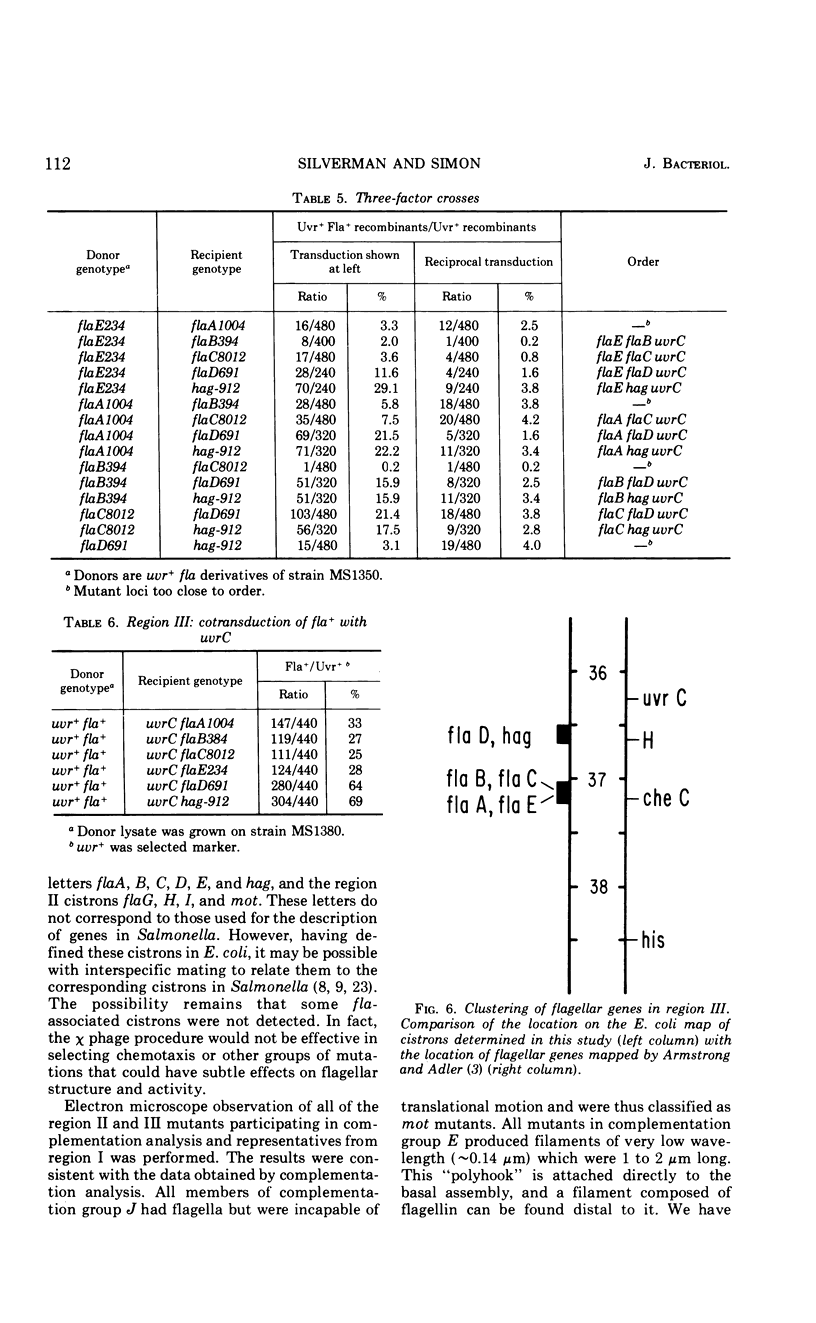
- Armstrong J. B., Adler J. Complementation of nonchemotactic mutants of Escherichia coli. Genetics. 1969 Jan;61(1):61–66. doi: 10.1093/genetics/61.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J. Genetics of motility in Escherichia coli: complementation of paralysed mutants. Genetics. 1967 Jul;56(3):363–373. doi: 10.1093/genetics/56.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J. Location of genes for motility and chemotaxis on the Escherichia coli genetic map. J Bacteriol. 1969 Jan;97(1):156–161. doi: 10.1128/jb.97.1.156-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONHOEFFER F., SCHALLER H. A METHOD FOR SELECTIVE ENRICHMENT OF MUTANTS BASED ON THE HIGH UV SENSITIVITY OF DNA CONTAINING 5-BROMOURACIL. Biochem Biophys Res Commun. 1965 Jun 18;20:93–97. [PubMed] [Google Scholar]
- Franklin N. C. Mutation in gal U gene of E. coli blocks phage P1 infection. Virology. 1969 May;38(1):189–191. doi: 10.1016/0042-6822(69)90144-5. [DOI] [PubMed] [Google Scholar]
- GAREN A., GAREN S. Complementation in vivo between structural mutants of alkaline phosphatase from E. coli. J Mol Biol. 1963 Jul;7:13–22. doi: 10.1016/s0022-2836(63)80015-7. [DOI] [PubMed] [Google Scholar]
- Grant W. D., Sutherland I. W., Wilkinson J. F. Exopolysaccharide colanic acid and its occurrence in the Enterobacteriaceae. J Bacteriol. 1969 Dec;100(3):1187–1193. doi: 10.1128/jb.100.3.1187-1193.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino T., Enomoto M. Genetical studies of non-flagellate mutants of Salmonella. J Gen Microbiol. 1966 Jun;43(3):315–327. doi: 10.1099/00221287-43-3-315. [DOI] [PubMed] [Google Scholar]
- Joys T. M., Stocker B. A. Complementation of non-flagellate Salmonella mutants. J Gen Microbiol. 1965 Oct;41(1):47–55. doi: 10.1099/00221287-41-1-47. [DOI] [PubMed] [Google Scholar]
- Kupor S. R., Fraenkel D. G. 6-phosphogluconolactonase mutants of Escherichia coli and a maltose blue gene. J Bacteriol. 1969 Dec;100(3):1296–1301. doi: 10.1128/jb.100.3.1296-1301.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG J. A duplication of the Hl (flagellar antigen) locus in Salmonella. Genetics. 1961 Nov;46:1475–1481. doi: 10.1093/genetics/46.11.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYNELL E. W. A phage, phi chi, which attacks motile bacteria. J Gen Microbiol. 1961 Jun;25:253–290. doi: 10.1099/00221287-25-2-253. [DOI] [PubMed] [Google Scholar]
- Pearce U. B., Stocker B. A. Phase variation of flagellar antigens in Salmonella: abortive transduction studies. J Gen Microbiol. 1967 Nov;49(2):335–349. doi: 10.1099/00221287-49-2-335. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER M. J., LEVINTHAL C. Hybrid protein formation of E. coli alkaline phosphatase leading to in vitro complementation. J Mol Biol. 1963 Jul;7:1–12. doi: 10.1016/s0022-2836(63)80014-5. [DOI] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade S. Z., Adler J., Ris H. How bacteriophage chi attacks motile bacteria. J Virol. 1967 Jun;1(3):599–609. doi: 10.1128/jvi.1.3.599-609.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D. E., Englesberg E. Further evidence for positive control of the L-arabinose system by gene araC. J Mol Biol. 1967 May 14;25(3):443–454. doi: 10.1016/0022-2836(67)90197-0. [DOI] [PubMed] [Google Scholar]
- Silverman M. R., Simon M. I. Flagellar assembly mutants in Escherichia coli. J Bacteriol. 1972 Nov;112(2):986–993. doi: 10.1128/jb.112.2.986-993.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- Wright M. Mutants of Escherichia coli lacking endonuclease I, ribonuclease I, or ribonuclease II. J Bacteriol. 1971 Jul;107(1):87–94. doi: 10.1128/jb.107.1.87-94.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Iino T., Horiguchi T., Ota K. Genetic analysis of fla and mot cistrons closely linked to H1 in Salmonella abortusequi and its derivatives. J Gen Microbiol. 1972 Apr;70(1):59–75. doi: 10.1099/00221287-70-1-59. [DOI] [PubMed] [Google Scholar]
- Yokota T., Gots J. S. Requirement of adenosine 3', 5'-cyclic phosphate for flagella formation in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1970 Aug;103(2):513–516. doi: 10.1128/jb.103.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



