Abstract
Aims/hypothesis
Long-term exposure to NEFAs leads to inhibition of glucose-induced insulin secretion. We tested whether the release of somatostatin and glucagon, the two other major islet hormones, is also affected.
Methods
Mouse pancreatic islets were cultured for 72 h at 4.5 or 15 mmol/l glucose with or without 0.5 mmol/l oleate or palmitate. The release of glucagon and somatostatin during subsequent 1 h incubations at 1 or 20 mmol/l glucose as well as the islet content of the two hormones were determined. Lipid-induced changes in islet cell ultrastructure were assessed by electron microscopy.
Results
Culture at 15 mmol/l glucose increased islet glucagon content by ∼50% relative to that observed following culture at 4.5 mmol/l glucose. Inclusion of oleate or palmitate reduced islet glucagon content by 25% (at 4.5 mmol/l glucose) to 50% (at 15 mmol/l glucose). Long-term exposure to the NEFA increased glucagon secretion at 1 mmol/l glucose by 50% (when islets had been cultured at 15 mmol/l glucose) to 100% (with 4.5 mmol/l glucose in the culture medium) and abolished the inhibitory effect of 20 mmol/l glucose on glucagon secretion. Somatostatin content was unaffected by glucose and lipids, but glucose-induced somatostatin secretion was reduced by ∼50% following long-term exposure to either of the NEFA, regardless of whether the culture medium contained 4.5 or 15 mmol/l glucose. Ultrastructural evidence of lipid deposition was seen in <10% of non-beta cells but in >80% of the beta cells.
Conclusions/interpretation
Long-term exposure to high glucose and/or NEFA affects the release of somatostatin and glucagon. The effects on glucagon secretion are very pronounced and in type 2 diabetes in vivo may aggravate the hyperglycaemic effects due to lack of insulin.
Keywords: Diabetes, Glucagon, Glucolipotoxicity, NEFA, Oleate, Palmitate, Somatostatin
Introduction
Obesity results in increased release of fatty acids from the adipocytes and thereby elevates circulating levels of NEFA. NEFA in turn affect insulin secretion and action. The effects of NEFA differ depending on the length of exposure. Short-term exposure (∼1 h) to lipids stimulates glucagon and insulin secretion, but inhibits somatostatin release [1]. Following long-term (72 h) exposure, however, glucose-induced insulin secretion is strongly reduced [2]. Recently, we reported that palmitate and oleate reduced glucose-evoked insulin secretion by ∼80%, while not affecting exocytosis of the secretory granules as monitored by capacitance measurements [3]. We therefore proposed that lipid pre-treatment somehow interferes with the emptying of insulin from the secretory granules [3]. Here we have analysed glucagon and somatostatin secretion in mouse pancreatic islets exposed to oleate or palmitate at low (4.5 mmol/l) or high (15 mmol/l) glucose for 72 h. We demonstrate that the suppression of insulin secretion is associated with hypersecretion of glucagon and a reduction of glucose-stimulated somatostatin secretion.
Methods
Animals and cell culture
All procedures were carried out in accordance with the principles of laboratory animal care as well as requirements of local and national authorities. NMRI mice were killed by cervical dislocation and pancreatic islets were isolated as previously described [3]. Islets were cultured in RPMI 1640 medium supplemented with fatty acid-free BSA (1%, wt/vol.) alone or with 0.5 mmol/l of oleate or palmitate at 4.5 and 15 mmol/l glucose. The NEFA stock solutions were prepared as described previously; the estimated unbound NEFA concentration was 26 nmol/l for palmitate and 47 nmol/l for oleate and was estimated by the stepwise equilibrium method [3]. The data reported here were obtained in islets treated identically to those previously used for the analysis of the long-term effects of glucose and NEFA on insulin secretion [3].
Hormone release measurements
Groups of ten to 12 size-matched islets were pre-incubated for 30 min in 1 ml Krebs Ringer solution, containing (in mmol/l): 140 NaCl, 3.6 KCl, 2 NaHCO3, 0.5 NaH2PO4, 0.5 MgSO4, 5 HEPES (pH 7.4 with NaOH), 2.6 CaCl2, 1 glucose and 1% BSA (wt/vol.). Islets were then incubated in 1 ml of the same solution containing 1 or 20 mmol/l glucose for 1 h. Islet hormone content was determined after extraction with acidic ethanol. Glucagon and somatostatin were determined using in-house radioimmunoassays [4]. All chemicals were of analytical grade and purchased from Sigma (Gillingham, UK).
Electron microscopy
Ultrastructural analysis was performed by electron microscopy as described previously [3].
Statistical analyses
Data or transformed data were analysed by one-way ANOVA and a least significant difference multiple comparison test using SPSS software (SPSS, Chicago, IL, USA).
Results
Glucagon
Glucagon content was reduced by ∼20% by palmitate or oleate when the islets were cultured at 4.5 mmol/l glucose. Culture in the presence of 15 mmol/l glucose for 72 h increased islet glucagon content by ∼50% relative to that observed following culture at 4.5 mmol/l glucose. The latter increase was antagonised by inclusion of oleate or palmitate in the culture medium (Fig. 1a).
Fig. 1.
Glucagon (a) and somatostatin (c) content in islets treated with low (4.5 mmol/l) or high (15 mmol/l) glucose, in the presence or absence of palmitate (P) or oleate (O). Data are presented as mean ± SEM of four experiments. *p < 0.05 for control islets at 15 mmol/l glucose vs all other groups. Glucagon (b) and somatostatin (d) hormone secretion in islets treated as indicated (a, c). Secretion was measured at 1 (black) and 20 mmol/l (grey) glucose during 1 h incubation following the 72 h culture at the indicated conditions. Data are presented as mean ± SEM of four experiments. *p < 0.05 (at least) for comparisons with secretion measured at 1 mmol/l glucose in the respective groups; † p < 0.05 (at least) for comparison with secretion measured at 1 mmol/l glucose in islets cultured at 4.5 mmol/l glucose; ‡ p < 0.05 (at least) for comparison of secretion measured in the presence of 20 mmol/l glucose in islets cultured for 72 h at 4.5 mmol/l glucose; § p < 0.05 (at least) for comparison of secretion measured in the presence of 20 mmol/l glucose in islets cultured for 72 h at 15 mmol/l glucose
The effects of oleate and palmitate on glucagon secretion, whether in the presence of low (4.5 mmol/l) or high (15 mmol/l) glucose, were identical and resulted in stimulation of basal (at 1 mmol/l glucose) glucagon secretion and complete loss of the inhibition that is normally produced by glucose at 20 mmol/l. Interestingly, culture at high glucose alone also abolished the inhibitory effect of 20 mmol/l glucose, whereas secretion at 1 mmol/l was unaffected (Fig. 1b).
Somatostatin
No significant differences in somatostatin contents were observed (Fig. 1c). Basal somatostatin secretion was marginally increased both by culture at high glucose and by the NEFA when applied at the lower glucose concentration (Fig. 1d). However, glucose-stimulated somatostatin release was reduced by oleate and palmitate exposure; the same inhibition was obtained regardless of the glucose concentration during the culture period. There were no statistically significant differences between the release rates measured in the presence of 20 mmol/l glucose in islets cultured at low or high glucose for any of the conditions (Fig. 1d).
Electron microscopy
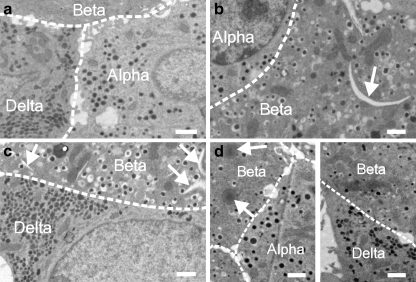
Figure 2 shows electron micrographs of islets cultured at 15 mmol/l glucose alone (Fig. 2a) and when the culture medium was supplemented with palmitate (Fig. 2b, c) or oleate (Fig. 2d). The alpha, beta and delta cells were identified on the basis of the appearance of the secretory granules. All islet cells appeared structurally intact with no obvious signs of apoptosis. However, exposure to oleate resulted in the appearance of translucent lipid droplets similar to those previously described in Ins1 cells exposed to lipids [5]. In oleate-treated islets, 80% of the beta cells (16 out of 20 cells), 9% of the alpha cells (one out of 11 cells) and 8% of the delta cells (one out of 12 cells) contained lipid droplets. Following exposure to palmitate, 92% of the beta cells (12 out of 13 cells) contained crescent-like structures similar to those previously documented in Ins1 cells and believed to represent tripalmitin deposition [5]; beta cells exposed to palmitate did not contain lipid droplets of the type seen in oleate-treated cells. No signs of lipid deposition were seen in non-beta cells exposed to palmitate (three delta cells and ten alpha cells were analysed).
Fig. 2.
Electron micrograph through mouse islets incubated for 72 h in 15 mmol/l glucose alone (a) and in 15 mmol/l glucose with 0.5 mmol/l palmitate (b, c) or 0.5 mmol/l oleate (d). Alpha, beta and delta cells are indicated. The presence of crescent-like structures in palmitate-treated cells (b, c) and oleate-induced lipid droplets are indicated by white arrows. The cell borders are indicated schematically by the dashed white lines. Micrographs and data presented in the text are representative of four to seven islets obtained from two different mice. Scale bar, 1 μm
Discussion
The adverse effects of long-term exposure to NEFA on glucose-stimulated insulin secretion are well established [2]. Much less is known about the consequences of a chronic elevation of NEFA on the release of somatostatin and glucagon. Here we demonstrate a dichotomy in the effects of long-term exposure to NEFA. Thus, whereas glucose-induced release of somatostatin, like that of insulin, was reduced following 72 h exposure to the NEFA, glucagon secretion was stimulated. In addition, the ability of high glucose concentrations to suppress glucagon release was abolished.
Following long-term exposure to the lipids, 80% to 90% of beta cells displayed signs of lipid deposition inside the cells in the form of lipid droplets and crescent-like structures, respectively. Many of the crescent-like structures were (at least partially) membrane-delimited, whereas the lipid droplets observed following exposure to oleate were without boundary membrane. The crescent-like structures might represent expansions of the endoplasmic reticulum [5], possibly an early sign of endoplasmic reticulum stress. Importantly, the ultrastructure of the non-beta cells was much less affected by long-term exposure to lipids, with the crescent-like structures being confined to the beta cells. Thus, alpha and delta cells would be expected to be less sensitive to lipids than beta cells and this may contribute to the hormonal abnormalities associated with obesity and diabetes.
The regulation of somatostatin secretion has traditionally been regarded to be similar to that of insulin secretion. Recently, however, it has become apparent that the mechanisms controlling glucose-induced insulin and somatostatin secretion are quite different. Thus, somatostatin release is highly dependent on Ca2+-induced Ca2+ release (CICR) [6]. It is possible that long-term exposure to NEFA might interfere with CICR and thus lower glucose-induced somatostatin secretion.
There have been few reports on the long-term effects of glucose and NEFA on glucagon secretion. Rat islets exposed for 24 h to 0.6 mmol/l palmitate have been reported to have 50% lower glucagon content than controls [7]. It has been argued that this effect is the result of insufficient biosynthesis in the face of higher secretion [8]. This may explain why glucagon content decreased following long-term exposure to NEFA (30–40%), a condition that led to stimulation of glucagon secretion. Conversely, the observed oversecretion of glucagon in islets cultured in the presence of NEFA cannot be accounted for by increased islet glucagon content. Thus, it seems justifiable to conclude that this stimulation results from a genuine effect on intracellular signalling within the alpha cell.
Glucagon secretion has been proposed to be under paracrine control. According to this concept, the reduction of insulin (and other factors co-released with insulin such as Zn2+) and somatostatin may underlie the hypersecretion of glucagon. However, it should be noted that culture at high glucose alone has only a marginal effect on glucose-induced insulin and somatostatin secretion and yet the suppressor effect of high glucose on glucagon secretion is abolished. Thus, the observed stimulation of glucagon release cannot be explained in terms of defective paracrine regulation and rather suggests that the defect occurs within the alpha cell itself. In this context it may be of relevance that NEFA metabolites like palmitoyl-CoA activates KATP-channels [9]. Recent data indicate that low concentrations of the KATP-channel opener diazoxide stimulate glucagon secretion and antagonise the inhibitory effect of glucose [10]. If enough acyl-CoAs are generated in alpha cells to exert a diazoxide-like effect, it may contribute to the loss of glucose regulation of glucagon secretion. The long-term effects of NEFA are rather similar to the acute actions previously reported [1]. Under acute conditions, application of NEFA did in fact exert a diazoxide-like effect on alpha cell [Ca2+]i and lead to the reappearance of [Ca2+]i oscillations in cells where they had been suppressed by high glucose.
In mouse pancreatic beta cells, long-term exposure to lipids does not result in any obvious functional impairment that explains the strong reduction of glucose-induced insulin secretion. Thus, metabolism, electrical activity and intracellular [Ca2+]i signalling all proceed as well as or better than in control cells and much of the effect on glucose induced insulin secretion is independent of cytotoxicity [3]. Instead, we proposed that lipids may interfere with insulin release at the level of fusion pore expansion (i.e. distal to the fusion of the granules with the plasma membrane), resulting in failure of the fusion pore to expand sufficiently to allow macromolecules like insulin to exit. The fact that glucagon secretion is enhanced rather than inhibited following lipid exposure suggests that there is some cell-specificity of the effect.
Oversecretion of glucagon and insufficient release of insulin are part of the aetiology of type 2 diabetes. The data presented here illustrate that the two defects may be interrelated. Thus, mild disturbances of insulin and glucagon secretion and/or a slight elevation of plasma NEFA levels can be envisaged to result in a vicious cycle of progressively impaired glucose regulation of insulin and glucagon release that eventually culminates in glucose intolerance and overt diabetes.
Acknowledgements
We thank A. Clark for valuable discussions and advice. This study was supported by Diabetes UK and the Wellcome Trust. P. Rorsman is a Wolfson Royal Society Merit Award Research Fellow. C. S. Olofsson holds a postdoctoral fellowship from the Swedish Research Council.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviation
- CICR
Ca2+-induced Ca2+ release
References
- 1.Olofsson CS, Salehi A, Gopel SO, Holm C, Rorsman P. Palmitate stimulation of glucagon secretion in mouse pancreatic alpha-cells results from activation of L-type calcium channels and elevation of cytoplasmic calcium. Diabetes. 2004;53:2836–2843. doi: 10.2337/diabetes.53.11.2836. [DOI] [PubMed] [Google Scholar]
- 2.Yaney GC, Corkey BE. Fatty acid metabolism and insulin secretion in pancreatic beta cells. Diabetologia. 2003;46:1297–1312. doi: 10.1007/s00125-003-1207-4. [DOI] [PubMed] [Google Scholar]
- 3.Olofsson CS, Collins S, Bengtsson M, et al. Long-term exposure to glucose and lipids inhibits glucose-induced insulin secretion downstream of granule fusion with plasma membrane. Diabetes. 2007;56:1888–1897. doi: 10.2337/db06-1150. [DOI] [PubMed] [Google Scholar]
- 4.Olofsson CS, Salehi A, Holm C, Rorsman P. Palmitate increases L-type Ca2+ currents and the size of the readily releasable granule pool in mouse pancreatic beta-cells. J Physiol. 2004;557:935–948. doi: 10.1113/jphysiol.2004.066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moffitt JH, Fielding BA, Evershed R, Berstan R, Currie JM, Clark A. Adverse physicochemical properties of tripalmitin in beta cells lead to morphological changes and lipotoxicity in vitro. Diabetologia. 2005;48:1819–1829. doi: 10.1007/s00125-005-1861-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Bengtsson M, Partridge C, et al. R-type Ca(2+)-channel-evoked CICR regulates glucose-induced somatostatin secretion. Nat Cell Biol. 2007;9:453–460. doi: 10.1038/ncb1563. [DOI] [PubMed] [Google Scholar]
- 7.Gremlich S, Bonny C, Waeber G, Thorens B. Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels. J Biol Chem. 1997;272:30261–30269. doi: 10.1074/jbc.272.48.30261. [DOI] [PubMed] [Google Scholar]
- 8.Dumonteil E, Magnan C, Ritz-Laser B, Ktorza A, Meda P, Philippe J. Glucose regulates proinsulin and prosomatostatin but not proglucagon messenger ribonucleic acid levels in rat pancreatic islets. Endocrinology. 2000;141:174–180. doi: 10.1210/en.141.1.174. [DOI] [PubMed] [Google Scholar]
- 9.Larsson O, Deeney JT, Branstrom R, Berggren PO, Corkey BE. Activation of the ATP-sensitive K+ channel by long chain acyl-CoA. A role in modulation of pancreatic beta-cell glucose sensitivity. J Biol Chem. 1996;271:10623–10626. doi: 10.1074/jbc.271.18.10623. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald PE, De Marinis YZ, Ramracheya R, et al. A K ATP channel-dependent pathway within alpha cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol. 2007;5:e143. doi: 10.1371/journal.pbio.0050143. [DOI] [PMC free article] [PubMed] [Google Scholar]