Abstract
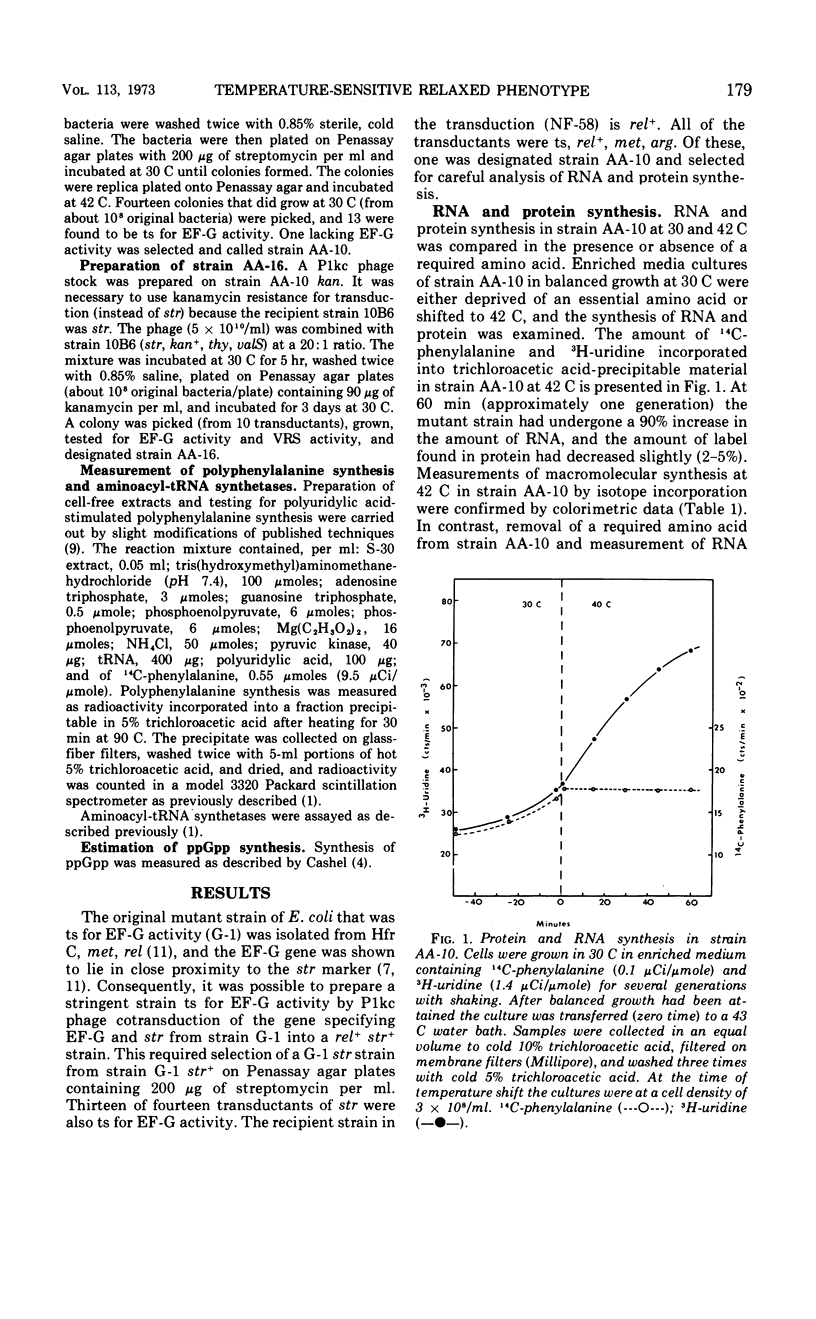
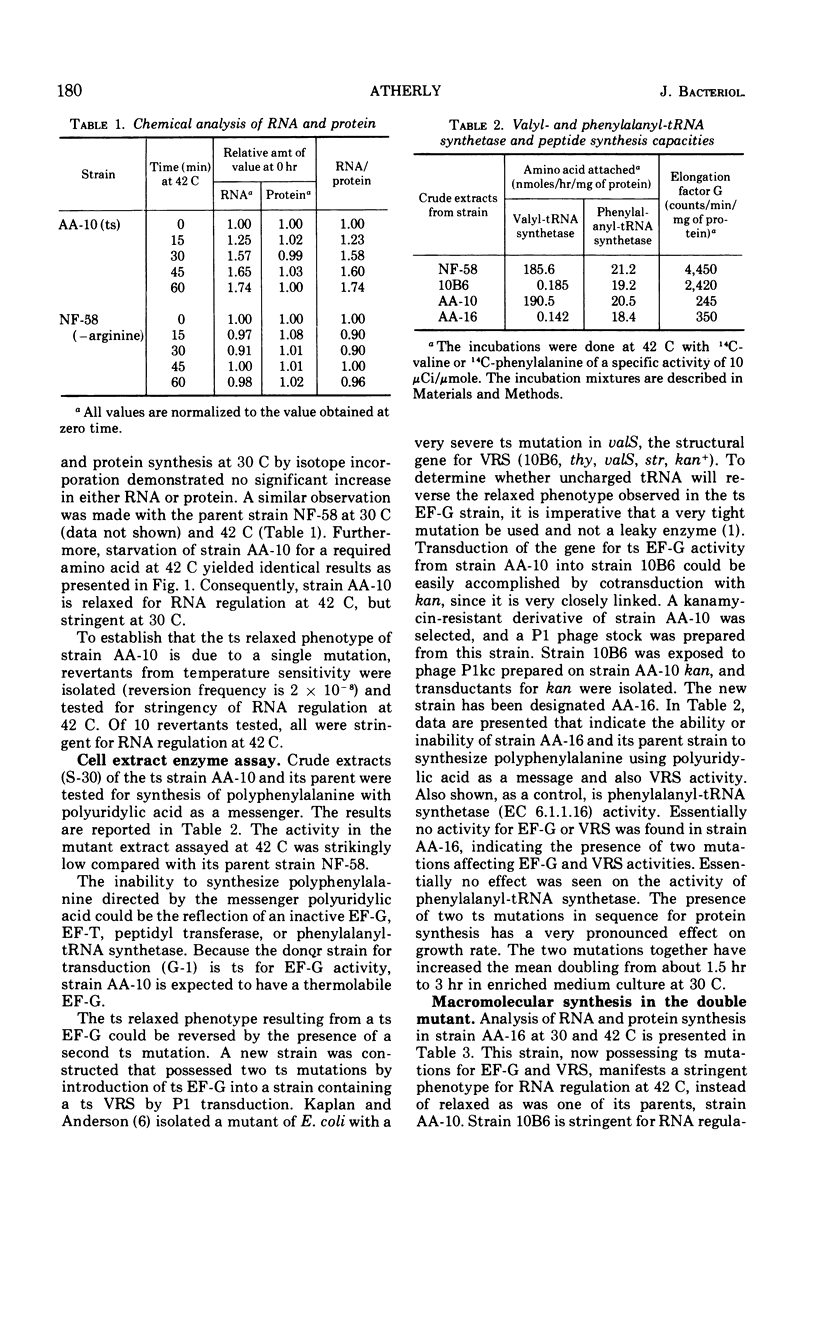
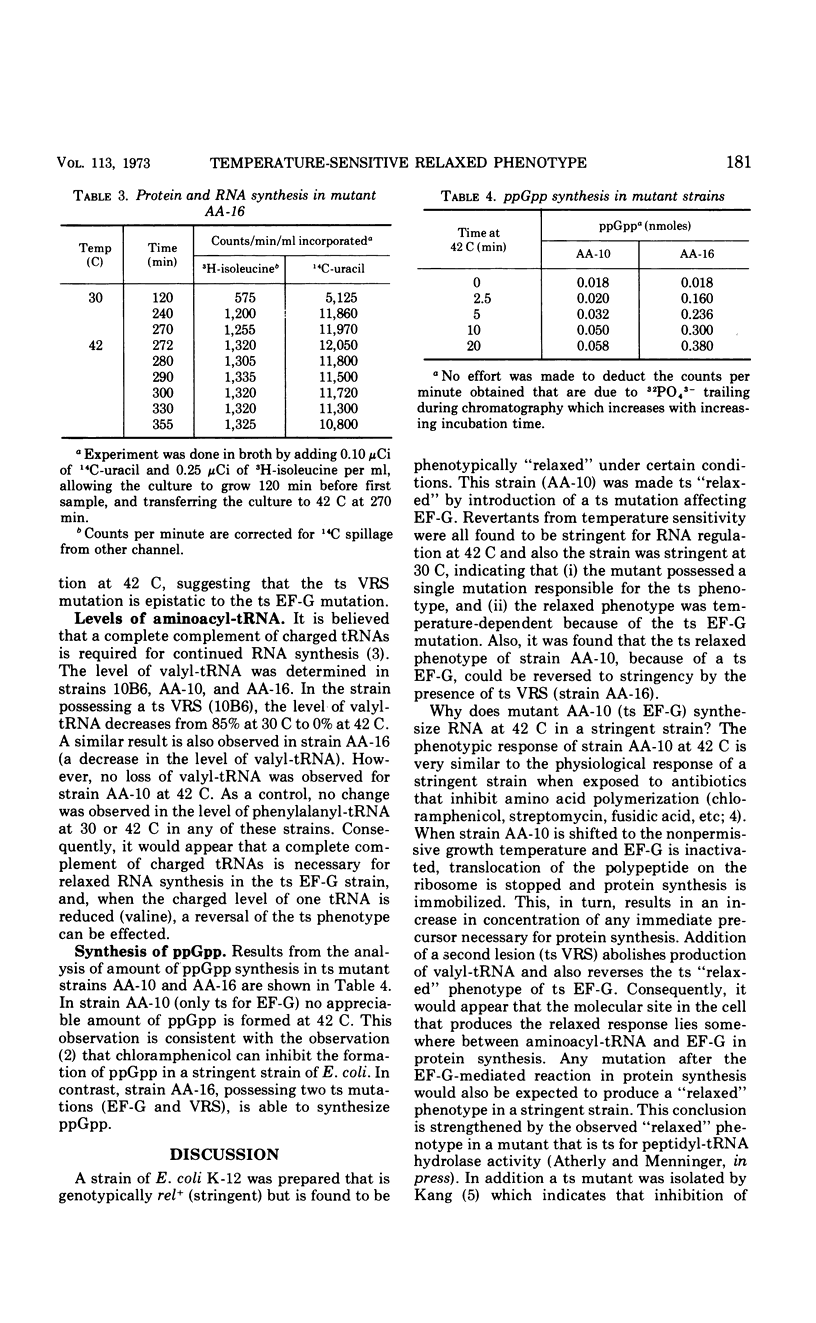
A temperature-sensitive elongation factor G (EF-G) mutation carried by a rel strain of Escherichia coli was transferred to a rel+ strain. The recombinant (AA-10) was found to be temperature-sensitive “relaxed” for ribonucleic acid (RNA) regulation. However, when a temperature-sensitive EF-G is present in a strain temperature-sensitive for valyl-transfer RNA (tRNA) synthetase, this new strain (AA-16) is no longer temperature-sensitive “relaxed” for RNA regulation. In strain AA-10, all tRNAs remain fully charged, and in AA-16 tRNAval becomes completely discharged at the nonpermissive growth temperature. Also, synthesis of ppGpp is not observed in strain AA-10 at 42 C but is observed at 42 C in strain AA-16.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherly A. G., Suchanek M. C. Characterization of mutants of Escherichia coli temperature-sensitive for ribonucleic acid regulation: an unusual phenotype associated with a phenylalanyl transfer ribonucleic acid synthetase mutant. J Bacteriol. 1971 Nov;108(2):627–638. doi: 10.1128/jb.108.2.627-638.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Edlin G., Broda P. Physiology and genetics of the "ribonucleic acid control" locus in escherichia coli. Bacteriol Rev. 1968 Sep;32(3):206–226. doi: 10.1128/br.32.3.206-226.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekiel D. H., Elkins B. N. The stimulation of ribonucleic acid synthesis by ribosome inhibitors in amino acid-starved Escherichia coli. Biochim Biophys Acta. 1968 Sep 24;166(2):466–474. doi: 10.1016/0005-2787(68)90234-7. [DOI] [PubMed] [Google Scholar]
- Kang S. S. A mutant of Escherichia coli with temperature-sensitive streptomycin protein. Proc Natl Acad Sci U S A. 1970 Mar;65(3):544–550. doi: 10.1073/pnas.65.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan S., Anderson D. Selection of temperature-sensitive activating enzyme mutants in Escherichia coli. J Bacteriol. 1968 Mar;95(3):991–997. doi: 10.1128/jb.95.3.991-997.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano M., Schlessinger D. G factor mutants of Escherichia coli: map location and properties. Biochem Biophys Res Commun. 1971 Feb 5;42(3):441–444. doi: 10.1016/0006-291x(71)90390-1. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- MATTHAEI J. H., NIRENBERG M. W. Characteristics and stabilization of DNAase-sensitive protein synthesis in E. coli extracts. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1580–1588. doi: 10.1073/pnas.47.10.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N., Kawano G., Kinoshita T. Chromosomal location of a fusidic acid resistant marker in Escherichia coli. Biochem Biophys Res Commun. 1971 Feb 5;42(3):564–567. doi: 10.1016/0006-291x(71)90408-6. [DOI] [PubMed] [Google Scholar]
- Tocchini-Valentini G. P., Mattoccia E. A mutant of E. coli with an altered supernatant factor. Proc Natl Acad Sci U S A. 1968 Sep;61(1):146–151. doi: 10.1073/pnas.61.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]


