The asymmetric construction of a quaternary center at C-3 of the indole core represents a great synthetic challenge as well as a powerful entry into the structurally diverse family of indole alkaloids.1 While many reports describe the asymmetric synthesis of 3,3-disubstituted oxindoles,2 there is only one example of an enantioselective process that takes advantage of the nucleophilicity of the C-3 of indoles to build a quaternary center directly from 3-substituted indoles.3,4 Recently, Tamaru and co-workers reported a C-3 selective palladium-catalyzed allylation of 1H -indoles promoted by triethylborane using allyl alcohols.5 Of particular interest to us was the reaction of 3-methylindole which provided the corresponding 3-methyl-3-allyl-indolenine. Since this reaction seems to proceed via a π-allylpalladium intermediate, we envisioned that our chiral ligands6 could be used to develop an enantioselective version. Here we report our progress in this area, which, to the best of our knowledge, is the first example of an enantioselective allylic alkylation using allyl alcohol as electrophile and reveals some insight into the mechanism of this interesting transformation.
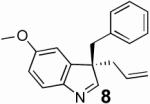
Preliminary experiments indicated that the anthracene derived ligand A gave the best enantioselectivities and that 1 eq of Et3B and 3 eq of allyl alcohol were necessary for the reaction to proceed in good yield. Solvent studies (Table 1) revealed that non-coordinating solvents provided higher selectivities with CH2Cl2 giving the best result. We then envisioned that since the high selectivity for C-3- versus N-allylation might result from the borane being tightly bound to the indole nitrogen during the allylation step,7 the nature of the borane should greatly influence the enantioselectivity. Indeed, we were pleased to observe an increase of the ee to 83% using the more bulky borane derived from the hydroboration of 1-hexene with 9-BBN (Table 1, entry 6). Further increasing the steric bulk of the borane using the dicyclohexylborane derivative was detrimental to the yield and ee, while using disiamylborane-C6H1 3 completely inhibited the reaction. Finally, optimal conditions (entry 9) were achieved with 1.05 eq of borane at 4°C, providing the indolenine in 92 % yield and 85 % ee. The product is then liberated from the borane by basic hydrolysis or treatment with ethanolamine in THF.8
Table 1.
Selected optimization studies for C-3 allylation 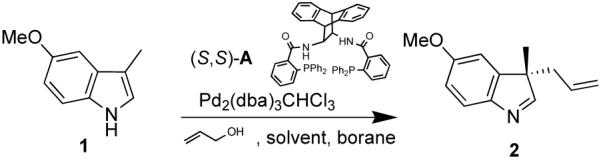
| Entry | Borane | T (°C) | Solvent | Yielda (%) | eeb (%) |
|---|---|---|---|---|---|
| 1 | Et3B | 25 | DME | 82 | 43 |
| 2 | Et3B | 25 | dioxane | 85 | 33 |
| 3 | Et3B | 25 | THF | 89 | 53 |
| 4 | Et3B | 25 | toluene | 85 | 61 |
| 5 | Et3B | 25 | CH2Cl2 | 88 | 66 |
| 6 | 9-BBN-C6H1 3 | 25 | CH2Cl 2 | 88 | 83 |
| 7 | (Chx)2B-C6H1 3 | 25 | CH2Cl 2 | 70 | 70 |
| 8 | Sia2B-C6H1 3 | 25 | CH2Cl 2 | - | - |
| 9 | 9-BBN-C6H1 3 | 4 | CH2Cl 2 | 92 | 85 |
All reactions were performed using 2.5 mol % of Pd2(dba)3CHCl3, 7.5 % of (S,S)-A, 1 (entry 1-6) or 1.05 eq (entry 7-9) of borane. and 3 eq of allyl alcohol.
Enantiomeric excess was determined by chiral HPLC after reduction of the imine using NaBH3CN.
We then examined the effect of substitution of the indole on the outcome of the reaction (Table 2). Surprisingly, the electronic character of the indole seems to influence the selectivity while the reactivity seems hardly affected (except for 3a which failed to react). The electron deficient indole 3 b reacts with moderate selectivity and the ee increases in correlation with the electron donating character of the C-5 substituent. Electron donating substituents in the 4- and 6-position also give high selectivities, while the 7-substituted indole failed to react most likely because it prevents binding of the boron to the indole nitrogen. This electronic effect could be ascribed to the stronger boron-nitrogen interaction in electron rich indoles.
Table 2.
Effect of the substitution of the indole benzene ring 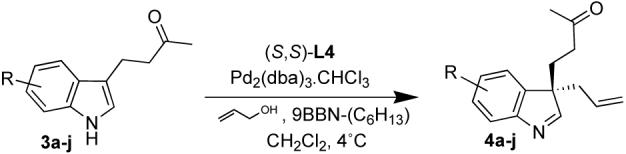
| Entry | R | Product | Yielda (%) | ee (%) |
|---|---|---|---|---|
| 1 | 5-NO2 | 4a | - | - |
| 2 | 5-Br | 4b | 89 | 60 |
| 3 | H | 4c | 92 | 74 |
| 4 | 5-MeO | 4d | 95 | 84 |
| 5 | 5-BnO | 4e | 87 | 83 |
| 6 | 5-HO | 4f | 88 | 86 |
| 7 | 5-Bn2N | 4g | 94 | 90 |
| 8 | 4-MeO | 4h | 92 | 83 |
| 9 | 6-MeO | 4i | 89 | 78 |
| 10 | 7-MeO | 4j | - | - |
All reactions were performed on a 0.2 mmol scale for 20h at 4 °C using 2.5 mol % of Pd2(dba)3CHCl3, 7.5 % of (S,S)-A, 1.05 eq of 9-BBN-(C6H13) and 3 eq of allyl alcohol.
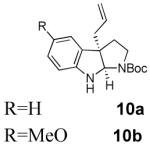
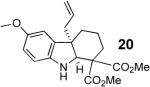
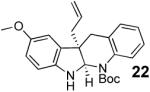
With these results in hand we examined the scope of this reaction using mainly readily available and synthetically relevant 5-methoxy-3-substituted indoles (Table 3). In all cases, high yield were achieved using the previously optimized conditions, with enantiomeric excess from 72% to 90%. Of particular interest are the substrates with a pendant nucleophile which cyclizes onto the imine under the reaction conditions (entry 3-11). In these cases, the corresponding indoline is obtained with a cis-5,5- or 5,6-fused ring system9 found in numerous natural products.1 These results also exemplify the very high chemoselectivity of this reaction since alcohol, phenol, carbamate10 and malonate functional groups are tolerated.11
Table 3.
Scope of the reaction
| Entry | Substrate | Product | Yielda (%) | ee (%) |
|---|---|---|---|---|
| 1 |  |
 |
85 | 84 |
| 2 | 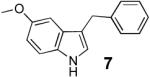 |
 |
89 | 85 |
| 3 | 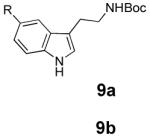 |
 |
87 | 72 |
| 4 | 91 | 82 | ||
| 5 | 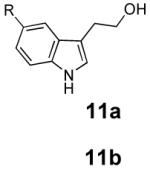 |
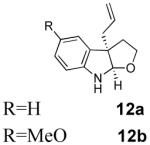 |
90 | 81 |
| 6 | 91 | 86 | ||
| 7 | 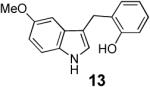 |
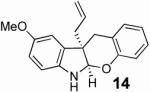 |
84 | 90 |
| 8 | 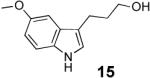 |
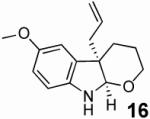 |
93 | 88 |
| 9 | 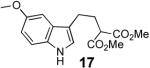 |
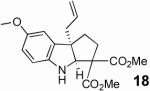 |
89b | 84 |
| 10 | 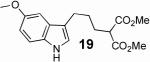 |
 |
85b | 81 |
| 11 | 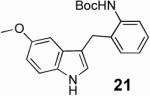 |
 |
91 | 85 |
All reactions were performed on a 0.2 mmol scale for 20h using 2.5 mol % of Pd2(dba)3CHCl3, 7.5 % of (S,S)-A, 1.1 eq of 9-BBN-(C6H13) and 3 eq of allyl alcohol.
Cyclization to the indoline occurred after addition of ethanolamine to the indolenine in THF (5 min stirring for 18 and 24h stirring for 20).
We were also interested in the oxidation of indolenines which could allow an easy access to the oxindole family of natural products. After examining various oxidative conditions, we found that treatement of 2 with tetrabutylammonium oxone®12 and acetic acid gave oxindole 23 in good yield (scheme 1). Recrystallization of 23 provided enantiopure material. Furthermore, after methylation and oxidative cleavage, aldehyde 24 was converted to (-)-esermethol by reductive amination-cyclization following Overman’s procedure.13 This intermediate has been previously used to synthesize (-)-phenserine,2b which is a drug candidate for treatment of Alzheimer’s disease.
Scheme 1.
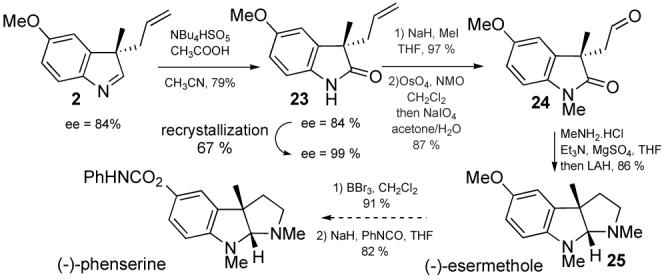
Synthesis of (-)-esermethole.
In summary, we have developed a new enantioselective C-3 allylation of 3-substituted indoles to give the corresponding 3,3-disubstituted indolenines and indolines. High enantioselectivities could be achieved using 9-BBN-C6H1 3 as the promoter of the reaction and electron rich indoles give higher selectivities. The dependence of the selectivity on the nature of the borane suggests that, in addition to promoting the ionization of allyl alcohol,14 the boron is directly involved in the enantiodiscriminating step. Studies to elucidate the mechanism of this reaction are underway and will be reported shortly.
Supplementary Material
Acknowledgment
We thank the National Science Foundation and the National Institutes of Health (GM33049) for their generous support of our programs. J.Q. thanks the Association pour la Recherche sur le Cancer (France) for a postdoctoral fellowship. We thank Johnson Matthey for a generous gift of palladium salts.
References
- (1)(a).Somei Masanori, S., Yamada Fumio, Y. Nat. Prod. Rep. 2005;22:73. doi: 10.1039/b316241a. [DOI] [PubMed] [Google Scholar]; (b) Rahman AU, Basha A. Indole alkaloids. Hawood Academic Publishers; Amsterdam: 1997. [Google Scholar]
- (2)(a).Trost BM, Frederiksen MU. Angew. Chem. Int. Ed. Engl. 2005;44:308. doi: 10.1002/anie.200460335.and reference cited theirein.Huang A, Kodanko JJ, Overman LE. J. Am. Chem. Soc. 2004;126:14043. doi: 10.1021/ja046690e. K.Adhikari S, Caille S, Hanbauer M, Ngo VX, Overman LE. Org. Lett. 2005;7:2795. doi: 10.1021/ol051172+.
- (3).Austin JF, Kim SG, Sinz CJ, Xiao WJ, MacMillan DWC. Proc. Natl. Acad. Sci. USA. 2004;101:5482. doi: 10.1073/pnas.0308177101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Other approaches to the formation of a quaternary center at C3 of the indole core include: (a) asymmetric Heck-iminium ion cyclization,Dounay AB, Overman LE, Wrobleski AD. J. Am. Chem. Soc. 2005;125:10186. doi: 10.1021/ja0533895.(b) enantioselective oxidative cyclisation of tryptophol,Hirose T, Sunazuka T, Yamamoto D, Kojima N, Shirahata T, Harigaya Y, Kuwajima I, Omura S. Tetrahedron. 2005;61:6015.and previous work cited theirein.
- (5).Kimura M, Futamata M, Mukai R, Tamaru Y. J. Am. Chem. Soc. 2005;127:4592. doi: 10.1021/ja0501161. [DOI] [PubMed] [Google Scholar]
- (6).Trost BM. Chem. Pharm. Bull. 2002;50:1. doi: 10.1248/cpb.50.1. [DOI] [PubMed] [Google Scholar]; (b) Trost BM, Crawley ML. Chem. Rev. 2003;103:2921. doi: 10.1021/cr020027w. [DOI] [PubMed] [Google Scholar]; (c) Trost BM. J. Org. Chem. 2004;69:5813. doi: 10.1021/jo0491004. [DOI] [PubMed] [Google Scholar]
- (7).A systematic study of base and solvent effect on the C3- versus N-selectivity in the palladium catalyzed allylation of indoles using allyl carbonates showed that the formation of a tight indolyl-metal ion favors the C3-allylation process.Bandini M, Melloni A, Umani-Ronchi A. Org. Lett. 2004;6:3199. doi: 10.1021/ol048663z.
- (8).Kol M, Bar-Haim G. Tetrahedron Lett. 1998;39:2643. [Google Scholar]
- (9).The relative stereochemistry was confirmed by NOE on 12a and 16.
- (10).Unprotected tryptamines failed to give clean reactions and high enantioselectivity.
- (11).Except for 4f which led to about 5 % of allylated phenol, no allylation of the pendant nucleophile nor of the indole nitrogen was detected.
- (12).Trost BM, Braslau R. J. Org. Chem. 1988;53:532. [Google Scholar]
- (13).23 was used to assign the absolute configuration of 2 and, by extension, of all indolenines and indolines.Matsuura T, Overman LE, Poon DJ. J. Am. Chem. Soc. 1998;120:6500.
- (14).Tamaru Y. Eur. J. Org. Chem. 2005:2647–2656. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


