Abstract
The CLC family of Cl−-transporting proteins includes both Cl− channels and Cl−/H+ exchange transporters. CLC-ec1, a structurally known bacterial homolog of the transporter subclass, exchanges two Cl− ions per proton with strict, obligatory stoichiometry. Point mutations at two residues, Glu148 and Tyr445, are known to impair H+ movement while preserving Cl− transport. In the x-ray crystal structure of CLC-ec1, these residues form putative “gates” flanking an ion-binding region. In mutants with both of the gate-forming side chains reduced in size, H+ transport is abolished, and unitary Cl− transport rates are greatly increased, well above values expected for transporter mechanisms. Cl− transport rates increase as side-chain volume at these positions is decreased. The crystal structure of a doubly ungated mutant shows a narrow conduit traversing the entire protein transmembrane width. These characteristics suggest that Cl− flux through uncoupled, ungated CLC-ec1 occurs via a channel-like electrodiffusion mechanism rather than an alternating-exposure conformational cycle that has been rendered proton-independent by the gate mutations.
Controlled movement of solutes across biological membranes is implemented by membrane proteins of two types: channels and transporters. These are commonly viewed as profoundly dissimilar in structure and function because of fundamental disparities in their modes of substrate transport. Channels, by definition, connect the two sides of the membrane via continuous watery pathways through which polar molecules passively diffuse down their thermodynamic gradients. Because energy barriers for aqueous diffusion are small, channel-mediated transport is fast (1), with ion-throughput rates of 106–108 s−1. Transporters, in contrast, operate by a cycle of conformational changes that expose substrate-binding sites alternately to the two sides of the membrane (2, 3). Like channels, they may be used to move solutes down their gradients, but in contrast to channels, many transporters are coupled to energy sources so as to drive substrates thermodynamically uphill. Regardless of whether they work as passive transmembrane facilitators or as energy-driven pumps, transporters are much slower than channels, with typical turnover rates (1–1,000 s−1) that reflect the larger energy barriers involved in protein rearrangements necessary for the transport cycle.
A growing collection of high-resolution crystal structures is currently unveiling the chemical basis for substrate binding, selectivity, and transmembrane movement in both classes of membrane-transport proteins (4). In many cases, function and structure are mutually enlightening, but some notable exceptions stand out, of which arguably the most peculiar is CLC-ec1, a bacterial homolog of the widespread CLC family of proteins that move Cl− ions across membranes in most organisms (5, 6). This protein was thought to be a Cl− channel when initially described (7–10), but on close electrophysiological scrutiny it turned out to be a Cl−/H+ exchange transporter (11). This surprising result led swiftly to the realization that the entire CLC family is split into channel and transporter subclasses (12–15); the human genome, for instance, encodes four CLC channels and five CLC transporters. The predictive utility of CLC-ec1, a prokaryotic transporter, as a structural model for eukaryotic channels verifies the sequence-based inference that the same structural scaffold has been adapted to support either diffusive or conformational mechanisms of Cl− movement in this single molecular family (16, 17).
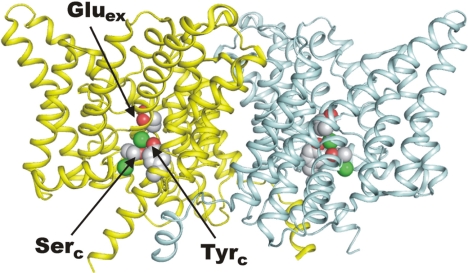
This work continues our examination of CLC-ec1, in which we have sought to identify regions of the protein responsible for the coordinated countermovements of Cl− and H+. Although the mechanism of exchange is unknown, several of its basic features are established. CLC-ec1 transports its substrate ions with a strict, obligatory stoichiometry of 2 Cl− for 1 H+ (11, 18). The protein is a homodimer (Fig. 1) in which each subunit acts as a full-fledged transporter (19). Each subunit contains multiple ion-binding sites located on structurally delineated transmembrane “pathways” along which Cl− and H+ move in opposite directions. These pathways overlap on the extracellular side of the protein and split apart approximately halfway across the membrane, emerging at distant points on the intracellular surface of the protein (20). The Cl− pathway, the subject of this work, is marked by “gates” on each end (Fig. 1). The extracellular gate is formed by a conserved glutamate side chain, Glu148, here denoted Gluex, that blocks off the Cl− pathway from the external solution when deprotonated; upon protonation, the side chain swings outward, opening the Cl− pathway to the outside (10). These two side-chain configurations have been observed crystallographically, the “closed” in wild-type (WT) protein, and the “outward-open” in a mutant with glutamine substituted to mimic a protonated carboxyl group. The intracellular gate is not so clearly understood, but crystal structures suggest that it is formed by the close apposition of two conserved hydroxyl-bearing side chains, Tyr445 and Ser107, denoted the “central” tyrosine and serine, Tyrc and Serc, respectively (9, 10). In crystal structures of CLC-ec1, this putative gate blocks off the Cl− pathway to the intracellular solution, except in mutants with small side chains substituted for Tyrc, where this “pinch point” becomes wider (21). We emphasize, however, that direct involvement of Tyrc in forming an inner gate has not been demonstrated functionally.
Fig. 1.
Structure of CLC-ec1. Ribbon diagram of WT CLC-ec1 is shown (PDB ID code 1OTS), extracellular side up, with Cl− ions as green spheres. (Left) Rendered in minimally cutaway view to visualize more easily side chains Gluex at the outer gate and Tyrc and Serc at the inner gate, as indicated.
Previous work identified hot spots on the Cl− and H+ pathways where mutations produce uncoupled CLC-ec1 transporters, such that H+ movement is impaired to varying degrees but Cl− movement persists (11, 20–22). We now focus on mutants at two such residues that flank the central Cl− ion, Gluex and Tyrc, in which H+ transport is abolished. These mutants transport Cl− via a conductive “leak” through the protein, but the detailed nature of this leak is not understood (22). By combining mutations at these two positions to produce uncoupled transporters with exceptionally high unitary turnover rates, we find that Cl− movement through these uncoupled transporters is by a channel-like diffusive mechanism; a corollary of this conclusion is that Tyrc is indeed part of the intracellular gate of the fully coupled WT exchanger.
Results
We recently determined unitary transport rates for CLC-ec1 and a panel of uncoupled mutants by measuring Cl− efflux from liposomes carrying only a single copy of the transporter (22). Under our standard assay conditions (Fig. 2), WT CLC-ec1 transports ≈2,000 Cl− ions per s, a rate near the high end of the range characteristic of transporter mechanisms. Mutants with Tyrc replaced by smaller residues (Gly, Ala, or Ser) are impaired for H+ movement, but their Cl− transport rates are not much different from WT values, as illustrated for Y445A in Fig. 2 [and reported, as for all mutants here, in supporting information (SI) Table S1]. This result might mean that the rate-determining step for Cl− transport occurs at a point in the conformational cycle that does not involve inner-gate opening or that Tyrc is not part of an inner gate.
Fig. 2.
Unitary Cl− efflux via CLC-ec1 gate mutants. Traces of Cl− appearance (normalized to final Cl− concentration) in a suspension of liposomes reconstituted with various CLC-ec1 constructs, under conditions in which liposomes contain ≈1 transporter on average are shown. Efflux was initiated by valinomycin/FCCP addition, and Cl− concentration was followed by a Ag/AgCl electrode. Liposomes were loaded with 300 mM Cl−, and external Cl− typically increased during the time course from 1.1 mM to 1.3 mM. Time courses of Cl− efflux were fit (red) by single exponentials with slow linear leak as described in ref. 22. Experiment was ended by addition of detergent to disrupt all liposomes, including those devoid of protein (abrupt increase in Cl− at end of red fits). Shown for illustrative purposes are: WT (E/Y), outer gate removed (A/Y), inner gate removed (E/A), and both gates removed (A/A). Unitary turnover rates derived from such traces are reported in Table S1 and Fig. 4.
To explore this question further, we studied a “single-gate” construct, E148A, devoid of the extracellular gate. This mutant has been described at both structural and functional levels. The x-ray crystal structure shows the Cl− pathway to be continuous with the extracellular solution because the Gluex side chain is absent (10). Moreover, this mutation nullifies the crucial function of Gluex to deliver extracellular protons into the transport machinery and thereby abolishes H+ transport (11). Cl−, however, still moves through this uncoupled mutant (Fig. 2) at a rate ≈5-fold lower than in WT. (E148Q, similarly uncoupled, is also 5-fold slower than WT.) This result is unsurprising for an exchange transporter, for which impairment of the movement of one substrate is often accompanied by inhibition of the movement of the other, possibly via a stalled conformation of the transport cycle. For E148A, it is natural to envision a Cl−-bound conformation awaiting a proton that fails to arrive. If there is any surprise at all here, it is that the mutant Cl− transport rate is depressed only 5-fold, i.e., that the transporter, being unable to move H+, is not stalled more severely than observed. The question thus arises of how the uncoupled protein moves Cl−: by a damaged conformational cycle proceeding at a low rate without proton involvement or by channel-like slippage through a fixed Cl− pathway, with a closed inner gate occasionally fluctuating open. We address this question below.
Combined Substitution of Gluex and Tyrc Side Chains in Uncoupled Mutants.
We showed (22) that removing the Cl− pathway presumptive inner gate by placing small side chains at Tyrc has only minimal effects on Cl− transport rate; analogous surgery on the outer gate at Gluex results in substantial inhibition of Cl− flux (Fig. 2). If both gates were to be widened simultaneously, the Cl− pathway might allow diffusion of Cl− ions across the entire membrane. In other words, if Tyrc and Gluex do in fact form physical gates of the Cl− pathway, as crystal structures suggest, then simultaneous substitution of these with small residues would be expected to create a persistently open Cl− channel. To test this possibility, we investigated ion-transport properties of a variety of double mutants, E148X1/Y445X2, hereafter denoted “X1/X2” to indicate residues at the outer- and inner-gate positions, respectively. These mutants are expected to be completely H+-uncoupled because they all lack the proton-transfer carboxylate at Gluex. We verified this expectation for A/A by recording currents in planar lipid bilayers, where current–voltage curves reverse close to the Cl− Nernst potential in Cl− gradients and fail to shift in pH gradients, two direct indicators of uncoupling (11) (Fig. S1).
A representative unitary Cl− efflux time course of a doubly ungated mutant, A/A, is compared with those of WT and the single-gate mutants in Fig. 2. This mutant is unusually fast, ≈20,000 s−1, 50-fold faster than the parental, uncoupled single-gate mutant A/Y and 10-fold faster than fully coupled WT protein, far and away the highest rate observed among all mutants tested since we began work on CLC-ec1 (7, 22). Thus, the doubly ungated mutant stands out as somehow distinct from the WT and singly mutated transporters. But is it a Cl− channel? That conclusion is at best soft; this rate is higher than for any previously studied transporter of any molecular family, but it is still 1–2 orders of magnitude lower than those typically observed in channels by electrical recording of unitary current fluctuations. Single-channel recordings of a human channel-type homolog, CLC-1, for instance, give turnover rates ≈20-fold higher than observed for A/S, our fastest double mutant (23).
Glu148 and Tyr445 Physically Cap the Cl− Pathway.
The dramatic rate enhancement in the combined gate mutants provides a functionally based suggestion that these positions cap a channel-like conduit through which Cl− ions diffuse. However, alternative explanations might account for the high Cl− flux of the double mutants. It is possible, for instance, that the substitutions merely lower energy barriers between intermediates visited in the normal transport cycle. This is admittedly an unattractive possibility because the single mutations have the opposite effect, but the idea that the combination of mutants adventitiously speeds the conformational cycle cannot be rejected solely on aesthetic grounds. A channel mechanism, with side chains at positions 148 and 445 acting sterically as occlusive doors, makes an additional and testable prediction: an inverse relationship between Cl− transport rate and side-chain volume at either gate when the other is kept open.
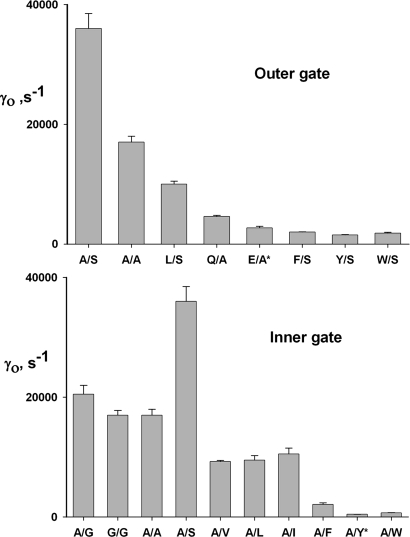
Accordingly, we performed two series of experiments on uncoupled CLC-ec1 mutants at these presumed gate positions. First, with the inner gate widened by small-residue substitutions, different side chains at Gluex were tested. Serine was used at the inner gate for most of these, because this residue produces the highest flux in the doubly ungated mutants above. As anticipated, the Cl− transport rate decreases with the outer-gate side-chain volume: A/S, A/A > L/S > Q/A, E/A > F/S, Y/S, W/S (illustrative raw traces in Fig. 3 and turnover rates for the full complement of mutants in Fig. 4). Conversely, with the outer gate removed (Ala substituted for Gluex), progressively larger residues at the inner gate produce a similarly graded impairment of Cl− transport (A/S > A/G > A/A > A/V, A/I, A/L > A/F > A/Y, A/W), the single exception to a strict pattern being the higher rate observed for A/S, which is almost twice the rate of A/A and 80-fold higher than the parental single-gate mutant A/Y. Only a few mutations at Ser107, the other residue thought to contribute to the inner gate, were studied because opportunities for shortening this side chain are limited. The single mutant S107G is strongly uncoupled (data not shown) and is 3-fold faster than WT; the triple mutant S107G on the A/G background is no faster than A/G and is actually 25% slower than this double mutant, in violation of our simple steric expectations.
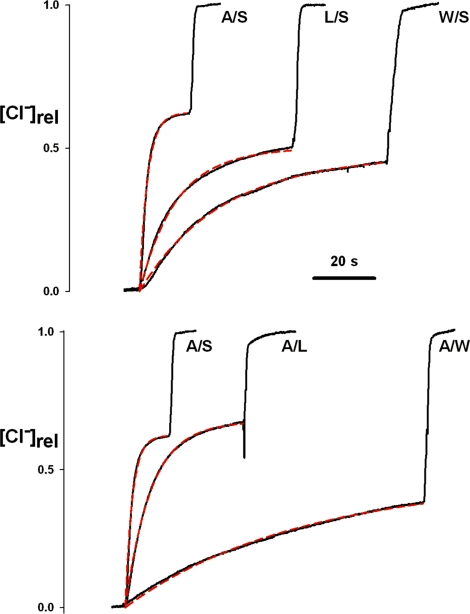
Fig. 3.
Cl− efflux traces of ungated mutants. Cl− efflux time courses were followed as in Fig. 2, for the indicated CLC-ec1 mutants. (Upper) Variation of the outer gate, with inner gate removed. (Lower) Variation of the inner gate, with outer gate removed.
Fig. 4.
Unitary Cl− transport rates for gate mutants. Cl− efflux traces as in Figs. 2 and 3 were analyzed to derive the unitary transport rates, γo, of the indicated mutants. (Upper) Variation of side chain at outer gate with inner gate removed. (Lower) Variation of inner gate with outer gate removed. Mutants with asterisks indicate single-gate mutants, i.e., with one WT gate residue.
Crystal Structure of an Ungated Mutant.
The enhanced Cl− transport rates observed do not reflect gross structural alteration in the mutants since the proteins are biochemically and electrophysiologically well behaved, homodimeric, and ideally Cl−-selective. The double mutants are somewhat refractory to crystallization, but we obtained a single dataset of A/A suitable for structural analysis at 2.8-Å resolution (Fig. S2 and Table S2). The crystal structure of this mutant is basically unaltered from WT except for an open pathway at each gate region; low-resolution anomalous difference maps from an A/A crystal grown in Br− (data not shown) confirm that ion occupancy of the anion pathway is as expected from the single-gate mutants E148A and Y445A (10, 21), with high halide occupancy of the inner and outer sites and low occupancy in the central site. The degree of “openness” of these gate regions was examined with the program HOLE (24), which visualizes conduits and cavities in proteins by a Monte Carlo algorithm that moves a probe of maximum radius through a static structure represented by a Protein Data Bank file. A narrow conduit worms its way into the WT protein from the cytoplasmic side, with a tight (≈1 Å) constriction near Tyrc and a complete blockage at Gluex (Fig. 5). This finding is as expected if these residues act as steric gates flanking the central Cl− ion. In the doubly ungated A/A structure, an unambiguous but exceedingly narrow conduit traverses the entire transmembrane width of the protein. The conduit is narrower, in places, even than Cl− ion itself. Electrodiffusive passage of ions through channels as narrow as this must rely on protein dynamics, thermally driven fluctuations in structure that transiently open a pathway for substrate diffusion (25). This may account for the fact that Cl− permeation in our fastest uncoupled mutants is still slow by ion channel standards.
Fig. 5.
Conduits through CLC-ec1. The HOLE program was used to visualize Cl− pathways through CLC-ec1 and a doubly ungated mutant. Images made in Pymol show translucent surface representation of CLC-ec1 homodimer, with Cl− ions (green spheres) marking the anion-binding sites and HOLE conduits (red dots). Each conduit shown was synthesized from ≈50 separate HOLE runs; only trajectories that coincided with the Cl−-binding sites were selected for display. (Left) WT protein (PDB ID code 1OTS), showing the gate residues Gluex and Tyrc in space-filled representation. (Right) Doubly ungated A/A mutant (PDB ID code 3DET). A third Cl− ion, located at the same position as the Gluex side chain of the WT, is crystallographically observed in all mutants with Gluex mutated to externally open residues.
Discussion
All transporters ultimately rely on a cycle of conformational changes in which substrate-binding sites switch access between the two sides of the membrane. This process is often schematized as a substrate-binding region flanked by two gates that open and close to coordinate substrate movements but must never be simultaneously open (26, 27). We have suggested that such gates in CLC transporters are formed by the Gluex and Tyrc residues conserved throughout the family and directly observed in CLC-ec1 crystal structures. The current work, motivated by the dual-mechanism character of the CLC family, aims to transform CLC-ec1, a Cl−/H+ exchanger, into a Cl−-selective channel by forcing both gates open via structure-guided mutagenesis.
Building a Channel by Removing Gates.
How can we know whether we have transformed CLC-ec1 into a Cl− channel? The very definition of a channel mechanism relies on a structural feature: a continuous, ion-occupied pore simultaneously exposed to both aqueous solutions. The crystal structure of A/A satisfies this fundamental criterion. In the absence of molecular structures, however, channels are often indirectly identified by three functional manifestations of pore-mediated ion diffusion that differ from those usually associated with the conformational mechanisms of transporters. First, ions move passively through channels, with no coupling to gradients of other ions. Second, unitary ion transport rates are high, often high enough (>105 s−1) to be detected by single-channel recording. Third, the activation enthalpy of transport, measured from the temperature coefficient of unitary rate, is typically low. Although these criteria do not rigorously distinguish channels from transporters, they serve as empirically reliable signposts of channel-mediated transport.
For the CLC-ec1 mutants here, the first functional criterion, uncoupling, is easily accomplished by removing the carboxylate of Gluex so as to abolish H+ movement (11). Mutants here (E148A) catalyze Cl− flux at ≈20% of the WT rate. We had initially reasoned that the uncoupled Y445A mutant lacking the inner gate alone would also satisfy the second hallmark of a channel: rapid Cl− transport; assuming that the outer gate opens when protonated (11), we expected to observe fast Cl− flux at low pH in Y445A. However, this hope was dashed by the simple fact that Y445A transports Cl− at rates only slightly greater than the WT value (Table S1), even at pH 4.5, where the Gluex outer gate should be open much of the time. Furthermore, we were unable to speed up the rate on this Y445A background >2-fold by substituting Gln, whose side chain adopts the outward-open configuration in the E148Q crystal structure (10). In desperation, therefore, we eliminated both gates by reducing the side-chain volume at Tyrc on a background lacking the outer gate. The resulting doubly ungated mutants A/G, A/A, and A/S display profoundly enhanced Cl− fluxes on the order of 20,000 s−1. With a turnover of 36,000 s−1, the A/S mutant is 3-fold faster than the fastest known transporter, a human Cl−/HCO3− exchanger (28), but ≈20-fold slower than CLC-1, a human CLC of the channel subclass (23). Thus, on the basis of unitary turnover rate, these uncoupled CLC-ec1 mutants occupy a gray area that deters us from asserting that the first two-channel mechanism criteria are unambiguously satisfied. Moreover, the third criterion, low-temperature coefficient of transport, cannot be used here because the temperature coefficient for fully coupled WT protein is already low (A.A. and F.W. unpublished data), as expected if Cl−/H+ exchange involves only small side-chain movements.
The mutants display an additional property expected for diffusion through a narrow pore: a systematic decrease in Cl− flux of single-gate mutants with side-chain volume at each gate position. As with all indirect indicators, such a pattern does not positively support an electrodiffusion mechanism, but this kind of variation naturally harmonizes with a channel picture, where a physical constriction may increase diffusive energy barriers experienced by the permeating ion. Moreover, there is no particular reason why transition rates of a conformational transport cycle should respond to side-chain volume in this way. The pattern is not perfect, however, an exception being the 2-fold higher rate of A/S among the small inner-gate substitutions; this anomaly may be explained away as reflecting the H-bonding or Cl−-coordination tendencies of the serine hydroxyl, which could slightly (<0.5 kcal/mol) lower the energy barrier for Cl− ions passing this narrow point at the inner end of the pathway. An additional departure from a purely steric view is the of the triple mutant S107G on the A/G background to increase Cl− flux beyond that seen in the double mutant. This failure may reflect a rate-determining step at the narrow external opening, or secondary effects of mutants at Ser107, which have not yet been examined closely.
The altered CLC-ec1 transporters described here thus display three functional characteristics pertinent to our experimental intentions: abolition of H+ coupling, Cl− throughput rate higher than that of any known transporter, and graded inhibition with physical size of the gate side chain. Although none of these is alone sufficient to identify the mechanism of Cl− transport as channel-like, the three together provide functional corroboration of the direct structural analysis showing a narrow, Cl−-occupied transmembrane pathway through the doubly ungated protein. We conclude, then, that these mutations transform a CLC Cl−/H+ exchange transporter into Cl− channels. It is not surprising that these are slow compared with familiar channels because the CLC-ec1 Cl− pathway evolved as a transporter rather than for the fast ion throughput required for the electrophysiological functions of channels. We hope eventually to identify the rate-limiting steps in Cl− transport, particularly whether protein breathing plays a part in allowing Cl− to diffuse through the unusually narrow ion pathway apparent in these structures. We note that the distinction between channel and transporter becomes fuzzy if the pore is so narrow that protein dynamics is required to permit diffusive ion movement. Such channel–transporter chimeras were envisioned theoretically by Läuger et al. many years ago (29).
Implications for Mechanisms of Exchange Transport and Channel Gating.
Several mechanistic inferences about both subclasses of CLC proteins, the exchange transporters and the Cl− channels, follow from these results. A fundamental requirement for understanding the workings of any transporter is to identify the regions within the protein that prevent access of the transported solute to aqueous solution in each conformation, inward- or outward-facing. These regions are traditionally called gates. Exchange transporters like CLC-ec1 must have two gates whose conformations are coordinated so that both are never open at the same time, lest a dissipative transmembrane channel is formed. X-ray crystal structures of this protein suggested the locations of gates for Cl− along a transmembrane trajectory (10). In the WT structure, the dehydrated central Cl− ion appears cut off from both extracellular and intracellular solutions at two distinct locales. The carboxyl group of Gluex blocks access to the extracellular side, and the Tyrc side chain appears to do the same on the inside. The function of Gluex as an extracellular gate necessary for Cl−/H+ exchange is well established in CLC-ec1 (10, 11). However, the role of Tyrc as the inner gate of the Cl− pathway has been only indirectly inferred from substitutions at this position, which uncouple Cl− from H+ transport to varying extents by lowering the H+/Cl− transport ratio, as though Cl−“slippage” occurs with smaller side chains (21, 22). The current work establishes Tyrc as an inner-gate residue by showing that it physically blocks off Cl− access to the intracellular solution in a mutant, E148A, in which the inner gate is the only gate present. Combining this conclusion with the known function of Gluex as the extracellular gate, we further infer that the x-ray crystal structure of the WT transporter reflects an “occluded” state in the transport cycle in which both gates are closed around a substrate Cl− ion. Whereas an outward-open state has been observed crystallographically (10), no inward-open state has been reported with an unblocked cytoplasmic pathway; such a state must exist in the transport cycle, however. We view the Y445A substitution here as a rough proxy for the inner-gate opening produced by Tyrc movement in conducting CLC channels. Recent work on CLC-ec1 labeled with fluorescent probes suggests pH-dependent conformational rearrangements occurring near Tyrc (30).
It has been argued that CLC channels arose in evolution as “degraded” CLC transporters in which transmembrane H+ movement is still coupled to the outer gate at Gluex, but the role of Tyrc as an inner gate has been lost (17). However, the doubly ungated mutants described here should not be taken as a model for transporter-to-channel evolution since Gluex and Tyrc are conserved in both channels and transporters. Fast gating in CLC channels involves opening of an extracellular gate formed by a protonated Gluex residue (10). However, because mutations at Tyrc have minimal effects on single-channel conductance (31, 32), we conclude that in open CLC channels, the Tyrc side chain neither blocks off nor interacts strongly with the pore. In other words, the WT CLC-ec1 occluded-state structure, despite its predictive virtues (16, 17, 33), fails to represent the cytoplasmic end of open CLC channels. This transporter, however, might approximate structures of closed channels; if this were the case, CLC channel opening would involve concerted movement of both Gluex and Tyrc, a circumstance consistent with electrophysiological features of drug block of CLC-0 (31, 34).
Examples in Nature of Channelized Transporters.
The present work, aimed at mechanistic metamorphosis of a transporter into a channel, is mirrored by features of two naturally occurring eukaryotic transporters: the cystic fibrosis transmembrane regulator (CFTR) and the Na+/K+ pump. CFTR is a member of the ABC transporter family, whose members hydrolyze ATP to pump solutes across membranes (35); but in this case, the conformational cycle produces a Cl− channel whose gating is driven by ATP hydrolysis as though at some point in the cycle, both gates become simultaneously open, or as if the protein contains only a single gate (36). A similar situation has been thoroughly documented in the Na+/K+ pump, a P-type ATPase universally found in animal plasma membranes (27, 37). A lethal marine toxin specifically converts this ATP-driven transporter into a cation channel by uncoupling the inner and outer gates, keeping the latter mostly open while the former opens and closes in the normal way. The resulting simultaneous opening of both gates at certain points in the conformational cycle thus produces a channel 10,000-fold faster in transporting ions than the intact pump. In both CFTR and the toxin-uncoupled Na+/K+ pump, ion-throughput rates are on the order of 106 s−1, substantially higher than the rates achieved here with CLC-ec1, which are still about an order of magnitude too low for direct single-channel recording.
Methods
Biochemical.
Expression, purification, and liposome-reconstitution of CLC-ec1 were performed as documented in detail (22, 38). Point mutations, introduced by PCR methods, were confirmed by sequencing the entire coding sequence. Proteoliposomes used for Cl− flux assays were formed from a mixture of 20 mg/ml Escherichia coli polar lipid, 4 μg of protein per ml, 300 mM KCl, 35 mM CHAPS, 25 mM citric acid-NaOH (pH 4.5), by overnight dialysis against the same solution without detergent. These ingredients were stored in aliquots at −80°C, and after thawing on the day of the flux assay they were passed through a 400-nm extrusion apparatus to form unilamellar vesicles. In some cases, samples were prepared for electrical recording in planar lipid bilayers, as described (19, 22); all mutants with substitutions at Gluex that were tested in planar bilayers were ideally Cl−-selective, as expected (38).
Unitary Cl− Flux Assay.
Unitary transport rates were determined by a Poisson dilution method (19, 22) with a concentration of protein so low that most of the transporting liposomes contained only a single CLC-ec1 homodimer. Most experiments were done on liposomes made from separate reconstitutions from the same protein preparation, but in some case several protein preparations were also used. Immediately before the Cl− flux assay, a 100-μl sample of liposomes loaded with 300 mM KCl was spun through a 1.5-ml Sephadex column equilibrated with 300 mM potassium isethionate, 1 mM KCl, 25 mM citrate-NaOH (pH 4.5) and diluted into 1.9 ml of this solution in a stirred cell at 25°C. Cl− efflux was initiated by valinomycin/carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) (1 μM each), and the Cl− concentration of the suspension was recorded continuously with a Ag/AgCl electrode; after several minutes, 50 mM octyl glucoside was added to disrupt all of the liposomes and record the total Cl− initially trapped within them. Transport rate was calculated from the initial rate of Cl− release (ions per second), determined in each experiment by calibrating with KCl addition. All fluxes are reported as Cl− ions per second per CLC-ec1 subunit, which yields values 50% of those reported previously because recent results demonstrate that the homodimeric complex contains two transporters, one in each subunit (19).
Supplementary Material
Acknowledgments.
We acknowledge the facilities and staff at GM/CA-CAT beamlines Advanced Photon Source, Argonne National Laboratory, and at beamline X29A, National Synchotron Light Source, Brookhaven National Laboratory.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 3DET).
This article contains supporting information online at www.pnas.org/cgi/content/full/0804503105/DCSupplemental.
References
- 1.Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 2.Gennis RB. Biomembranes: Molecular Structure and Function. New York: Springer; 1989. [Google Scholar]
- 3.Majumdar DS, et al. Single-molecule fret reveals sugar-induced conformational dynamics in Lacy. Proc Natl Acad Sci USA. 2007;104:12640–12645. doi: 10.1073/pnas.0700969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gouaux E, MacKinnon R. Principles of selective ion transport in channels and pumps. Science. 2005;310:1461–1465. doi: 10.1126/science.1113666. [DOI] [PubMed] [Google Scholar]
- 5.Matulef K, Maduke M. The CLC “chloride channel” family: Revelations from prokaryotes. Mol Membr Biol. 2007;24:342–350. doi: 10.1080/09687680701413874. [DOI] [PubMed] [Google Scholar]
- 6.Zifarelli G, Pusch M. CLC chloride channels and transporters: A biophysical and physiological perspective. Rev Physiol Biochem Pharmacol. 2007;158:23–76. doi: 10.1007/112_2006_0605. [DOI] [PubMed] [Google Scholar]
- 7.Maduke M, Pheasant DJ, Miller C. High-level expression, functional reconstitution, and quaternary structure of a prokaryotic CLC-type chloride channel. J Gen Physiol. 1999;114:713–722. doi: 10.1085/jgp.114.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mindell JA, Maduke M, Miller C, Grigorieff N. Projection structure of a CLC-type chloride channel at 6.5-Å resolution. Nature. 2001;409:219–223. doi: 10.1038/35051631. [DOI] [PubMed] [Google Scholar]
- 9.Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a CLC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature. 2002;415:287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- 10.Dutzler R, Campbell EB, MacKinnon R. Gating the selectivity filter in CLC chloride channels. Science. 2003;300:108–112. doi: 10.1126/science.1082708. [DOI] [PubMed] [Google Scholar]
- 11.Accardi A, Miller C. Secondary active transport mediated by a prokaryotic homolog of CLC Cl− channels. Nature. 2004;427:803–807. doi: 10.1038/nature02314. [DOI] [PubMed] [Google Scholar]
- 12.Picollo A, Pusch M. Chloride/proton antiporter activity of mammalian CLC proteins CLC-4 and CLC-5. Nature. 2005;436:420–423. doi: 10.1038/nature03720. [DOI] [PubMed] [Google Scholar]
- 13.Scheel O, Zdebik AA, Lourdel S, Jentsch TJ. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature. 2005;436:424–427. doi: 10.1038/nature03860. [DOI] [PubMed] [Google Scholar]
- 14.DeAngeli A, et al. AtCLCa, a proton/nitrate antiporter, mediates nitrate accumulation in plant vacuoles. Nature. 2006;442:939–942. doi: 10.1038/nature05013. [DOI] [PubMed] [Google Scholar]
- 15.Graves AR, Curran PK, Mindell JA. The Cl−/H+ antiporter CLC-7 is the primary chloride permeation pathway in lysosomes. Nature. 2008;453:788–792. doi: 10.1038/nature06907. [DOI] [PubMed] [Google Scholar]
- 16.Engh AM, Maduke M. Cysteine accessibility in CLC-0 supports conservation of the CLC intracellular vestibule. J Gen Physiol. 2005;125:601–617. doi: 10.1085/jgp.200509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller C. CLC chloride channels viewed through a transporter lens. Nature. 2006;440:484–489. doi: 10.1038/nature04713. [DOI] [PubMed] [Google Scholar]
- 18.Nguitragool W, Miller C. Uncoupling of a CLC Cl−/H+ exchange transporter by polyatomic anions. J Mol Biol. 2006;362:682–690. doi: 10.1016/j.jmb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Nguitragool W, Miller C. CLC Cl−/H+ transporters constrained by covalent cross-linking. Proc Natl Acad Sci USA. 2007;104:20659–20665. doi: 10.1073/pnas.0708639104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Accardi A, et al. Separate ion pathways in a Cl−/H+ exchanger. J Gen Physiol. 2005;126:563–570. doi: 10.1085/jgp.200509417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Accardi A, Lobet S, Williams C, Miller C, Dutzler R. Synergism between halide binding and proton transport in a CLC-type exchanger. J Mol Biol. 2006;362:691–699. doi: 10.1016/j.jmb.2006.07.081. [DOI] [PubMed] [Google Scholar]
- 22.Walden M, et al. Uncoupling and turnover in a Cl−/H+ exchange transporter. J Gen Physiol. 2007;129:317–329. doi: 10.1085/jgp.200709756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saviane C, Conti F, Pusch M. The muscle chloride channel CLC-1 has a double-barreled appearance that is differentially affected in dominant and recessive myotonia. J Gen Physiol. 1999;113:457–468. doi: 10.1085/jgp.113.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smart OS, Neduvelil JG, Wang X, Wallace BA, Sansom MS. Hole: A program for the analysis of the pore dimensions of ion channel structural models. J Mol Graphics. 1996;14:354–360. doi: 10.1016/s0263-7855(97)00009-x. [DOI] [PubMed] [Google Scholar]
- 25.Tilton RR, Kuntz ID, Petsko GA. Cavities in proteins: Structure of a metmyoglobin–xenon complex solved to 1.9 Å. Biochemistry. 1984;23:2849–2857. doi: 10.1021/bi00308a002. [DOI] [PubMed] [Google Scholar]
- 26.Patlak CS. Contributions to the theory of active transport. II. The gate-type noncarrier mechanism and generalizations concerning tracer flow, efficiency, and measurement of energy expenditure. Bull Math Biophys. 1957;19:209–235. [Google Scholar]
- 27.Artigas P, Gadsby DC. Na+/K+-pump ligands modulate gating of palytoxin-induced ion channels. Proc Natl Acad Sci USA. 2003;100:501–505. doi: 10.1073/pnas.0135849100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brahm J. Temperature-dependent changes of chloride transport kinetics in human red cells. J Gen Physiol. 1977;70:283–306. doi: 10.1085/jgp.70.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Läuger P, Stephan W, Frehland E. Fluctuations of barrier structure in ionic channels. Biochim Biophys Acta. 1980;602:167–180. doi: 10.1016/0005-2736(80)90299-0. [DOI] [PubMed] [Google Scholar]
- 30.Bell SP, Curran PK, Choi S, Mindell JA. Site-directed fluorescence studies of a prokaryotic CLC antiporter. Biochemistry. 2006;45:6773–6782. doi: 10.1021/bi0523815. [DOI] [PubMed] [Google Scholar]
- 31.Accardi A, Pusch M. Conformational changes in the pore of CLC-0. J Gen Physiol. 2003;122:277–294. doi: 10.1085/jgp.200308834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen MF, Chen TY. Side-chain charge effects and conductance determinants in the pore of CLC-0 chloride channels. J Gen Physiol. 2003;122:133–145. doi: 10.1085/jgp.200308844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin CW, Chen TY. Probing the pore of CLC-0 by substituted cysteine accessibility method using methane thiosulfonate reagents. J Gen Physiol. 2003;122:147–159. doi: 10.1085/jgp.200308845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Traverso S, Elia L, Pusch M. Gating competence of constitutively open CLC-0 mutants revealed by the interaction with a small organic inhibitor. J Gen Physiol. 2003;122:295–306. doi: 10.1085/jgp.200308784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hollenstein K, Dawson RJ, Locher KP. Structure and mechanism of ABC transporter proteins. Curr Opin Struct Biol. 2007;17:412–418. doi: 10.1016/j.sbi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Baukrowitz T, Hwang T-C, Nairn AC, Gadsby DC. Coupling of CFTR Cl− channel gating to an ATP hydrolysis cycle. Neuron. 1994;12:473–482. doi: 10.1016/0896-6273(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 37.Reyes N, Gadsby DC. Ion permeation through the Na+,K+-ATPase. Nature. 2006;443:470–474. doi: 10.1038/nature05129. [DOI] [PubMed] [Google Scholar]
- 38.Accardi A, Kolmakova-Partensky L, Williams C, Miller C. Ionic currents mediated by a prokaryotic homolog of CLC Cl− channels. J Gen Physiol. 2004;123:109–119. doi: 10.1085/jgp.200308935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.