Abstract
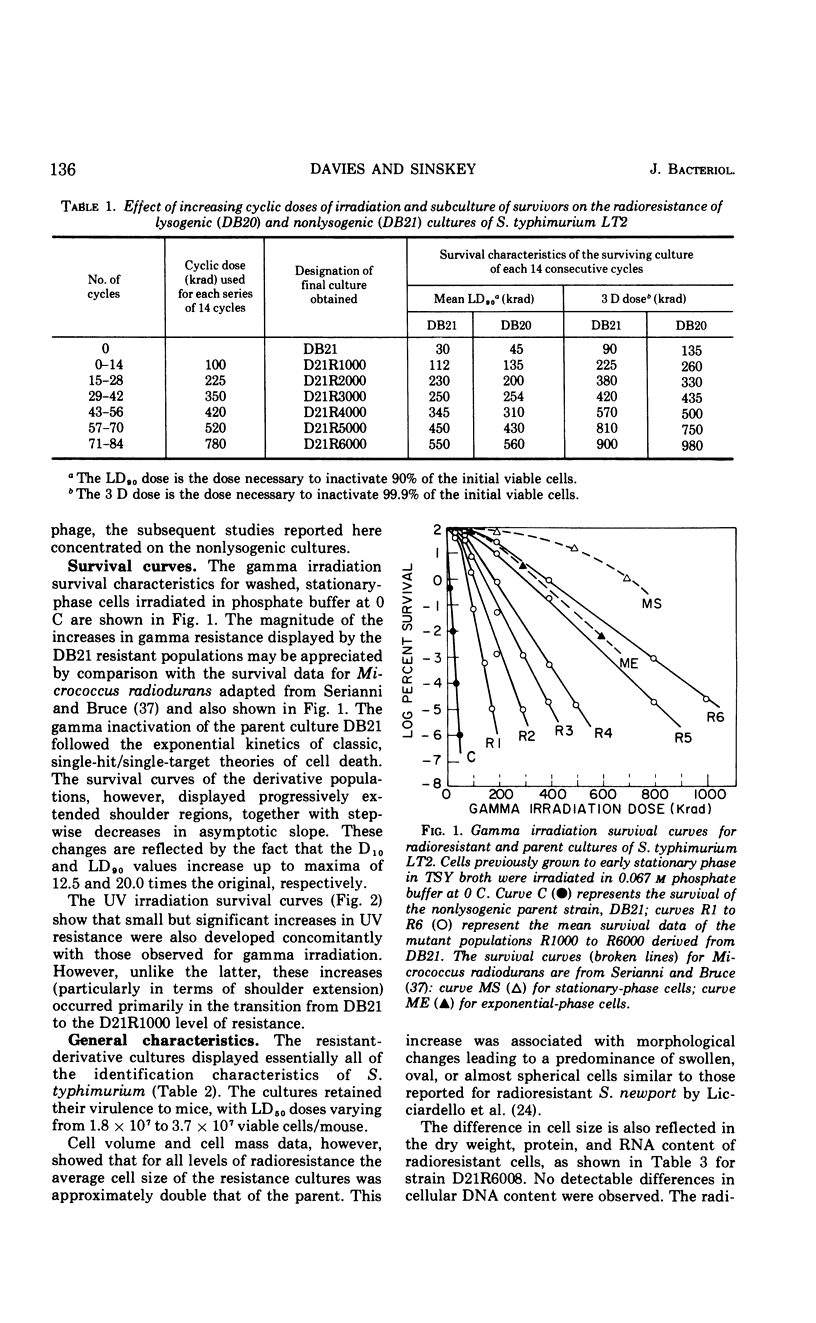
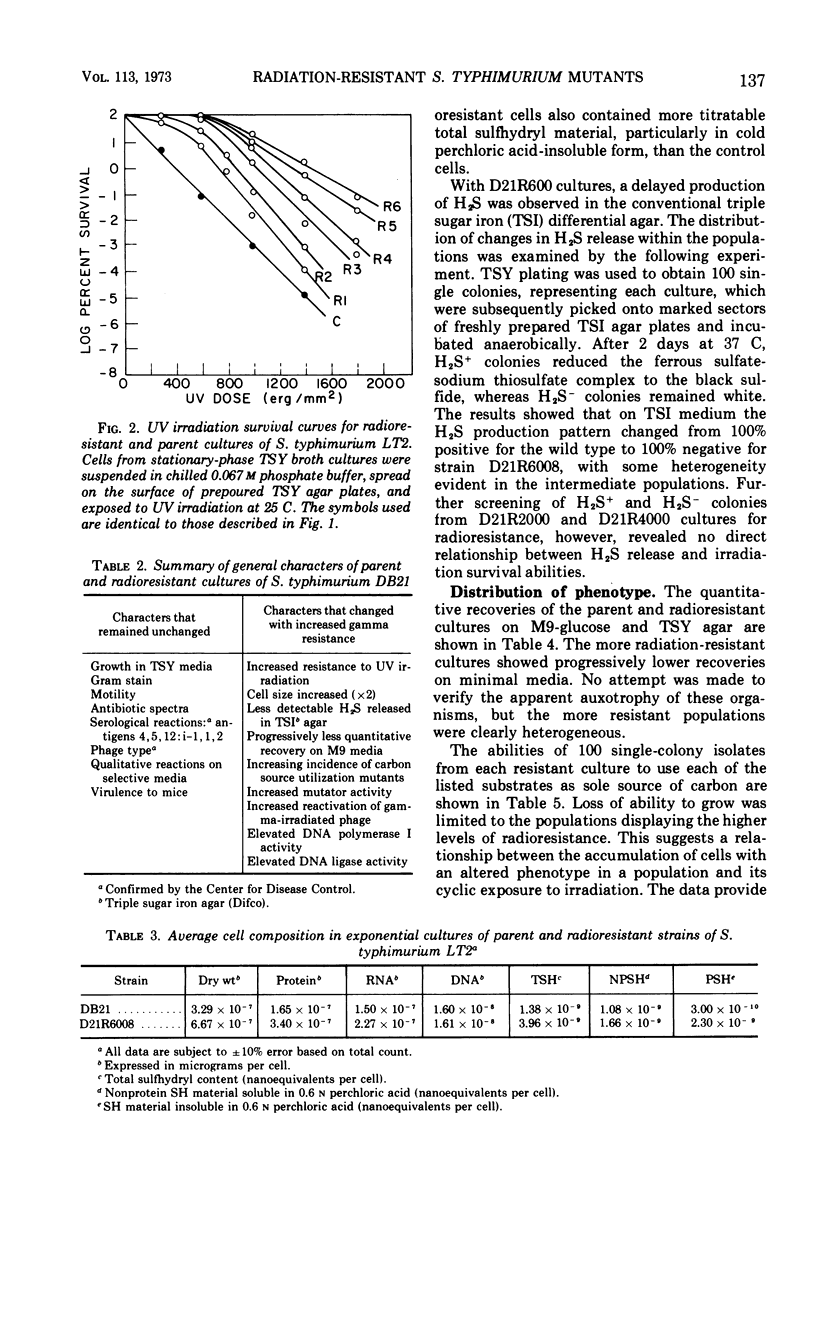
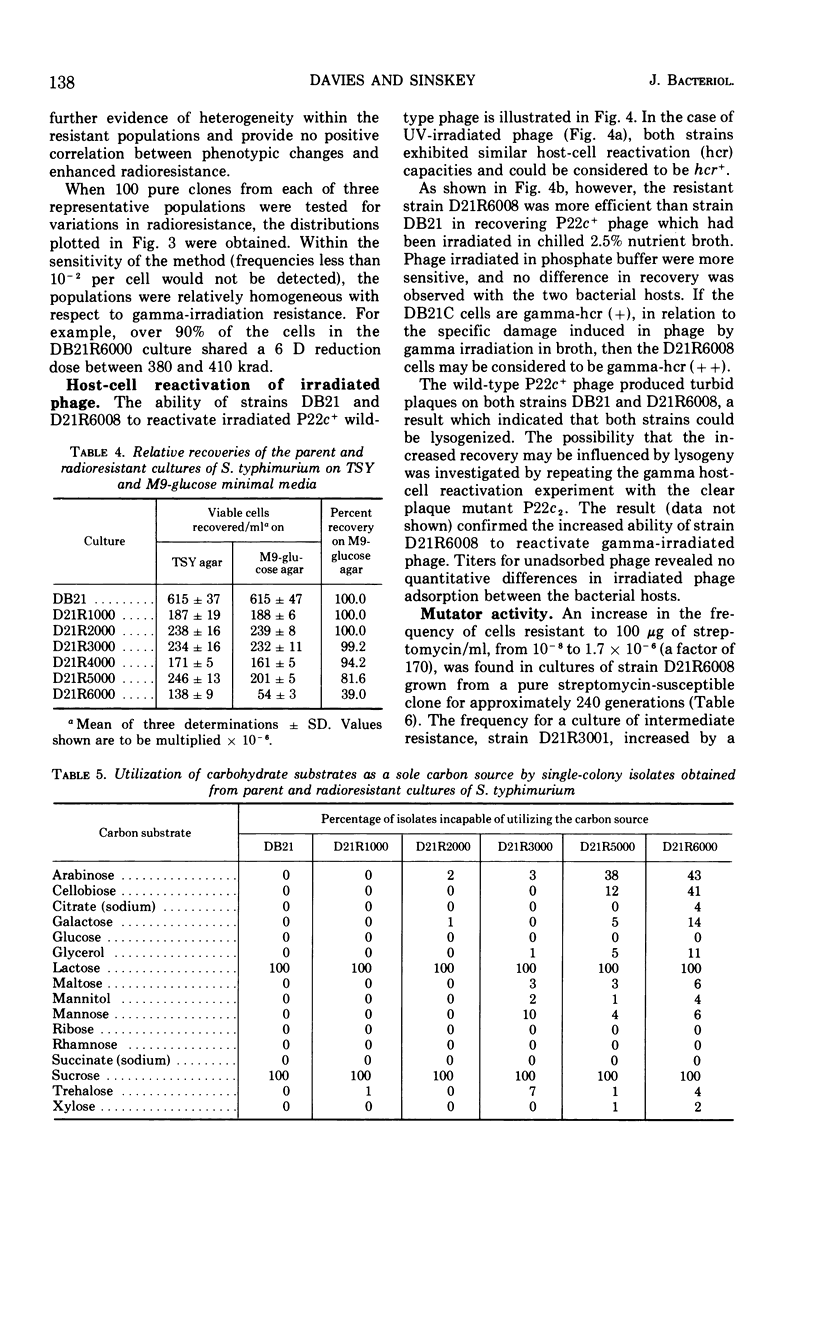
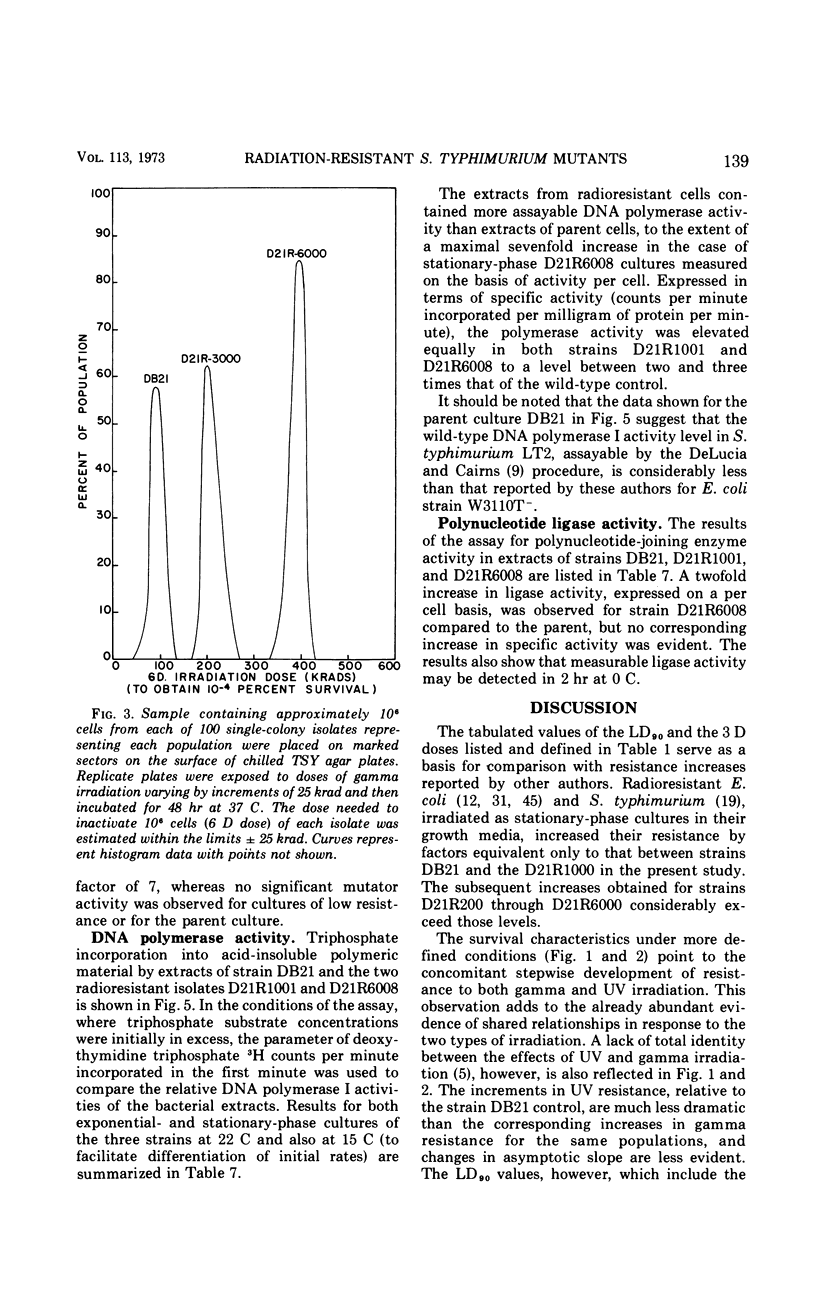
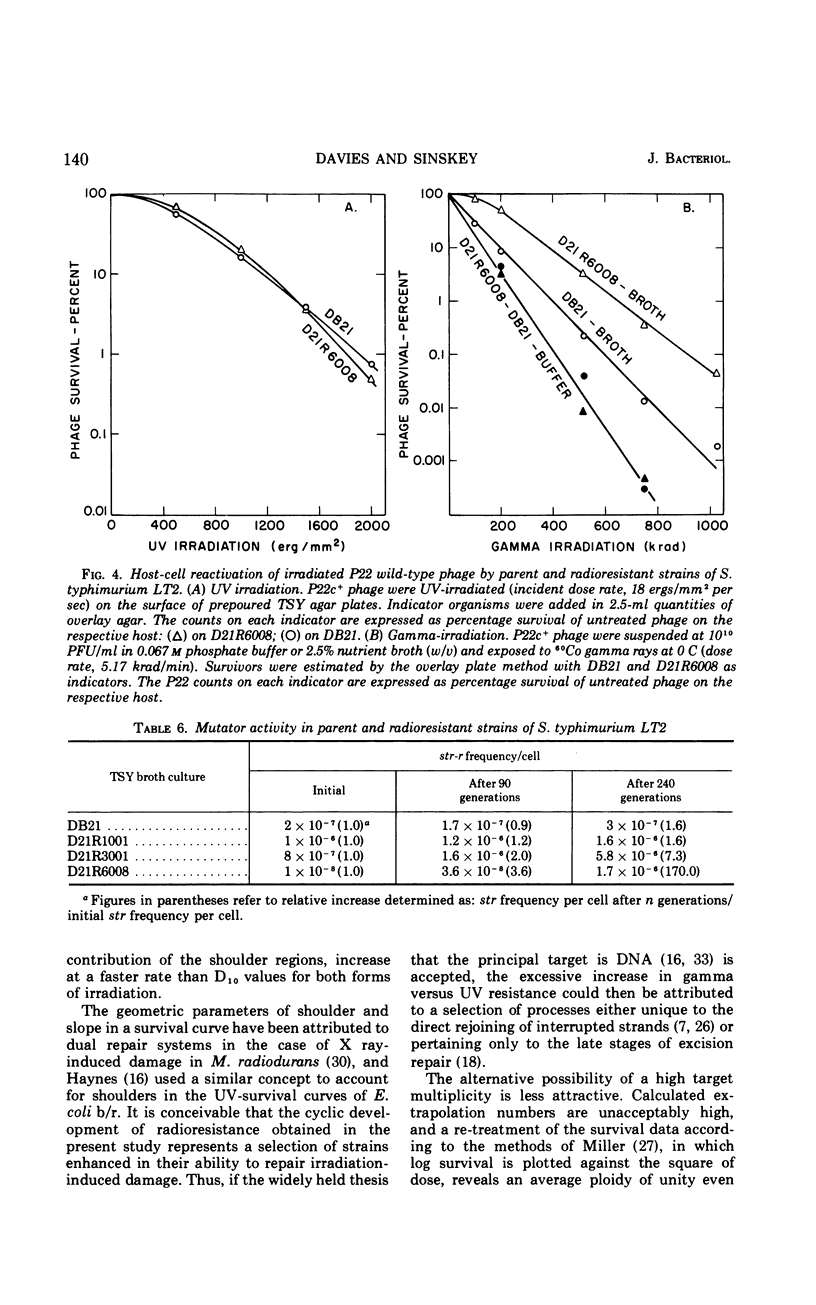
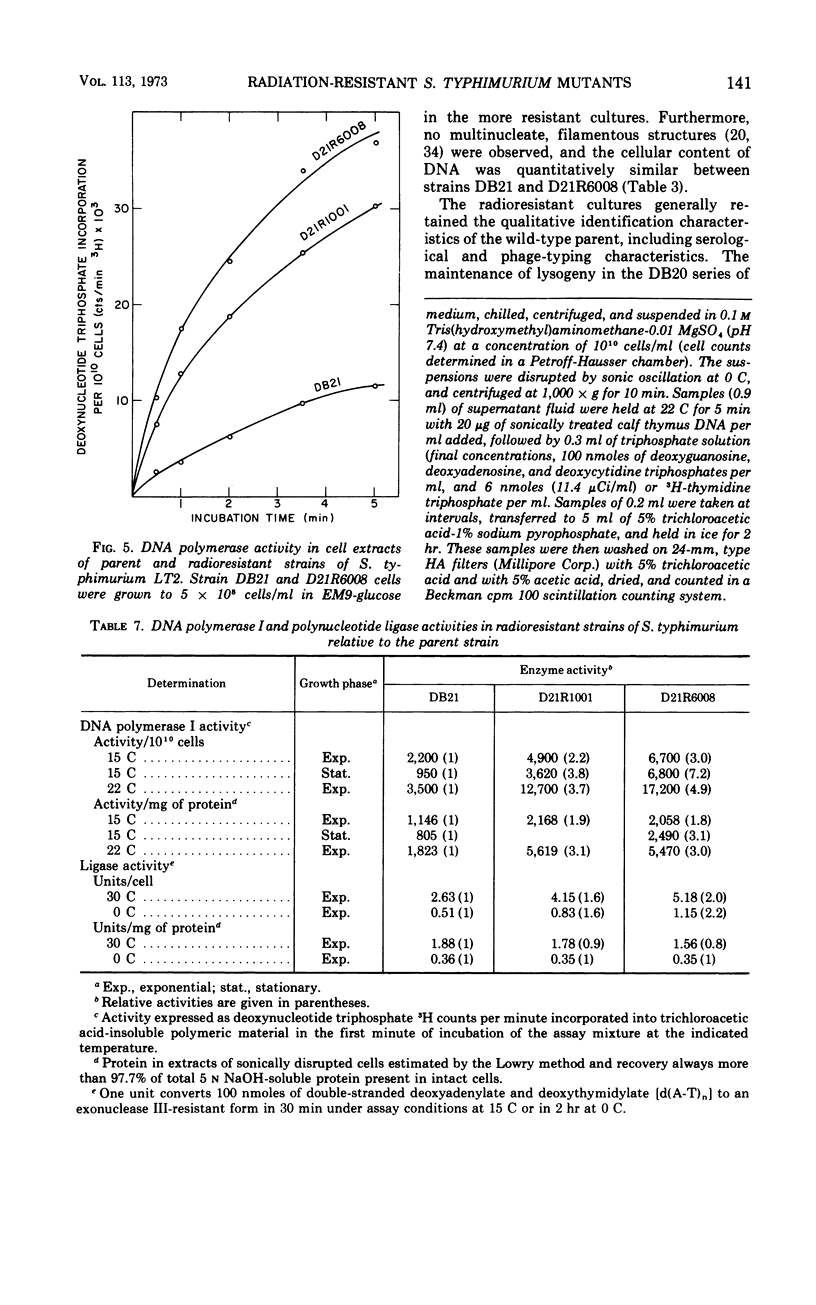
A series of repeated exposures to gamma irradiation with intervening outgrowth of survivors was used to develop radioresistant cultures of Salmonella typhimuium LT2. Stepwise increases in resistance to both ionizing and ultraviolet irradiation were obtained independently of the presence or absence of integrated P22 prophage. Single clonal isolates, representing parent and radioresistant populations, retained the general characteristics of the LT2 parent, including serological properties, phage typing, antibiotic sensitivities, mouse virulence, and most biochemical test reactions. Resistant cells were generally larger and contained 1.8 to 2.1 times more ribonucleic acid and protein than parent cells, but deoxyribonucleic acid (DNA) contents were similar. Heterogeneity in the populations with respect to release of H2S, utilization of carbon sources, and growth on minimal medium is considered to be ancillary, rather than causally related, to increased radioresistance. The resistant isolates displayed an increased ability to reactivate gamma-irradiated P22 phage. DNA polymerase I and polynucleotide-joining enzyme activities were elevated in extracts of radioresistant cells relative to parent cells. It is suggested that the observed increases in radioresistance result from a selection of mutations leading to an increased capacity to repair DNA.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohne L., Coquerelle T., Hagen U. Radiation sensitivity of bacteriophage DNA. II. Breaks and cross-links after irradiation in vivo. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(3):205–215. doi: 10.1080/09553007014550241. [DOI] [PubMed] [Google Scholar]
- Botstein D. Synthesis and maturation of phage P22 DNA. I. Identification of intermediates. J Mol Biol. 1968 Jun 28;34(3):621–641. doi: 10.1016/0022-2836(68)90185-x. [DOI] [PubMed] [Google Scholar]
- Boyle J. M., Paterson M. C., Setlow R. B. Excision-repair properties of an Escherichia coli mutant deficient in DNA polymerase. Nature. 1970 May 23;226(5247):708–710. doi: 10.1038/226708a0. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Munson R. J. Excision-repair of DNA damage in an auxotrophic strain of Escherichia coli. Biochem Biophys Res Commun. 1966 Feb 3;22(3):268–273. doi: 10.1016/0006-291x(66)90476-1. [DOI] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Dean C. J., Ormerod M. G., Serianni R. W., Alexander P. DNA strand breakage in cells irradiated with x-rays. Nature. 1969 Jun 14;222(5198):1042–1044. doi: 10.1038/2221042a0. [DOI] [PubMed] [Google Scholar]
- Dean C., Pauling C. Properties of a deoxyribonucleic acid ligase mutant of Escherichia coli: x-ray sensitivity. J Bacteriol. 1970 May;102(2):588–589. doi: 10.1128/jb.102.2.588-589.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. A colorimetric method for determining low concentrations of mercaptans. Arch Biochem Biophys. 1958 Apr;74(2):443–450. doi: 10.1016/0003-9861(58)90014-6. [DOI] [PubMed] [Google Scholar]
- ERDMAN I. E., THATCHER F. S., MACQUEEN K. F. Studies on the irradiation of microorganisms in relation to food preservation. II. Irradiation resistant mutants. Can J Microbiol. 1961 Apr;7:207–215. doi: 10.1139/m61-027. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Mechanism of inactivation of coliphage T7 by x-rays. Proc Natl Acad Sci U S A. 1965 Jul;54(1):128–134. doi: 10.1073/pnas.54.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J., Gross M. Genetic analysis of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1166–1168. doi: 10.1038/2241166a0. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- IDZIAK E. S., THATCHER F. S. SOME PHYSIOLOGICAL ASPECTS OF MUTANTS OF ESCHERICHIA COLI RESISTANT TO GAMMA IRRADIATION. Can J Microbiol. 1964 Oct;10:683–697. doi: 10.1139/m64-088. [DOI] [PubMed] [Google Scholar]
- Idziak E. S., Incze K. Radiation treatment of foods. I. Radurization of fresh eviscerated poultry. Appl Microbiol. 1968 Jul;16(7):1061–1066. doi: 10.1128/am.16.7.1061-1066.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L., Hanawalt P. Repair deficiency in a bacterial mutant defective in DNA polymerase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):149–155. doi: 10.1016/0006-291x(70)90770-9. [DOI] [PubMed] [Google Scholar]
- Kapp D. S., Smith K. C. Lack of in vitro repair of x-ray-induced chain breaks in DNA by the polynucleotide-joining enzyme. Int J Radiat Biol Relat Stud Phys Chem Med. 1969 Jan 17;14(6):567–571. doi: 10.1080/09553006914551741. [DOI] [PubMed] [Google Scholar]
- Kato T., Kondo S. Genetic and molecular characteristics of X-ray-sensitive mutants of Escherichia coli defective in repair synthesis. J Bacteriol. 1970 Nov;104(2):871–881. doi: 10.1128/jb.104.2.871-881.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Licciardello J. J., Nickerson J. T., Goldblith S. A., Shannon C. A., Bishop W. W. Development of radiation resistance in Salmonella cultures. Appl Microbiol. 1969 Jul;18(1):24–30. doi: 10.1128/am.18.1.24-30.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- MOSELEY B. E., LASER H. REPAIR OF X-RAY IN MICROCOCCUS RADIODURANS. Proc R Soc Lond B Biol Sci. 1965 Apr 13;162:210–222. doi: 10.1098/rspb.1965.0035. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Miller D. R. Theoretical survival curves for radiation damage in bacteria. J Theor Biol. 1970 Mar;26(3):383–398. doi: 10.1016/0022-5193(70)90091-3. [DOI] [PubMed] [Google Scholar]
- Miyake T. Mutator Factor in Salmonella Typhimurium. Genetics. 1960 Jan;45(1):11–14. doi: 10.1093/genetics/45.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P., Lehman I. R. Enzymatic joining of polynucleotides. IX. A simple and rapid assay of polynucleotide joining (ligase) activity by measurement of circle formation from linear deoxyadenylate-deoxythymidylate copolymer. J Biol Chem. 1970 Jul 25;245(14):3626–3631. [PubMed] [Google Scholar]
- Mouton R. F., Trémeau O., Dupuy P. Evolution microbiologique sous rayonnement: phénotype d'un mutant radiorésistant d'Escherichia coli K12 induit et sélectionné par expositions successives au rayonnement gamma du 60 C0. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(3):237–248. doi: 10.1080/09553007014550271. [DOI] [PubMed] [Google Scholar]
- Munro H. N., Fleck A. Recent developments in the measurement of nucleic acids in biological materials. A supplementary review. Analyst. 1966 Feb;91(79):78–88. doi: 10.1039/an9669100078. [DOI] [PubMed] [Google Scholar]
- PONTEFRACT R. D., THATCHER F. S. A CYTOLOGICAL STUDY OF NORMAL AND RADIATION-RESISTANT ESCHERICHIA COLI. Can J Microbiol. 1965 Apr;11:271–278. doi: 10.1139/m65-033. [DOI] [PubMed] [Google Scholar]
- Robern H., Thatcher F. S. Nutritional requirements of mutants of Escherichia coli resistant to gamma irradiation. Can J Microbiol. 1968 Jun;14(6):711–715. doi: 10.1139/m68-118. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E. Current linkage map of Salmonella typhimurium. Bacteriol Rev. 1970 Jun;34(2):176–193. doi: 10.1128/br.34.2.176-193.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serianni R. W., Bruce A. K. Radioresistance of Micrococcus radiodurans during the growth cycle. Radiat Res. 1968 Nov;36(2):193–207. [PubMed] [Google Scholar]
- Skavronskaya A. G., Pokrovsky V. N., Zlatev V. I., Andreeva I. V. Isolation and some properties of the radiation-sensitive mutant of S. typhimurium LT 2. Mutat Res. 1969 Mar-Apr;7(2):248–251. doi: 10.1016/0027-5107(69)90038-4. [DOI] [PubMed] [Google Scholar]
- Stavrić S., Dickie N., Thatcher F. S. Effects of gamma-irradiation on Escherichia coli wild type and its radiation-resistant mutants. II. Post-irradiation degradation of DNA. Int J Radiat Biol Relat Stud Phys Chem Med. 1968;14(5):403–410. doi: 10.1080/09553006814551281. [DOI] [PubMed] [Google Scholar]
- TAKEBE H., HARTMAN P. E. Effects of x-rays on transduction by Salmonella phage P22. Virology. 1962 Jun;17:295–300. doi: 10.1016/0042-6822(62)90120-4. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town C. D., Smith K. C., Kaplan H. S. DNA polymerase required for rapid repair of x-ray--induced DNA strand breaks in vivo. Science. 1971 May 21;172(3985):851–854. doi: 10.1126/science.172.3985.851. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Mount D. W. Genetic analysis of recombination-deficient mutants of Escherichia coli K-12 carrying rec mutations cotransducible with thyA. J Bacteriol. 1969 Nov;100(2):923–934. doi: 10.1128/jb.100.2.923-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Genetics of Resistance to Radiation in ESCHERICHIA COLI. Genetics. 1947 May;32(3):221–248. doi: 10.1093/genetics/32.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. J., Hill E. C. The development of radiation-resistant cultures of Escherichia coli I by a process of 'growth-irradiation cycles'. J Gen Microbiol. 1968 Apr;51(1):97–106. doi: 10.1099/00221287-51-1-97. [DOI] [PubMed] [Google Scholar]
- Yamamoto N. Damage, repair, and recombination. I. Contribution of the host to recombination between the superinfecting phage and the prophage. Virology. 1969 Jul;38(3):447–456. doi: 10.1016/0042-6822(69)90157-3. [DOI] [PubMed] [Google Scholar]


