Abstract
The potential of early interventions for dementia has increased interest in cognitive impairments less severe than dementia. However, predictors of the trajectory from intact cognition to dementia have not yet been clearly identified. The purpose of this study was to determine whether known risk factors for dementia increased the risk of mild cognitive impairments or progression from mild cognitive impairments to dementia. A polytomous logistic regression model was used, with parameters governing transitions within transient states (intact cognition, mild cognitive impairments, global impairment) estimated separately from parameters governing the transition from transient to absorbing state (dementia or death). Analyses were based on seven annual examinations (1991–2002) of 470 Nun Study participants aged ≥75 years at baseline and living in the United States. Odds of developing dementia increased with age primarily for those with low educational levels. In these women, presence of an apolipoprotein E gene *E4 allele increased the odds more than fourfold by age 95 years. Age, education, and the apolipoprotein E gene were all significantly associated with mild cognitive impairments. Only age, however, was associated with progression to dementia. Thus, risk factors for dementia may operate primarily by predisposing individuals to develop mild cognitive impairments; subsequent progression to dementia then depends on only time and competing mortality.
Keywords: aged, 80 and over; apolipoproteins E; cognition disorders; cohort studies; dementia; disease progression; Markov chains; risk factors
Dementia and its subtypes, including Alzheimer's disease, have long been a focus of clinical and epidemiologic research. More recently, however, the potential of early interventions for dementia has increased interest in cognitive impairments less severe than dementia, with lively debate as to whether these mild cognitive impairments inevitably progress to dementia (1-3). The relation of these impairments to dementia and Alzheimer's disease has not yet been firmly established, although research suggests they may share a neuropathologic substrate (2, 4-6). Despite intense study of these mild cognitive impairments, there is as yet no standard terminology or definition, aside from the consensus that the condition reflects acquired cognitive impairments in older adults that do not meet criteria for dementia (7-12). Mild cognitive impairments are of substantial public health interest because of their association with increased risk of dementia (3, 7, 10, 13), mortality (14-16), and institutionalization (16).
Risk factors for these mild cognitive impairments, as well as predictors of the trajectory from intact cognition to dementia, have not yet been clearly identified. Traditional epidemiologic research has focused on risk factors for the development of dementia from intact cognition. Although knowledge of established risk factors for dementia can logically contribute to the search for predictors of the progression of cognitive impairment, it is still unclear where in the dementing process these risk factors for dementia exert their effects. If the risk factors for mild cognitive impairments are the same as those for dementia, and if there are no unique risk factors for the progression of mild cognitive impairments to dementia, then mild cognitive impairments may indeed simply reflect early dementia.
The purposes of this study were to determine whether known risk factors for dementia (i.e., older age, less education, and presence of an apolipoprotein E gene (APOE)*E4 allele) 1) increase the risk of mild cognitive impairments or progression from mild cognitive impairments to dementia and 2) influence the odds of developing dementia across the long-term trajectory from intact cognition to dementia and death. The analytic approach takes into consideration the transient nature of cognitive status and that transitions in cognitive status may occur in both directions. Thus, mild cognitive impairments are a transient state, whereby some people improve (to intact cognition), some worsen (to global impairment or dementia), and some stay the same (persistent mild cognitive impairments). In this paper, we define intact cognition, mild cognitive impairments, and global impairment as nonabsorbing (i.e., transient) states and dementia and death as absorbing ones. We use a multistate Markov process for the analyses, a useful approach to explain risk factors associated with progressive diseases such as Alzheimer's disease.
MATERIALS AND METHODS
Study population
The design of the Nun Study has been described elsewhere (17-20). Briefly, from 1991 to 1993, all members of the School Sisters of Notre Dame born before 1917 and living in communities in the midwestern, eastern, and southern United States were invited to join the Nun Study. Of 1,031 eligible Catholic sisters aged 75 years or older, 678 (66 percent) agreed to participate, a high percentage given that each sister consented to collection of archival and medical records, annual cognitive and physical assessments, and brain donation after death. Participants did not differ significantly from nonparticipants in mean age, mortality rate, race, or country of birth. Analyses were based on data from the first seven annual examinations (1991–2002). After we excluded those for whom examinations were missing (n = 58), data on APOE were missing (n = 35), or dementia was present at the first examination (n = 115), the final analytic sample consisted of 470 nondemented participants at baseline (table 1). Of these 470, 192 provided complete data (six transitions) for all seven annual examinations: 149 who survived without dementia to the seventh examination and 43 who developed dementia (n = 10) or died (n = 33) between the sixth and seventh examinations.
TABLE 1.
Age, education, and apolipoprotein E allele status in the Nun Study, United States, 1991–2002 (n = 470 subjects)
| Characteristic | No. | % |
|---|---|---|
| Age (years) at baseline (mean (standard deviation)) | 84.3 (5.0) | |
| Education | ||
| ≤High school | 47 | 10.0 |
| Undergraduate degree | 193 | 48.9 |
| Graduate degree | 230 | 41.1 |
| Apolipoprotein E*E4 allele | ||
| Present | 92 | 19.6 |
| Absent | 378 | 80.4 |
Participants in the Nun Study have had relatively comparable lifestyles and environments throughout their adult lives. Although their unique characteristics may limit the generalizability of findings, the high level of homogeneity between participants minimizes or eliminates many factors that may confound other epidemiologic studies, such as tobacco use, heavy alcohol consumption, marital status, reproductive history, and access to health care.
Cognitive states
Previous Nun Study work (4, 21) has defined a set of mutually exclusive cognitive states that captures the full range of cognitive function from intact status through dementia. In addition to a category for intact function, categories for cognitive and functional decline include mild cognitive impairments, global impairment, and dementia. Participants classified as intact had scores within normal limits on four cognitive tests in the Consortium to Establish a Registry for Alzheimer's Disease neuropsychologic battery of tests. They also had intact global cognitive ability as measured by the Mini-Mental State Examination (22, 23) and were intact regarding activities of daily living (i.e., dressing, walking, standing [transferring], feeding, and toileting) (24, 25).
Participants who met our criteria for dementia (4, 19, 21) had a decline in function, impairments in memory and at least one other area of cognitive function, and impaired activities of daily living. Those with mild cognitive impairments (4, 21) had at least one specific area of impaired cognitive function, such as memory or naming, but had intact global cognitive ability and activities of daily living. Participants with global impairment (4, 21) were impaired regarding global cognitive ability, activities of daily living, or both; other impairments in a specific area of cognitive function could also have been present. Participants with global impairment did not meet criteria for dementia because only one area of cognitive function was impaired or, if two areas of cognition were impaired, activities of daily living were intact.
Statistical methods
The status of a participant at each examination was recorded as being in one of five states: intact cognition, mild cognitive impairments, global impairment, dementia, or dead. The conditional distribution of the status of an individual participant at an arbitrary examination given her status at previous examinations was assumed to have the Markov property (i.e., that status at this examination depended on the status at only the most recent previous examination and was independent of status at other previous examinations) (26). Hence, a Markov chain was used to model transitions from one state to another. In this chain, intact cognition, mild cognitive impairments, and global impairment were considered transient states, whereas dementia and death were absorbing states (figure 1). For simplicity and because they were not of interest in these analyses, transitions from dementia to death were ignored although they could be incorporated into the chain. It was assumed that parameters governing transitions within the transient, nondemented states were estimated separately from parameters governing the transition from nondemented to absorbing states. The likelihood function for this chain was thus factored as the product of four independent functions: one for the transitions among the nondemented states conditioned on nonabsorption (the quasi-stationary distribution of the three nondemented, living states) and one for the transitions from each nondemented state to the absorbing states. Each function was assumed to be a polytomous logistic regression model that depended on the risk factors age, education, and APOE allele status. The CATMOD procedure in PC-SAS, version 9.1 software (SAS Institute Inc., Cary, North Carolina) was used to fit each polytomous logistic model to the data. Statistical significance was determined at the 0.05 level.
FIGURE 1.
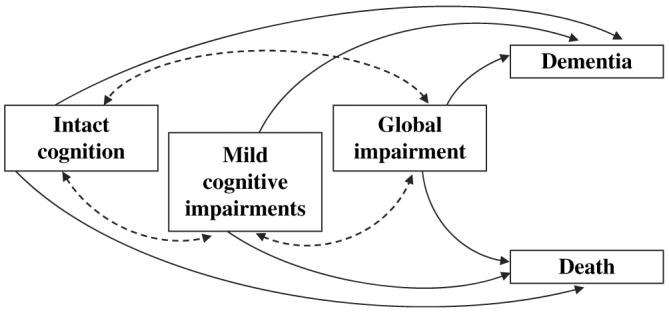
Possible cognitive transitions between transient states (intact cognition, mild cognitive impairments, global impairment) and absorbing states (dementia, death).
The procedure also yielded two sets of estimated odds ratios. The first set of odds ratios and their standard errors were derived from polytomous logistic regression models and were used to assess the contribution of each risk factor to the probability of transition. The second set of odds ratios refers to the odds of becoming demented before death and can be obtained from the canonical representation of the one-step transition matrix by using a result given by Bhat (27). Finally, the standard error associated with this last set of odds can be estimated by using a bootstrap procedure (28). Further details are provided in the Appendix.
RESULTS
Table 2 summarizes the 1,905 transitions of the 470 subjects that form the basis of the subsequent analyses. For each of the transient states, subjects were more likely to remain in that cognitive state at the next cognitive assessment than to transition to another cognitive state (e.g., 65.3 percent of those cognitively intact at the previous examination remained cognitively intact at their subsequent examination). Thus, a total of 1,100 (57.7 percent) one-step transitions (between cognitive status at two consecutive examinations) reflected cognitive states that remained the same. However, the remaining 805 (42.3 percent) transitions reflected movement either forward or backward, including 180 backward transitions (improvements in cognitive status) in 155 subjects.
TABLE 2.
Number and frequency of transitions between cognitive states in the Nun Study, United States, 1991–2002 (1,905 transitions in 470 subjects)
| Prior cognitive state |
||||||
|---|---|---|---|---|---|---|
| Current cognitive state |
Intact cognition |
Mild cognitive impairments |
Global impairment |
|||
| No. | % | No. | % | No. | % | |
| Intact cognition | 436 | 65.3 | 138 | 14.5 | 12 | 4.2 |
| Mild cognitive impairments | 155 | 23.2 | 551 | 58.1 | 30 | 10.4 |
| Global impairment | 39 | 5.8 | 110 | 11.6 | 113 | 39.2 |
| Dementia | 6 | 0.9 | 71 | 7.5 | 60 | 20.8 |
| Dead | 32 | 4.8 | 79 | 8.3 | 73 | 25.4 |
| Total | 668 | 100 | 949 | 100 | 288 | 100 |
Table 3 summarizes the effects of the risk factors on transitions within the transient states, that is, between intact cognition, mild cognitive impairments, and global impairment. Age, education, and APOE were all significant predictors of the transition from intact cognition to mild cognitive impairments. A similar pattern was seen for transitions from intact cognition to global impairment. Prior state was also a significant risk factor, for transitions to both mild cognitive impairments and global impairment. For example, a subject who was globally impaired at the previous examination was 50 (1/0.02) times more likely to still be globally impaired at the next examination than to recover to intact cognition. (In the polytomous logistic regression model, the base state was intact cognition. Thus, the model computed the log odds that a participant transitioned to either mild cognitive impairments or global impairment as opposed to intact cognition.) These adjusted odds ratios in table 3 parallel the crude results in table 2. Consistent with the above multivariate results, table 2 results for the same example showed that if the cognitive state at the previous examination was global impairment, then the cognitive state at the next examination was much more likely to remain global impairment (39.2 percent of transitions) than to improve to intact cognition (4.2 percent) (the comparison group was intact cognition at the previous examination declining to global impairment at the next examination (5.8 percent) rather than remaining intact (65.3 percent)).
TABLE 3.
Effects of age, education, apolipoprotein E *E4 allele, and prior cognitive state on the risk of transitions from intact cognition to mild cognitive impairments or global impairment in the Nun Study, United States, 1991–2002 (1,584 transitions in 392 subjects)*
| Characteristic | Mild cognitive impairments |
Global impairment |
||
|---|---|---|---|---|
| OR† | 95% CI† | OR | 95% CI | |
| Age (years) | 1.06 | 1.03, 1.09 | 1.15 | 1.10, 1.20 |
| Education | ||||
| ≤High school | 2.36 | 1.26, 4.42 | 2.79 | 1.32, 5.91 |
| Undergraduate degree | 1.53 | 1.17, 2.00 | 1.62 | 1.10, 2.38 |
| Graduate degree | 1.00 | Reference | 1.00 | Reference |
| Apolipoprotein E *E4 allele | ||||
| Present | 1.87 | 1.27, 2.73 | 3.02 | 1.87, 4.89 |
| Absent | 1.00 | Reference | 1.00 | Reference |
| Prior cognitive state | ||||
| Intact cognition | 0.18 | 0.09, 0.38 | 0.02 | 0.01, 0.03 |
| Mild cognitive impairments | 1.82 | 0.90, 3.68 | 0.11 | 0.06, 0.21 |
| Global impairment | 1.00 | Reference | 1.00 | Reference |
Number of transitions excludes transitions to dementia or death; number of subjects excludes participants who experienced these transitions to dementia or death only.
OR, odds ratio; CI, confidence interval.
Table 4 summarizes the risk of transition from each transient nondemented state to dementia or death in comparison to remaining in the transient state. Only age was a significant risk factor for transition from mild cognitive impairments to dementia or death, with each additional year of age increasing the risk 7 percent for both outcomes. APOE was a significant predictor of transitions from intact cognition to both dementia and death. However, the effect was much stronger for dementia (odds ratio = 9.43, 95 percent confidence interval: 1.27, 69.90) than for death (odds ratio = 2.58, 95 percent confidence interval: 1.06, 6.28).
TABLE 4.
Risk of transition from a given transient state (intact cognition, mild cognitive impairments, or global impairment) to dementia or death in the Nun Study, United States, 1991–2002 (1,905 transitions in 470 subjects)
| Characteristic | Dementia |
Death |
||
|---|---|---|---|---|
| OR* | 95% CI* | OR | 95% CI | |
| Transition from intact cognition | ||||
| Age (years) | 1.13 | 0.93, 1.37 | 1.11 | 1.02, 1.22 |
| Education | ||||
| ≤High school | 41.48 | 4.00, 42.40 | 1.24 | 0.15, 10.2 |
| Undergraduate degree | 2.07 | 0.28, 15.10 | 0.92 | 0.42, 2.01 |
| Graduate degree | 1.00 | Reference | 1.00 | Reference |
| Apolipoprotein E *E4 allele | ||||
| Present | 9.43 | 1.27, 69.90 | 2.58 | 1.06, 6.28 |
| Absent | 1.00 | Reference | 1.00 | Reference |
| Transition from mild cognitive impairments | ||||
| Age (years) | 1.07 | 1.02, 1.12 | 1.07 | 1.02, 1.12 |
| Education | ||||
| ≤High school | 1.11 | 0.49, 2.53 | 1.38 | 0.64, 2.97 |
| Undergraduate degree | 0.76 | 0.45, 1.29 | 0.92 | 0.55, 1.52 |
| Graduate degree | 1.00 | Reference | 1.00 | Reference |
| Apolipoprotein E *E4 allele | ||||
| Present | 1.12 | 0.60, 2.08 | 1.10 | 0.60, 2.00 |
| Absent | 1.00 | Reference | 1.00 | Reference |
| Transition from global impairment | ||||
| Age (years) | 1.05 | 0.99, 1.11 | 1.03 | 0.97, 1.00 |
| Education | ||||
| ≤High school | 0.61 | 0.23, 1.59 | 0.44 | 0.19, 1.02 |
| Undergraduate degree | 1.00 | 0.50, 2.00 | 0.56 | 0.30, 1.06 |
| Graduate degree | 1.00 | Reference | 1.00 | Reference |
| Apolipoprotein E *E4 allele | ||||
| Present | 2.32 | 1.17, 4.58 | 0.92 | 0.45, 1.88 |
| Absent | 1.00 | Reference | 1.00 | Reference |
OR, odds ratio; CI, confidence interval.
Table 5 summarizes the influence of factors on the long-term trajectory of cognitive changes from intact cognition to dementia, in contrast to the previous analyses of predictors of one-step transitions (between two consecutive assessments). The odds of developing dementia before death increased with age primarily for those with low educational levels. Participants with medium levels of education had odds of dementia intermediate between those for subjects with high and low levels of education. The presence of an APOE*E4 allele increased the risk of dementia regardless of educational level but had its strongest effect on those with low levels of education. For these subjects, the odds of dementia increased by more than twofold at age 75 years (2.23 for APOE*E4+ vs. 0.92 for APOE*E4−) and four-fold by age 95 years (4.88 for APOE*E4+ vs. 1.17 for APOE*E4−).
TABLE 5.
Odds of developing dementia before death for women with intact cognition in the Nun Study, United States, 1991–2002 (n = 470 subjects)
| Presence of an apolipoprotein E gene *E4 allele |
Age (years) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Educational level* | 75 |
80 |
85 |
90 |
95 |
||||||
| Odds | SE† | Odds | SE | Odds | SE | Odds | SE | Odds | SE | ||
| No | Low | 0.92 | 0.065 | 0.92 | 0.062 | 0.96 | 0.054 | 1.04 | 0.046 | 1.17 | 0.050 |
| Medium | 0.56 | 0.035 | 0.59 | 0.022 | 0.61 | 0.016 | 0.64 | 0.018 | 0.64 | 0.020 | |
| High | 0.45 | 0.040 | 0.47 | 0.027 | 0.47 | 0.019 | 0.47 | 0.019 | 0.47 | 0.022 | |
| Yes | Low | 2.23 | 0.319 | 2.57 | 0.287 | 3.17 | 0.243 | 4.00 | 0.201 | 4.88 | 0.186 |
| Medium | 0.89 | 0.060 | 1.00 | 0.054 | 1.04 | 0.050 | 1.08 | 0.049 | 1.08 | 0.059 | |
| High | 0.64 | 0.045 | 0.67 | 0.034 | 0.69 | 0.030 | 0.69 | 0.027 | 0.67 | 0.026 | |
Low, ≤high school; medium, undergraduate degree; high, graduate degree.
SE, standard error.
DISCUSSION
Much of the research on risk factors for progression from mild cognitive impairments to dementia has focused on clinical predictors, such as neuropsychologic test performance, despite the recommendation that inclusion of factors other than cognitive test performance would increase the utility of clinical predictive models (29). In contrast, relatively little has been done to examine more traditional epidemiologic risk factors for progression from mild cognitive impairments to dementia, with even fewer studies of risk factors for developing mild cognitive impairments (30).
In our study, a novel analytic strategy was used to determine predictors of transitions in cognitive status through the full spectrum from intact cognition to dementia and death. Age, education, and APOE influenced the odds of developing dementia, with those who had the lowest level of education most influenced by APOE status. In addition, age, education, and APOE were all significant predictors of the transition from intact cognition to mild cognitive impairments. However, only age was a significant risk factor for transition from mild cognitive impairments to dementia. Thus, these established risk factors for dementia appear to operate primarily by starting persons on the road to dementia, with subsequent progression to dementia depending on only time (age) and competing mortality.
Our analytic approach showed face validity, producing results consistent with those of other studies using more traditional analytic strategies. Age (31-33), education (31-33), and APOE (31, 32) have been associated with the development of mild cognitive impairments. Studies of the progression from mild cognitive impairments to dementia have found age to be a significant risk factor (29, 34-36), but educational level (29, 34-36) and APOE (34, 35) did not predict progression to dementia. In contrast, APOE status was found to be a significant predictor of progression to Alzheimer's disease in some studies (36-38), but this finding may be related to the particular definition of mild cognitive impairments used and the specific dementia subtype (Alzheimer's disease) examined. There may also be a complex interaction with age (13, 38, 39).
A Markov chain was used as the foundation of our analytic strategy. Thus, cognitive status at a given examination was assumed to depend on only the most recent previous examination. This Markov property was illustrated in another study of cognitive status (26), where the probability of advancing to more severe Alzheimer's disease was found to be independent of the person's previous severity of cognitive impairments. Studies of the progression of cognitive impairments have typically modeled it as a requisite right-shift process with progression inexorably to poorer cognitive status, despite many studies documenting improvements in cognitive status (refer to the review by Palmer et al. (3), for example). A strength of the study by Neumann et al. (26) was inclusion of backward transitions. However, their analysis, based on a Cox proportional hazards model, was limited by the small number of transitions in some subgroups and, as presented, is applicable to only advanced Alzheimer's disease (40). Fuh et al. (41) replicated their method to estimate transition probabilities but studied transitions between only Alzheimer's disease severity states and death. Our method, however, allows for both forward and backward transitions within the entire spectrum of cognition, from intact cognition to dementia and death. In addition, the analytic strategy developed is simple to implement because it uses a standard software package (SAS), an advantage over similar approaches that require customized software (42).
Our study shares the disadvantage of other such studies in investigating a condition (mild cognitive impairments) that lacks a standard definition. In addition, a limitation of this study, common to many others, is the inherent arbitrariness of dividing a continuous process of cognitive decline into discrete categories of cognitive status. The Markov property used as the basis for the analytic strategy assumes that cognitive status depends on status at only the previous examination and thus does not account for heterogeneity in the cognitive status history of a person. Cognitive status at earlier examinations may, however, also play a role. Finally, although gender does not appear to be a consistent predictor of development of mild cognitive impairments (31, 32) or progression to dementia (29, 34, 35), we were unable to investigate its effects in this study because all of our participants were women. Similarly, homogeneity in participants' lifestyles and environments precluded investigation of the potential influence of these factors on disease progression.
However, strengths of our study are inclusion of seven rounds of annual examinations in a well-characterized, longitudinal cohort of very old women. The high level of homogeneity in the participants' lifestyles and environments minimizes or eliminates many factors that may confound other epidemiologic studies. In addition, although information can be lost when categorizing continuous data, we divided cognitive status into multiple categories and used more categories of cognitive impairment than have usually been included. Finally, our statistical strategy more closely modeled reality by allowing for transitions in both directions: to poorer or to better cognitive status.
This study examined the influence of established risk factors for dementia on transitions between various cognitive states in the progression from intact cognition to dementia. The analytic strategy used standard software to provide a novel and effective method to determine predictors of transitions in cognitive status. We defined dementia as an absorbing state because predictors of transitions from dementia were not of interest in this study. Our analytic model, however, could be expanded to determine predictors of transitions from dementia to death as well as to model the influence of other risk factors for progression to dementia. In addition, this approach could be applied to other progressive conditions where transitions between states are of interest.
If risk factors for mild cognitive impairments are the same as those for dementia, and if there are no unique risk factors for the progression of mild cognitive impairments to dementia, then mild cognitive impairments may simply reflect an early stage of dementia. The results of the current study suggest that some risk factors for dementia may operate primarily by predisposing persons to develop mild cognitive impairments. Once they are so affected, the development of dementia may depend on only the passage of time and competing mortality. Thus, our findings support the position that mild cognitive impairments indeed reflect early dementia.
ACKNOWLEDGMENTS
This study was funded by National Institute on Aging grants R01 AG09862, K04 AG00553, and P50 AG05144 and by a grant from the Kleberg Foundation.
This study would not have been possible without the support of the members, leaders, and health care providers of the School Sisters of Notre Dame religious congregation. The authors also thank Jeanne Sturgill and Mary Roycraft, members of the Nun Study team who provided invaluable assistance on this project.
Abbreviation
- APOE
apolipoprotein E gene
APPENDIX
Let Y = (Y1, Y2, … , YT) be the random vector representing the observed states for a typical person whose cognitive status is assessed on T equally spaced occasions. Following the method of Diggle et al. (43), we adopted a transition model for Y by focusing on the conditional distribution of Y given Y1, which depends on a vector of unknown parameters θ. Assume that the Markov property holds. Rather than simply expressing h(y | y1, θ), the conditional distribution, as the product of elements from the one-step transition matrix P(θ)5x5, as suggested by Guo and Marshall (42), we chose instead to rewrite this distribution as follows:
| (1) |
Here, δ is the Kronecker's delta function, psl(θ) is the one-step transition probability from state s to state l, and gsl (θ) is the conditional probability of a transition from the transient state s to the transient state l given that the subject has not been absorbed. In this expression, we have assumed that the person transitions among transient states during the first T – 1 examinations and, on examination T, transitions to either one of the absorbing states. It is possible that the person makes all transitions among only transient states, in which case the latter two terms do not appear in equation 1. It is also possible that the person makes no transitions among transient states, in which case the first terms do not appear in equation 1.
We assume that the vector of unknown parameters satisfies a separability property described as follows: θ = (θ0, θ1, θ2, θ3), where θ0 measures the effect of the covariates on transitions among the transient states, whereas θs for s = 1, 2, 3 measures the effect of the covariates on transitions from transient state s to the absorbing states (notice that 1 = intact cognition, 2 = mild cognitive impairments and 3 = global impairment). When this separability property is used, the likelihood in equation 1 becomes the product of four likelihood functions, each depending on a separate subvector of θ.
Polytomous logistic regression models are used to express the probabilities in equation 1 as functions of the covariates. If b represents the “base” state, then the transition probabilities among the transient states conditioned on nonabsorption can be expressed as
| (2) |
Here, ω1 and ω2 are defined as
Also in equation 2, z represents the vector of covariates, and θ0 = (α2, α3, λ12, λ22, λ13, λ23, β2, β3.
On the other hand, the transition probabilities from the transient state s to absorption are given by
| (3) |
Here, θs = (αs4, αs5, βs4, βs5).
The conditional probabilities in equation 2 are linked to the unconditional transition probabilities through the following equation:
| (4) |
The one-step transition matrix is obtained from equations 3 and 4 and can be written in canonical form as follows:
Here, Q(θ) is a (3 × 3) matrix containing the probabilities of transitions among transient states, and R(θ) is a (3 × 2) matrix containing the probabilities of transitions from transient to absorbing states. To obtain the odds of developing dementia before dying, we use a well-known result from the theory of stochastic process (27) that enables us to calculate the probability of eventual passage to the absorbing class by means of the following matrix:
When this matrix and the values of the covariates of interest are used, the odds of eventual absorption into dementia before death can be obtained by dividing the entry in the first column of F(θ) by the corresponding entry in the second column. The standard error for these odds can be estimated by using a bootstrap procedure (28). In this procedure, 200 replicates of the observed data were obtained by sampling with replacement (dropping one randomly selected subject per replicate). The variability in the resulting estimates of the odds ratios across these replicates yields the desired standard errors.
Footnotes
Conflict of interest: none declared.
REFERENCES
- 1.Celsis P. Age-related cognitive decline, mild cognitive impairment or preclinical Alzheimer's disease? Ann Med. 2000;32:6–14. doi: 10.3109/07853890008995904. [DOI] [PubMed] [Google Scholar]
- 2.Morris JC, Storandt M, Miller P, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 3.Palmer K, Fratiglioni L, Winblad B. What is mild cognitive impairment? Variations in definitions and evolution of nondemented persons with cognitive impairment. Acta Neurol Scand. 2003;107:14–20. doi: 10.1034/j.1600-0404.107.s179.2.x. [DOI] [PubMed] [Google Scholar]
- 4.Riley KP, Snowdon DA, Markesbery WR. Alzheimer's neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002;51:567–77. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DA, Schneider JA, Bienias JL, et al. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–41. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 6.Petersen RC, Parisi JE, Dickson DW. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–72. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 8.Milwain E. Mild cognitive impairment: further caution. (Letter) Lancet. 2000;355:1018. doi: 10.1016/S0140-6736(05)74764-4. [DOI] [PubMed] [Google Scholar]
- 9.Ritchie K, Touchon J. Mild cognitive impairment: conceptual basis and current nosological status. Lancet. 2000;355:225–8. doi: 10.1016/S0140-6736(99)06155-3. [DOI] [PubMed] [Google Scholar]
- 10.Petersen RC, Stevens JC, Ganguli M, et al. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review): report of the Quality Standards Sub-committee of the American Academy of Neurology. Neurology. 2001;56:1133–42. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 11.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 12.Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367:1262–70. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 13.Luis C, Loewenstein DA, Acevedo A, et al. Mild cognitive impairment: directions for future research. Neurology. 2003;61:438–44. doi: 10.1212/01.wnl.0000080366.90234.7f. [DOI] [PubMed] [Google Scholar]
- 14.Hogan DB, Ebly EM. Predicting who will develop dementia in a cohort of Canadian seniors. Can J Neurol Sci. 2000;27:18–24. doi: 10.1017/s0317167100051921. [DOI] [PubMed] [Google Scholar]
- 15.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 16.Storandt M, Grant EA, Miller JP, et al. Rates of progression in mild cognitive impairment and early Alzheimer's disease. Neurology. 2002;59:1034–41. doi: 10.1212/wnl.59.7.1034. [DOI] [PubMed] [Google Scholar]
- 17.Snowdon DA, Kemper SJ, Mortimer JA, et al. Linguistic ability in early life and cognitive function and Alzheimer's disease in late life. JAMA. 1996;275:528–32. [PubMed] [Google Scholar]
- 18.Snowdon DA. Aging and Alzheimer's disease: lessons from the Nun Study. Gerontologist. 1997;37:150–6. doi: 10.1093/geront/37.2.150. [DOI] [PubMed] [Google Scholar]
- 19.Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of Alzheimer disease: the Nun Study. JAMA. 1997;277:813–17. [PubMed] [Google Scholar]
- 20.Snowdon DA. Aging with grace: what the Nun Study teaches us about leading longer, healthier, and more meaningful lives. Bantam Books; New York, NY: 2001. [Google Scholar]
- 21.Riley KP, Snowdon DA, Desrosiers MF, et al. Early life linguistic ability, late life cognitive function, and neuropathology: findings from the Nun Study. Neurobiol Aging. 2005;26:341–7. doi: 10.1016/j.neurobiolaging.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–65. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 23.Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–14. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 24.Potvin AR, Tourtellotte WW, Dailey JS, et al. Simulated activities of daily living examination. Arch Phys Med Rehabil. 1972;53:476–86. [PubMed] [Google Scholar]
- 25.Kuriansky J, Gurland B. The performance test of activities of daily living. Int J Aging Hum Dev. 1976;7:343–52. doi: 10.2190/x45l-tww7-wxxy-ka6k. [DOI] [PubMed] [Google Scholar]
- 26.Neumann PJ, Araki SS, Arcelus A, et al. Measuring Alzheimer's disease progression with transition probabilities. Neurology. 2001;57:957–64. doi: 10.1212/wnl.57.6.957. [DOI] [PubMed] [Google Scholar]
- 27.Bhat UN. Elements of applied stochastic processes. Wiley; New York, NY: 1984. [Google Scholar]
- 28.Efron B, Tibshirani RJ. An introduction to the bootstrap. Chapman & Hall/CRC; New York, NY: 1993. [Google Scholar]
- 29.Tian J, Bucks RS, Haworth J, et al. Neuropsychological prediction of conversion to dementia from questionable dementia: statistically significant but not yet clinically useful. J Neurol Neurosurg Psychiatry. 2003;74:433–8. doi: 10.1136/jnnp.74.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritchie K. Mild cognitive impairment: an epidemiologic perspective. Dialogues Clin Neurosci. 2004;6:401–8. doi: 10.31887/DCNS.2004.6.4/kritchie. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 2. Arch Neurol. 2003;60:1394–9. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 32.Tervo S, Kivipelto M, Hänninen T, et al. Incidence and risk factors for mild cognitive impairment: a population-based three-year follow-up study of cognitively healthy elderly subjects. Dement Geriatr Cogn Disord. 2004;17:196–203. doi: 10.1159/000076356. [DOI] [PubMed] [Google Scholar]
- 33.Solfrizzi V, Panza F, Colacicco AM, et al. Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology. 2004;63:1882–91. doi: 10.1212/01.wnl.0000144281.38555.e3. [DOI] [PubMed] [Google Scholar]
- 34.Amieva H, Letenneur L, Dartigues JF, et al. Annual rate and predictors of conversion to dementia in subjects presenting mild cognitive impairment criteria defined according to a population-based study. Dement Geriatr Cogn Disord. 2004;18:87–93. doi: 10.1159/000077815. [DOI] [PubMed] [Google Scholar]
- 35.Kryscio RJ, Schmitt FA, Salazar JC, et al. Risk factors for transitions from normal to mild cognitive impairment and dementia. Neurology. 2006;66:828–32. doi: 10.1212/01.wnl.0000203264.71880.45. [DOI] [PubMed] [Google Scholar]
- 36.Aggarwal NT, Wilson RS, Beck TL, et al. The apolipoprotein E ε4 allele and incident Alzheimer's disease in persons with mild cognitive impairment. Neurocase. 2005;11:3–7. doi: 10.1080/13554790490903038. [DOI] [PubMed] [Google Scholar]
- 37.Petersen RC, Smith GE, Ivnik RJ, et al. Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory-impaired individuals. JAMA. 1995;273:1274–8. [PubMed] [Google Scholar]
- 38.Hsiung GY, Sadovnick AD, Feldman H. Apolipoprotein E-ε4 genotype as a risk factor for cognitive decline and dementia: data from the Canadian Study of Health and Aging. Can Med Assoc J. 2004;171:863–7. doi: 10.1503/cmaj.1031789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devanand DP, Pelton GH, Zamora D, et al. Predictive utility of apolipoprotein E genotype for Alzheimer disease in outpatients with mild cognitive impairment. Arch Neurol. 2005;62:975–80. doi: 10.1001/archneur.62.6.975. [DOI] [PubMed] [Google Scholar]
- 40.Mendiondo MS, Kryscio RJ, Schmitt FA. Models of progression in AD: predicting disability and costs. Neurology. 2001;57:943–4. doi: 10.1212/wnl.57.6.943. [DOI] [PubMed] [Google Scholar]
- 41.Fuh JL, Pwu RF, Wang SJ, et al. Measuring Alzheimer's disease progression with transition probabilities in the Taiwanese population. Int J Geriatr Psychiatry. 2004;19:266–70. doi: 10.1002/gps.1076. [DOI] [PubMed] [Google Scholar]
- 42.Guo W, Marshall G. ORDMKV: a computer program fitting proportional odds model for multi-state Markov process. Comput Methods Programs Biostat. 1995;46:257–63. doi: 10.1016/0169-2607(95)01625-4. [DOI] [PubMed] [Google Scholar]
- 43.Diggle PJ, Heagerty P, Liang KY, et al. Analysis of longitudinal data. Oxford University Press; New York, NY: 2002. [Google Scholar]


